Aging-Related Metabolome Analysis of the Masseter Muscle in Senescence-Accelerated Mouse-Prone 8
Abstract
1. Introduction
2. Results
2.1. Body Weight
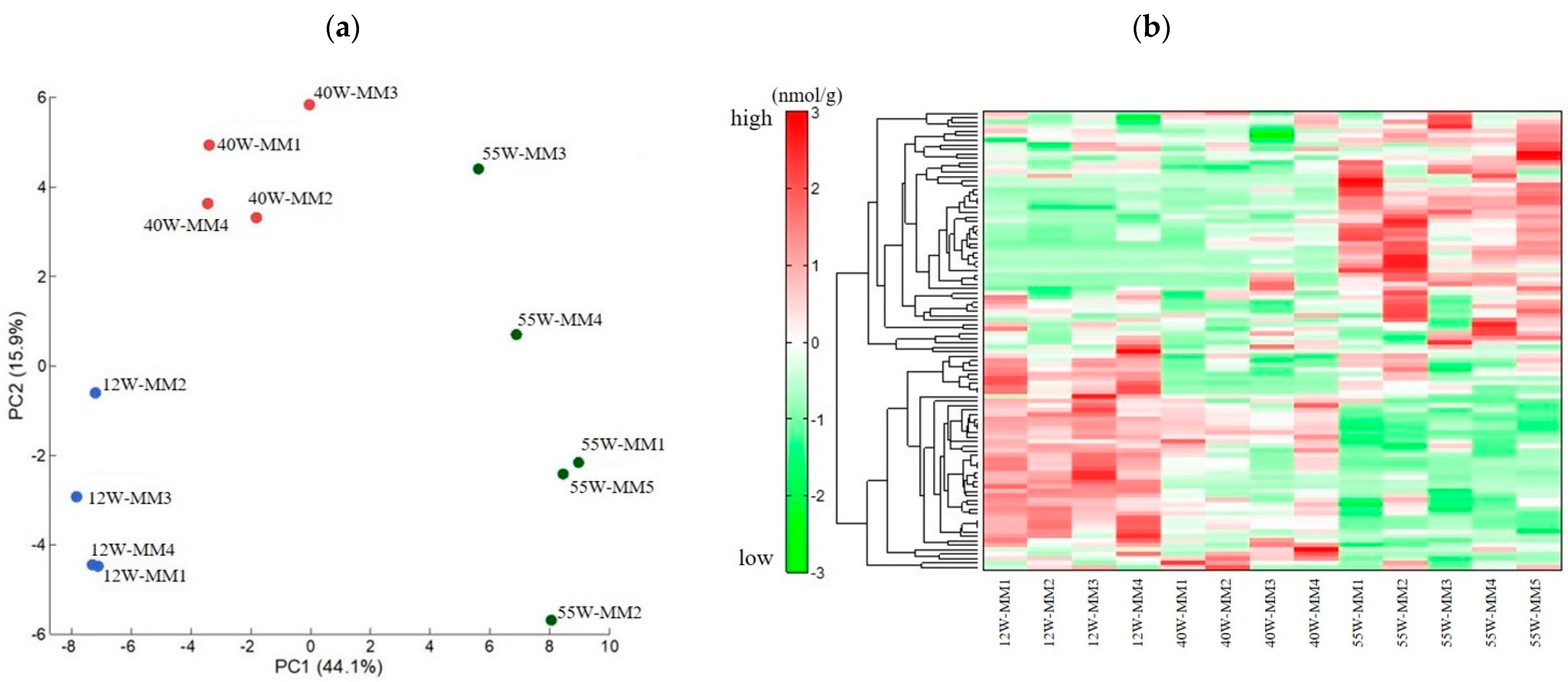
2.2. Metabolome Analysis
2.2.1. Change in Metabolite Levels between 12 and 40−Week−Old Mice
2.2.2. Change in Metabolite Levels between 12 and 55−Week−Old Mice
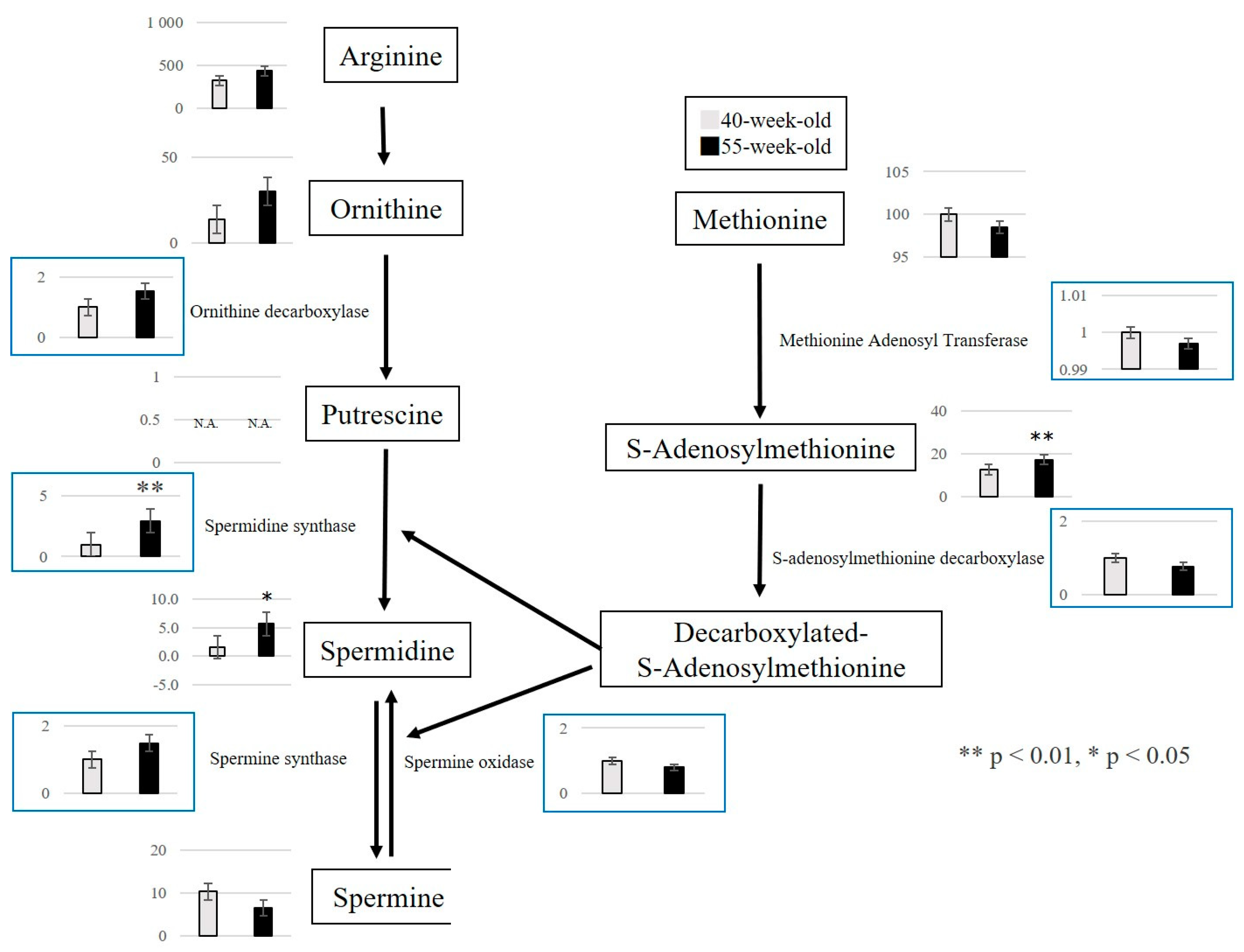
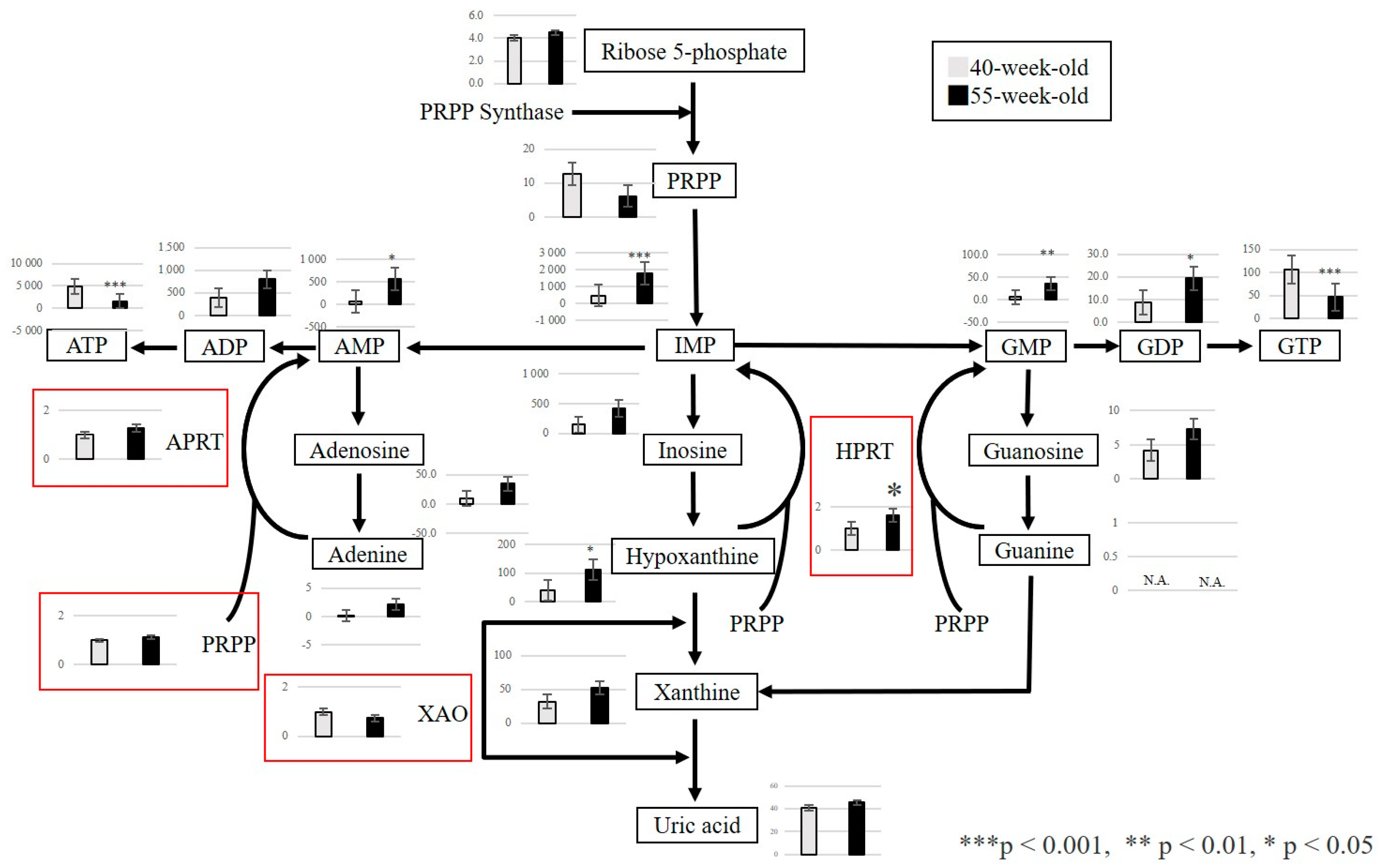
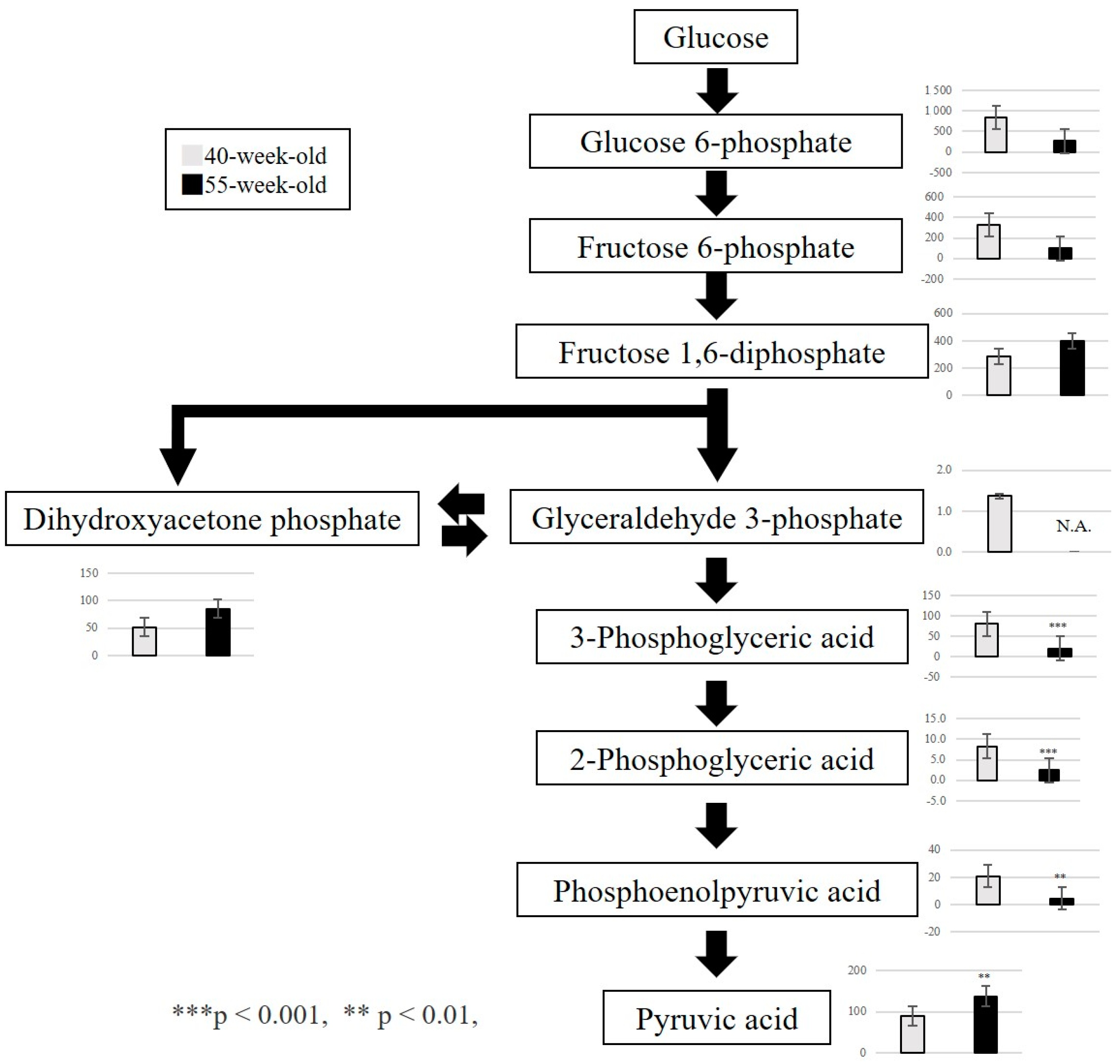
2.2.3. Changes in Metabolite Levels between 40 and 55−Week−Old Mice
2.3. Quantitative Analysis of Metabolites Using RT-PCR
3. Discussion
4. Materials and Methods
4.1. Animal Experiment
4.2. Metabolite Extraction and Capillary Electrophoresis–Mass Spectrometry (CE-MS) Metabolome Analysis
4.3. Quantitative Analysis of Gene Expression in the Masseter Muscle with Aging
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, H.; Watanabe, Y.; Sakai, K.; Kim, H.; Katakura, A. Relationship between chewing ability and sarcopenia in Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 2015, 15, 1007–1012. [Google Scholar] [CrossRef]
- Iwasaki, M.; Yoshihara, A.; Sato, N.; Sato, M.; Minagawa, K.; Shimada, M.; Nishimuta, M.; Ansai, T.; Yoshitake, Y.; Ono, T.; et al. A 5-year longitudinal study of association of maximum bite force with development of frailty in community-dwelling older adults. J. Oral. Rehabil. 2018, 45, 17–24. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef]
- Snow, L.M.; McLoon, L.K.; Thompson, L.V. Adult and developmental myosin heavy chain isoforms in soleus muscle of aging Fischer Brown Norway rat. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 286, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.Y.; Leung, K.S.; Siu, P.M.; Qin, J.H.; Chow, S.K.; Qin, L.; Li, C.Y.; Cheung, W.H. Muscle mass, structural and functional investigations of senescence-accelerated mouse P8 (SAMP8). Exp. Anim. 2015, 64, 425–433. [Google Scholar] [PubMed]
- Hoshino, T.; Kato, Y.; Sugahara, K.; Katakura, A. Aging-related metabolic changes in the extensor digitorum longus muscle of senescence-accelerated mouse-prone 8. Geriatr. Gerontol. Int. 2022, 22, 160–167. [Google Scholar] [CrossRef]
- Uchitomi, R.; Hatazawa, Y.; Senoo, N.; Yoshioka, K.; Fujita, M.; Shimizu, T.; Miura, S.; Ono, Y.; Kamei, Y. Metabolomic analysis of skeletal muscle in aged mice. Sci. Rep. 2019, 9, 10425. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Iijima, K.; Watanabe, Y.; Tanaka, T.; Iwasa, Y.; Edahiro, A.; Ohara, Y.; Motokawa, K.; Shirobe, M.; Hirano, H. Development of a simple method to measure masseter musclemass. Gerodontology 2020, 4, 384–388. [Google Scholar]
- Umeki, K.; Watanabe, Y.; Hirano, H.; Edahiro, E.; Ohara, Y.; Yoshida, H.; Obuchi, S.; Kawai, H.; Murakami, M.; Takagi, D.; et al. The relationship between masseter muscle thickness and appendicular skeletal muscle mass in Japanese community-dwelling elders: A cross-sectional study. Arch. Gerintol Geriatr. 2018, 78, 18–22. [Google Scholar] [CrossRef]
- Hoshino, T.; Yamamoto, M.; Kasahara, K.; Katakura, A. Comparison of age-related morphological changes in the masseter muscles of senescence-accelerated mouse (SAM). J. Oral. Maxillofac. Surg. Med. Pathol. 2018, 30, 44–49. [Google Scholar] [CrossRef]
- Onishi, S.; Ishino, M.; Kitazawa, H.; Yoto, A.; Shimba, Y.; Mochizuki, Y.; Unno, K.; Meguro, S.; Tokimitsu, I.; Miura, S. Green tea extracts ameliorate high-fat diet-induced muscle atrophy in senescence-accelerated mouse prone-8 mice. PLoS ONE 2018, 13, e0195753. [Google Scholar] [CrossRef]
- Morley, J.E. Decreased food intake with aging. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, 81–88. [Google Scholar] [CrossRef]
- Tuxen, A.; Kirkeby, S. An animal model for human masseter muscle: Histochemical characterization of mouse, rat, rabbit, cat, dog, pig, and cow masseter muscle. J. Oral. Maxillofac. Surg. 1990, 48, 1063–1067. [Google Scholar] [CrossRef]
- Yamashita, K.; Yoshioka, T. Profiles of creatine kinase isoenzyme compositions in single muscle fibres of different types. J. Muscle Res. Cell. Motil. 1991, 12, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Arundel, C.M.; Nishioka, K.; Tofilon, P.J. Effects of alpha-difluoromethylornithine-induced polyamine depletion on the radiosensitivity of a human colon carcinoma cell line. Radiat. Res. 1988, 114, 634–640. [Google Scholar] [CrossRef]
- Pietrocola, F.; Lachkar, S.; Enot, D.P.; Niso-Santano, M.; Bravo-San Pedro, J.M.; Sica, V.; Izzo, V.; Maiuri, M.C.; Madeo, F.; Mariño, G.; et al. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2015, 22, 509–516. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Carmona-Gutierrez, D.; Bernier, M.; Hall, M.N.; Madeo, F. The search for antiaging interventions: From elixirs to fasting regimens. Cell 2014, 157, 1515–1526. [Google Scholar] [CrossRef]
- Madeo, F.; Bauer, M.A.; Carmona-Gutierrez, D.; Kroemer, G. Spermidine: A physiological autophagy inducer acting as an anti-aging vitamin in humans. Autophagy 2019, 15, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Iida, R.H.; Kanko, S.; Suga, T.; Morito, M.; Yamane, A. Autophagic-lysosomal pathway functions in the masseter and tongue muscles in the klotho mouse, a mouse model for aging. Mol. Cell Biochem. 2011, 348, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Norton, M.; Verstegeden, A.; Maxwell, L.C.; McCarter, R.M. Constancy of masseter muscle structure and function with age in F344 rats. Arch. Oral. Biol. 2001, 46, 139–146. [Google Scholar] [CrossRef]
- Pegg, A.E. The function of spermine. IUBMB Life 2014, 66, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Obata, F.; Miura, M. Enhancing S-adenosyl-methionine catabolism extends Drosophila lifespan. Nat. Commun. 2015, 6, 8332. [Google Scholar] [CrossRef] [PubMed]
- Zykovich, A.; Hubbard, A.; Flynn, J.M.; Tarnopolsky, M.; Fraga, M.F.; Kerksick, C.; Ogborn, D.; MacNeil, L.; Mooney, S.D.; Melov, S. Genome-wide DNA methylation changes with age in disease-free human skeletal muscle. Aging Cell 2014, 13, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.J.; Park, H.K.; Cho, Y.M.; Pak, Y.K.; Lee, K.U.; Kim, M.S.; Friso, S.; Choi, S.W.; Park, K.S.; Lee, H.K. S-adenosyl-L-methionine increases skeletal muscle mitochondrial DNA density and whole body insulin sensitivity in OLETF rats. J. Nutr. 2007, 137, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Day, K.; Waite, L.L.; Thalacker-Mercer, A.; West, A.; Bamman, M.M.; Brooks, J.D.; Myers, R.M.; Absher, D. Differential DNA methylation with age displays both common and dynamic features across human tissues that are influenced by CpG landscape. Genome Biol. 2013, 14, R102. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Karir, P.; Garg, V.K. Role of DNA methylation in human age prediction. Mech. Ageing Dev. 2017, 166, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, W.; Yuan, T.; Bai, R.; Liu, G.H.; Zhang, W.; Qu, J. DNA methylome: Unveiling your biological age. Protein Cell 2013, 4, 723–725. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guimarães-Ferreira, L. Role of the phosphocreatine system on energetic homeostasis in skeletal and cardiac muscles. Einstein 2014, 12, 126–131. [Google Scholar] [CrossRef]
- Jacek, Z.; Krzystof, K. Pathways of purine metabolism: Effects of exercise and training in competitive athletes. Trends Sport. Sci. 2015, 3, 103–112. [Google Scholar]
- Otsuka, S.; Fukumaru, K.; Tani, A.; Takada, S.; Kikuchi, K.; Norimatsu, K.; Matsuzaki, R.; Matsuoka, T.; Sakakima, H.; Omiya, Y.; et al. Analysis of the effects of Ninjin’yoeito on physical frailty in mice. Int. J. Mol. Sci. 2022, 23, 11183. [Google Scholar] [CrossRef]
- Derave, W.; Eijnde, B.O.; Ramaekers, M.; Hespel, P. Soleus muscles of SAMP8 mice provide an accelerated model of skeletal muscle senescence. Exp. Gerontol. 2005, 40, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Ueno, Y.; Tomita, M.; Soga, T. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol. Biosyst. 2008, 4, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol. Biosyst. 2011, 7, 1217–1223. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, Y.; Hoshino, T.; Ogawa, Y.; Sugahara, K.; Katakura, A. Aging-Related Metabolome Analysis of the Masseter Muscle in Senescence-Accelerated Mouse-Prone 8. Int. J. Mol. Sci. 2024, 25, 9684. https://doi.org/10.3390/ijms25179684
Kato Y, Hoshino T, Ogawa Y, Sugahara K, Katakura A. Aging-Related Metabolome Analysis of the Masseter Muscle in Senescence-Accelerated Mouse-Prone 8. International Journal of Molecular Sciences. 2024; 25(17):9684. https://doi.org/10.3390/ijms25179684
Chicago/Turabian StyleKato, Yoshiaki, Teruhide Hoshino, Yudai Ogawa, Keisuke Sugahara, and Akira Katakura. 2024. "Aging-Related Metabolome Analysis of the Masseter Muscle in Senescence-Accelerated Mouse-Prone 8" International Journal of Molecular Sciences 25, no. 17: 9684. https://doi.org/10.3390/ijms25179684
APA StyleKato, Y., Hoshino, T., Ogawa, Y., Sugahara, K., & Katakura, A. (2024). Aging-Related Metabolome Analysis of the Masseter Muscle in Senescence-Accelerated Mouse-Prone 8. International Journal of Molecular Sciences, 25(17), 9684. https://doi.org/10.3390/ijms25179684






