Abstract
Predictive biomarkers for immune checkpoint inhibitors (ICIs) in solid tumors such as melanoma, hepatocellular carcinoma (HCC), colorectal cancer (CRC), non-small cell lung cancer (NSCLC), endometrial carcinoma, renal cell carcinoma (RCC), or urothelial carcinoma (UC) include programmed cell death ligand 1 (PD-L1) expression, tumor mutational burden (TMB), defective deoxyribonucleic acid (DNA) mismatch repair (dMMR), microsatellite instability (MSI), and the tumor microenvironment (TME). Over the past decade, several types of ICIs, including cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors, anti-programmed cell death 1 (PD-1) antibodies, anti-programmed cell death ligand 1 (PD-L1) antibodies, and anti-lymphocyte activation gene-3 (LAG-3) antibodies have been studied and approved by the Food and Drug Administration (FDA), with ongoing research on others. Recent studies highlight the critical role of the gut microbiome in influencing a positive therapeutic response to ICIs, emphasizing the importance of modeling factors that can maintain a healthy microbiome. However, resistance mechanisms can emerge, such as increased expression of alternative immune checkpoints, T-cell immunoglobulin (Ig), mucin domain-containing protein 3 (TIM-3), LAG-3, impaired antigen presentation, and alterations in the TME. This review aims to synthesize the data regarding the interactions between microbiota and immunotherapy (IT). Understanding these mechanisms is essential for optimizing ICI therapy and developing effective combination strategies.
1. Introduction
ICIs offer a groundbreaking approach to treating various malignant solid tumors, contrasting with traditional cytotoxic therapies []. As immune cell surface receptors, ICIs have the capacity to either activate or inhibit the immune response. In the context of IT, they function as blockers of T-lymphocyte cell surface receptors, thereby enhancing the antitumor immune response [].
For a long time, the progress in IT has been eclipsed by the rapid advancements in radiotherapy (RT) and chemotherapy [,]. However, recent research focused on cancer immunology and IT has enhanced our understanding of the antitumor response of T cells, as well as their interactions with cancer cells, antigen-presenting cells (APCs), immunosuppressive cells, and other immune mechanisms [,]. Studies by Bretcher and Cohn and Jenkins and Schwartz highlight that T-cell activation occurs via the T-cell receptor (TCR), which recognizes specific antigens, and through the costimulatory interaction between a T-cell costimulatory receptor and its ligand on an APC [,]. The inhibitory signals in T cells ensure tolerance to self-antigens, while the stimulatory signals promote an adaptive immune response to foreign antigens, collectively regulating immune activation []. Immune checkpoints allow inhibitory stimuli to modulate the inflammatory response following T-cell activation and protect normal cells from T-cell-mediated cytotoxicity []. This mechanism is crucial as it prevents the T-cell immune response from targeting cancer cells within the TME, serving as a protective measure [].
Over the past decade, several types of ICIs have been studied and approved by the FDA. The first of these was the CTLA-4 inhibitor, Ipilimumab, approved in 2011 for metastatic malignant melanoma []. CTLA-4 exerts a negative regulatory effect on T cells, and its blockade can inhibit tumor formation, making it useful in cancer IT []. Additionally, the FDA has approved three anti-PD-1 antibodies—Pembrolizumab, Nivolumab, and Cemiplimab—and three anti-PD-L1 antibodies—Atezolizumab, Durvalumab, and Avelumab []. PD-1 and PD-L1 induce T-cell exhaustion, and blocking these pathways has been shown to enhance tumor-specific T-cell responses and promote tumor regression []. Ongoing research is exploring other potential ICI targets, including inhibitory receptors like TIM-3, V-domain Ig suppressor of T-cell activation (VISTA), and LAG-3, as well as activating molecules such as OX40 (CD134) and glucocorticoid-induced TNFR-related protein (GITR) []. However, studies have yielded mixed results, with some patients experiencing sustained therapeutic responses and others showing diminished responses over time []. This variability has highlighted the need to identify molecular biomarkers to predict treatment response and resistance mechanisms to checkpoint inhibitors, which will be discussed further in this article.
Malignant cells possess immunosuppressive properties, such as the expression of PD-L1 and the secretion of suppressive cytokines, which help them reduce their immunogenicity []. The resistance mechanisms of malignant cells can be categorized into two main strategies: evading immune recognition and creating an immunosuppressive TME []. These mechanisms rely on tumor cell-intrinsic and -extrinsic factors that interfere with antigen processing and presentation or enable tumors to recruit immune-suppressing cells that counteract the activity of effector cells []. Recent studies highlight the pivotal role of the TME in tumor-induced immunosuppression, as it is involved in downregulating effector T-cell activity and recruiting immunosuppressive cells [].
Biomarkers can be categorized as either predictive or prognostic markers. Predictive markers, like the BRAF mutation status in melanoma, help determine the likelihood of a patient’s response to specific treatments, such as BRAF/MEK inhibitors [,]. Prognostic markers, such as the overall stage of disease in solid cancers and prostate-specific antigen levels at prostate cancer diagnosis, are independent of cancer interventions and can predict disease recurrence or patient survival [,]. The hormonal status of breast cancer serves a dual purpose as both a prognostic and predictive biomarker. Recent studies suggest that ongoing research and a deeper understanding of biological processes will enhance existing biomarkers and aid in developing new ones. PD-L1 expression and MSI are the two FDA-approved biomarkers used with ICIs. However, these biomarkers have shown limitations. Due to the genomic variability of humans and tumor cells, future approaches will likely require the use of multiple biomarkers to predict responses to ICIs accurately [].
Recently, significant interest has emerged in studying the role of the gut microbiota in shaping responses to ICIs []. Several studies have identified bacterial species linked to favorable responses to ICIs, such as Akkermansia muciniphila, and others associated with resistance, like Ruminococcus obeum [,,]. In patients with NSCLC or RCC, the use of antibiotics during ICI initiation has been associated with reduced microbiome diversity, leading to decreased overall survival and progression-free survival [,]. Additionally, a high-fiber diet has been shown to have a more beneficial effect on ICI response than a low-fiber diet [].
2. Immune Checkpoint Inhibition in Cancer—Mechanisms of Action and Resistance Mechanisms
A class of medications known as ICIs is used to treat cancer []. These medications stimulate the body’s immune system to target cancer cells more successfully. It is useful to have some understanding of the interactions between cancer cells and the immune system to comprehend how they function [].
The immune system’s mission is to identify aberrant cells, including cancerous ones, and eliminate them. Immune cells, known as T cells, are essential to this process []. But to keep the immune system from attacking normal, healthy cells, it has “checkpoints” that function as brakes []. These are T-cell surface proteins, and they must be activated (or inactivated) to initiate an immunological response []. These checkpoints can be used by cancer cells to deter immune system attacks []. They possess the ability to express proteins that interact with these checkpoints and essentially shut down T cells, thus permitting the malignancy to spread unrestrained [].
Checkpoint inhibitors have been approved for the treatment of a wide range of malignancies, particularly those that have proven to be resistant to conventional therapies like radiation or chemotherapy []. They are categorized based on the target molecule they each have, and they were first licensed for distinct malignancies in the European Union (EU) and the United States of America (USA) at different points in time, just as Table 1 describes below, but later on, it was discovered that they were also effective in other locations [].

Table 1.
Classification of checkpoint inhibitors by target molecule, their brand names, their first approval dates in the USA and EU, and the first tumor type and location in which they were determined as efficient and approved for use.
Numerous new immune checkpoint targets have been discovered recently, including VISTA, TIM-3, LAG-3, and T-cell Ig and immunoreceptor tyrosine-based inhibitory motif (ITIM) domain (TIGIT) []. These studies suggested that inhibiting one immunological checkpoint could cause the TME to upregulate other checkpoint receptors in a compensatory manner []. Lung cancer was found to have a comparable compensatory mechanism between TIM-3 and PD-1 [].
One of the earliest and most successful checkpoint inhibitor applications was in melanoma. ICIs like Pembrolizumab, Nivolumab, and Ipilimumab have significantly improved survival rates for patients with advanced melanoma [,].
Regarding other cutaneous malignancies, ICIs such as Avelumab and Pembrolizumab are used to treat Merkel cell carcinoma, a rare and aggressive skin cancer, particularly in advanced stages, while Cemiplimab is used in the treatment of cutaneous CSCC [,,].
ICIs like Pembrolizumab, Nivolumab, Atezolizumab, or Durvalumab are also widely used in advanced NSCLC, either as a first-line treatment or after chemotherapy [,]. Some patients are tested for PD-L1 expression to determine the likelihood of response to treatment. Atezolizumab and Durvalumab are often used in combination with chemotherapy as a first-line treatment for extensive-stage small cell lung cancer (SCLC) [].
In RCC, ICIs like Nivolumab, Pembrolizumab, and Atezolizumab are used both alone and in combination with other therapies like tyrosine kinase inhibitors (e.g., Axitinib) to treat advanced kidney cancer [].
ICIs like Atezolizumab, Pembrolizumab, Nivolumab, Durvalumab, and Avelumab are often used in patients with advanced or metastatic bladder cancer (urothelial carcinoma) who are not eligible for chemotherapy or after chemotherapy has failed [].
Another use for ICIs such as Nivolumab, Pembrolizumab, and Atezolizumab (in combination with Bevacizumab) is advanced HCC, especially when other treatments like surgery or local therapies are not an option [].
Pembrolizumab and Atezolizumab are ICIs that are used in combination with chemotherapy for advanced triple-negative breast cancer (TNBC), especially in patients whose tumors express PD-L1 [].
Pembrolizumab and Nivolumab are two ICIs particularly effective in patients with microsatellite instability-high (MSI-H) or dMMR tumors, which are subtypes of CRC []. They are also effective in advanced or metastatic gastric and esophageal cancers, often in patients whose tumors express PD-L1 [].
Pembrolizumab is also approved for use in advanced or metastatic cervical cancer with PD-L1 expression []. Pembrolizumab and Dostarlimab are typically used in advanced or recurrent cases of endometrial cancer, particularly for tumors with MSI-H or dMMR [].
ICIs can be used in lymphatic malignancies other than solid tumors []. In primary mediastinal large B-cell lymphoma (PMBCL), Pembrolizumab is approved for use in this rare type of non-Hodgkin lymphoma, especially after other treatments have failed []. In Hodgkin lymphoma, ICIs like Pembrolizumab and Nivolumab have been highly effective in treating it, particularly in cases where the disease has relapsed or is resistant to other treatments [].
Research indicates that progression-free survival and duration of response to Nivolumab (an anti-PD-1 antibody), Ipilimumab (an anti-CTLA-4 antibody), or Pembrolizumab (another anti-PD-1 antibody) are greater in individuals with high tumor mutational burdens (TMBs) compared to those with lower mutational burdens [].
ICIs can interact with tumor cells and have antitumoral effects through a variety of mechanisms, such as the following:
2.1. Blocking PD-1/PD-L1 Interaction
PD-1 is a receptor on T cells, and PD-L1 is its ligand, which can be expressed in cancer cells [,,]. When PD-1 on T cells binds to PD-L1 on cancer cells, it sends an inhibitory signal that reduces the T cell’s ability to kill the cancer cell [,,]. This is a way for cancer cells to “turn off” the immune response [,,]. Drugs like Pembrolizumab, Nivolumab, and Atezolizumab work by blocking this interaction. This blockage prevents the inhibitory signal from being sent, thereby allowing T cells to remain active and attack the cancer cells [,,].
The way ICIs strengthen the body’s defenses against cancer is depicted in Figure 1.
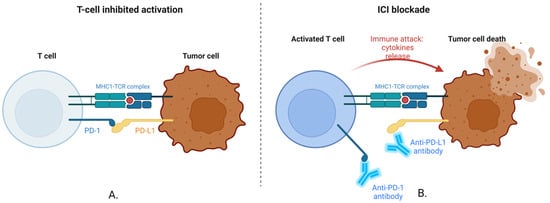
Figure 1.
Inhibition of PD-L1/PD-1 checkpoint. (A). T-cell-Inhibited Activation. T Cell: This immune cell is essential for locating and eliminating cancer cells. Tumor Cell: A malignant cell that uses inhibitory pathways to avoid detection by the immune system. MHC-TCR Complex: The tumor cell’s major histocompatibility complex (MHC) binds an antigen to the TCR. For T cells to identify the tumor cell, this contact is necessary. When activated, a T-cell receptor known as PD-1 transmits an inhibitory signal that lessens the T cell’s capacity to assault the tumor. PD-L1 is a ligand expressed on the surface of tumor cells and attaches itself to T-cell PD-1. By blocking T-cell activation, this interaction enables the tumor cell to avoid immune recognition and elimination. Since the tumor cell, in this instance, expresses PD-L1, which binds to the T cell’s PD-1 receptor, the tumor cell’s capacity to attack the T cell is hindered. The T cell receives a “stop” signal from this contact, which stops it from destroying the tumor cell. (B). ICI Blockade. T cells that have been fully activated can mount an immunological response. Anti-PD-1/PD-L1 Antibodies: These antibodies prevent the interaction between the tumor cell’s PD-L1 and the T cell’s PD-1. The inhibitory signal is stopped by obstructing this contact, which keeps the T cell activated. Tumor Cell Death and Immune Attack: As a result of the antibodies blocking the inhibitory signals, the T cell releases cytokines and other cytotoxic chemicals, which ultimately cause the tumor cell to be destroyed. Anti-PD-1 or anti-PD-L1 antibodies, which are ICIs, in this case, block the interaction that would typically inhibit the T cell. As a result, the tumor cell is successfully attacked and killed by the T cell, which still functions. Created with BioRender.com (accessed on 18 August 2024).
Providing a “brake” on the immune system, the PD-1/PD-L1 relationship normally stops T cells from attacking cells—including cancer cells—too aggressively [,,]. To evade immune destruction, many tumors do, however, take advantage of this mechanism [,,]. The immune system’s “brakes” are released by employing antibodies to obstruct this connection, which enables T cells to target and eliminate cancer cells more successfully [,,]. The foundation of contemporary IT for several malignancies is this mechanism [,,].
2.2. Blocking CTLA-4 Interaction
CTLA-4 is another inhibitory receptor found on T cells. It competes with the stimulatory receptor CD28 to bind to B7 molecules (B7-1/CD80 and B7-2/CD86) on APCs [,,]. When CTLA-4 binds to B7, it sends an inhibitory signal to the T-cell, dampening the immune response. Drugs like Ipilimumab block CTLA-4, which prevents it from binding to B7 molecules [,,]. This blockage allows CD28 to bind to B7 instead, promoting T-cell activation and enhancing the immune response against cancer cells [,,]. Figure 2 shows how an anti-CTLA-4 antibody can control the ratio of T-cell activation to inhibition.
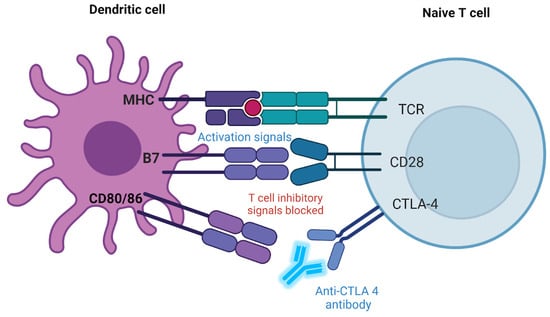
Figure 2.
CTLA-4 checkpoint inhibition mechanism. Dendritic cell (DC): The APC type plays a crucial role in activating naive T cells. MHC: The MHC on the DC presents an antigen to the TCR on the naive T cell. This interaction is essential for T-cell activation. B7 molecules (CD80/CD86): These molecules on the DC surface bind to receptors on the T cell to either provide activation signals or engage in inhibitory signaling. Naive T cell: This T cell has not yet encountered its specific antigen. TCR: This receptor recognizes the antigen the MHC presents on the DC, initiating activation. CD28: A costimulatory receptor on the T cell that binds to B7 molecules (CD80/CD86) on the DC. When CD28 binds to B7, it provides a necessary costimulatory signal for T-cell activation, enhancing its response. CTLA-4: This receptor is also on the T cell and competes with CD28 for binding to B7. However, when CTLA-4 binds to B7, it sends an inhibitory signal, dampening the T cell’s activation to prevent excessive immune responses. Anti-CTLA-4 antibody: The figure shows an anti-CTLA-4 antibody blocking the CTLA-4 receptor. This prevents CTLA-4 from binding to B7 molecules, thereby blocking the inhibitory signal normally downregulating T-cell activation. By blocking CTLA-4, the antibody enhances the activation signal from the CD28-B7 interaction, promoting a stronger immune response. Created with BioRender.com (accessed on 10 August 2024).
Typically, CTLA-4 acts as a “checkpoint” to prevent T-cell activation and limit the immune system’s overreaction. Nonetheless, using an antibody to suppress CTLA-4 can strengthen and prolong T-cell activation in cancer treatment, boosting the immune response against tumor cells []. Certain immunotherapies used to treat cancer are based on this process [,].
2.3. Enhanced T-Cell Activation and Proliferation
T cells, when not inhibited by checkpoint pathways, can proliferate more effectively and exert stronger antitumor activity []. The immune response is more robust, with a higher number of active T cells available to target and destroy cancer cells []. By blocking the inhibitory signals (like PD-1/PD-L1 or CTLA-4), checkpoint inhibitors enhance the activation and proliferation of T cells. This leads to a more powerful immune response against the tumor [].
2.4. Induction of Immunological Memory
A successful immune response against cancer can lead to the generation of memory T cells, which can remember and respond more quickly to cancer cells if they return []. Because checkpoint inhibitors can enhance the initial immune response, they may also promote the development of immunological memory []. This means the immune system can remain vigilant and potentially prevent cancer recurrence [].
2.5. Reactivation of Exhausted T Cells
In the tumor microenvironment, T cells can become “exhausted” due to chronic exposure to cancer antigens and continuous inhibitory signaling []. Exhausted T cells are less effective at attacking cancer cells []. Checkpoint inhibitors can rejuvenate these exhausted T cells by blocking the inhibitory signals (such as PD-1/PD-L1), restoring their function and enabling them to resume their attack on cancer cells [].
2.6. Overcoming Tumor-Induced Immunosuppression
Tumors can create an immunosuppressive environment by secreting various factors such as transforming growth factor beta (TGF-β) and interleukin 10 (IL-10) or by recruiting regulatory cells (like regulatory T cells and myeloid-derived suppressor cells (MDSCs) that inhibit T-cell function []. While not directly targeting these factors, checkpoint inhibitors can help overcome some of the tumor-induced immunosuppression by reinvigorating T cells and reducing the influence of the tumor’s immunosuppressive strategies [,].
2.7. Synergy with Other Immune Cells
Besides T cells, other immune cells like DCs (which present antigens) and natural killer (NK) cells also play roles in antitumor immunity []. By enhancing T-cell function, checkpoint inhibitors may also indirectly improve the activity of other immune cells, leading to a more coordinated and effective immune attack on the tumor [].
ICIs, which allow the immune system to identify and target tumor cells, have completely changed the treatment of cancer []. But not every patient responds to these treatments, and resistance may arise from internal (primary) or external sources (secondary) []. There are several ways in which tumors may resist the effects of ICIs [].
Several factors, including lack of tumor antigen presentation, low TMB, absence of infiltrating T cells (“Cold tumors”), inhibitory immune checkpoints beyond PD-1/PD-L1 and CTLA-4, or immunosuppressive cytokines, can lead to intrinsic (primary) resistance, which exists before the start of treatment and prevents the therapy from being effective at all [,].
- Lack of tumor antigen presentation: Cancer cells must present antigens via the MHC for T lymphocytes to identify and destroy them []. T cells become resistant when tumors downregulate or stop expressing MHC molecules, which prevents the T cells from identifying the cancer cells [].
- TMB: Neoantigens, or novel antigens that the immune system can target, are produced in smaller quantities by tumors with fewer mutations [,]. ICIs are less effective when the TMB is low because the immune system has fewer targets [,].
- Absence of infiltrating T cells (“Cold tumors”): Often called “cold” tumors, some cancers have an immunosuppressive microenvironment that hinders T cells from penetrating the tumor []. ICIs are ineffective without T-cell infiltration [].
- Inhibitory immune checkpoints beyond PD-1/PD-L1 and CTLA-4: Other immune checkpoint molecules that can similarly suppress T-cell function, such as TIM-3, LAG-3, or VISTA, may also be expressed by tumors [,]. If these other pathways are activated, it might not be enough to use ICIs that only target CTLA-4 or PD-1/PD-L1 [,].
- Immunosuppressive cytokines: Certain tumors release cytokines, such as vascular endothelial growth factor (VEGF), IL-10, or TGF-β, that impair the immune system and produce an environment that is unfavorable to T-cell activation [,,].
- After a first response to the therapy, acquired (secondary) resistance arises, which ultimately results in treatment failure []. It can arise from a variety of mechanisms, including signaling pathway mutations, decreased neoantigen expression, upregulation of alternative immune checkpoints, elevated PD-L1 expression, T-cell exhaustion, metabolic changes in the tumor microenvironment, or immunosuppressive cell recruitment [].
- Mutations in signaling pathways: Signaling pathway abnormalities in tumor cells can enable them to evade immune recognition [,,]. A tumor’s capacity to react to interferon-gamma (IFN-γ), a cytokine essential for immune-mediated tumor elimination, can be hindered by mutations in the JAK1/2 gene, for instance [,,].
- Loss of neoantigen expression: Neoantigens that were first identified by the immune system may disappear from tumors through evolution, preventing T cells from identifying and eliminating them [,].
- Upregulation of alternative immune checkpoints: Tumors may upregulate additional inhibitory molecules such as TIM-3, LAG-3, or VISTA after PD-1/PD-L1 or CTLA-4 pathways are inhibited, offering other possibilities to suppress the immune response [,].
- Increased expression of PD-L1: In reaction to the immune attack, tumor cells might adapt by upregulating their expression of PD-L1, which can overcome the blockage offered by anti-PD-1/PD-L1 antibodies [,].
- T-cell exhaustion: The process in which T cells lose their capacity to operate normally, even in the presence of ICIs, might result from prolonged contact with the tumor environment. Tired T cells do not make enough cytokines and display a lot of inhibitory receptors [,].
- Metabolic alterations in the tumor microenvironment: Tumors can produce metabolic wastes like lactic acid that inhibit immune cell activity or alter their microenvironment to deprive immune cells of vital resources like glucose and amino acids [,].
- Recruitment of immunosuppressive cells: The action of ICIs can be countered by tumors attracting regulatory T cells (Tregs), MDSCs, and tumor-associated macrophages (TAMs), which together generate an immunosuppressive environment [,].
The TME influences acquired and inherent mechanisms, which is vital in resistance []. As previously noted, the TME contains Tregs, MDSCs, and TAMs that can suppress effector T-cell activity and neutralize the effects of ICIs []. Due to the creation of barriers to immune cell infiltration and the promotion of molecules like VEGF production, hypoxia within the tumor might result in an immunosuppressive environment [,]. ICI efficacy may be restricted by dense stromal tissue in the TME, which may physically prevent immune cells from penetrating the tumor [,].
When it comes to ICIs, there are various ways to overcome resistance []. One such tactic is to combine ICIs with other therapies like radiation, chemotherapy, targeted therapy, or other immune-modulating medicines. This way, the tumor will be attacked from multiple angles, helping to overcome resistance [,]. To overcome resistance to CTLA-4 or PD-1/PD-L1 inhibitors, drugs targeting other immune checkpoints such as LAG-3, TIM-3, or TIGIT are also being studied []. Conversely, treatments that alter the TME to promote immune cell activity—such as those that target MDSCs or Tregs, lower hypoxia, or weaken stromal barriers—can aid in overcoming resistance [].
In conclusion, the interaction between checkpoint inhibitors and cancer cells is primarily focused on removing the inhibitory signals that prevent the immune system from attacking the cancer []. By blocking proteins like PD-1, PD-L1, and CTLA-4, these drugs enable a stronger and more sustained immune response, leading to the destruction of cancer cells []. This process involves multiple mechanisms, including direct T-cell activation, overcoming immunosuppression, and potentially inducing long-term immunological memory []. Despite the great potential of ICIs, resistance remains a significant issue. Understanding the reasons causing this resistance is crucial to developing strategies to improve the efficacy of these medicines.
3. Exploratory Biomarkers for Immune Checkpoint Inhibition
As discussed in the next subchapter, several predictive biomarkers have already received FDA approval and are utilized to monitor the therapeutic response to ICIs. Recent research is concentrating on evaluating the effectiveness of new biomarkers, including DNA damage response (DDR) gene alterations, MHC genotypes, beta-2-microglobulin (B2M) deficiency, polymerase epsilon (POLE) mutations, Janus kinase 1/2 (JAK1/2) mutations, tumor-infiltrating T cells (TILs), peripheral T cells, and the gut microbiome []. The latter will be discussed separately in a separate section of this article.
The DDR represents the cellular response to both endogenous and exogenous DNA damage, being implicated in repair processes through different pathways: dMMR, direct reversal, homologous recombination repair, nucleotide excision repair, base excision repair, and non-homologous end joining [,,]. Recent studies have shown that immune checkpoint blockade holds great promise for NSCLCs. In a study by Chae et al., the authors examined a predefined list of 812 immune metagenes, derived from 813 microarrays across 36 studies, to accurately predict the presence of up to 28 intratumoral immune cell types in a large cohort of lung adenocarcinoma patients from The Cancer Genome Atlas. It was demonstrated that tumors with DNA repair deficiencies (n = 230), particularly in the dMMR pathway, which can accelerate mutation rates and impact prognosis, have significant potential to be used as biomarkers for TMB, neoantigen load, and tumor-infiltrating lymphocytes in patients undergoing anti-PD-1/PD-L1 therapy [,]. In urothelial cancer (UC), recent studies have indicated reduced response rates to IT, yet still an improvement overall compared to previous systemic therapies. In a study by Teo et al., the researchers hypothesized that DDR gene mutations could serve as biomarkers for anti-PD-1/PD-L1 therapy in patients with metastatic UC []. They analyzed sixty patients with metastatic UC treated with anti-PD-1/PD-L1 antibodies, enrolled in three separate prospective trials between April 2014 and December 2016, and identified seventy-four DDR gene alterations, including twenty-seven deleterious DDR gene alterations, before treatment initiation []. Patients with deleterious DDR gene alterations exhibited a lower incidence of hepatic involvement and were associated with a higher TMB []. Their findings showed that DDR gene alterations were linked to clinical benefits in patients receiving anti-PD-1/PD-L1 therapy, with more pronounced effects observed in those with deleterious DDR alterations (80%) and in those whose tumors had wild-type DDR genes (19%; p = 0.001) []. Additionally, Teo et al. emphasized the significance of deleterious DDR alterations beyond MMR deficiency, which constituted only 5% of their study cohort, in contrast to findings by Chae et al. [,].
MHC-I molecules present intracellular proteins to T cells, enabling them to identify mutated or foreign peptides, which influences antigenicity []. Recent research indicates that the insufficient presentation of driver mutation neoantigens by MHC-I may explain why certain tumors do not respond to ICIs []. MHC-II molecules, found specifically in APCs such as macrophages and DCs (DC), have also been studied. Recent findings suggest that human leukocyte antigen-DR isotype (HLA-DR), a component of MHC-II, is elevated in malignant cells and is associated with favorable prognosis and improved response to ICI treatment [,]. In a study conducted by Johnson et al., the authors analyzed two independent cohorts of melanoma patients treated with anti-PD-1 therapy, and they highlighted that malignant cells, particularly melanoma cells, with high levels of HLA-DR are more responsive to PD-1 antibodies due to their inflammatory signals, even in patients with poor prognosis, such as those with liver metastases []. In their study, 75% of HLA-DR-positive patients responded to anti-PD-1 therapy, compared to only 27% of HLA-DR-negative patients (p = 0.025), and they also demonstrated a significantly better survival rate (median not reached versus 27.5 months, log-rank p = 0.003) []. Additionally, a study by Jiang et al. evaluated the IT response in 85 NSCLC patients, using HLA typing, revealing that those expressing HLA-DRB4 had an improved overall survival of 26.3 months compared to 10.2 months in untreated patients. This finding was significant in both univariate (p = 0.022; 26.3 months vs. 10.2 months) and multivariate analysis (p = 0.011, HR 0.49, 95% CI = 0.29–0.85), emphasizing the significance of MHC-II in the antitumor immune response during ICI treatment []. However, Jiang et al. also noted an increased risk of endocrine adverse reactions in patients with HLA-DRB4 undergoing ICI treatment (11 patients, 81.8% had HLA-DRB4, p = 0.0139), although the cause remains unknown []. Importantly, Jiang et al. found that patients who developed adverse reactions to ICIs had increased overall survival rates, underscoring the complex relationship between treatment response and adverse reactions [].
Recent studies have shown that B2M deficiency, a subunit of MHC-1, can downregulate MHC-1 antigen presentation, thereby increasing cancer immune evasion []. In a study by Sade-Feldman, the authors analyzed longitudinal tumor biopsies from 17 metastatic melanoma patients and observed that B2M deficiency was more prevalent in non-responder patients (29.4%) compared to responders (10%) treated with anti-CTLA-4 or anti-PD-1 therapy. This deficiency was associated with poorer survival rates, suggesting that B2M could be a potential biomarker for therapy resistance []. The study highlighted that one of the main reasons for this resistance is the absence of proteins that can substitute for B2M in HLA class I presentation [].
POLE is a crucial eukaryotic DNA polymerase involved in DNA replication and repair. A study by Rousseau et al. discovered that POLE mutations can serve as predictive biomarkers for patients with CRC or endometrial carcinoma treated with PD-1 antibodies, as these mutations are associated with increased TMB and tumor-infiltrating lymphocytes []. The study highlighted that POLE mutations are primarily found in young male patients with CRC and young female patients with endometrial carcinoma, often indicating a favorable prognosis []. Their analysis revealed that tumors harboring POLE mutations exhibited higher levels of cytotoxic T helper (Th) 1 cell infiltrates and PD-1 expression, along with significant upregulation of IFN-γ, PD-L1, and CTLA-4, which are critical for patients undergoing ICI therapy []. Using Nivolumab, an anti-PD-1 antibody therapy, the study observed therapeutic responses exclusively in CRC and endometrial carcinoma patients with POLE mutations, reporting a survival rate of 5.4 months []. Remarkably, they noted a near-complete response in a 42-year-old patient with unresectable CRC and POLE mutations []. The study also emphasized the correlation between dMMR and POLE mutations, suggesting that DNA repair defects can be effective biomarkers for ICI treatment in certain cancer types []. Furthermore, they underscored the importance of associating POLE mutations with low-TMB tumors, which do not benefit from PD-1 blockade, in contrast to high-TMB tumors [].
JAK1/2-inactivating mutations have been shown to increase tumor resistance to Pembrolizumab, an anti-PD-1 therapy, by disabling the IFN-γ responsive pathway []. In a study by Shin et al., these mutations were analyzed in patients with melanoma or CRC and high TMB that did not respond to anti-PD-1 therapy due to the diminished IFN-γ pathway and consequent lack of PD-L1 expression []. This finding is intriguing, as many studies have shown that patients with high TMB generally respond better to IT []. Biopsies from non-responder patients with advanced melanoma and high TMB revealed JAK1 domain mutations, along with low or absent CD8 infiltrates and PD-1/PD-L1 expression []. Conversely, in responder patients, JAK2 mutations were observed but lacked the same functional impact []. The results indicated that patients with JAK1 mutations did not respond to IFN-α, IFN-β, or IFN-γ, and consequently to anti-PD-1 therapy, whereas patients with JAK2 mutations were unresponsive only to IFN-γ but showed PD-L1 upregulation when exposed to IFN-α or IFN-β []. Nonetheless, survival rates for patients with tumors harboring JAK1/2 mutations were significantly lower []. The study concluded that JAK1/2-inactivating mutations are both acquired and primary causes of resistance to anti-PD-1 therapy, serving as effective predictive markers for this resistance [].
TILs, especially tumor-reactive cytotoxic CD8+ T cells, have been linked to better responses in patients with various tumor types treated with ICIs [,,]. Tissue-resident memory T cells (TRM), a recently discovered subtype of CD8+ memory T cells, have shown promise in improving outcomes for patients with NSCLC, oral, or breast cancer treated with anti-PD-1 therapy [,,]. These cells enhance responses by blocking PD-1 or CTLA-4 expression, making them potential biomarkers for ICI treatment [,,].
Corgnac et al. conducted a retrospective study on a cohort of 111 patients with NSCLC, discovering that those with higher pre-IT levels of CD8+ TRM cells, characterized by the CD103+CD49a+CD69+ phenotype, had increased TRM levels during anti-PD-1 treatment. This was associated with improved outcomes compared to non-responders who had low CD8+ levels (HR = 0.40, 95% confidence interval [CI], 0.20–0.77, p = 0.006). The median immunotherapy progression-free survival for these patients was 30 months (95% CI, 2.73, not reached) []. This improvement was attributed to the higher presence of activated tumor antigen-reactive T cells. Thus, TRM cells were suggested as potential biomarkers for ICI treatment in NSCLC patients [].
Another study by Lee et al. demonstrated that TRM cells with the CD39+ phenotype contributed to favorable responses in patients with triple-negative breast cancer treated with ICIs and predicted patient survival, primarily due to their functional restoration capability []. Importantly, CD39+ TRM cells influenced both local and systemic antitumor immunity. Their study also showed that combining anti-CTLA-4 and anti-PD-1 therapy yielded better outcomes than anti-PD-1 therapy alone in these patients [].
Chronic antigen stimulation leads to the exhaustion of tumor-reactive cytotoxic CD8+ T cells, giving rise to exhausted T cells and their precursors (TPEX) []. These TPEX cells have been recently studied in melanoma and NSCLC patients undergoing anti-PD-1 therapy, being correlated with enhanced response duration, suggesting their potential as biomarkers for ICI treatment [,].
Miller et al. found that TPEX cells were more effective than terminally exhausted T cells in controlling tumor growth []. In their study of melanoma patients who responded to ICI therapy, they observed higher expression of TPEX cells compared to terminally exhausted T cells, concluding that TPEX cells could be biomarkers for longer response duration to ICIs in melanoma patients []. Similarly, Liu et al. observed higher TPEX cell levels and lower terminally exhausted T-cell levels in NSCLC responders compared to non-responders following anti-PD-1 therapy, reinforcing the potential of TPEX cells as biomarkers [].
Immuno-oncology has undergone significant changes over time due to rapid advancements in research. Today, thousands of potential therapies are available in immuno-oncology, thanks to robust drug development pipelines. However, despite promising preclinical data, some studies have observed hyperprogressive disease in patients during later clinical trials when treated with a combination of immune checkpoint inhibitors (ICIs) and other immuno-oncology therapies [].
Chen et al. conducted a comprehensive pan-cancer analysis, examining the prevalence of Kelch-like ECH-associated protein 1 (KEAP1) mutations in a cohort of 40,167 patients with various cancer types (including lung cancer, endometrial cancer, hepatocellular carcinoma, head and neck cancer, bladder cancer, colorectal cancer, and esophagogastric cancer) using data from the cBioPortal online database []. They found that the overall prevalence of KEAP1 mutations was 2.7%, with the highest prevalence observed in patients with NSCLC (15.8%) []. The study also revealed that patients with KEAP1 missense mutations exhibited the highest TMB levels, indicating that KEAP1 mutations could serve as negative predictive biomarkers for immunotherapy outcomes []. The authors demonstrated the prognostic significance of KEAP1 mutations in both early-stage (p = 0.0099) and advanced-stage cancers (p < 0.0001) []. Moreover, when correlating KEAP1 mutations with overall survival, they found that patients with these mutations had a significantly reduced overall survival rate (39 months) compared to those without KEAP1 mutations (109 months) (p < 0.0001) and shorter disease-free survival (97 vs. 158 months, p = 0.0677) []. A contributing factor to these outcomes was the significantly lower infiltration of CD8+ T cells in patients with KEAP1 mutations, particularly in endometrial cancer, breast cancer, bladder cancer, colorectal cancer, lung adenocarcinoma, and lung squamous cell carcinoma, with the lowest levels observed in lung adenocarcinoma [].
In a retrospective study by Papillon-Cavanagh et al., the researchers analyzed mutations in KEAP1 and serine/threonine kinase 11 (STK11) among 2276 patients with stage IIIB, IIIC, IVA, or IVB NCSLC, including 574 patients who received anti-PD-1/anti-PD-L1 inhibitor treatment. The study found that both STK11 and KEAP1 mutations were associated with a poor response across multiple therapeutic classes []. However, this poor response was not specifically observed in the cohort treated with ICIs, nor was there a significant impact on progression-free survival or overall survival. For STK11 mutations, the hazard ratios were HR 1.05 (95% CI 0.76 to 1.44, p = 0.785) for progression-free survival and HR 1.13 (95% CI 0.76 to 1.67, p = 0.540) for overall survival []. For KEAP1 mutations, the hazard ratios were HR 0.93 (95% CI 0.67 to 1.28, p = 0.653) for progression-free survival and HR 0.98 (95% CI 0.66 to 1.45, p = 0.913) for overall survival []. The authors concluded that these mutations serve more as prognostic biomarkers rather than predictive ones for anti-PD-1/anti-PD-L1 therapy [].
In contrast, many studies have highlighted the poor prognosis of patients with STK11 gene codes for liver kinase B1 (STK11/LKB1) and KEAP1 mutations treated with ICIs. However, recent research evaluating their impact in KRAS wild-type patients, or independent of KRAS mutations, suggests that the poor prognosis is primarily linked to concurrent KRAS mutations []. These contradictory findings largely stem from study limitations, such as the inclusion of other gene mutations and the lack of comparison arms involving patients who received alternative treatments [].
Recent studies have emphasized the significance of targeting the nuclear factor erythroid 2-related factor 2 (NRF2)–KEAP1 axis in various diseases, including cancer, due to the cytoprotective functions of proteins encoded by NRF2-target genes, such as those involved in antioxidant defense, detoxification, and anti-inflammatory processes []. However, targeting the NRF2-KEAP1 axis was not previously considered a priority, largely because of its extensive range of biochemical activities that extend beyond the regulation of antioxidant systems, many of which are governed either directly or indirectly by NRF2, leading to nonspecific effects []. The benefits of targeting the NRF2-KEAP1 axis have only recently gained attention, including the maintenance of redox signaling, enhanced biotransformation of xenobiotics, regulation and resolution of inflammation, suppression of gluconeogenesis and hepatic lipogenesis, support of proteostasis, and inhibition of fibrosis []. Recent research has identified numerous effective drugs targeting the NRF2-KEAP1 axis for both neoplastic and non-neoplastic diseases, with many already approved and in use, and several others still under investigation []. Table 2 summarizes drugs targeting the NRF2-KEAP1 axis that have shown efficacy in certain types of cancer.

Table 2.
Predictive biomarkers approved or being studied for specific treatment of different types of cancer with KRAS mutations.
In a comprehensive pan-cancer analysis by Lee et al., researchers examined the genomic landscape of Kirsten rat sarcoma viral oncogene homolog (KRAS)-altered solid and hematologic malignancies in 426,706 patients treated with chemotherapy, chemotherapy combined with ICIs, or ICI monotherapy []. They found that KRAS alterations were present in 23% of the cases, with 88% being mutations, most commonly observed in CRC with KRAS G12D and G12V mutations, followed by NSCLC with KRAS G12C mutations []. The study also evaluated co-altered genes related to immunotherapy response, including TMB and PD-L1 expression, as well as co-mutations in STK11 and KEAP1, which were associated with low (28% and 16%) or negative (53% and 27%) PD-L1 expression, respectively []. The researchers observed similar overall survival rates in NSCLC patients with KRAS G12C and KRAS G12V mutations (11 vs. 10 months, HR 1.0, 95% CI 0.86–1.20, p = 0.88) and KRAS G12D mutations (11 vs. 12 months, HR 0.91, 95% CI 0.75–1.11, p = 0.36) []. Notably, patients with KRAS alterations had decreased overall survival compared to those without KRAS alterations but with other oncogenic mutations (11 vs. 27 months, HR 0.55, 95% CI 0.49–0.63, p < 0.001) []. Similar findings were reported in CRC and other cancer types, emphasizing the significant implications for developing KRAS inhibitors, particularly targeting G12C, G12D, and G12V mutations []. The authors acknowledged study limitations, including the lack of detailed clinical and treatment information for patients in the database and the selection bias introduced by using comprehensive genomic profiling [].
Selective KRAS G12C inhibitors are the only treatments that have shown clinical benefits in patients with NSCLC and KRAS G12C mutations who have undergone at least one prior systemic therapy []. Sotorasib, an approved selective KRAS G12C inhibitor, has demonstrated clinical efficacy and a tolerable safety profile in these patients []. Similarly, Adagrasib, another approved KRAS G12C inhibitor, has proven effective in pretreated NSCLC patients []. However, some newer inhibitors are still under investigation and face limitations due to intrinsic and acquired resistance mechanisms. KRAS mutations are also present in pancreatic ductal adenocarcinoma and CRC [].
Sotorasib is a KRAS G12C inhibitor that binds irreversibly to the S-IIP pocket, locking KRAS in its inactive GDP-bound state and inhibiting KRAS oncogenic signaling []. Additionally, Sotorasib enhances its interaction with KRAS G12C and increases potency by binding to a surface groove created by an alternative orientation of histidine at position 95, surpassing the effectiveness of AMG-1620, another KRAS G12C inhibitor []. Studies using xenograft models have demonstrated that Sotorasib can inhibit ERK phosphorylation and tumor cell growth in KRAS G12C-mutant cells, leading to durable tumor regression as a monotherapy []. It can also be used in combination with chemotherapy [].
Adagrasib is another KRAS G12C inhibitor with a mechanism similar to that of Sotorasib, irreversibly binding to the mutant protein and locking it in its inactive GDP-bound form []. However, Adagrasib offers some advantages over Sotorasib, including high oral bioavailability, a long half-life (approximately 24 h), extensive tissue distribution, and the ability to penetrate the central nervous system []. Notably, studies have shown that Adagrasib exhibits synergistic effects when combined with inhibitors of the EGFR family, SHP2, mTOR, or CDK4/6 []. Table 2 provides a summary of the key drugs used to inhibit the KRAS G12C mutation across various cancer types.
Additionally, other mutations, such as mesenchymal–epithelial transition (MET), human epidermal growth factor receptor 2 (HER2), rearranged during transfection (RET), and neurotrophic tyrosine receptor kinase (NTRK), are still under study and have shown promising clinical potential for the development of specific inhibitors [].
4. Approved Biomarkers for Immune Checkpoint Inhibition
Despite the remarkable efficacy of ICIs, many patients (~70%) do not respond well or develop resistance to these drugs []. The response rate to ICIs varies between 15% and 30% in most solid tumors and 45% and 60% in MSI-H cancers and malignant melanoma [].
Currently, the FDA has only approved three predictive biomarkers for ICI therapy in malignancies []. These biomarkers are PD-L1, MSI or dMMR, and TMB [].
PD-L1, also known as programmed death ligand 1, has gained recognition as a significant biomarker in breast cancer in recent times []. There is a correlation between this factor and poorer outcomes in patients with hormone receptor-positive breast cancer. However, it has a predictive function in guiding the response to systemic treatment specifically in the TNBC subtype, particularly in cases where the cancer has spread []. ICIs are now being incorporated into the treatment regimen for a growing number of patients with TNBC [].
Monoclonal antibody IT that targets PD-1 andvPD-L1 has become a widely accepted treatment for patients with lung cancer (NSCLC) []. It is used in both initial and subsequent treatment stages, and has shown long-lasting positive responses in around 10-20% of treated patients [].
In 1992, T. Honjo and his colleagues at Kyoto University discovered that PD-1 is a gene related to apoptosis []. However, the process of apoptosis does not necessitate the overexpression of PD-1 [].
PD-1 function requires tyrosine in T and B lymphocytes []. The orderliness in the cytoplasmic tail of PD-1 can be bound by protein tyrosine phosphatase 1 and 2 (SHP-1, SHP-2) []. However, it is unknown whether PD-1 takes SHP-1 and/or SHP-2 in a physiological setting. Cysteine is responsible for the homodimerization of CD28, CTLA-4, and inducible costimulatory molecules []. On the other hand, PD-1 is monomeric on the cell surface and is unable to form covalent homodimers because it lacks the extracellular surface [].
Subsequent research conducted in 1996 by the same group revealed that PD-1 expression was stimulated by antigen receptors on T and B cells [] and had a role in suppressing immunological responses, since PD-1-deficient mice displayed autoimmune symptoms [,].
Nobel prize laureate Tasuku Honjo and his team have used J43 mAb immunoprecipitation and flow cytometry to determine the PD-1 gene product, which is a 50–55 kDa membrane protein produced on the cell surface of 2B4.11 cells, and various PD-1 cDNA transfectants [].
The PD-1 antigen is present in specific subsets of thymocytes and spleen T cells, which may cause T-cell activation or death []. In a similar manner, in vitro treatment with an anti-Ig M antibody caused PD-1 expression on spleen B cells []. PD-1, on the other hand, was not substantially expressed on lymphocytes after treatment with lipopolysaccharide, dexamethasone, or growth factor deprivation []. The findings indicate that PD-1 antigen expression is closely controlled and stimulated by signal transduction via the antigen receptor []. While PD-1 expression is not necessary for the common process of apoptosis, it remains possible that the PD-1 antigen contributes to lymphocyte clonal selection [].
PD-1 functions as PD-L1’s T-cell-inhibitory receptor, and both PD-L1 (CD274) and PD-L2 (CD273; also known as B7-DC) are expressed in cancer cells and APCs in the TME [,,].
The goal of targeting PD-1 and its ligands is to attack tumors with the help of the body’s immune system [,]. The development and use of such blocking antibodies fundamentally altered the course of cancer IT [,].
PD-1 is activated in several peripheral hematopoietic cells by cytokines and antigen receptor signaling; its expression occurs throughout thymic development []. During TCR β rearrangement, immature CD4−CD8− (double negative) thymocytes express PD-1 []. Upon their activation, peripheral CD4+ and CD8+ T cells, B cells, monocytes, NK T cells, and certain subsets of DCs express PD-1 through induction [].
PD-1 on T cells and APCs can be induced by estrogen []. As a result of being activated, peripheral T cells (CD4+ and CD8+), B cells, NK T cells, monocytes, and certain types of DCs (DC) show PD-1 expression. BCR or TCR activation makes PD-1 function [].
Macrophages, memory B cells, and mast cells exhibit inducible PD-L2; PD-L2 is expressed in fewer cell types compared with PD-L1 [,]. Hematopoietic and non-hematopoietic cells generally express PD-L1 []. Although produced constitutively in T cells, PD-L1 levels are raised after the activation of B cells, DCs, macrophages, and mast cells originating from bone marrow [].
Mice naturally express more PD-L1 than humans []. Neurons, pancreatic islet cells, endothelial cells, epithelial cells, fibroblastic reticular cells, and cells identified in immune-privileged regions, such as retinal pigment epithelial cells and the placenta, all contain PD-L1 [].
Due to the widespread expression of PD-L1 on both hematological and non-hematopoietic cells, and the expression of PD-1 on B cells, T cells, some DCs, and macrophages, there are several potential interactions in both directions between PD-1 and PD-L1 []. T cells excel in facilitating the function of PD-1 [].
PD-1 interacts with one of its ligands during TCR signaling, reducing T-cell survival and inhibiting T-cell proliferation, cytokine generation, and cytolytic activity [,].
PD-Ls have been shown in several studies to have the ability to inhibit T-cell cytokine production and cell proliferation. However, they have also been seen to stimulate T-cell activation. The origins of these divergent results are now unclear [,].
Immune cells that target particular antigens and do not have PD-1 receptors respond more strongly to antigen-containing resting DCs, mostly via CD8+ T cells. It is the antigen itself that causes this reaction []. A negative impact on effector T-cell reactivation, proliferation, and function may also be exerted via the PD-1/PD-L pathway []. Some tumor-penetrant cancer cells and APCs may also display PD-1/PD-L1 interaction in cis when these cells express PD-1 and PD-L1, according to recent studies []. This interaction is called cis expression []. Researchers found that when PD-1 and PD-L1 are produced on the same cancer or antigen-presenting cell (APC), they touch each other directly on the cell surface. Hence, this contact, called cis interaction, reduces the capacity of PD-L1 to bind to PD-1 on T cells in a trans manner [,]. This goal was achieved by the creation of new methods for re-creating lipid bilayer systems in vitro and by conducting experiments in cell cultures [].
4.1. MSI or dMMR
Repetitive DNA sequences (repeats) are scattered across the human genomes, e.g., repeats of mononucleotides, dinucleotides, or higher-order nucleotide repeats like (A)n or (CA)n make microsatellites. Because polymerases slip during DNA synthesis, these sequence motifs are particularly vulnerable to the accumulation of mutations []. The MMR system is in charge of monitoring and fixing mistakes made in microsatellites []. Microsatellites, also known as short tandem repeats, are genome-wide repeating DNA sequences that are dispersed throughout both coding and noncoding areas []. Their unit lengths range from one (mononucleotides) to six bases (tetra-, penta-, esa-, and trinucleotides) []. Although they vary greatly throughout themes, they are constant in every single person [,]. MSI, which is easily detected by the study of polyA microsatellites, is the result of the accumulation of mutations resulting in repeat length variations in dMMR [,,]. These areas are especially sensitive to mismatch errors because of their repeating nature. MSI thus indicates a hypermutable condition of cells and serves as a hallmark of dMMR [,,].
Maintaining genomic integrity is largely dependent on the highly conserved biological system known as dMMR []. Base–base mismatches and insertion/deletion mispairs produced during DNA replication and recombination are the main sources of MMR’s specificity []. Additionally, MMR inhibits homologous recombination and has been implicated in the signaling of DNA damage in eukaryotic cells []. The primary errors associated with microsatellites are insertion–deletion loops, which refer to extrahelical nucleotides that form DNA hairpins, and base–base mismatches, which bypass the intrinsic proofreading mechanism of DNA polymerases []. When the starting nucleotide and template strand split and recombine incorrectly in a microsatellite, it leads to the formation of partnerless nucleotides []. Frameshift mutations that lead to protein truncations are generated by insertions or deletions in microsatellites located in coding regions of DNA [].
When DNA replication, iatrogenic damage, or recombination leads to a mismatch, the process of dMMR comes into play to resolve the issue [,,]. Four genes—MLH1, MSH2, MSH6, and PMS2—control the mitotic spindle region (MMR) process [,,]. Two genes, MLH1/PMS2 and MSH2/MSH6, encode separate proteins [,,]. The mismatch repair genes MLH1, MSH2, MSH6, and PMS2 have germline mutations or epigenetic silencing of MLH1 and account for approximately 15% of CRC with MSI []. The mismatch repair genes MLH1, MSH2, MSH6, and PMS2 have germline mutations or epigenetic silencing of MLH1, and account for approximately 15% of CRC with MSI [].
The idea of an MSI phenotype in CRC originated from the description of the clinical and molecular characteristics of MSI tumors []. Research has shown that MSI tumors are more likely to respond favorably to chemotherapy than microsatellite-stable CRC; however, there may be differences in how MSI cancers react to these treatments [,].
Lynch syndrome (LS) or Hereditary Non-Polyposis Colorectal Cancer (HNPCC) is brought on by a germline heterozygous mutation in one of the four MMR genes []. LS is the most common inherited cancer predisposition syndrome. Germline mutations in MLH1 (42%) or MSH2 (33%), followed by MSH6 (18%) and PMS2 (7%), generally occur in a significant number of these individuals []. In most cases, tumors manifest themselves in the uterus and colon, but they may also manifest in other organs []. Patients with this condition often have a mean age lower than 45 years []. It is estimated that LS, an inherited genetic condition, is responsible for around two to three percent of all incidences of CRC [].
The cumulative risk of acquiring CRC is 60–70% for men and 30–40% for women if germline mutations in MMR genes are present; the cumulative risk of developing endometrial cancer is 40–80% [,,].
Compared to stage III (~12%) and stage IV (~4%) CRC, MSI is more common in stage II (~20%) than in stage III (~12%) [,,,,]. Furthermore, it was found in two small retrospective investigations that the Egyptian population (37%) and African American population (20–45%) had higher rates of MSI malignancies [,,,,]. It is evident that the MSI phenotype constitutes a clinically significant fraction of CRC, even if the frequency may differ throughout populations [,,,,]. It is evident that the MSI phenotype constitutes a clinically significant fraction of CRC, even if the frequency may differ throughout populations [,,,,].
There are some biochemical changes that are seen in families who have HNPCC, with the epigenetic suppression of MSH2 being a particularly significant example []. The deletion of exon 3 of the TACSTD1 gene in the germline of a heterozygous individual is the root cause of the heritable epigenetic silencing of gene MSH2 []. The protein known as epithelial cell adhesion molecule (EpCAM) is encoded by this gene, which is responsible for its production [].
APC, MLH1, or PMS2 germline mutations are the source of Turcot’s syndrome, which is clinically defined by the early emergence of primary brain and colorectal tumors [].
Roughly 15% of sporadic CRCs have MSI, most of which are associated with hypermethylation of the MLH1 promoter, which results in the loss of protein production and silencing of gene transcription [,].
According to the Association for Molecular Pathology, all newly diagnosed colorectal tumors should undergo MSI analysis to be divided into three subgroups: Lynch dMMR15, sporadic dMMR, and sporadic MMR-proficient []. The recognized clinical features and predominately right-sided localization of MSI-H CRCs predominantly affect those with female gender, advanced age, high grade, mucinous differentiation, medullary histology or signet ring, peritumoral lymphocytic infiltration, Crohn’s disease-like inflammation, diploid status, lower stage, and improved prognosis [,,,,,].
4.2. TMB
Another type of biomarker approved by the FDA for ICI therapy in cancers is the TMB, also referred to as tumor mutational load (TML) []. The TMB represents the total amount of modifications, or mutations, discovered in cancer cell DNA []. Having an understanding of the TMB could aid in therapy planning []. For instance, it appears that certain forms of IT may be more effective against cancers with a high number of mutations [].
There is a variation in the link between the mutational load of tumors and the expression of PD-1/PD-L1 depending on the kind of tumor [].
Pembrolizumab (anti-PD-1) was approved by the FDA for use in the treatment of all types of cancer due to its high TMB [].
There have been some meta-analyses that have supported this conclusion by suggesting that the TMB is an effective predictor of response to ICI treatment [,]. These meta-analyses have been conducted throughout the whole cancer spectrum.
Concerns have been raised about the possibility that the FDA may have allowed Pembrolizumab for an inappropriately broad indication based on TMB []. Additionally, investigations that contradict each other have shown that TMB may not serve as an accurate indication of the success rate of IT [].
5. Microbiota as an Immune Adjuvant for Immune Checkpoint Blockade
The microbiota, alongside PD-L1 expression, TMB, dMMR, MSI, and TME, has emerged as a crucial biomarker under continuous investigation for its role in the therapeutic response of solid cancers. It significantly contributes to the development of the intestinal immune system [,].
Recent studies have demonstrated a strong association between the microbiota and the efficacy of therapeutic responses in solid tumors, including ICIs []. The microbiota induces an immune response against malignant cells by activating T cells and increasing the levels of human IL-2 and IFN-γ []. Figure 3 depicts the enhancement of treatment with ICIs through the influence of the intestinal microbiota.
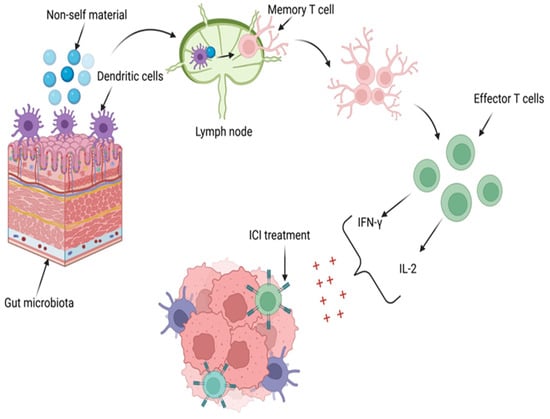
Figure 3.
The influence of intestinal microbiota in ICI treatment. DCs in the gut microbiome identify non-self-material and present it to T cells in the lymph nodes, activating memory T cells (TCMs). These TCMs differentiate into effector T cells and proliferate, increasing levels of human IL-2 and IFN-γ. Through their primary mechanisms, IL-2 and IFN-γ enhance the effectiveness of the therapeutic response to ICIs. Created with BioRender.com (accessed on 10 August 2024).
The role of IFN-γ in producing a favorable response to ICI therapy is achieved through its regulation of 30 genes, stimulation of NK cell activity, enhancement of antigen presentation, and ability to increase macrophage lysosomal activity, thereby exerting antitumor and immunoregulatory effects [,]. Similarly, IL-2 contributes to a favorable response to ICI therapy by regulating leukocyte activity, interacting with antibodies, promoting hematopoiesis and tumor surveillance, and inducing NK cells to produce cytokines such as IFN-γ and tumor necrosis factor-α (TNF-α) [,].
Dysbiosis, an altered state of the gut microbiota, has been linked to chronic health conditions and cancer, but also a poor response to ICIs [,,]. Dysbiosis caused by antibiotics and various diets can influence antitumor immune responses by reducing the production of deoxycholate and short-chain fatty acids []. Bacterial species most associated with effective ICI responses include Bifidobacterium, Faecalibacterium, Lactobacillus, and Akkermansia (HR = 2.92, 95% CI = 1.08–7.89), particularly in cases of melanoma or HCC, modulating tumor responses to checkpoint blockade IT [,].
A study by Gopalakrishnan et al. found significant differences in the gut microbiota of patients with melanoma. Responders to ICI treatment (CTLA-4 inhibitors and PD-1 antibodies) exhibited higher alpha diversity and an abundance of Ruminococcaceae bacteria (p < 0.01), along with enrichment of anabolic pathways and enhanced systemic and antitumor immunity []. The gut microbiota of responder patients was enriched with Clostridiales, Faecalibacterium, and Ruminococcaceae (p < 0.01), whereas non-responders had increased amounts of Bacteroidales, Escherichia coli, and Anaerotruncus colihominis (p < 0.01) [].
The study also observed that responder patients had elevated levels of CD4+ and CD8+ T cells, with preserved cytokine response and a higher density of immune cells and markers of antigen processing and presentation, which can modulate antitumor immune responses []. In contrast, non-responders exhibited higher levels of regulatory T cells and MDSCs, along with a reduced cytokine response [].
Unfortunately, some studies have highlighted the risk of immune-related adverse events in patients treated with ICIs. Despite Faecalibacterium being associated with a positive effect on ICI efficacy, an increased risk of immune-related adverse events has been observed []. The gut microbiome can influence the local immune system and the trafficking of bacterial peptide-primed T cells distally, potentially leading to increased toxicity from ICI treatment [].
In a study by Xu X et al., the efficacy of PD-1 antibody IT was evaluated in CT26 tumor-bearing mice with CRC, treated with different types of antibiotics (vancomycin and colistin) and different gut microbiota compositions, compared to a control group given non-antibiotic sterile drinking water []. Initially, the antibiotic-treated mice showed reduced tumor growth, but this trend reversed significantly after introducing PD-1 antibody treatment []. Tumor growth progressed more in the vancomycin group (moderately) and the colistin group (significantly), compared to the control group, which responded favorably to the PD-1 antibody []. The bacterial species observed were Bacteroides spp. (18.76%) and Bacteroidales (4.6%) in the control group, Bacteroides spp. (20.83%), Prevotella spp. (7.75%), and Bacteroidales in the colistin-treated group, and Prevotella spp. and Akkermansia muciniphila (3.85%) in the vancomycin-treated group []. Prevotella and Akkermansia muciniphila were associated with a favorable response to IT due to their roles in the biosynthesis of steroid hormones, mannose, phenylpropane, beet red, and carotene []. In contrast, Bacteroides and Bacteroidales were linked to a poor therapeutic response through tumor signaling pathways, antigen recognition and presentation, Th17 cell differentiation, IL-17 secretion, estrogen signaling pathways, pyrimidine metabolism, and nucleic acid shearing and repair [].
Routy et al. [] also highlighted the positive effects of Akkermansia muciniphila in epithelial tumors (p = 0.004). Studies have shown that Akkermansia muciniphila enhances gut barrier function, normalizes metabolic endotoxemia and adipose tissue metabolism, improves immune responses, and decreases serum ALT, AST, pro-inflammatory cytokines, and chemokines [,,].
Significant differences in metabolic and immune processes were observed when comparing the two antibiotic-treated groups. In the colistin-treated mice, abnormal T-cell glucose metabolism and chronic inflammation induced by type I diabetes mellitus, antigen processing and presentation, cancer pathways, Th 17 cell differentiation, and IL-17 signaling pathways were noted [,,]. In contrast, vancomycin-treated mice showed increased glycerolipid metabolism and glycosphingolipid biosynthesis, which were associated with a more favorable response to PD-1 antibody treatment [].
An important aspect analyzed by Xu et al. was the similarity of bacterial species before and after PD-1 antibody treatment in the three groups, indicating that the microbiota did not change post-IT []. The authors also noted changes in IFN-γ and IL-2 cytokines in the TME of antibiotic-treated mice, secondary to changes in gut microbiota, leading to different efficacies of PD-1 antibody treatment [].
Recent studies have demonstrated that anti-PD-1 antibodies exhibit some efficacy in treating HCC [,]. Zheng et al. conducted a study analyzing eight patients with stage C HCC, according to the Barcelona Clinic Liver Cancer classification, who were treated with anti-PD-1 antibodies without any concurrent antibiotic treatment []. At the beginning of ICI treatment, the patients exhibited no dysbiosis, with an enrichment of Gram-positive Firmicutes, Gram-negative Bacteroidetes, and Gram-negative Proteobacteria in their fecal samples []. Throughout the treatment, patients who responded did not show significant changes in gut microbiota composition []. After 12 weeks, Proteobacteria, particularly Escherichia coli, became dominant in non-responding patients, while Klebsiella pneumoniae increased in responding patients []. Additionally, responding patients exhibited elevated levels of Lactobacillus species, Bifidobacterium dentium, and Streptococcus thermophilus, which inhibit pathogenic microorganisms []. They also had higher levels of Lachnospiraceae, Ruminococcaceae species, and Akkermansia muciniphila, which help maintain normal intestinal permeability and systemic immunosuppression []. In conclusion, Zheng et al. highlighted that the gut microbiome could serve as a biomarker for early detection of the 6-month prognosis of patients treated with PD-1 antibodies, as early as 3-6 weeks into the treatment [].
Some studies emphasize the importance of probiotics in the intestinal flora, noting that they can increase the concentration of bacterial metabolites such as propionate and butyrate, which in turn enhance the activity of immune cells [,,]. Other studies have demonstrated that the oral administration of Bifidobacterium can enhance the efficacy of PD-L1 antibody therapy and that oral administration of Akkermansia muciniphila can increase the effectiveness of PD-1 antibodies [,]. Table 3 summarizes the types of bacteria and their effects on the efficacy of ICI treatment responses.

Table 3.
The effect of different types of bacteria on ICI treatment response.
Many recent studies highlight the critical importance of controlling the gut microbiota before and during treatment with PD-1 antibodies, emphasizing that maintaining a normal gut microbiota is crucial for a favorable therapeutic response []. Therefore, all factors that can alter the taxonomic composition, functional metagenomics, and metabolic profiles of the microbiota must be considered before starting treatment with ICIs, as these factors can significantly influence the therapeutic response to ICIs [].
6. Conclusions and Future Directions
ICIs have significantly transformed cancer IT, particularly in metastatic melanoma, by improving overall survival rates []. These therapies target immune checkpoints like CTLA-4 and PD-1, which play essential roles in T-cell activation and maintaining immunological tolerance []. Despite their success, ICIs present challenges, including treatment resistance, immune-related adverse events (irAEs), and limited safety data in pediatric patients []. Adverse events are notably more severe with CTLA-4 inhibitors, especially at higher doses, while PD-1/PD-L1 inhibitors generally have milder toxicities, with fatigue being the most common side effect []. The potential for life-threatening off-target effects in children is particularly concerning [,]. Ongoing research is exploring novel combinations of ICIs with other therapies—such as chemotherapy, targeted agents, RT, and T-cell-based treatments—to enhance outcomes, especially in patients who do not respond favorably to ICI monotherapy []. Additionally, the TME plays a crucial role in treatment response, posing further complexities that require continued investigation to optimize therapeutic strategies across various cancer types []. Combining RT and IT may enhance their effectiveness through synergistic mechanisms, offering hope against cancers resistant to conventional treatments []. Additionally, photodynamic therapy (PDT), which disrupts tumor vasculature and activates the immune system, holds promise but requires carefully designed photosensitizers to work effectively in the challenging tumor microenvironment, particularly under hypoxic conditions [].
In conclusion, the gut microbiota has emerged as a significant biomarker influencing the therapeutic response to ICIs in solid cancers. Research shows that the microbiota can enhance immune responses against tumors by activating T cells and increasing cytokine levels, such as IL-2 and IFN-γ, thereby improving ICI efficacy. However, dysbiosis—an imbalance in the gut microbiota—can lead to poor therapeutic outcomes []. Specific bacterial species, like Akkermansia muciniphila and Bifidobacterium, are associated with favorable responses to ICIs, while others are linked to negative effects. The gut microbiome’s composition can also predict patient prognosis early in treatment []. Probiotics have shown the potential to enhance the efficacy of ICIs by modulating the gut microbiota, highlighting the importance of microbiota in cancer IT.
One limitation of our study is the heterogeneity of the included trials, particularly in the definition of microbiome diversity, which may limit the generalizability of these findings. Additionally, most studies did not control for antibiotic use, which is a significant confounder in microbiome research.
Therefore, many promising future studies should consider these variables regarding anti-PD-1 and anti-CTLA-4 treatments and the improvement of their efficacy.
Author Contributions
Conceptualization, L.A.P., O.A.R., S.M.C. and B.S.G.; methodology, R.M.E., O.A.R. and M.P.; software and image design, O.A.R. and R.M.E.; data curation, O.A.R., M.P. and B.S.G.; writing—original draft preparation, L.A.P., R.M.E., O.A.R., M.P., S.M.C. and B.S.G.; supervision, S.M.C.; project administration, S.M.C.; critically review and revision of the full article, S.M.C. and B.S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| APCs | antigen-presenting cells |
| B2M | beta-2-microglobulin |
| CRC | colorectal cancer |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| DDR | DNA damage response |
| DNA | deoxyribonucleic acid |
| dMMR | defective DNA mismatch repair |
| EMA | European Medicines Agency |
| ER | estrogen receptor |
| EU | European Union |
| EpCAM | epithelial cell adhesion molecule |
| FDA | Food and Drug Administration |
| GITR | glucocorticoid-induced TNFR-related protein |
| HCC | hepatocellular carcinoma |
| HER2 | human epidermal growth factor receptor 2 |
| HLA-DR | human leukocyte antigen-DR isotype |
| HNPCC | Hereditary Non-Polyposis Colorectal Cancer |
| ICI | immune checkpoint inhibitor |
| IFN-γ | interferon-gamma |
| Ig | immunoglobulin |
| IL-10 | interleukin 10 |
| irAEs | immune-related adverse events |
| IT | immunotherapy |
| ITIM | immunoreceptor tyrosine-based inhibitory motif |
| JAK1/2 | Janus kinases |
| KEAP 1 | Kelch-like ECH-associated protein 1 |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
| LAG-3 | lymphocyte activation gene-3 |
| LS | Lynch syndrome |
| MDSCs | myeloid-derived suppressor cells |
| MET | mesenchymal–epithelial transition |
| MHC | major histocompatibility complex |
| MSI | microsatellite instability |
| MSI-H | microsatellite instability-high |
| NK | natural killer |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| NSCLC | non-small cell lung cancer |
| NTRK | neurotrophic tyrosine receptor kinase |
| PD-1 | programmed cell death 1 |
| PD-L1 | programmed cell death ligand 1 |
| PDT | photodynamic therapy |
| PMBCL | primary mediastinal large B-cell lymphoma |
| POLE | polymerase epsilon |
| RCC | renal cell carcinoma |
| RET | rearranged during transfection |
| RT | radiotherapy |
| SCLC | small cell lung cancer |
| SHP-1, SHP-2 | protein tyrosine phosphatase 1 and 2 |
| STK11 | serine/threonine kinase 11 |
| STK11/LKB1 | STK11 gene codes for liver kinase B1 |
| TAMs | tumor-associated macrophages |
| TCMs | memory T cells |
| TCR | T-cell receptor |
| TGF-β | transforming growth factor beta |
| Th | helper T cell |
| TIGIT | T-cell immunoglobulin and ITIM domain |
| TILs | tumor-infiltrating T cells |
| TIM-3 | T-cell immunoglobulin and mucin domain-containing protein 3 |
| TMB | tumor mutational burden |
| TML | tumor mutational load |
| TME | tumor microenvironment |
| TNBC | triple-negative breast cancer |
| TNF-α | tumor necrosis factor-α |
| TPEX | exhausted T cells and their precursors |
| TRM | tissue-resident memory T cells |
| Tregs | regulatory T cells |
| UC | urothelial carcinoma |
| USA | United States of America |
| VEGF | vascular endothelial growth factor |
| VISTA | V-domain Ig suppressor of T-cell activation |
References
- Fujiwara, Y.; Mittra, A.; Naqash, A.R.; Takebe, N. A review of mechanisms of resistance to immune checkpoint inhibitors and potential strategies for therapy. Cancer Drug Resist. 2020, 3, 252–275. [Google Scholar] [CrossRef] [PubMed]
- Basudan, A.M. The Role of Immune Checkpoint Inhibitors in Cancer Therapy. Clin. Pract. 2022, 13, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P.; Cohn, M. A Theory of self-nonself discrimination. Science 1970, 169, 1042–1049. [Google Scholar] [CrossRef]
- Jenkins, M.K.; Schwartz, R.H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 1987, 165, 302–319. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Linhares, A.; Leitner, J.; Grabmeier-Pfistershammer, K.; Steinberger, P. Not All Immune Checkpoints Are Created Equal. Front. Immunol. 2018, 9, 1909. [Google Scholar] [CrossRef]
- Sambi, M.; Bagheri, L.; Szewczuk, M.R. Current Challenges in Cancer Immunotherapy: Multimodal Approaches to Improve Efficacy and Patient Response Rates. J. Oncol. 2019, 2019, 45087941. [Google Scholar] [CrossRef]
- Koustas, E.; Sarantis, P.; Papavassiliou, A.G.; Karamouzis, M.V. The Resistance Mechanisms of Checkpoint Inhibitors in Solid Tumors. Biomolecules 2020, 10, 666. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Shum, B.; Larkin, J.; Turajlic, S. Predictive biomarkers for response to immune checkpoint inhibition. Semin. Cancer Biol. 2021, 79, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, A.V.; Whittington, R.; Malkowicz, S.B.; Schultz, D.; Blank, K.; Broderick, G.A.; Tomaszewski, J.E.; Renshaw, A.A.; Kaplan, I.; Beard, C.J.; et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998, 280, 969–974. [Google Scholar] [CrossRef]
- Routy, B.; le Chatelier, E.; DeRosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Elkrief, A.; Derosa, L.; Zitvogel, L.; Kroemer, G.; Routy, B. The intimate relationship between gut microbiota and cancer immunotherapy. Gut Microbes 2019, 10, 424–428. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Alturki, N.A. Review of the Immune Checkpoint Inhibitors in the Context of Cancer Treatment. J. Clin. Med. 2023, 12, 4301. [Google Scholar] [CrossRef] [PubMed]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Fife, B.T.; Bluestone, J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008, 224, 166–182. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Burns, M.C.; O’donnell, A.; Puzanov, I. Pembrolizumab for the treatment of advanced melanoma. Expert Opin. Orphan Drugs 2016, 4, 867–873. [Google Scholar] [CrossRef]
- Raedler, L.A. Opdivo (Nivolumab): Second PD-1 Inhibitor Receives FDA Approval for Unresectable or Metastatic Melanoma. Am. Health Drug Benefits 2015, 8, 180–183. [Google Scholar] [PubMed]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.F.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef]
- Alkholifi, F.K.; Alsaffar, R.M. Dostarlimab an Inhibitor of PD-1/PD-L1: A New Paradigm for the Treatment of Cancer. Medicina 2022, 58, 1572. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, N.; Wang, X.; Ravindranathan, S.; Chin, D.J.; Tseng, S.-Y.; Klakamp, S.L.; Widmann, K.; Kapoor, V.N.; Vexler, V.; Keegan, P.; et al. Toripalimab, a therapeutic monoclonal anti-PD-1 antibody with high binding affinity to PD-1 and enhanced potency to activate human T cells. Cancer Immunol. Immunother. 2024, 73, 60. [Google Scholar] [CrossRef] [PubMed]
- Suzman, D.L.; Agrawal, S.; Ning, Y.-M.; Maher, V.E.; Fernandes, L.L.; Karuri, S.; Tang, S.; Sridhara, R.; Schroeder, J.; Goldberg, K.B.; et al. FDA Approval Summary: Atezolizumab or Pembrolizumab for the Treatment of Patients with Advanced Urothelial Carcinoma Ineligible for Cisplatin-Containing Chemotherapy. Oncologist 2018, 24, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Spira, A.; Raben, D.; Planchard, D.; Cho, B.; Özgüroğlu, M.; Daniel, D.; Villegas, A.; Vicente, D.; Hui, R.; et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann. Oncol. 2020, 31, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Avelumab: A Review in Metastatic Merkel Cell Carcinoma. Target. Oncol. 2018, 13, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Mansh, M. Ipilimumab and cancer immunotherapy: A new hope for advanced stage melanoma. Yale J. Biol. Med. 2011, 84, 381–389. [Google Scholar]
- Keam, S.J. Tremelimumab: First Approval. Drugs 2023, 83, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sheng, I.Y.; Ornstein, M.C. Ipilimumab and Nivolumab as First-Line Treatment of Patients with Renal Cell Carcinoma: The Evidence to Date. Cancer Manag. Res. 2020, 12, 4871–4881. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadegan, M.; Bavandpour, P.; Nowroozi, M.R.; Amini, E.; Kourosh-Arami, M.; Momeni, S.A.; Bokaie, S.; Sharifi, L. The Potential of T Cell Immunoglobulin and Mucin-Domain Containing-3 (Tim-3) in Designing Novel Immunotherapy for Bladder Cancer. Endocrine Metab. Immune Disord. Drug Targets 2021, 21, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Toor, S.M.; Khalaf, S.; Elkord, E. Breast Cancer Cells and PD-1/PD-L1 Blockade Upregulate the Expression of PD-1, CTLA-4, TIM-3 and LAG-3 Immune Checkpoints in CD4+ T Cells. Vaccines 2019, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Fojnica, A.; Ljuca, K.; Akhtar, S.; Gatalica, Z.; Vranic, S. An Updated Review of the Biomarkers of Response to Immune Checkpoint Inhibitors in Merkel Cell Carcinoma: Merkel Cell Carcinoma and Immunotherapy. Cancers 2023, 15, 5084. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Hernández, A.; González Del Portillo, E.; Tamayo-Velasco, Á.; Figuero-Pérez, L.; Zhilina-Zhilina, S.; Fonseca-Sánchez, E.; Miramontes-González, J.P. Immune checkpoint inhibitors in non-small cell lung cancer: From current perspectives to future treatments—A systematic review. Ann. Transl. Med. 2023, 11, 354. [Google Scholar] [CrossRef]
- Fitzpatrick, O.; Naidoo, J. Immunotherapy for Stage III NSCLC: Durvalumab and Beyond. Lung Cancer Targets Ther. 2021, 12, 123–131. [Google Scholar] [CrossRef]
- Zou, Y.; Ren, X.; Zhang, H.; Wang, Y.; Wang, H.; Bai, R.; Zhang, Z.; Sun, G.; Xu, L. Efficacy and safety of durvalumab + chemotherapy vs. atezolizumab + chemotherapy in the treatment of small-cell lung cancer: A retrospective comparative cohort study. J. Thorac. Dis. 2023, 15, 3339–3349. [Google Scholar] [CrossRef]
- Semenescu, L.E.; Kamel, A.; Ciubotaru, V.; Baez-Rodriguez, S.M.; Furtos, M.; Costachi, A.; Dricu, A.; Tătăranu, L.G. An Overview of Systemic Targeted Therapy in Renal Cell Carcinoma, with a Focus on Metastatic Renal Cell Carcinoma and Brain Metastases. Curr. Issues Mol. Biol. 2023, 45, 7680–7704. [Google Scholar] [CrossRef]
- Tovoli, F.; De Lorenzo, S.; Trevisani, F. Immunotherapy with Checkpoint Inhibitors for Hepatocellular Carcinoma: Where Are We Now? Vaccines 2020, 8, 578. [Google Scholar] [CrossRef]
- Kwapisz, D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol. Immunother. 2021, 70, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Lizardo, D.Y.; Kuang, C.; Hao, S.; Yu, J.; Huang, Y.; Zhang, L. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside. Biochim. Biophys. Acta (BBA) Rev. Cancer 2020, 1874, 188447. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.D.; Mathias-Machado, M.C.; Jácome, A.; Gil, M.; Fogacci, J.; Sodré, B.; Passarini, T.; Chaves, A.; Diniz, P.H.; Lino, F.; et al. PD-L1 testing in advanced gastric cancer—What physicians who treat this disease must know—A literature review. J. Gastrointest. Oncol. 2023, 14, 1560–1575. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, J.; Xu, K.; Chen, H.; Bian, C. PD-1/PD-L1 inhibitors for advanced or metastatic cervical cancer: From bench to bed. Front. Oncol. 2022, 12, 849352. [Google Scholar] [CrossRef]
- Corr, B.; Cosgrove, C.; Spinosa, D.; Guntupalli, S. Endometrial cancer: Molecular classification and future treatments. BMJ Med. 2022, 1, e000152. [Google Scholar] [CrossRef]
- Lin, A.Y.; Schnitter, J.M.; Gordon, L.I. I. Immune Checkpoint Blockade for the Treatment of Hodgkin Lymphoma. ImmunoTargets Ther. 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Armand, P.; Rodig, S.; Melnichenko, V.; Thieblemont, C.; Bouabdallah, K.; Tumyan, G.; Özcan, M.; Portino, S.; Fogliatto, L.; Caballero, M.D.; et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 3291–3299. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.R.; Platero, S.; Saini, K.S.; Curigliano, G.; Anderson, S. Immuno-oncology trends: Preclinical models, biomarkers, and clinical development. J. Immunother. Cancer 2022, 10, e003231. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wu, H.; Wu, J.; Ding, P.; He, J.; Sang, M.; Liu, L. Mechanisms of immune checkpoint inhibitors: Insights into the regulation of circular RNAS involved in cancer hallmarks. Cell Death Dis. 2024, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022, 20, 44. [Google Scholar] [CrossRef]
- Qiu, J.; Cheng, Z.; Jiang, Z.; Gan, L.; Zhang, Z.; Xie, Z. Immunomodulatory Precision: A Narrative Review Exploring the Critical Role of Immune Checkpoint Inhibitors in Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5490. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Mahmud, A.R.; Siddiquee, M.F.-.R.-S.; Shahriar, A.; Biswas, P.; Shimul, E.K.; Ahmed, S.Z.; Ema, T.I.; Rahman, N.; Khan, A.; et al. Role of T cells in cancer immunotherapy: Opportunities and challenges. Cancer Pathog. Ther. 2023, 1, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Khatwani, N.; Searles, T.G.; Turk, M.J.; Angeles, C.V. Memory CD8+ T cell responses to cancer. Semin. Immunol. 2020, 49, 101435. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Luo, S.; Ye, P.; Hwang, W.-L.; Cha, J.-H.; Yan, X.; Yang, W.-H. T-cell exhaustion and stemness in antitumor immunity: Characteristics, mechanisms, and implications. Front. Immunol. 2023, 14, 1104771. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ahn, E.; Kissick, H.T.; Ahmed, R. Reinvigorating Exhausted T Cells by Blockade of the PD-1 Pathway. Forum Immunopathol. Dis. Ther. 2015, 6, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Wei, Y.-Q.; Wei, X.-W. Immunosuppressive cells in cancer: Mechanisms and potential therapeutic targets. J. Hematol. Oncol. 2022, 15, 61. [Google Scholar] [CrossRef]
- Tang, T.; Huang, X.; Zhang, G.; Hong, Z.; Bai, X.; Liang, T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 72. [Google Scholar] [CrossRef]
- Wang, M.M.; Coupland, S.E.; Aittokallio, T.; Figueiredo, C.R. Resistance to immune checkpoint therapies by tumour-induced T-cell desertification and exclusion: Key mechanisms, prognostication and new therapeutic opportunities. Br. J. Cancer 2023, 129, 1212–1224. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Yang, F.; Huang, Z.-Q.; Li, Y.-Y.; Shi, H.-Y.; Sun, Q.; Ma, Y.; Wang, Y.; Zhang, Y.; Yang, S.; et al. T cells, NK cells, and tumor-associated macrophages in cancer immunotherapy and the current state of the art of drug delivery systems. Front. Immunol. 2023, 14, 1199173. [Google Scholar] [CrossRef]
- Olbryt, M.; Rajczykowski, M.; Widłak, W. Biological Factors behind Melanoma Response to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 4071. [Google Scholar] [CrossRef]
- Khosravi, G.; Mostafavi, S.; Bastan, S.; Ebrahimi, N.; Gharibvand, R.S.; Eskandari, N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun. 2024, 44, 521–553. [Google Scholar] [CrossRef] [PubMed]
- Kallingal, A.; Olszewski, M.; Maciejewska, N.; Brankiewicz, W.; Baginski, M. Cancer immune escape: The role of antigen presentation machinery. J. Cancer Res. Clin. Oncol. 2023, 149, 8131–8141. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Das, B.; Shin, S.; Chen, A. Challenges and Future Directions in the Management of Tumor Mutational Burden-High (TMB-H) Advanced Solid Malignancies. Cancers 2023, 15, 5841. [Google Scholar] [CrossRef]
- Yu, G.; Pang, Y.; Merchant, M.; Kesserwan, C.; Gangalapudi, V.; Abdelmaksoud, A.; Ranjan, A.; Kim, O.; Wei, J.S.; Chou, H.-C.; et al. Tumor Mutation Burden, Expressed Neoantigens and the Immune Microenvironment in Diffuse Gliomas. Cancers 2021, 13, 6092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, T.; Jiang, C. Recent Advancements in Nanomedicine for ‘Cold’ Tumor Immunotherapy. Nano-Micro Lett. 2021, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shao, C.; Shi, Y.; Han, W. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J. Hematol. Oncol. 2018, 11, 31. [Google Scholar] [CrossRef]
- Ziogas, D.C.; Theocharopoulos, C.; Lialios, P.-P.; Foteinou, D.; Koumprentziotis, I.-A.; Xynos, G.; Gogas, H. Beyond CTLA-4 and PD-1 Inhibition: Novel Immune Checkpoint Molecules for Melanoma Treatment. Cancers 2023, 15, 2718. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Molina, A.M.; Santana, T.T.; Redondo, M.; Romero, M.J.B. The Crucial Role of Inflammation and the Immune System in Colorectal Cancer Carcinogenesis: A Comprehensive Perspective. Int. J. Mol. Sci. 2024, 25, 6188. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Bai, R.; Chen, N.; Li, L.; Du, N.; Bai, L.; Lv, Z.; Tian, H.; Cui, J. Mechanisms of Cancer Resistance to Immunotherapy. Front. Oncol. 2020, 10, 1290. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Huang, Y.Y.; Hurlstone, A. The role of IFN-γ-signalling in response to immune checkpoint blockade therapy. Essays Biochem. 2023, 67, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, R.; Martin, A.; Bommarito, D.; Wang, K.; Hansen, S.H.; Freeman, G.J.; Ritz, J. Interferon-γ-induced activation of JAK1 and JAK2 suppresses tumor cell susceptibility to NK cells through upregulation of PD-L1 expression. OncoImmunology 2015, 4, e1008824. [Google Scholar] [CrossRef] [PubMed]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Lang, F.; Schrörs, B.; Löwer, M.; Türeci, Ö.; Sahin, U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat. Rev. Drug Discov. 2022, 21, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, M.; Qin, Y.; Gao, W.; Tao, L.; Su, W.; Zhong, J. Neoantigen: A New Breakthrough in Tumor Immunotherapy. Front. Immunol. 2021, 12, 672356. [Google Scholar] [CrossRef] [PubMed]
- Barrueto, L.; Caminero, F.; Cash, L.; Makris, C.; Lamichhane, P.; Deshmukh, R.R. Resistance to Checkpoint Inhibition in Cancer Immunotherapy. Transl. Oncol. 2020, 13, 100738. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Li, X.; Zhang, X.; Xue, S.; Cao, Y.; Niedermann, G.; Lu, Y.; Xue, J. Development of pharmacological immunoregulatory anti-cancer therapeutics: Current mechanistic studies and clinical opportunities. Signal Transduct. Target. Ther. 2024, 9, 126. [Google Scholar] [CrossRef]
- Reiss, K.A.; Forde, P.M.; Brahmer, J.R. Harnessing the Power of the Immune System Via Blockade of PD-1 and PD-L1: A Promising New Anticancer Strategy. Immunotherapy 2014, 6, 459–475. [Google Scholar] [CrossRef]
- Cui, J.-W.; Li, Y.; Yang, Y.; Yang, H.-K.; Dong, J.-M.; Xiao, Z.-H.; He, X.; Guo, J.-H.; Wang, R.-Q.; Dai, B.; et al. Tumor immunotherapy resistance: Revealing the mechanism of PD-1/PD-L1-mediated tumor immune escape. Biomed. Pharmacother. 2024, 171, 116203. [Google Scholar] [CrossRef]
- Brunell, A.E.; Lahesmaa, R.; Autio, A.; Thotakura, A.K. Exhausted T cells hijacking the cancer-immunity cycle: Assets and liabilities. Front. Immunol. 2023, 14, 1151632. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, H.E.; Zamora, A.E.; Thomas, P.G.; Youngblood, B.A. Cell-Intrinsic Barriers of T Cell-Based Immunotherapy. Trends Mol. Med. 2016, 22, 1000–1011. [Google Scholar] [CrossRef]
- Wu, Q.; Yu, X.; Li, J.; Sun, S.; Tu, Y. Metabolic regulation in the immune response to cancer. Cancer Commun. 2021, 41, 661–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, W.; Gao, Y.; Zhang, Y.; Zhao, Y.; Hao, S.; Ni, T. The Role of Tumor Metabolic Reprogramming in Tumor Immunity. Int. J. Mol. Sci. 2023, 24, 17422. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.M.; George, S. Emerging resistance vs. losing response to immune check point inhibitors in renal cell carcinoma: Two differing phenomena. Cancer Drug Resist. 2023, 6, 642–655. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Avalle, L.; Natalini, D.; Piccolantonio, A.; Arina, P.; Morellato, A.; Ala, U.; Taverna, D.; Turco, E.; et al. The role of tumor microenvironment in drug resistance: Emerging technologies to unravel breast cancer heterogeneity. Front. Oncol. 2023, 13, 1170264. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Luo, Y.; Rao, D.; Wang, T.; Lei, Z.; Chen, X.; Zhang, B.; Li, Y.; Liu, B.; Xia, L.; et al. Myeloid-derived suppressor cells in cancer: Therapeutic targets to overcome tumor immune evasion. Exp. Hematol. Oncol. 2024, 13, 39. [Google Scholar] [CrossRef]
- Khouzam, R.A.; Brodaczewska, K.; Filipiak, A.; Zeinelabdin, N.A.; Buart, S.; Szczylik, C.; Kieda, C.; Chouaib, S. Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies. Front. Immunol. 2021, 11, 613114. [Google Scholar] [CrossRef]
- Zhang, Y.; Brekken, R.A. Direct and indirect regulation of the tumor immune microenvironment by VEGF. J. Leukoc. Biol. 2022, 111, 1269–1286. [Google Scholar] [CrossRef]
- Moeckel, C.; Bakhl, K.; Georgakopoulos-Soares, I.; Zaravinos, A. The Efficacy of Tumor Mutation Burden as a Biomarker of Response to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2023, 24, 6710. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Escudero, M.; Arias-González, N.; Martínez-Cáceres, E. Regulatory cells and the effect of cancer immunotherapy. Mol. Cancer 2023, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jin, J.; Guo, D.; Tao, Z.; Hu, X. Immune Checkpoint Inhibitors Combined with Targeted Therapy: The Recent Advances and Future Potentials. Cancers 2023, 15, 2858. [Google Scholar] [CrossRef]
- Chyuan, I.-T.; Chu, C.-L.; Hsu, P.-N. Targeting the Tumor Microenvironment for Improving Therapeutic Effectiveness in Cancer Immunotherapy: Focusing on Immune Checkpoint Inhibitors and Combination Therapies. Cancers 2021, 13, 1188. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, Z.Z.; Zhong, N.N.; Cao, L.M.; Liu, B.; Bu, L.L. Charting new frontiers: Co-inhibitory immune checkpoint proteins in therapeutics, biomarkers, and drug delivery systems in cancer care. Transl. Oncol. 2023, 38, 101794. [Google Scholar] [CrossRef]
- Lao, Y.; Shen, D.; Zhang, W.; He, R.; Jiang, M. Immune Checkpoint Inhibitors in Cancer Therapy—How to Overcome Drug Resistance? Cancers 2022, 14, 3575. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hsu, J.-M.; Sun, L.; Wang, S.-C.; Hung, M.-C. Advances and prospects of biomarkers for immune checkpoint inhibitors. Cell Rep. Med. 2024, 5, 101621. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Jalal, S.; Earley, J.N.; Turchi, J.J. DNA repair: From genome maintenance to biomarker and therapeutic target. Clin. Cancer Res. 2011, 17, 6973–6984. [Google Scholar] [CrossRef]
- Chae, Y.K.; Anker, J.F.; Bais, P.; Namburi, S.; Giles, F.J.; Chuang, J.H. Mutations in DNA repair genes are associated with increased neo-antigen load and activated T cell infiltration in lung adenocarcinoma. Oncotarget 2018, 9, 7949–7960. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.Y.; Seier, K.; Ostrovnaya, I.; Regazzi, A.M.; Kania, B.E.; Moran, M.M.; Cipolla, C.K.; Bluth, M.J.; Chaim, J.; Al-Ahmadie, H.; et al. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from pd-1/pd-l1 blockade in advanced urothelial cancers. J. Clin. Oncol. 2018, 36, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Amrane, K.; Le Meur, C.; Besse, B.; Hemon, P.; Le Noac’h, P.; Pradier, O.; Berthou, C.; Abgral, R.; Uguen, A. HLA-DR expression in melanoma: From misleading therapeutic target to potential immunotherapy biomarker. Front. Immunol. 2023, 14, 1285895. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.Y.; Zhao, L.; Green, M.D.; Ravishankar, S.; Towlerton, A.M.H.; Scott, A.J.; Raghavan, M.; Cusick, M.F.; Warren, E.H.; Ramnath, N. Class II HLA-DRB4 is a predictive biomarker for survival following immunotherapy in metastatic non-small cell lung cancer. Sci. Rep. 2024, 14, 345. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Estrada, M.V.; Salgado, R.; Sanchez, V.; Doxie, D.B.; Opalenik, S.R.; Vilgelm, A.E.; Feld, E.; Johnson, A.S.; Greenplate, A.R.; et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat. Commun. 2016, 7, 10582. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.-P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, B.; Bieche, I.; Pasmant, E.; Hamzaoui, N.; Leulliot, N.; Michon, L.; de Reynies, A.; Attignon, V.; Foote, M.B.; Masliah-Planchon, J.; et al. PD-1 Blockade in Solid Tumors with Defects in Polymerase Epsilon. Cancer Discov. 2022, 12, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef] [PubMed]
- Corgnac, S.; Malenica, I.; Mezquita, L.; Auclin, E.; Voilin, E.; Kacher, J.; Halse, H.; Grynszpan, L.; Signolle, N.; Dayris, T.; et al. CD103+CD8+ TRM Cells Accumulate in Tumors of Anti-PD-1-Responder Lung Cancer Patients and Are Tumor-Reactive Lymphocytes Enriched with Tc17. Cell Rep. Med. 2020, 1, 100127. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, J.Y.; Jeon, S.H.; Nam, H.; Jung, J.H.; Jeon, M.; Kim, E.-S.; Bae, S.J.; Ahn, J.; Yoo, T.-K.; et al. CD39 + tissue-resident memory CD8 + T cells with a clonal overlap across compartments mediate antitumor immunity in breast cancer. Sci. Immunol. 2022, 7, eabn8390. [Google Scholar] [CrossRef]
- Miller, B.C.; Sen, D.R.; Al Abosy, R.; Bi, K.; Virkud, Y.V.; LaFleur, M.W.; Yates, K.B.; Lako, A.; Felt, K.; Naik, G.S.; et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019, 20, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hu, X.; Feng, K.; Gao, R.; Xue, Z.; Zhang, S.; Zhang, Y.; Corse, E.; Hu, Y.; Han, W.; et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat. Cancer 2020, 3, 108–121. [Google Scholar] [CrossRef]
- Chen, X.; Su, C.; Ren, S.; Zhou, C.; Jiang, T. Pan-cancer analysis of KEAP1 mutations as biomarkers for immunotherapy outcomes. Ann. Transl. Med. 2020, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Papillon-Cavanagh, S.; Doshi, P.; Dobrin, R.; Szustakowski, J.; Walsh, A.M. STK11 and KEAP1 mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open 2022, 5, e000706. [Google Scholar] [CrossRef]
- Di Federico, A.; De Giglio, A.; Parisi, C.; Gelsomino, F. STK11/LKB1 and KEAP1 mutations in non-small cell lung cancer: Prognostic rather than predictive? Eur. J. Cancer 2021, 157, 108–113. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, M.; Ciappina, G.; Spagnolo, C.C.; Squeri, A.; Passalacqua, M.I.; Aguilar, A.; Gonzalez-Cao, M.; Giovannetti, E.; Silvestris, N.; Rosell, R. Targeted therapies for KRAS-mutant non-small cell lung cancer: From preclinical studies to clinical development—A narrative review. Transl. Lung Cancer Res. 2023, 12, 346–368. [Google Scholar] [CrossRef]
- Taguchi, K.; Yamamoto, M. The KEAP1–NRF2 System in Cancer. Front. Oncol. 2017, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Sivakumar, S.; Schrock, A.B.; Madison, R.; Fabrizio, D.; Gjoerup, O.; Ross, J.S.; Frampton, G.M.; Napalkov, P.; Montesion, M.; et al. Comprehensive pan-cancer genomic landscape of KRAS altered cancers and real-world outcomes in solid tumors. npj Precis. Oncol. 2022, 6, 91. [Google Scholar] [CrossRef]
- Vranic, S.; Gatalica, Z. PD-L1 testing by immunohistochemistry in Immuno-Oncology. Bosn. J. Basic Med. Sci. 2022, 23, 15–25. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.-H.; Duhon, M.; et al. FDA-Approved and emerging next generation predictive biomarkers for immune checkpoint inhibitors in cancer patients. Front. Oncol. 2021, 11, 683419. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.N.; Calabuig-Fariñas, S.; de Mena, M.L.; Torres-Martínez, S.; González, C.G.; García, J.G.; González-Cruz, V.I.; Herrero, C.C. Programmed death-ligand 1 (PD-L1) as immunotherapy biomarker in breast cancer. Cancers 2022, 14, 307. [Google Scholar] [CrossRef]
- Li, J.-X.; Huang, J.-M.; Jiang, Z.-B.; Li, R.-Z.; Sun, A.; Leung, E.L.-H.; Yan, P.-Y. Current clinical progress of PD-1/PD-L1 immunotherapy and potential combination treatment in non–small cell lung cancer. Integr. Cancer Ther. 2019, 18, 1534735419890020. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Chemnitz, J.M.; Parry, R.V.; Nichols, K.E.; June, C.H.; Riley, J.L. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human t cell stimulation, but Only receptor ligation prevents t cell activation. J. Immunol. 2004, 173, 945–954. [Google Scholar] [CrossRef]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Schwartz, J.C.; Guo, X.; Bhatia, S.; Cao, E.; Lorenz, M.; Cammer, M.; Chen, L.; Zhang, Z.Y.; Edidin, M.A.; et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity 2004, 20, 337–347. [Google Scholar] [CrossRef]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubat, T.; Yagita, H.; Honjo, T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar] [CrossRef]
- Wang, J.; Yoshida, T.; Nakaki, F.; Hiai, H.; Okazaki, T.; Honjo, T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc. Natl. Acad. Sci. USA 2005, 102, 11823–11828. [Google Scholar] [CrossRef]
- Wang, J.; Okazaki, I.-M.; Yoshida, T.; Chikuma, S.; Kato, Y.; Nakaki, F.; Hiai, H.; Honjo, T.; Okazaki, T. PD-1 deficiency results in the development of fatal myocarditis in MRL mice. Int. Immunol. 2010, 22, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Honjo, T. PD-1 and PD-1 ligands: From discovery to clinical application. Int. Immunol. 2007, 19, 813–824. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar] [CrossRef]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7-H1 improves myeloid dendritic cell–mediated antitumor immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non–small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Agata, Y.; Kawasaki, A.; Sato, M.; Imamura, S.; Minato, N.; Yagita, H.; Nakano, T.; Honjo, T. Developmentally regulated expression of the PD-1 protein on the surface of double-negative(CD4−CD8−) thymocytes. Int. Immunol. 1996, 8, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, M.J.; Hopke, C.; Vandenbark, A.A.; Offner, H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of treg cells, and enhanced expression of the PD-1 costimulatory pathway. J. Neurosci. Res. 2006, 84, 370–378. [Google Scholar] [CrossRef]
- Boussiotis, V.A.; Chatterjee, P.; Li, L. Biochemical Signaling of PD-1 on T Cells and Its Functional Implications. Cancer J. 2014, 20, 265–271. [Google Scholar] [CrossRef]
- Cho, H.Y.; Lee, S.W.; Seo, S.K.; Choi, I.W.; Choi, I.; Lee, S.W. Interferon-sensitive response element (ISRE) is mainly responsible for IFN-α-induced upregulation of programmed death-1 (PD-1) in macrophages. Biochim. Biophys. Acta 2008, 1779, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2016, 8, 2171–2186. [Google Scholar] [CrossRef] [PubMed]
- Butte, M.J.; Keir, M.E.; Phamduy, T.B.; Sharpe, A.H.; Freeman, G.J. Programmed Death-1 Ligand 1 Interacts Specifically with the B7-1 Costimulatory Molecule to Inhibit T Cell Responses. Immunity 2007, 27, 111–122. [Google Scholar] [CrossRef]
- Mueller, S.N.; Vanguri, V.K.; Ha, S.-J.; West, E.E.; Keir, M.E.; Glickman, J.N.; Sharpe, A.H.; Ahmed, R. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J. Clin. Investig. 2010, 120, 2508–2515. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Hu, L.; Zhang, X.; Jiang, S.; Li, J.; Zhang, Z.; Wang, X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019, 10, 2298. [Google Scholar] [CrossRef]
- Riley, J.L. PD-1 signaling in primary T cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Akiba, H.; Koyanagi, A.; Azuma, M.; Yagita, H.; Okumura, K. Blockade of B7-H1 on Macrophages Suppresses CD4+ T Cell Proliferation by Augmenting IFN-γ-Induced Nitric Oxide Production. J. Immunol. 2005, 175, 1586–1592. [Google Scholar] [CrossRef]
- Keir, M.E.; Liang, S.C.; Guleria, I.; Latchman, Y.E.; Qipo, A.; Albacker, L.A.; Koulmanda, M.; Freeman, G.J.; Sayegh, M.H.; Sharpe, A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006, 203, 883–895. [Google Scholar] [CrossRef]
- Probst, H.C.; McCoy, K.; Okazaki, T.; Honjo, T.; van den Broek, M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 2005, 6, 280–286. [Google Scholar] [CrossRef]
- Nava, S.; Lisini, D.; Frigerio, S.; Bersano, A. Dendritic Cells and Cancer Immunotherapy: The Adjuvant Effect. Int. J. Mol. Sci. 2021, 22, 12339. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-H.; Chan, L.-C.; Li, C.-W.; Hsu, J.L.; Hung, M.-C. Mechanisms Controlling PD-L1 Expression in Cancer. Mol. Cell 2019, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Schöniger, S.; Jasani, B. The PD-1/PD-L1 pathway: A perspective on comparative immuno-oncology. Animals 2022, 12, 2661. [Google Scholar] [CrossRef]
- Zhao, Y.; Harrison, D.L.; Song, Y.; Ji, J.; Huang, J.; Hui, E. Antigen-Presenting Cell-Intrinsic PD-1 Neutralizes PD-L1 in cis to Attenuate PD-1 Signaling in T Cells. Cell Rep. 2018, 24, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Viguera, E.; Canceill, D.; Ehrlich, S. Replication slippage involves DNA polymerase pausing and dissociation. EMBO J. 2001, 20, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Angelis, G.L.D.; Bottarelli, L.; Azzoni, C.; Angelis, N.D.; Leandro, G.; Di Mario, F.; Gaiani, F.; Negri, F. Microsatellite instability in colorectal cancer. Acta Biomed. 2018, 89, 97–101. [Google Scholar] [CrossRef]
- Weissenbach, J.; Gyapay, G.; Dib, C.; Vignal, A.; Morissette, J.; Millasseau, P.; Vaysseix, G.; Lathrop, M. A second-generation linkage map of the human genome. Nature 1992, 359, 794–801. [Google Scholar] [CrossRef]
- Arzimanoglou, I.I.; Gilbert, F.; Barber, H.R. Microsatellite instability in human solid tumors. Cancer 1998, 82, 1808–1820. [Google Scholar] [CrossRef]
- Gatalica, Z.; Vranic, S.; Xiu, J.; Swensen, J.; Reddy, S. High microsatellite instability (MSI-H) colorectal carcinoma: A brief review of predictive biomarkers in the era of personalized medicine. Fam. Cancer 2016, 15, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Jiricny, J. The multifaceted mismatch-repair system. Nat. Rev. Mol. Cell Biol. 2006, 7, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Modrich, P.; Lahue, R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu. Rev. Biochem. 1996, 65, 101–133. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Zaanan, A.; Sinicrope, F.A. Implications of mismatch repair-deficient status on management of early stage colorectal cancer. J. Gastrointest. Oncol. 2015, 6, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancer—The stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087.e2073. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.-S.; Carethers, J.M. Chemotherapeutic implications in microsatellite unstable colorectal cancer1. Cancer Biomarkers 2006, 2, 51–60. [Google Scholar] [CrossRef]
- Profir, M.; Roşu, O.A.; Creţoiu, S.M.; Gaspar, B.S. Friend or foe: Exploring the relationship between the gut microbiota and the pathogenesis and treatment of digestive cancers. Microorganisms 2024, 12, 955. [Google Scholar] [CrossRef]
- Imai, K.; Yamamoto, H. Carcinogenesis and microsatellite instability: The interrelationship between genetics and epigenetics. Carcinogenesis 2007, 29, 673–680. [Google Scholar] [CrossRef]
- Plazzer, J.P.; Sijmons, R.H.; Woods, M.O.; Peltomäki, P.; Thompson, B.; Dunnen, J.T.D.; Macrae, F. The InSiGHT database: Utilizing 100 years of insights into lynch syndrome. Fam. Cancer 2013, 12, 175–180. [Google Scholar] [CrossRef]
- Hampel, H.; Frankel, W.L.; Martin, E.; Arnold, M.; Khanduja, K.; Kuebler, P.; Nakagawa, H.; Sotamaa, K.; Prior, T.W.; Westman, J.; et al. Screening for the lynch syndrome (hereditary nonpolyposis colorectal cancer). N. Engl. J. Med. 2005, 352, 1851–1860. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Lee, S.; Nafa, K.; Lee, J.; Romans, K.; Watson, P.; Gruber, S.B.; Euhus, D.; Kinzler, K.W.; et al. Prediction of germline mutations and cancer risk in the lynch syndrome. JAMA 2006, 296, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, E.; Mukherjee, B.; Raymond, V.M.; Tayob, N.; Kastrinos, F.; Sparr, J.; Wang, F.; Bandipalliam, P.; Syngal, S.; Gruber, S.B. Calculation of risk of colorectal and endometrial cancer among patients with lynch syndrome. Gastroenterology 2009, 137, 1621–1627. [Google Scholar] [CrossRef]
- Roth, A.D.; Tejpar, S.; Yan, P.; Fiocca, R.; Dietrich, D.; Delorenzi, M.; Labianca, R.; Cunningham, D.; Van Cutsem, E.; Bosman, F. Stage-specific prognostic value of molecular markers in colon cancer: Results of the translational study on the PETACC 3-EORTC 40993-SAKK 60–00 trial. J. Clin. Oncol. 2009, 27, 4002. [Google Scholar] [CrossRef]
- Koopman, M.; Kortman, G.A.M.; Mekenkamp, L.; Ligtenberg, M.J.L.; Hoogerbrugge, N.; Antonini, N.F.; Punt, C.J.A.; van Krieken, J.H.J.M. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br. J. Cancer 2009, 100, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Ashktorab, H.; Smoot, D.T.; Carethers, J.M.; Rahmanian, M.; Kittles, R.; Vosganian, G.; Doura, M.; Nidhiry, E.; Naab, T.; Momen, B.; et al. High incidence of microsatellite instability in colorectal cancer from African Americans. Clin. Cancer Res. 2003, 9, 1112–1117. [Google Scholar] [PubMed]
- Kumar, K.; Brim, H.; Giardiello, F.; Smoot, D.T.; Nouraie, M.; Lee, E.L.; Ashktorab, H. Distinct BRAF (V600E) and KRAS mutations in high microsatellite instability sporadic colorectal cancer in african americans. Clin. Cancer Res. 2009, 15, 1155–1161. [Google Scholar] [CrossRef]
- Soliman, A.S.; Bondy, M.L.; El-Badawy, S.A.; Mokhtar, N.; Eissa, S.; Bayoumy, S.; Seifeldin, I.A.; Houlihan, P.S.; Lukish, J.R.; Watanabe, T.; et al. Contrasting molecular pathology of colorectal carcinoma in Egyptian and Western patients. Br. J. Cancer 2001, 85, 1037–1046. [Google Scholar] [CrossRef]
- Ligtenberg, M.J.; Kuiper, R.P.; Chan, T.L.; Goossens, M.; Hebeda, K.M.; Voorendt, M.; Lee, T.Y.; Bodmer, D.; Hoenselaar, E.; Hendriks-Cornelissen, S.J.; et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat. Genet. 2009, 41, 112–117. [Google Scholar] [CrossRef]
- Lawes, D.; SenGupta, S.; Boulos, P. The clinical importance and prognostic implications of microsatellite instability in sporadic cancer. Eur. J. Surg. Oncol. (EJSO) 2003, 29, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Poynter, J.N.; Siegmund, K.D.; Weisenberger, D.J.; Long, T.I.; Thibodeau, S.N.; Lindor, N.; Young, J.; Jenkins, M.A.; Hopper, J.L.; Baron, J.A.; et al. Molecular characterization of msi-h colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 3208–3215. [Google Scholar] [CrossRef]
- Funkhouser, W.K.; Lubin, I.M.; Monzon, F.A.; Zehnbauer, B.A.; Evans, J.P.; Ogino, S.; Nowak, J.A. Relevance, pathogenesis, and testing algorithm for mismatch repair–defective colorectal carcinomas: A report of the association for molecular pathology. J. Mol. Diagn. 2012, 14, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Watanabe, T.; Wu, T.-T.; Rashid, A.; Li, S.; Hamilton, S.R. Histopathological identification of colon cancer with microsatellite instability. Am. J. Pathol. 2001, 158, 527–535. [Google Scholar] [CrossRef]
- Greenson, J.K.; Bonner, J.D.; Ben-Yzhak, O.; Cohen, H.I.; Miselevich, I.; Resnick, M.B.; Trougouboff, P.; Tomsho, L.D.; Kim, E.; Low, M.; et al. Phenotype of microsatellite unstable colorectal carcinomas: Well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am. J. Surg. Pathol. 2003, 27, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Shia, J.; Ellis, N.A.; Paty, P.B.; Nash, G.M.; Qin, J.; Offit, K.; Zhang, X.-M.; Markowitz, A.J.; Nafa, K.; Guillem, J.G.; et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am. J. Surg. Pathol. 2003, 27, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Yearsley, M.; Hampel, H.; Lehman, A.; Nakagawa, H.; de la Chapelle, A.; Frankel, W.L. Histologic features distinguish microsatellite-high from microsatellite-low and microsatellite-stable colorectal carcinomas, but do not differentiate germline mutations from methylation of the MLH1 promoter. Hum. Pathol. 2006, 37, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Greenson, J.K.; Huang, S.-C.; Herron, C.B.; Moreno, V.; Bonner, J.D.; Tomsho, L.P.B.; Ben-Izhak, O.; Cohen, H.I.; Trougouboff, P.; Bejhar, J.; et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am. J. Surg. Pathol. 2009, 33, 126–133. [Google Scholar] [CrossRef]
- Zlobec, I.; Bihl, M.P.; Foerster, A.; Rufle, A.; Lugli, A. The impact of CpG island methylator phenotype and microsatellite instability on tumour budding in colorectal cancer. Histopathology 2012, 61, 777–787. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Said, S.S.; Ibrahim, W.N. Cancer Resistance to Immunotherapy: Comprehensive Insights with Future Perspectives. Pharmaceutics 2023, 15, 1143. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Albacker, L.A.; Hopkins, A.C.; Montesion, M.; Murugesan, K.; Vithayathil, T.T.; Zaidi, N.; Azad, N.S.; Laheru, D.A.; Frampton, G.M.; et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. J. Clin. Investig. 2019, 4, 126908. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden–High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef] [PubMed]
- Samstein, R.M.; Lee, C.-H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef]
- Rousseau, B.; Foote, M.B.; Maron, S.B.; Diplas, B.H.; Lu, S.; Argilés, G.; Cercek, A.; Diaz, L.A. The Spectrum of Benefit from Checkpoint Blockade in Hypermutated Tumors. New Engl. J. Med. 2021, 384, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Gurjao, C.; Tsukrov, D.; Imakaev, M.; Luquette, L.; Mirny, L. Limited evidence of tumour mutational burden as a biomarker of response to immunotherapy. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ionescu, R.F.; Cozma, E.C.; Enache, R.M.; Cretoiu, S.M.; Iancu, M.A.; Mandea, M.; Profir, M.; Roşu, O.A.; Gaspar, B.S. Intestinal Microbiomics in Physiological and Pathological Conditions. In Advances in Probiotics for Health and Nutrition; Vasudeo, Z., Mohd, F.M.D., Puja, G., Bhupendra Gopalbhai, P., Eds.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Yi, M.; Qin, S.; Chu, Q.; Wu, K. The role of gut microbiota in immune checkpoint inhibitor therapy. HepatoBiliary Surg. Nutr. 2018, 7, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lv, J.; Guo, F.; Li, J.; Jia, Y.; Jiang, D.; Wang, N.; Zhang, C.; Kong, L.; Liu, Y.; et al. Gut Microbiome Influences the Efficacy of PD-1 Antibody Immunotherapy on MSS-Type Colorectal Cancer via Metabolic Pathway. Front. Microbiol. 2020, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Naqash, A.R.; Kihn-Alarcón, A.J.; Stavraka, C.; Kerrigan, K.; Vareki, S.M.; Pinato, D.J.; Puri, S. The role of gut microbiome in modulating response to immune checkpoint inhibitor therapy in cancer. Ann. Transl. Med. 2021, 9, 1034. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential Modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. mBio 2014, 5, e01438-14. [Google Scholar] [CrossRef]
- Ottman, N.; Huuskonen, L.; Reunanen, J.; Boeren, S.; Klievink, J.; Smidt, H.; Belzer, C.; de Vos, W.M. Characterization of Outer Membrane Proteome of Akkermansia muciniphila Reveals Sets of Novel Proteins Exposed to the Human Intestine. Front. Microbiol. 2016, 7, 1157. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, R.F.; Enache, R.M.; Cretoiu, S.M.; Gaspar, B.S. Gut Microbiome Changes in Gestational Diabetes. Int. J. Mol. Sci. 2022, 23, 12839. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, T.; Tu, X.; Huang, Y.; Zhang, H.; Tan, D.; Jiang, W.; Cai, S.; Zhao, P.; Song, R.; et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Gomes-Neto, J.C.; Lagkouvardos, I.; Ramer-Tait, A.E. Deciphering interactions between the gut microbiota and the immune system via microbial cultivation and minimal microbiomes. Immunol. Rev. 2017, 279, 8–22. [Google Scholar] [CrossRef]
- Everts, B. Metabolomics in Immunology Research. Methods Mol. Biol. 2018, 1730, 29–42. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Younis, A.; Gribben, J. Immune Checkpoint Inhibitors: Fundamental Mechanisms, Current Status and Future Directions. Immuno 2024, 4, 186–210. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Choi, J.; Lee, S.Y. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 2020, 20, e9. [Google Scholar] [CrossRef] [PubMed]
- Spiers, L.; Coupe, N.; Payne, M. Toxicities associated with checkpoint inhibitors—An overview. Rheumatology 2019, 58, vii7–vii16. [Google Scholar] [CrossRef] [PubMed]
- Shalitin, S. Endocrine-related adverse conditions in pediatric patients treated with immune checkpoint inhibition for malignancies. Horm. Res. Paediatr. 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ciurej, A.; Lewis, E.; Gupte, A.; Al-Antary, E. Checkpoint Immunotherapy in Pediatric Oncology: Will We Say Checkmate Soon? Vaccines 2023, 11, 1843. [Google Scholar] [CrossRef] [PubMed]
- Dagar, G.; Gupta, A.; Shankar, A.; Chauhan, R.; Macha, M.A.; Bhat, A.A.; Das, D.; Goyal, R.; Bhoriwal, S.; Pandita, R.K.; et al. The future of cancer treatment: Combining radiotherapy with immunotherapy. Front. Mol. Biosci. 2024, 11, 1409300. [Google Scholar] [CrossRef]
- Warszyńska, M.; Repetowski, P.; Dąbrowski, J.M. Photodynamic therapy combined with immunotherapy: Recent advances and future research directions. Coord. Chem. Rev. 2023, 495, 215350. [Google Scholar] [CrossRef]
- Profir, M.; Roşu, O.A.; Gaspar, B.S.; Cretoiu, S.M. Gut Microbiome and the Role of Its Metabolites as Promoters or Inhibitors in Gastrointestinal Cancers. In Interdisciplinary Cancer Research; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).