From iPSCs to Pancreatic β Cells: Unveiling Molecular Pathways and Enhancements with Vitamin C and Retinoic Acid in Diabetes Research
Abstract
:1. Introduction
2. Results
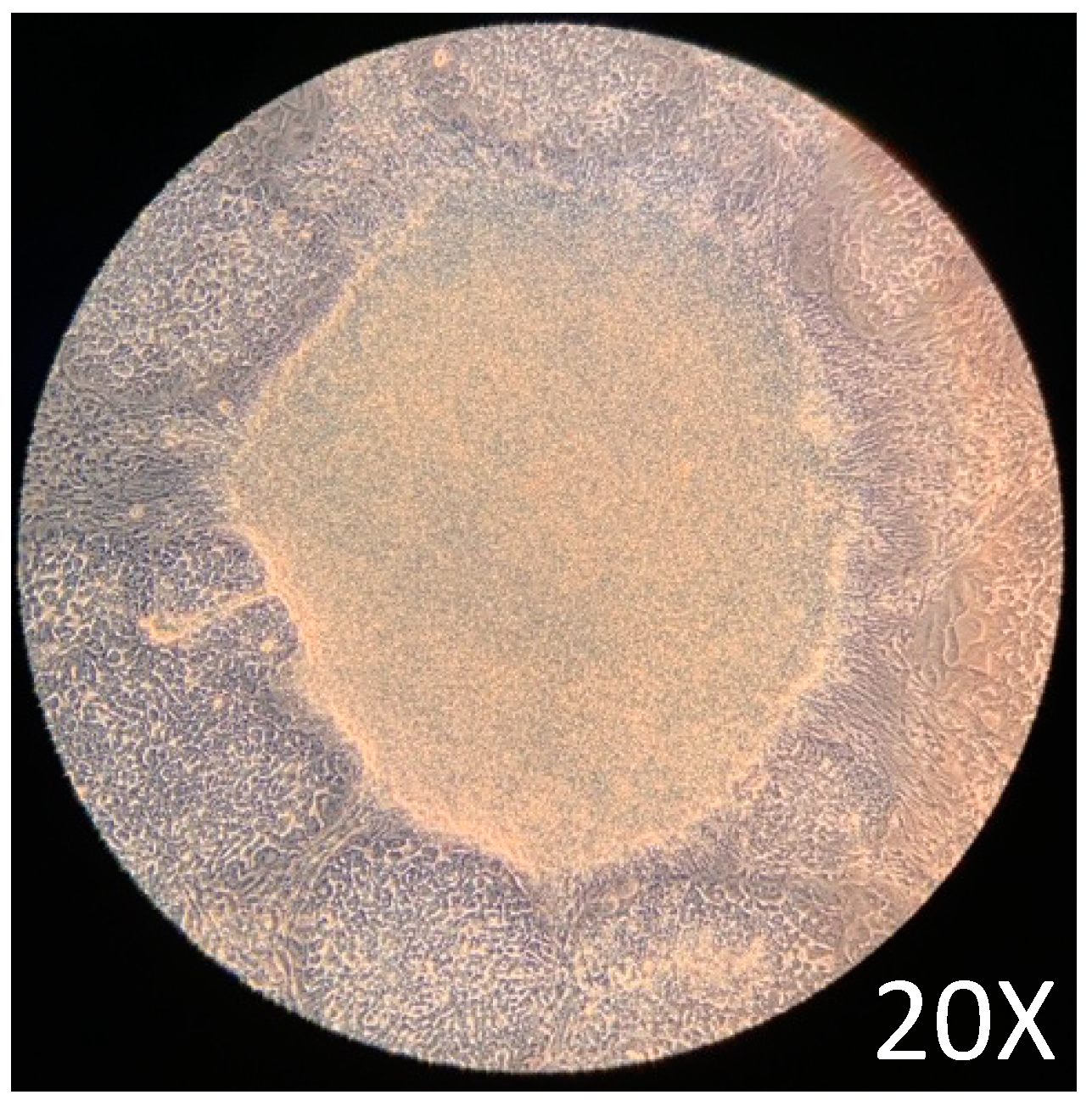
2.1. iPSCs Growth and Maintenance
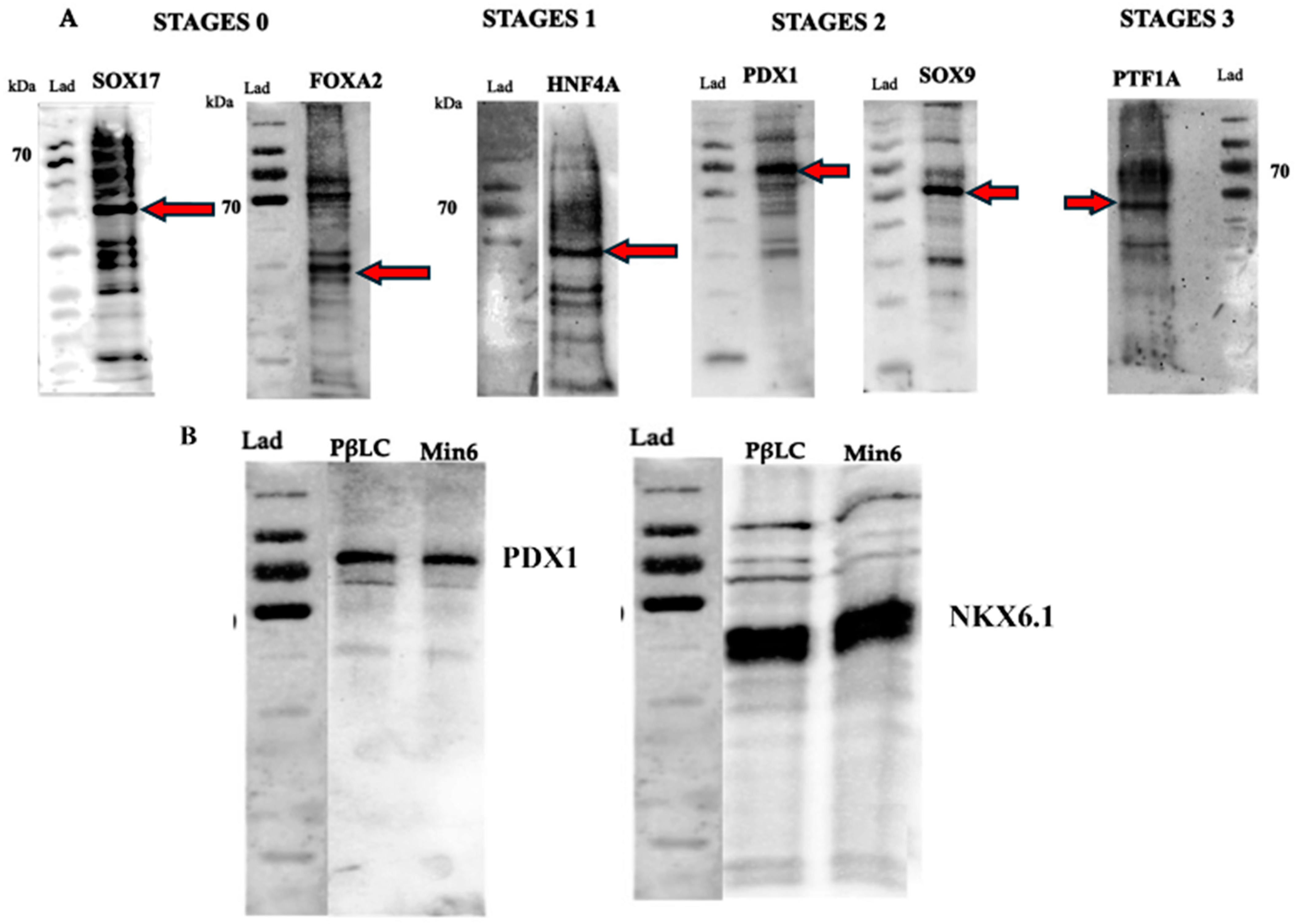
2.2. Differentiation of Definitive Endoderm from iPSCs
2.3. Differentiation of Definitive Endoderm into Pancreatic Progenitors
2.4. Differentiation of Pancreatic Progenitors into Pancreatic Beta-like Cells
2.5. Glucose-Stimulate Insulin Secretion
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. iPSC Culture and Maintenance
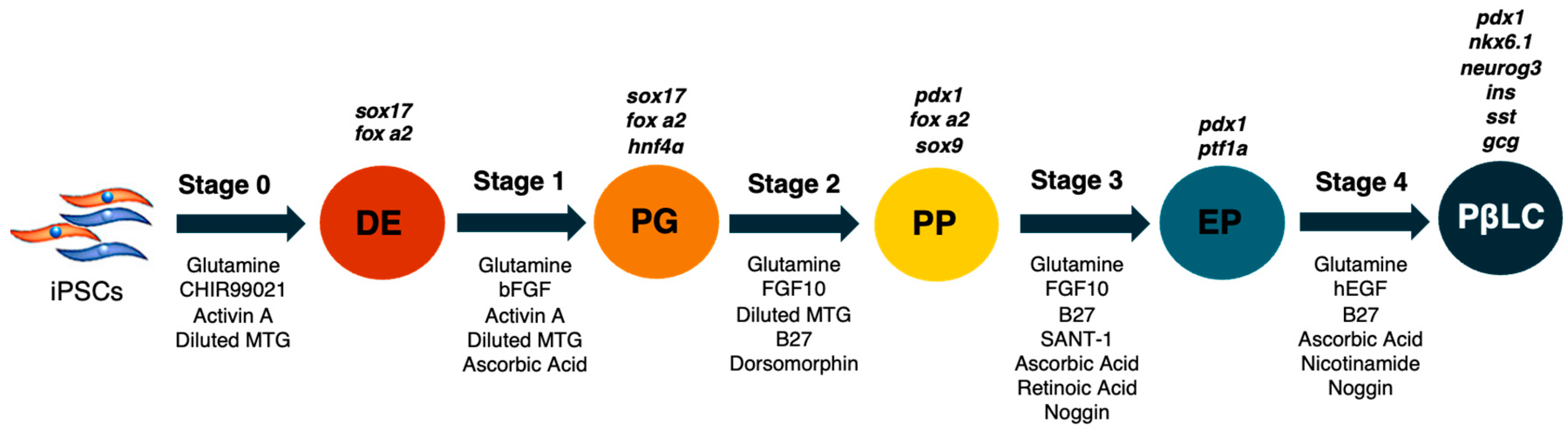
4.3. Differentiation of iPSCs
- (1)
- (2)
- Day 0: Stage 0 medium (S0) was supplemented with glutamine (1%), CHIR99021 (2 µM, activin A (100 ng/mL), and diluted MTG (3 µL/mL). The cells were incubated with an S0 medium for 24 h.
- (3)
- Days 1–2: The S1 medium was removed, and the cells were washed twice with DPBS w/o Ca++ and Mg++. S1 medium was supplemented with glutamine (1%), activin A (100 ng/mL), diluted MTG (3 µL/mL), heat-stable recombinant human basic fibroblast growth factor (bFGF) (5 ng/mL), and ascorbic acid (50 µg/mL). The medium was changed each day during stage 1.
- (4)
- Days 3–5: The S2 medium was removed, and the cells were rewashed with DPBS w/o Ca++ and Mg++ twice. S2 medium was supplemented with glutamine (1%), diluted MTG (3 µL/mL), dorsomorphin (0.75 µM), human fibroblastic growth factor-10 (FGF10) (50 ng/mL), and B27 supplement minus vitamin A (1%). Subsequently, the cells were incubated with the S2 medium, and the medium was changed each day during stage 2.
- (5)
- Days 6–7: The S3 medium was removed, and the cells were washed twice. S3 medium was supplemented with glutamine (1%), ascorbic acid (50 µg/mL), FGF10 (50 ng/mL), B27 (1%), SANT-1 (0,25 µM), retinoic acid (2 µM) and recombinant human noggin (Noggin) (50 ng/mL). Cells during stage 3 were incubated at 37 °C, 5% and CO2, and the medium was changed daily.
- (6)
- Finally, on days 8–12, the cells were cultivated with S4 medium supplemented with glutamine (1%), ascorbic acid (50 µg/mL), B27 (1%), Noggin (50 ng/mL), recombinant human epidermal growth factor (hEGF) (100 ng/mL), retinoic acid (1 µM), and nicotinamide (10 mM).
4.4. Western Blot
4.5. Total RNA Extraction RT-PCR and Quantitative PCR (qPCR)
4.6. Glucose Stimulation Insulin Secretion (GSIS)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arroyave, F.; Montano, D.; Lizcano, F. Diabetes Mellitus Is a Chronic Disease that Can Benefit from Therapy with Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2020, 21, 8685. [Google Scholar] [CrossRef]
- Saadi, H.; Nagelkerke, N.; Carruthers, S.G.; Benedict, S.; Abdulkhalek, S.; Reed, R.; Lukic, M.; Nicholls, M.G. Association of TCF7L2 polymorphism with diabetes mellitus, metabolic syndrome, and markers of beta cell function and insulin resistance in a population-based sample of Emirati subjects. Diabetes Res. Clin. Pract. 2008, 80, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besancon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef]
- Zinman, B.; Skyler, J.S.; Riddle, M.C.; Ferrannini, E. Diabetes Research and Care Through the Ages. Diabetes Care 2017, 40, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, N.; Sahay, R.; Kalra, S.; Bajaj, S.; Dasgupta, A.; Shrestha, D.; Dhakal, G.; Tiwaskar, M.; Sahay, M.; Somasundaram, N.; et al. Consensus on Medical Nutrition Therapy for Diabesity (CoMeND) in Adults: A South Asian Perspective. Diabetes Metab. Syndr. Obes. 2021, 14, 1703–1728. [Google Scholar] [CrossRef]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Boland, B.B.; Rhodes, C.J.; Grimsby, J.S. The dynamic plasticity of insulin production in beta-cells. Mol. Metab. 2017, 6, 958–973. [Google Scholar] [CrossRef]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Fonseca, V.A.; et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2019 Executive Summary. Endocr. Pract. 2019, 25, 69–100. [Google Scholar] [CrossRef]
- Phillip, M.; Bergenstal, R.M.; Close, K.L.; Danne, T.; Garg, S.K.; Heinemann, L.; Hirsch, I.B.; Kovatchev, B.P.; Laffel, L.M.; Mohan, V.; et al. The Digital/Virtual Diabetes Clinic: The Future Is Now-Recommendations from an International Panel on Diabetes Digital Technologies Introduction. Diabetes Technol. Ther. 2021, 23, 146–154. [Google Scholar] [CrossRef]
- Isobe, K.; Cheng, Z.; Nishio, N.; Suganya, T.; Tanaka, Y.; Ito, S. iPSCs, aging and age-related diseases. New Biotechnol. 2014, 31, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Stirban, A.O.; Tschoepe, D. Cardiovascular complications in diabetes: Targets and interventions. Diabetes Care 2008, 31 (Suppl. S2), S215–S221. [Google Scholar] [CrossRef] [PubMed]
- Suchy, F.; Yamaguchi, T.; Nakauchi, H. iPSC-Derived Organs In Vivo: Challenges and Promise. Cell Stem Cell 2018, 22, 21–24. [Google Scholar] [CrossRef]
- Rickels, M.R.; Robertson, R.P. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr. Rev. 2019, 40, 631–668. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liang, Z.; Wang, S.; Sun, D.; Wang, X.; Liew, S.Y.; Lu, S.; Wu, S.; Jiang, Y.; Wang, Y.; et al. Human pluripotent stem-cell-derived islets ameliorate diabetes in non-human primates. Nat. Med. 2022, 28, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Melton, D.A. Pancreas regeneration. Nature 2018, 557, 351–358. [Google Scholar] [CrossRef]
- Ma, H.; Wert, K.J.; Shvartsman, D.; Melton, D.A.; Jaenisch, R. Establishment of human pluripotent stem cell-derived pancreatic beta-like cells in the mouse pancreas. Proc. Natl. Acad. Sci. USA 2018, 115, 3924–3929. [Google Scholar] [CrossRef]
- Apostolou, E.; Stadtfeld, M. Cellular trajectories and molecular mechanisms of iPSC reprogramming. Curr. Opin. Genet. Dev. 2018, 52, 77–85. [Google Scholar] [CrossRef]
- Malik, N.; Rao, M.S. A review of the methods for human iPSC derivation. Methods Mol. Biol. 2013, 997, 23–33. [Google Scholar] [CrossRef]
- Pagliuca, F.W.; Millman, J.R.; Gurtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic beta cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef]
- Korytnikov, R.; Nostro, M.C. Generation of polyhormonal and multipotent pancreatic progenitor lineages from human pluripotent stem cells. Methods 2016, 101, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Leite, N.C.; Sintov, E.; Meissner, T.B.; Brehm, M.A.; Greiner, D.L.; Harlan, D.M.; Melton, D.A. Modeling Type 1 Diabetes In Vitro Using Human Pluripotent Stem Cells. Cell Rep. 2020, 32, 107894. [Google Scholar] [CrossRef]
- Sim, E.Z.; Shiraki, N.; Kume, S. Recent progress in pancreatic islet cell therapy. Inflamm. Regen. 2021, 41, 1. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, A.; Mizuno, K.; Tsuda, K.; Yamamoto, T.; Michiue, T. A simple method of hiPSCs differentiation into insulin-producing cells is improved with vitamin C and RepSox. PLoS ONE 2021, 16, e0254373. [Google Scholar] [CrossRef] [PubMed]
- Lorberbaum, D.S.; Kishore, S.; Rosselot, C.; Sarbaugh, D.; Brooks, E.P.; Aragon, E.; Xuan, S.; Simon, O.; Ghosh, D.; Mendelsohn, C.; et al. Retinoic acid signaling within pancreatic endocrine progenitors regulates mouse and human beta cell specification. Development 2020, 147, dev189977. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Hale, C.; Mukhopadhyay, S. A Simple Multistep Protocol for Differentiating Human Induced Pluripotent Stem Cells into Functional Macrophages. Methods Mol. Biol. 2018, 1784, 13–28. [Google Scholar] [CrossRef]
- Chandrasekaran, V.; Carta, G.; da Costa Pereira, D.; Gupta, R.; Murphy, C.; Feifel, E.; Kern, G.; Lechner, J.; Cavallo, A.L.; Gupta, S.; et al. Generation and characterization of iPSC-derived renal proximal tubule-like cells with extended stability. Sci. Rep. 2021, 11, 11575. [Google Scholar] [CrossRef]
- Kunisada, Y.; Tsubooka-Yamazoe, N.; Shoji, M.; Hosoya, M. Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells. Stem Cell Res. 2012, 8, 274–284. [Google Scholar] [CrossRef]
- Kroon, E.; Martinson, L.A.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.; Smart, N.G.; Cunningham, J.; et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008, 26, 443–452. [Google Scholar] [CrossRef]
- Andreasson, L.; Evenbratt, H.; Mobini, R.; Simonsson, S. Differentiation of induced pluripotent stem cells into definitive endoderm on Activin A-functionalized gradient surfaces. J. Biotechnol. 2021, 325, 173–178. [Google Scholar] [CrossRef]
- Millman, J.R.; Xie, C.; Van Dervort, A.; Gurtler, M.; Pagliuca, F.W.; Melton, D.A. Generation of stem cell-derived beta-cells from patients with type 1 diabetes. Nat. Commun. 2016, 7, 11463. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, K.A.; Bang, A.G.; Eliazer, S.; Kelly, O.G.; Agulnick, A.D.; Smart, N.G.; Moorman, M.A.; Kroon, E.; Carpenter, M.K.; Baetge, E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006, 24, 1392–1401. [Google Scholar] [CrossRef]
- Cuesta-Gomez, N.; Verhoeff, K.; Jasra, I.T.; Pawlick, R.; Dadheech, N.; Shapiro, A.M.J. Characterization of stem-cell-derived islets during differentiation and after implantation. Cell Rep. 2022, 40, 111238. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, K.L.; Marikawa, Y. Regulation of developmental competence and commitment towards the definitive endoderm lineage in human embryonic stem cells. Stem Cell Res. 2013, 10, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chen, C.; Randolph, L.N.; Ye, S.; Zhang, X.; Bao, X.; Lian, X.L. Generation of pancreatic progenitors from human pluripotent stem cells by small molecules. Stem Cell Rep. 2021, 16, 2395–2409. [Google Scholar] [CrossRef]
- Payne, C.; King, J.; Hay, D. The role of activin/nodal and Wnt signaling in endoderm formation. Vitam. Horm. 2011, 85, 207–216. [Google Scholar] [CrossRef]
- Ndlovu, R.; Deng, L.C.; Wu, J.; Li, X.K.; Zhang, J.S. Fibroblast Growth Factor 10 in Pancreas Development and Pancreatic Cancer. Front. Genet. 2018, 9, 482. [Google Scholar] [CrossRef]
- Watson, J.; Francavilla, C. Regulation of FGF10 Signaling in Development and Disease. Front. Genet. 2018, 9, 500. [Google Scholar] [CrossRef]
- Jin, W.; Jiang, W. Stepwise differentiation of functional pancreatic beta cells from human pluripotent stem cells. Cell Regen. 2022, 11, 24. [Google Scholar] [CrossRef]
- Diedisheim, M.; Oshima, M.; Albagli, O.; Huldt, C.W.; Ahlstedt, I.; Clausen, M.; Menon, S.; Aivazidis, A.; Andreasson, A.C.; Haynes, W.G.; et al. Modeling human pancreatic beta cell dedifferentiation. Mol. Metab. 2018, 10, 74–86. [Google Scholar] [CrossRef]
- Jennings, R.E.; Berry, A.A.; Gerrard, D.T.; Wearne, S.J.; Strutt, J.; Withey, S.; Chhatriwala, M.; Piper Hanley, K.; Vallier, L.; Bobola, N.; et al. Laser Capture and Deep Sequencing Reveals the Transcriptomic Programmes Regulating the Onset of Pancreas and Liver Differentiation in Human Embryos. Stem Cell Rep. 2017, 9, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.C.; Alves, T.C.; Helman, A.; Chen, J.C.; Kenty, J.H.; Cardone, R.L.; Liu, D.R.; Kibbey, R.G.; Melton, D.A. Glucose Response by Stem Cell-Derived beta Cells In Vitro Is Inhibited by a Bottleneck in Glycolysis. Cell Rep. 2020, 31, 107623. [Google Scholar] [CrossRef]
- Thakur, G.; Lee, H.J.; Jeon, R.H.; Lee, S.L.; Rho, G.J. Small Molecule-Induced Pancreatic beta-Like Cell Development: Mechanistic Approaches and Available Strategies. Int. J. Mol. Sci. 2020, 21, 2388. [Google Scholar] [CrossRef] [PubMed]
- Russ, H.A.; Parent, A.V.; Ringler, J.J.; Hennings, T.G.; Nair, G.G.; Shveygert, M.; Guo, T.; Puri, S.; Haataja, L.; Cirulli, V.; et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015, 34, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Bastidas-Ponce, A.; Roscioni, S.S.; Burtscher, I.; Bader, E.; Sterr, M.; Bakhti, M.; Lickert, H. Foxa2 and Pdx1 cooperatively regulate postnatal maturation of pancreatic beta-cells. Mol. Metab. 2017, 6, 524–534. [Google Scholar] [CrossRef]
- Bastidas-Ponce, A.; Scheibner, K.; Lickert, H.; Bakhti, M. Cellular and molecular mechanisms coordinating pancreas development. Development 2017, 144, 2873–2888. [Google Scholar] [CrossRef]
- Aydin, S.; Sagrac, D.; Sahin, F. Differentiation Potential of Mesenchymal Stem Cells into Pancreatic beta-Cells. Adv. Exp. Med. Biol. 2020, 1247, 135–156. [Google Scholar] [CrossRef]
- Hashemitabar, M.; Heidari, E. Redefining the signaling pathways from pluripotency to pancreas development: In vitro beta-cell differentiation. J. Cell Physiol. 2019, 234, 7811–7827. [Google Scholar] [CrossRef]
- Vegas, A.J.; Veiseh, O.; Gurtler, M.; Millman, J.R.; Pagliuca, F.W.; Bader, A.R.; Doloff, J.C.; Li, J.; Chen, M.; Olejnik, K.; et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat. Med. 2016, 22, 306–311. [Google Scholar] [CrossRef]
- Marotta, D.; Rao, C.; Fossati, V. Human Induced Pluripotent Stem Cell (iPSC) Handling Protocols: Maintenance, Expansion, and Cryopreservation. Methods Mol. Biol. 2022, 2454, 1–15. [Google Scholar] [CrossRef]
- Kim, E.; Hyun, S.H. Apoptosis in Porcine Pluripotent Cells: From ICM to iPSCs. Int. J. Mol. Sci. 2016, 17, 1533. [Google Scholar] [CrossRef] [PubMed]
- Sola, S.; Morgado, A.L.; Rodrigues, C.M. Death receptors and mitochondria: Two prime triggers of neural apoptosis and differentiation. Biochim. Biophys. Acta 2013, 1830, 2160–2166. [Google Scholar] [CrossRef] [PubMed]
- Demine, S.; Schiavo, A.A.; Marin-Canas, S.; Marchetti, P.; Cnop, M.; Eizirik, D.L. Pro-inflammatory cytokines induce cell death, inflammatory responses, and endoplasmic reticulum stress in human iPSC-derived beta cells. Stem Cell Res. Ther. 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Naujok, O.; Diekmann, U.; Lenzen, S. The generation of definitive endoderm from human embryonic stem cells is initially independent from activin A but requires canonical Wnt-signaling. Stem Cell Rev. Rep. 2014, 10, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Yabe, S.G.; Fukuda, S.; Takeda, F.; Nashiro, K.; Shimoda, M.; Okochi, H. Efficient generation of functional pancreatic beta-cells from human induced pluripotent stem cells. J. Diabetes 2017, 9, 168–179. [Google Scholar] [CrossRef]
- Sui, L.; Bouwens, L.; Mfopou, J.K. Signaling pathways during maintenance and definitive endoderm differentiation of embryonic stem cells. Int. J. Dev. Biol. 2013, 57, 1–12. [Google Scholar] [CrossRef]
- Maehr, R.; Chen, S.; Snitow, M.; Ludwig, T.; Yagasaki, L.; Goland, R.; Leibel, R.L.; Melton, D.A. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc. Natl. Acad. Sci. USA 2009, 106, 15768–15773. [Google Scholar] [CrossRef]
- Huang, H.; Bader, T.N.; Jin, S. Signaling Molecules Regulating Pancreatic Endocrine Development from Pluripotent Stem Cell Differentiation. Int. J. Mol. Sci. 2020, 21, 5867. [Google Scholar] [CrossRef]
- Salisbury, R.J.; Blaylock, J.; Berry, A.A.; Jennings, R.E.; De Krijger, R.; Piper Hanley, K.; Hanley, N.A. The window period of NEUROGENIN3 during human gestation. Islets 2014, 6, e954436. [Google Scholar] [CrossRef]
- Hussein, S.M.; Batada, N.N.; Vuoristo, S.; Ching, R.W.; Autio, R.; Narva, E.; Ng, S.; Sourour, M.; Hamalainen, R.; Olsson, C.; et al. Copy number variation and selection during reprogramming to pluripotency. Nature 2011, 471, 58–62. [Google Scholar] [CrossRef]
- Bray, S.J. Notch signalling in context. Nat. Rev. Mol. Cell Biol. 2016, 17, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Apelqvist, A.; Li, H.; Sommer, L.; Beatus, P.; Anderson, D.J.; Honjo, T.; Hrabe de Angelis, M.; Lendahl, U.; Edlund, H. Notch signalling controls pancreatic cell differentiation. Nature 1999, 400, 877–881. [Google Scholar] [CrossRef]
- Hart, A.; Papadopoulou, S.; Edlund, H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev. Dyn. 2003, 228, 185–193. [Google Scholar] [CrossRef]
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.S.; Li, X. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Browning, V.L.; Odorico, J.S. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech. Dev. 2011, 128, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Apelqvist, A.; Ahlgren, U.; Edlund, H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr. Biol. 1997, 7, 801–804. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, X.; Zhu, S. Recent advances and potential applications of human pluripotent stem cell-derived pancreatic beta cells. Acta Biochim. Biophys. Sin. 2020, 52, 708–715. [Google Scholar] [CrossRef]
- Serup, P. Signaling pathways regulating murine pancreatic development. Semin. Cell Dev. Biol. 2012, 23, 663–672. [Google Scholar] [CrossRef]
- Martin, M.; Gallego-Llamas, J.; Ribes, V.; Kedinger, M.; Niederreither, K.; Chambon, P.; Dolle, P.; Gradwohl, G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev. Biol. 2005, 284, 399–411. [Google Scholar] [CrossRef]
- Tulachan, S.S.; Doi, R.; Kawaguchi, Y.; Tsuji, S.; Nakajima, S.; Masui, T.; Koizumi, M.; Toyoda, E.; Mori, T.; Ito, D.; et al. All-trans retinoic acid induces differentiation of ducts and endocrine cells by mesenchymal/epithelial interactions in embryonic pancreas. Diabetes 2003, 52, 76–84. [Google Scholar] [CrossRef]
- Shen, C.N.; Marguerie, A.; Chien, C.Y.; Dickson, C.; Slack, J.M.; Tosh, D. All-trans retinoic acid suppresses exocrine differentiation and branching morphogenesis in the embryonic pancreas. Differentiation 2007, 75, 62–74. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, W.; Liu, M.; Sui, X.; Yin, X.; Chen, S.; Shi, Y.; Deng, H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009, 19, 429–438. [Google Scholar] [CrossRef]
- Meng, Y.; Ren, Z.; Xu, F.; Zhou, X.; Song, C.; Wang, V.Y.; Liu, W.; Lu, L.; Thomson, J.A.; Chen, G. Nicotinamide Promotes Cell Survival and Differentiation as Kinase Inhibitor in Human Pluripotent Stem Cells. Stem Cell Rep. 2018, 11, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Otonkoski, T.; Beattie, G.M.; Mally, M.I.; Ricordi, C.; Hayek, A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J. Clin. Investig. 1993, 92, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Aigha, I.I.; Abdelalim, E.M. NKX6.1 transcription factor: A crucial regulator of pancreatic beta cell development, identity, and proliferation. Stem Cell Res. Ther. 2020, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Nostro, M.C.; Sarangi, F.; Yang, C.; Holland, A.; Elefanty, A.G.; Stanley, E.G.; Greiner, D.L.; Keller, G. Efficient generation of NKX6-1+ pancreatic progenitors from multiple human pluripotent stem cell lines. Stem Cell Rep. 2015, 4, 591–604. [Google Scholar] [CrossRef]
- Veres, A.; Faust, A.L.; Bushnell, H.L.; Engquist, E.N.; Kenty, J.H.; Harb, G.; Poh, Y.C.; Sintov, E.; Gurtler, M.; Pagliuca, F.W.; et al. Charting cellular identity during human in vitro beta-cell differentiation. Nature 2019, 569, 368–373. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, C.C. Vitamin C’s essential role in DNA and histone demethylation and a preclinical rationale for its therapeutic high-dose potential in renal cell carcinoma. Ann. Transl. Med. 2019, 7, S117. [Google Scholar] [CrossRef]
- Lee Chong, T.; Ahearn, E.L.; Cimmino, L. Reprogramming the Epigenome With Vitamin C. Front. Cell Dev. Biol. 2019, 7, 128. [Google Scholar] [CrossRef]
- Bergsten, P.; Moura, A.S.; Atwater, I.; Levine, M. Ascorbic acid and insulin secretion in pancreatic islets. J. Biol. Chem. 1994, 269, 1041–1045. [Google Scholar] [CrossRef]
- Agrawal, A.; Narayan, G.; Gogoi, R.; Thummer, R.P. Recent Advances in the Generation of beta-Cells from Induced Pluripotent Stem Cells as a Potential Cure for Diabetes Mellitus. Adv. Exp. Med. Biol. 2021, 1347, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Diaz, F.L.; Osorio-Quintero, C.; Diaz-Miranda, M.A.; Kishore, S.; Leavens, K.; Jobaliya, C.; Stanescu, D.; Ortiz-Gonzalez, X.; Yoon, C.; Chen, C.S.; et al. Modeling Monogenic Diabetes using Human ESCs Reveals Developmental and Metabolic Deficiencies Caused by Mutations in HNF1A. Cell Stem Cell 2019, 25, 273–289.e5. [Google Scholar] [CrossRef] [PubMed]
- Rovira, M.; Huang, W.; Yusuff, S.; Shim, J.S.; Ferrante, A.A.; Liu, J.O.; Parsons, M.J. Chemical screen identifies FDA-approved drugs and target pathways that induce precocious pancreatic endocrine differentiation. Proc. Natl. Acad. Sci. USA 2011, 108, 19264–19269. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, M.; Loffler, K.A.; Edfalk, S.; Selander, L.; Dahl, U.; Ricordi, C.; Jeon, J.; Correa-Medina, M.; Diez, J.; Edlund, H. Retinoic acid promotes the generation of pancreatic endocrine progenitor cells and their further differentiation into beta-cells. PLoS ONE 2008, 3, e2841. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Joo, J.H.; Oh, J.Y.; Seo, E.H.; Kim, Y.H.; Jun, E.; Shim, I.K.; Kim, S.C. Continuous Inhibition of Sonic Hedgehig Signaling Leads to Differentiation of Human-Induced Pluripotent Cells into Functional Insulin-Producring b Cells. Stem Cells Int. 2021, 2021, 6681257. [Google Scholar] [CrossRef]
- Pellegrini, S.; Zamarian, V.; Sordi, V. Strategies to Improve the Safety of iPSC-Derived beta Cells for beta Cell Replacement in Diabetes. Transpl. Int. 2022, 35, 10575. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef]




| Name | Forward | Reverse |
|---|---|---|
| foxA2 | GGAACACCACTACGCCTTCAAC | AGTGCATCACCTGTTCGTAGGC |
| sox17 | ACGCTTTCATGGTGTGGGCTAAG | GTCAGCGCCTTCCACGACTTG |
| hnf4A | GGTGTCCATACGCATCCTTGAC | AGCCGCTTGATCTTCCCTGGAT |
| pdx1 | GAAGTCTACCAAAGCTCACGCG | GGAACTCCTTCTCCAGCTCTAG |
| sox9 | AGGAAGCTCGCGGACCAGTAC | GGTGGTCCTTCTTGTGCTGCAC |
| ptf1A | GAAGGTCATCATCTGCCATCGG | CCTTGAGTTGTTTTTCATCAGTCC |
| nkx6.1 | CCTATTCGTTGGGGATGACAGAG | TCTGTCTCCGAGTCCTGCTTCT |
| ngn3 | CCTAAGAGCGAGTTGGCACTGA | AGTGCCGAGTTGAGGTTGTGCA |
| ins | ACGAGGCTTCTTCTACACACCC | TCCACAATGCCACGCTTCTGCA |
| gcg | CGTTCCCTTCAAGACACAGAGG | ACGCCTGGAGTCCAGATACTTG |
| sst | CCAGACTCCGTCAGTTTCTGCA | TTCCAGGGCATCATTCTCCGTC |
| actB | CACCATTGGCAATGAGCGGTTC | AGGTCTTTGCGGATGTCCACGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroyave, F.; Uscátegui, Y.; Lizcano, F. From iPSCs to Pancreatic β Cells: Unveiling Molecular Pathways and Enhancements with Vitamin C and Retinoic Acid in Diabetes Research. Int. J. Mol. Sci. 2024, 25, 9654. https://doi.org/10.3390/ijms25179654
Arroyave F, Uscátegui Y, Lizcano F. From iPSCs to Pancreatic β Cells: Unveiling Molecular Pathways and Enhancements with Vitamin C and Retinoic Acid in Diabetes Research. International Journal of Molecular Sciences. 2024; 25(17):9654. https://doi.org/10.3390/ijms25179654
Chicago/Turabian StyleArroyave, Felipe, Yomaira Uscátegui, and Fernando Lizcano. 2024. "From iPSCs to Pancreatic β Cells: Unveiling Molecular Pathways and Enhancements with Vitamin C and Retinoic Acid in Diabetes Research" International Journal of Molecular Sciences 25, no. 17: 9654. https://doi.org/10.3390/ijms25179654








