Hydroxychloroquine as an Adjunct Therapy for Diabetes in Pregnancy
Abstract
:1. Introduction
2. HCQ Mediated Effects on the Pathophysiology of Diabetes Mellitus
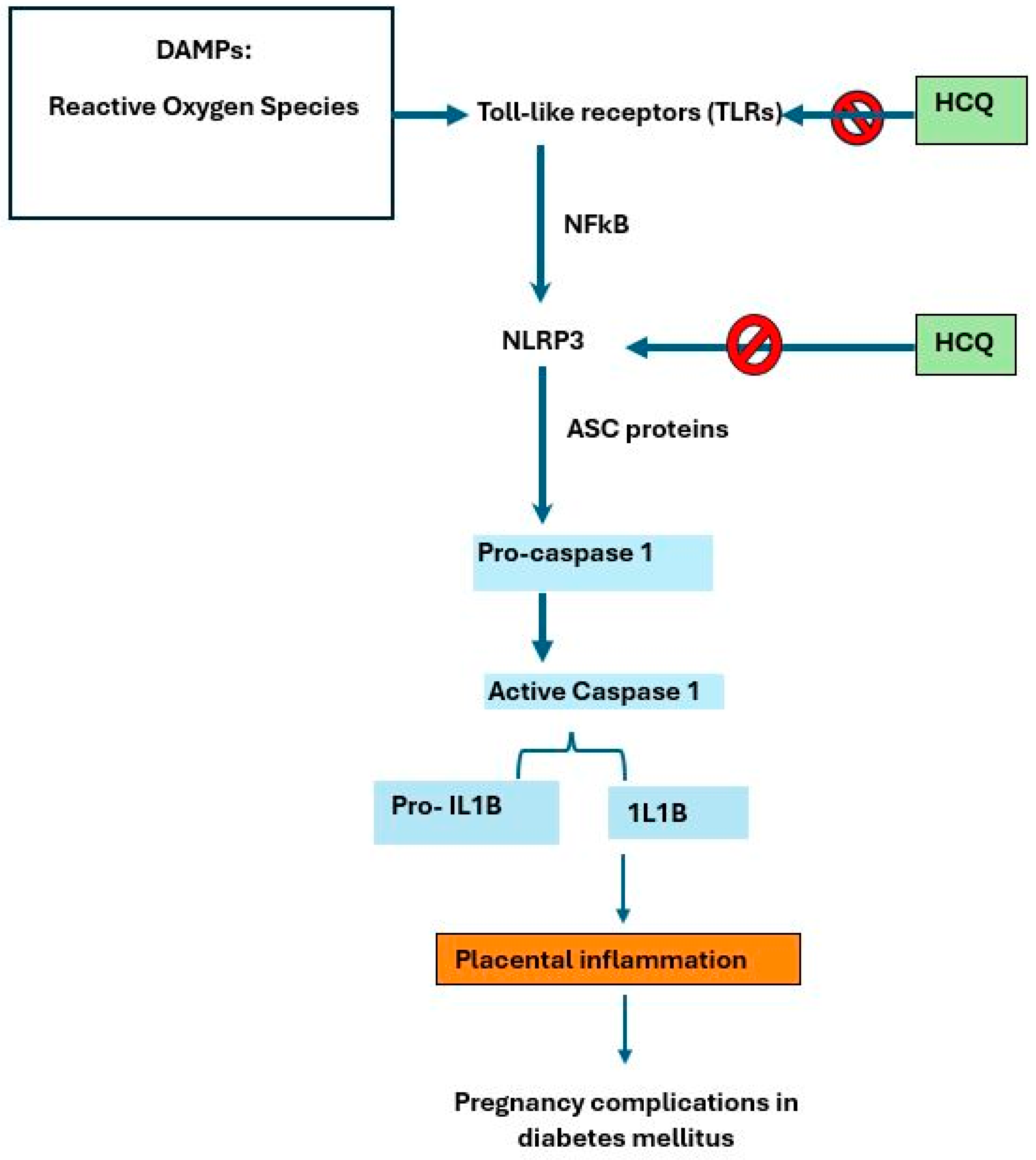
2.1. Inflammasome Complex
2.2. Inflammatory Cytokines
2.3. Oxidative Stress and Modulatory Effect
2.4. Hyperglycemia
3. Potential Use of HCQ in Pregnancy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0140673623013016 (accessed on 15 April 2024). [CrossRef] [PubMed]
- Schellenberg, E.S.; Dryden, D.M.; Vandermeer, B.; Ha, C.; Korownyk, C. Lifestyle Interventions for Patients with and at Risk for Type 2 Diabetes. Ann. Intern. Med. 2013, 159, 543–551. Available online: https://www.acpjournals.org/doi/abs/10.7326/0003-4819-159-8-201310150-00007 (accessed on 15 April 2024). [CrossRef]
- Yang, J.; Qian, F.; Chavarro, J.E.; Ley, S.H.; Tobias, D.K.; Yeung, E.; Hinkle, S.N.; Bao, W.; Li, M.; Liu, A.; et al. Modifiable risk factors and long term risk of type 2 diabetes among individuals with a history of gestational diabetes mellitus: Prospective cohort study. BMJ 2022, 378, e070312. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.P.H.; Papachristou Nadal, I.; Rysinova, V.; Basri, N.I.; Samsudin, I.N.; Forbes, A.; Noor, N.M.; Supian, Z.A.; Hassan, H.; Paimin, F.; et al. Study protocol on risk factors for the diagnosis of gestational diabetes mellitus in different trimesters and their relation to maternal and neonatal outcomes (GDM-RIDMAN). BMJ Open 2022, 12, e052554. [Google Scholar] [CrossRef]
- Idris, N.; Hatikah, C.C.; Munizah, M.; Rushdan, M. Universal versus selective screening for detection of GDM in Malaysian population. Malays. Fam. Physician 2009, 4, 87. [Google Scholar]
- Basri, N.I.; Mahdy, Z.A.; Ahmad, S.; Abdul Karim, A.K.; Shan, L.P.; Abdul Manaf, M.R.; Ismail, N.A.M. The World Health Organization (WHO) versus the International Association of Diabetes and Pregnancy Study Group (IADPSG) diagnostic criteria of gestational diabetes mellitus (GDM) and their associated maternal and neonatal outcomes. Horm. Mol. Biol. Clin. Investig. 2018, 34, 20170077. [Google Scholar] [CrossRef]
- Khanna, D.; Welch, B.S.; Rehman, A. Pathophysiology of Obesity. [Updated 2022 Oct 20]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572076/ (accessed on 20 April 2024).
- Sanches, J.M.; Zhao, L.N.; Salehi, A.; Wollheim, C.B.; Kaldis, P. Pathophysiology of type 2 diabetes and the impact of altered metabolic interorgan crosstalk. FEBS J. 2023, 290, 620–648. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev. Radcl. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Leutner, M.; Harreiter, J. Sex differences in type 2 diabetes. Diabetologia 2023, 66, 986–1002. [Google Scholar] [CrossRef]
- Goossens, G.H.; Jocken, J.W.E.; Blaak, E.E. Sexual dimorphism in cardiometabolic health: The role of adipose tissue, muscle and liver. Nat. Rev. Endocrinol. 2021, 17, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Marsh, M.L.; Oliveira, M.N.; Vieira-Potter, V.J. Adipocyte Metabolism and Health after the Menopause: The Role of Exercise. Nutrients 2023, 15, 444. [Google Scholar] [CrossRef]
- Gado, M.; Tsaousidou, E.; Bornstein, S.R.; Perakakis, N. Sex-based differences in insulin resistance. J. Endocrinol. 2024, 261, e230245. Available online: https://joe.bioscientifica.com/view/journals/joe/261/1/JOE-23-0245.xml (accessed on 21 April 2024). [CrossRef]
- Huynh, J.; Dawson, D.; Roberts, D.; Bentley-Lewis, R. A systematic review of placental pathology in maternal diabetes mellitus. Placenta 2015, 36, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Sina, R.E.; Keyes, D. Lifestyle Modification for Diabetes and Heart Disease Prevention; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Vajje, J.; Khan, S.; Kaur, A.; Kataria, H.; Sarpoolaki, S.; Goudel, A.; Bhatti, A.H.; Allahwala, D. Comparison of the Efficacy of Metformin and Lifestyle Modification for the Primary Prevention of Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Cureus 2023, 15, e47105. [Google Scholar] [CrossRef] [PubMed]
- Celli, A.; Barnouin, Y.; Jiang, B.; Blevins, D.; Colleluori, G.; Mediwala, S.; Armamento-Villareal, R.; Qualls, C.; Villareal, D.T. Lifestyle Intervention Strategy to Treat Diabetes in Older Adults: A Randomized Controlled Trial. Diabetes Care 2022, 45, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Timothy Garvey, W.; Karen Lau, K.H.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition therapy for adults with diabetes or prediabetes: A consensus report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Pollack, R.M.; Donath, M.Y.; LeRoith, D.; Leibowitz, G. Anti-inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care 2016, 39, S244–S252. [Google Scholar] [CrossRef]
- Rajput, R.; Upadhyay, P.; Rajput, S.; Saini, S.; Kharab, S. Effect of hydroxychloroquine on beta cell function, insulin resistance, and inflammatory markers in type 2 diabetes patients uncontrolled on glimepiride and metformin therapy. Int. J. Diabetes Dev. Ctries. 2023, 43, 955–960. [Google Scholar] [CrossRef]
- Li, D.; Zhong, J.; Zhang, Q.; Zhang, J. Effects of anti-inflammatory therapies on glycemic control in type 2 diabetes mellitus. Front. Immunol. 2023, 14, 1125116. [Google Scholar] [CrossRef]
- Agrawal, S.N. Chloroquine and Hydroxychloroquine: The History Revisited. Afr. J. Biol. Med. Res. 2021, 4, 1–7. [Google Scholar] [CrossRef]
- Wong, S.K. Repurposing new use for old drug chloroquine against metabolic syndrome: A review on animal and human evidence. Int. J. Med. Sci. 2021, 18, 2673–2688. [Google Scholar] [CrossRef]
- Wondafrash, D.Z.; Desalegn, T.Z.; Yimer, E.M.; Tsige, A.G.; Adamu, B.A.; Zewdie, K.A. Potential Effect of Hydroxychloroquine in Diabetes Mellitus: A Systematic Review on Preclinical and Clinical Trial Studies. J. Diabetes Res. 2020, 2020, 5214751. [Google Scholar] [CrossRef]
- Abd Rahman, R.; Min Tun, K.; Kamisan Atan, I.; Mohamed Said, M.S.; Mustafar, R.; Zainuddin, A.A. New Benefits of Hydroxychloroquine in Pregnant Women with Systemic Lupus Erythematosus: A Retrospective Study in a Tertiary Centre. Rev. Bras. Ginecol. Obstet. 2020, 42, 705–711. [Google Scholar] [CrossRef]
- Nori, W.; Akram, N.N.; Al-Ani, R.M. Update on hydroxychloroquine use in pregnancy. World J. Exp. Med. 2023, 13, 99–101. [Google Scholar] [CrossRef]
- Abd Rahman, R.; Murthi, P.; Singh, H.; Gurungsinghe, S.; Leaw, B.; Mockler, J.C.; Lim, R.; Wallace, E.M. Hydroxychloroquine mitigates the production of 8-isoprostane and improves vascular dysfunction: Implications for treating preeclampsia. Int. J. Mol. Sci. 2020, 21, 2504. [Google Scholar] [CrossRef] [PubMed]
- Murthi, P.; Pinar, A.A.; Dimitriadis, E.; Samuel, C.S. Inflammasomes—A molecular link for altered immunoregulation and inflammation mediated vascular dysfunction in preeclampsia. Int. J. Mol. Sci. 2020, 21, 1406. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, H.; Liu, B.; Zhang, Y.; Pan, X.; Yu, X.Y.; Shen, Z.; Song, Y.H. Inflammasomes as therapeutic targets in human diseases. Sig. Transduct. Target. Ther. 2021, 6, 247. [Google Scholar] [CrossRef]
- Yu, Z.-W.; Zhang, J.; Li, X.; Wang, Y.; Fu, Y.-H.; Gao, X.-Y. A new research hot spot: The role of NLRP3 inflammasome activation, a key step in pyroptosis, in diabetes and diabetic complications. Life Sci. 2020, 240, 117138. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Eugenia Schroeder, M.; Russo, S.; Costa, C.; Hori, J.; Tiscornia, I.; Bollati-Fogolín, M.; Zamboni, D.S.; Ferreira, G.; Cairoli, E.; Hill, M. Pro-inflammatory Ca++-activated K+ channels are inhibited by hydroxychloroquine. Sci. Rep. 2017, 7, 1892. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Hong, P.; Li, Z.; Lin, J.; Wu, X.; Nie, K.; Zhang, X.; Wan, J. Chloroquine inhibits NLRP3 inflammasomes activation and alleviates renal fibrosis in mouse model of hyperuricemic nephropathy with aggravation by a high-fat-diet. Int. Immunopharmacol. 2023, 120, 110353. [Google Scholar] [CrossRef]
- Yehualashet, A.S. Toll-like receptors as a potential drug target for diabetes mellitus and diabetes-associated complications. Diabetes Metab. Syndr. Obes. 2020, 13, 4763–4777. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Wen, Y.; Lv, L.-L.; Liu, H.; Ma, K.; Liu, B. Hydroxychloroquine Attenuates Renal Ischemia Reperfusion Injury By Inhibiting Cathepsin Mediated Nlrp3 Inflammasome Activation. Nephrol. Dial. Transplant. 2017, 32, 158. [Google Scholar] [CrossRef]
- Sepehri, Z.; Kiani, Z.; Nasiri, A.A.; Kohan, F. Toll-like receptor 2 and type 2 diabetes. Cell Mol. Biol. Lett. 2016, 21, 2. [Google Scholar] [CrossRef]
- Bahadoram, M.; Keikhaei, B.; Saeedi-Boroujeni, A.; Mahmoudian-Sani, M.R. Chloroquine/hydroxychloroquine: An inflammasome inhibitor in severe COVID-19? Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 997–1001. [Google Scholar] [CrossRef]
- Tang, W.; Cao, Z.; Han, M.; Wang, Z.; Chen, J.; Sun, W.; Wu, Y.; Xiao, W.; Liu, S.; Chen, E.; et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: Open label, randomised controlled trial. BMJ 2020, 369, 1849. [Google Scholar] [CrossRef]
- Toledo, F.G.S.; Miller, R.G.; Helbling, N.L.; Zhang, Y.; DeLany, J.P. The effects of hydroxychloroquine on insulin sensitivity, insulin clearance and inflammation in insulin-resistant adults: A randomized trial. Diabetes Obes. Metab. 2021, 23, 1252–1261. [Google Scholar] [CrossRef]
- Freeman, D.J.; Norrie, J.; Caslake, M.J.; Gaw, A.; Ford, I.; Lowe, G.D.; O’Reilly, D.S.J.; Packard, C.J.; Sattar, N.; West of Scotland Coronary Prevention Study Group. C-Reactive Protein Is an Independent Predictor of Risk for the Development of Diabetes in the West of Scotland Coronary Prevention Study. Diabetes 2002, 51, 1596–1600. [Google Scholar] [CrossRef]
- Carstensen, M.; Herder, C.; Kivimäki, M.; Jokela, M.; Roden, M.; Shipley, M.J.; Witte, D.R.; Brunner, E.J.; Tabák, A.G. Accelerated increase in serum interleukin-1 receptor antagonist starts 6 years before diagnosis of type 2 diabetes: Whitehall II prospective cohort study. Diabetes 2010, 59, 1222–1227. [Google Scholar] [CrossRef]
- Maedler, K.; Sergeev, P.; Ris, F.; Oberholzer, J.; Joller-Jemelka, H.I.; Spinas, G.A.; Witte, D.R.; Brunner, E.J.; Tabák, A.G. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Investig. 2002, 110, 851–860. [Google Scholar] [CrossRef]
- Matsumori, A. Novel Biomarkers of Inflammation for the Management of Diabetes: Immunoglobulin-Free Light Chains. Biomedicines 2022, 10, 666. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Zhou, Z.C.; Niu, R.; Su, Y.T.; Sun, Y.; Liu, H.L.; Teng, J.L.; Ye, J.N.; Shi, H.; Yang, C.D.; et al. Hydroxychloroquine improves obesity-associated insulin resistance and hepatic steatosis by regulating lipid metabolism. Front. Pharmacol. 2019, 10, 855. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, A.A.M.; El-Firgany, A.E.D.L. Hydroxychloroquine hindering of diabetic isletopathy carries its signature on the inflammatory cytokines. J. Mol. Histol. 2016, 47, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Chandurkar, N.; Thomas, N.; Viswanathan, V.; Deshpande, A.; Gupta, O.P.; Shah, A.; Kakrani, A.; Bhandari, S.; Thulasidharan, N.K.; et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: A double blind, randomized comparison with pioglitazone. Curr. Med. Res. Opin. 2014, 30, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dixit, P.K.; Ghana, P.; Abhisheka, K.; Khurana, H.; Jha, V.K.; Mahapatra, D.; Goel, J.; Ahmed, S.; Varadaraj, G. Open-label randomized control trial of hydroxychloroquine in patients with moderate to severe coronavirus disease 2019 infection. Med. J. Armed Forces India 2021, 77, S305–S311. [Google Scholar] [CrossRef]
- Wasko, M.C.M.; McClure, C.K.; Kelsey, S.F.; Huber, K.; Orchard, T.; Toledo, F.G.S. Antidiabetogenic effects of hydroxychloroquine on insulin sensitivity and beta cell function: A randomised trial. Diabetologia 2015, 58, 2336–2343. [Google Scholar] [CrossRef]
- McCarthy, C.G.; Wenceslau, C.F.; Goulopoulou, S.; Ogbi, S.; Matsumoto, T.; Webb, R.C. Autoimmune therapeutic chloroquine lowers blood pressure and improves endothelial function in spontaneously hypertensive rats. Pharmacol. Res. 2016, 113, 384–394. [Google Scholar] [CrossRef]
- Hirata, Y.Y.; Yamamoto, H.; Atta, M.S.; Mahmoud, S.; Oh-hashi, K.; Kiuchi, K. Chloroquine inhibits glutamate-induced death of a neuronal cell line by reducing reactive oxygen species through sigma-1 receptor. J. Neurochem. 2011, 119, 839–847. [Google Scholar] [CrossRef]
- Zannah, S.; Islam, M.S.; Rahman, A.F.M.; Asaduzzaman, M.; Al Bari, A.A.; Ali, Y.; Jahirul Islam, G.; Alam, A.H.M.; Ali, H.; Rashid, M. Antidiabetic Drugs in Combination with Hydroxychloroquine Improve Glycemic Control in Alloxan Induced Diabetic Rats. Pharmacol. Pharm. 2014, 5, 725–735. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Haro Girón, S.; Monserrat Sanz, J.; Ortega, M.A.; Garcia-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Boaru, D.L.; de Leon-Oliva, D.; Guijarro, L.G.; Atienza-Perez, M.; et al. Prognostic Value of Malondialdehyde (MDA) in the Temporal Progression of Chronic Spinal Cord Injury. J. Pers. Med. 2023, 13, 626. [Google Scholar] [CrossRef]
- Zannah, S.; Islam, M.; Ali, Y.; Asaduzzaman, M.; Sarwar, M.S.; Al Bari, A.A.; Rashid, M. Antidiabetics in Combination with Hydroxychloroquine Improve Antioxidant and Hepatoprotective Activities in Alloxan-Induced Diabetic Rats. Bangladesh Pharm. J. 2015, 18, 72–77. [Google Scholar] [CrossRef]
- Emami, J.; Pasutto, F.M.; Mercer, J.R.; Jamali, F. Inhibition of insulin metabolism by hydroxychloroquine and its enantiomers in cytosolic fraction of liver homogenates from healthy and diabetic rats. Life Sci. 1999, 64, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Rezabakhsh, A.; Montazersaheb, S.; Nabat, E.; Hassanpour, M.; Montaseri, A.; Malekinejad, H.; Movassaghpour, A.A.; Rahbarghazi, R.; Garjani, A. Effect of hydroxychloroquine on oxidative/nitrosative status and angiogenesis in endothelial cells under high glucose condition. BioImpacts 2017, 7, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Shalash, M.; Badra, M.; Imbaby, S.; ElBanna, E. Malondialdehyde in Type 2 Diabetics And Association with Cardiovascular Risk Factors. J. Med. Res. Inst. 2020, 41, 21–30. [Google Scholar] [CrossRef]
- Arias de la Rosa, I.; Escudero-Contreras, A.; Ruiz-Ponce, M.; Román-Rodríguez, C.; Pérez-Sánchez, C.; Ábalos-Aguilera, M.D.C.; Ortega-Castro, R.; Alcaide, J.; Murri, M.; Font, P.; et al. Molecular Changes in the Adipose Tissue Induced by Rheumatoid Arthritis: Effects of Disease-Modifying Anti-Rheumatic Drugs. Front. Immunol. 2021, 12, 744022. [Google Scholar] [CrossRef]
- Rekedal, L.R.; Massarotti, E.; Garg, R.; Bhatia, R.; Gleeson, T.; Lu, B.; Solomon, D.H. Changes in glycosylated hemoglobin after initiation of hydroxychloroquine or methotrexate treatment in diabetes patients with rheumatic diseases. Arthritis Care Res. 2010, 62, 3569–3573. [Google Scholar] [CrossRef]
- Puvvada, R.K.; Adusumilli, P.; Maddukuri, R.K.; Samaksha PB, S.; Annam, M.G.; Undela, K. Efficacy and Safety of Hydroxychloroquine in the Treatment of Type-2 Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Young Pharm. 2022, 14, 402–407. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Simental-Mendía, M.; Sánchez-García, A.; Linden-Torres, E. Effect of hydroxychloroquine on glucose control in patients with and without diabetes: A systematic review and meta-analysis of randomized controlled clinical trials. Eur. J. Clin. Pharmacol. 2021, 77, 1705–1712. [Google Scholar] [CrossRef]
- McGill, J.B.; Johnson, M.; Hurst, S.; Cade, W.T.; Yarasheski, K.E.; Ostlund, R.E.; Schechtman, K.B.; Razani, B.; Kastan, M.B.; McClain, D.A.; et al. Low dose chloroquine decreases insulin resistance in human metabolic syndrome but does not reduce carotid intima-media thickness. Diabetol. Metab. Syndr. 2019, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Rifas-Shiman, S.L.; Perng, W.; Oken, E.; Gillman, M.W. Maternal inflammation during pregnancy and childhood adiposity. Obesity 2016, 24, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Hauguel-De Mouzon, S.; Lepercq, J.; Challier, J.C.; Huston-Presley, L.; Friedman, J.E.; Kalhan, S.C.; Catalano, P.M. TNF-Is a Predictor of Insulin Resistance in Human Pregnancy. Diabetes 2002, 51, 2207–2213. [Google Scholar] [CrossRef]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Review Article Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 2019, 5320156. [Google Scholar] [CrossRef]
- Abell, S.K.; De Courten, B.; Boyle, J.A.; Teede, H.J. Inflammatory and other biomarkers: Role in pathophysiology and prediction of gestational diabetes mellitus. Int. J. Mol. Sci. 2015, 16, 13442–13473. [Google Scholar] [CrossRef] [PubMed]
- Mackin, S.T.; Nelson, S.M.; Wild, S.H.; Colhoun, H.M.; Wood, R.; Lindsay, R.S. Factors associated with stillbirth in women with diabetes. Diabetologia 2019, 62, 1938–1947. [Google Scholar] [CrossRef]
- Oros Ruiz, M.; Perejón López, D.; Serna Arnaiz, C.; Siscart Viladegut, J.; Àngel Baldó, J.; Sol, J. Maternal and foetal complications of pregestational and gestational diabetes: A descriptive, retrospective cohort study. Sci. Rep. 2024, 14, 9017. [Google Scholar] [CrossRef]
- Murthi, P.; Rajaraman, G. Inflammasomes in the Pathophysiology of Maternal Obesity: Potential Therapeutic Targets to Reduce Long-Term Adverse Health Outcomes in the Mother and Offspring. Curr. Vasc. Pharmacol. 2021, 19, 165–175. [Google Scholar] [CrossRef]
- Kohli, S.; Isermann, B. Placental hemostasis and sterile inflammation: New insights into gestational vascular disease. Thromb. Res. 2017, 151, S30–S33. [Google Scholar] [CrossRef]
- Ravikumar, G.; Liza, V. Clinical and Histological Associations of Chronic Inflammatory Lesions in Preterm Placentas: Uncovering the Hidden Dangers. Pediatr. Dev. Pathol. 2023, 27, 59–66. [Google Scholar] [CrossRef]
- Huppertz, B. Placental Development with Histological Aspects; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–27. [Google Scholar] [CrossRef]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, E.; Talton, O.O.; Schust, D.J.; Schulz, L.C. Placental structural abnormalities in gestational diabetes and when they develop: A scoping review. Placenta 2021, 116, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Berceanu, C.; Tetileanu, A.V.; Brǎtilǎ, E.; Navolan, D.; Ciortea, R.; Berceanu, S.; Cîrstoiu, M.M.; Ofițeru, A.M.; Bohîlțea, R.E.; Stepan, A.E.; et al. Morphologic and ultrasound survey in type 2 diabetic placenta. Rom. J. Morphol. Embryol. 2018, 59, 175–186. [Google Scholar] [PubMed]
- Mulla, M.J.; Weel, I.C.; Potter, J.A.; Gysler, S.M.; Salmon, J.E.; Peraçoli, M.T.S.; Rothlin, C.V.; Chamley, L.W.; Abrahams, V.M. Antiphospholipid Antibodies Inhibit Trophoblast Toll-Like Receptor and Inflammasome Negative Regulators. Arthritis Rheumatol. 2018, 70, 891–902. [Google Scholar] [CrossRef]
- Lai, Y.M.; Tan, G.C.; Shah, S.A.; Abd Rahman, R.; Mohd Saleh, M.F.; Mansor, S.; Khong, T.Y.; Wong, Y.P. Non-hypertensive gestational diabetes mellitus: Placental histomorphology and its association with perinatal outcomes. Placenta 2024, 147, 21–27. Available online: https://www.sciencedirect.com/science/article/pii/S0143400424000213 (accessed on 12 May 2024). [CrossRef]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M.F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef]
- Escudero, C.; Gonzlez, M.; Acurio, J.; Valenzuela, F.; Sobrevi, L. The Role of Placenta in the Fetal Programming Associated to Gestational Diabetes. In Gestational Diabetes—Causes, Diagnosis and Treatment; InTech: London, UK, 2013. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Fabbri, A. Antihyperglycemic properties of hydroxychloroquine in patients with diabetes: Risks and benefits at the time of COVID-19 pandemic. J. Diabetes 2020, 12, 659–667. [Google Scholar] [CrossRef]
- Targońska-Stępniak, B. Antidiabetic effect of disease-modifying antirheumatic drugs. Rheumatol. Forum 2022, 8, 122–128. [Google Scholar] [CrossRef]
- Canti, V.; Scarrone, M.; De Lorenzo, R.; Ramirez, G.A.; Erra, R.; Bordoli, S.; Cella, S.; Schmit, E.; Rosa, S.; Castiglioni, M.T.; et al. Low incidence of intrauterine growth restriction in pregnant patients with systemic lupus erythematosus taking hydroxychloroquine. Immunol. Med. 2021, 44, 204–210. [Google Scholar] [CrossRef]
- Chambers, M.A.; Mecham, C.; Arreola, E.V.; Sinha, M. Increase in the Number of Pediatric New-Onset Diabetes and Diabetic Ketoacidosis Cases During the COVID-19 Pandemic. Endocr. Pract. 2022, 28, 479–485. [Google Scholar] [CrossRef]
- Mathiesen, E.R.; Ali, N.; Alibegovic, A.C.; Anastasiou, E.; Cypryk, K.; De Valk, H.; Dores, J.; Dunne, F.; Gall, M.A.; Garcia, S.D.; et al. Risk of major congenital malformations or perinatal or neonatal death with insulin detemir versus other basal insulins in pregnant women with preexisting diabetes: The real-world evolve study. Diabetes Care 2021, 44, 2069–2077. [Google Scholar] [CrossRef]
- Costedoat-Chalumeau, N.; Amoura, Z.; Duhaut, P.; Huong, D.L.T.; Sebbough, D.; Wechsler, B.; Vauthier, D.; Denjoy, I.; Lupoglazoff, J.M.; Piette, J.C.; et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: A study of one hundred thirty-three cases compared with a control group. Arthritis Rheum. 2003, 48, 3207–3211. [Google Scholar] [CrossRef]
- Clowse, M.E.B.; Eudy, A.M.; Balevic, S.; Sanders-Schmidler, G.; Kosinski, A.; Fischer-Betz, R.; Gladman, D.D.; Molad, Y.; Nalli, C.; Mokbel, A.; et al. Hydroxychloroquine in the pregnancies of women with lupus: A meta-analysis of individual participant data. Lupus Sci. Med. 2022, 9, e000651. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Liu, Y.; Zhao, X.; Ma, Y.; Wang, Y. Hydroxychloroquine improves pregnancy outcomes of women with positive antinuclear antibody spectrum test results. Front. Med. 2023, 10, 1113127. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis Neto, E.T.; Kakehasi, A.M.; de Medeiros Pinheiro, M.; Ferreira, G.A.; Lopes Marques, C.D.; da Mota, L.M.H.; Paiva, E.D.S.; Pileggi, G.C.S.; Sato, E.I.; Reis, A.P.M.G. Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Adv. Rheumatol. 2020, 60, 32. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Gayed, M.; Khamashta, M.A.; Leone, F.; Toescu, V.; Bruce, I.N.; Giles, I.; Teh, L.S.; McHugh, N.; Akil, M.; et al. Outcomes of children born to mothers with systemic lupus erythematosus exposed to hydroxychloroquine or azathioprine. Rheumatology 2023, 62, 1124–1135. [Google Scholar] [CrossRef]
- Huybrechts, K.F.; Bateman, B.T.; Zhu, Y.; Straub, L.; Mogun, H.; Kim, S.C.; Desai, R.J.; Hernandez-Diaz, S. Hydroxychloroquine early in pregnancy and risk of birth defects. Am. J. Obstet. Gynecol. 2021, 224, 290.e1–290.e22. [Google Scholar] [CrossRef]
- Fasano, S.; Messiniti, V.; Iudici, M.; Coscia, M.A.; Ciccia, F. Hydroxychloroquine daily dose, hydroxychloroquine blood levels and the risk of flares in patients with systemic lupus erythematosus. Lupus Sci. Med. 2023, 10, e000841. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.Y.; Melles, R.B.; Mieler, W.F. American Academy of Ophthalmology Statement: Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef]
- Dima, A.; Jurcut, C.; Chasset, F.; Felten, R.; Arnaud, L. Hydroxychloroquine in systemic lupus erythematosus: Overview of current knowledge. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X211073001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basri, N.I.; Murthi, P.; Abd Rahman, R. Hydroxychloroquine as an Adjunct Therapy for Diabetes in Pregnancy. Int. J. Mol. Sci. 2024, 25, 9681. https://doi.org/10.3390/ijms25179681
Basri NI, Murthi P, Abd Rahman R. Hydroxychloroquine as an Adjunct Therapy for Diabetes in Pregnancy. International Journal of Molecular Sciences. 2024; 25(17):9681. https://doi.org/10.3390/ijms25179681
Chicago/Turabian StyleBasri, Nurul Iftida, Padma Murthi, and Rahana Abd Rahman. 2024. "Hydroxychloroquine as an Adjunct Therapy for Diabetes in Pregnancy" International Journal of Molecular Sciences 25, no. 17: 9681. https://doi.org/10.3390/ijms25179681
APA StyleBasri, N. I., Murthi, P., & Abd Rahman, R. (2024). Hydroxychloroquine as an Adjunct Therapy for Diabetes in Pregnancy. International Journal of Molecular Sciences, 25(17), 9681. https://doi.org/10.3390/ijms25179681





