A Hypoxia–Decidual Macrophage Regulatory Axis in Normal Pregnancy and Spontaneous Miscarriage
Abstract
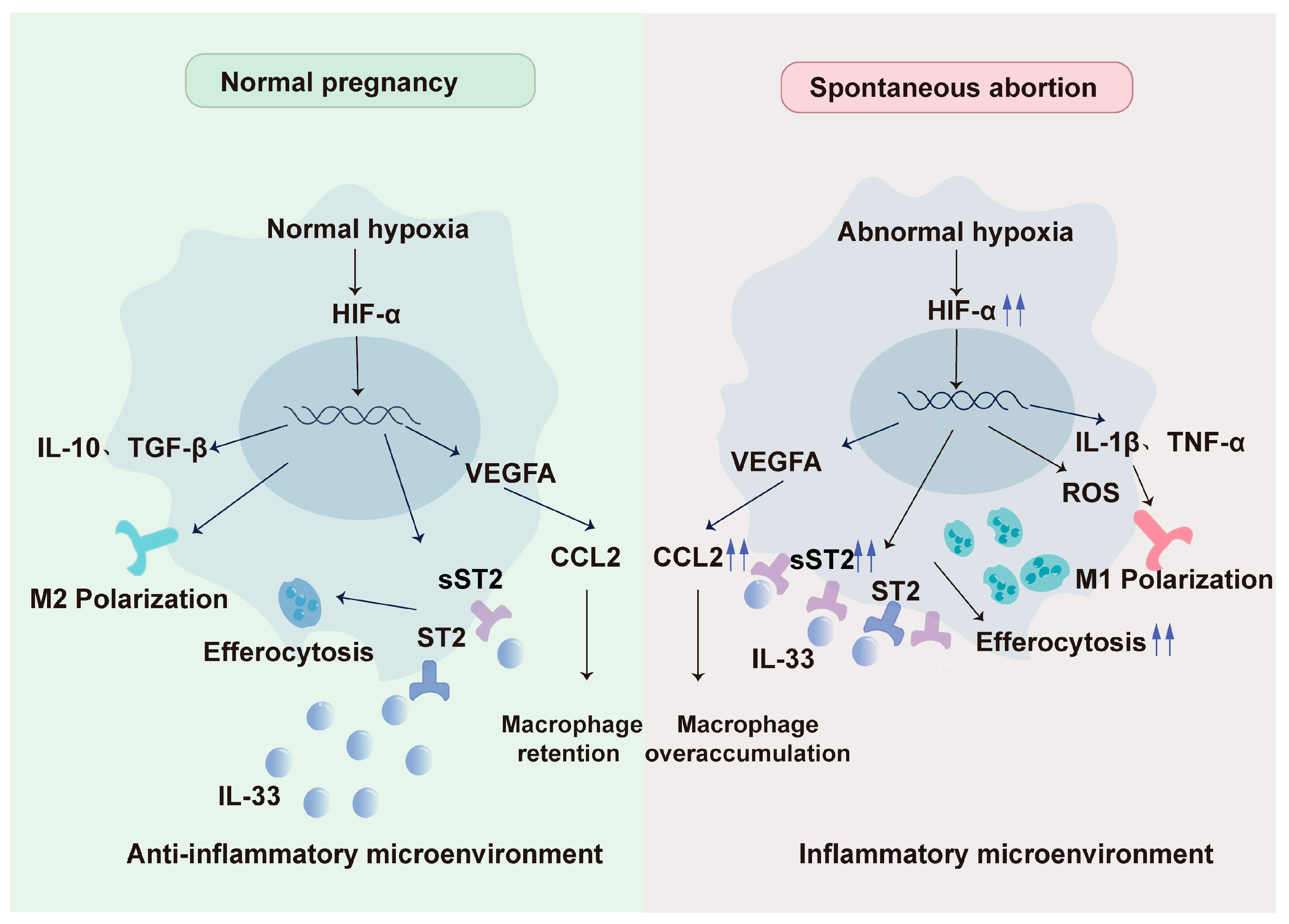
1. Introduction
2. Dynamic Changes of Blood Oxygen Concentration at the Maternal–Fetal Interface during Gestation
3. The Pivotal Function of Dmφs in Normal Pregnancy
4. Hypoxia Promotes DMφs Infiltration and Residence
5. Hypoxia Induces the Polarization of DMφs
6. Hypoxia Regulates Efferocytosis of DMφs to Maintain Tissue Homeostasis
7. Abnormality of Hypoxia-DMφ Regulatory Axis Impels Spontaneous Abortion
8. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dimitriadis, E.; Menkhorst, E.; Saito, S.; Kutteh, W.H.; Brosens, J.J. Recurrent pregnancy loss. Nat. Rev. Dis. Primers 2020, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Bulletti, C.; Flamigni, C.; Giacomucci, E. Reproductive failure due to spontaneous abortion and recurrent miscarriage. Human Reprod. Update 1996, 2, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Regan, L.; Braude, P.R.; Trembath, P.L. Influence of past reproductive performance on risk of spontaneous abortion. BMJ 1989, 299, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Garrido-Gimenez, C. Current Concepts and New Trends in the Diagnosis and Management of Recurrent Miscarriage. Obstet. Gynecol. Surv. 2013, 68, 445–466. [Google Scholar] [CrossRef]
- Lash, G.E.; Pitman, H.; Morgan, H.L.; Innes, B.A.; Agwu, C.N.; Bulmer, J.N. Decidual macrophages: Key regulators of vascular remodeling in human pregnancy. J. Leukoc. Biol. 2016, 100, 315–325. [Google Scholar] [CrossRef]
- Abrahams, V.M.; Kim, Y.M.; Straszewski, S.L.; Romero, R.; Mor, G. Macrophages and apoptotic cell clearance during pregnancy. Am. J. Reprod. Immunol. 2004, 51, 275–282. [Google Scholar] [CrossRef]
- Egashira, M.; Hirota, Y.; Shimizu-Hirota, R.; Saito-Fujita, T.; Haraguchi, H.; Matsumoto, L.; Matsuo, M.; Hiraoka, T.; Tanaka, T.; Akaeda, S.; et al. F4/80+ Macrophages Contribute to Clearance of Senescent Cells in the Mouse Postpartum Uterus. Endocrinology 2017, 158, 2344–2353. [Google Scholar] [CrossRef]
- Ning, F.; Liu, H.; Lash, G.E. The Role of Decidual Macrophages During Normal and Pathological Pregnancy. Am. J. Reprod. Immunol. 2016, 75, 298–309. [Google Scholar] [CrossRef]
- Liu, X.; Fei, H.; Yang, C.; Wang, J.; Zhu, X.; Yang, A.; Shi, Z.; Jin, X.; Yang, F.; Wu, D.; et al. Trophoblast-Derived Extracellular Vesicles Promote Preeclampsia by Regulating Macrophage Polarization. Hypertension 2022, 79, 2274–2287. [Google Scholar] [CrossRef]
- Bezemer, R.E.; Schoots, M.H.; Timmer, A.; Scherjon, S.A.; Erwich, J.; van Goor, H.; Gordijn, S.J.; Prins, J.R. Altered Levels of Decidual Immune Cell Subsets in Fetal Growth Restriction, Stillbirth, and Placental Pathology. Front. Immunol. 2020, 11, 1898. [Google Scholar] [CrossRef]
- Xu, Y.; Romero, R.; Miller, D.; Kadam, L.; Mial, T.N.; Plazyo, O.; Garcia-Flores, V.; Hassan, S.S.; Xu, Z.; Tarca, A.L.; et al. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J. Immunol. 2016, 196, 2476–2491. [Google Scholar] [CrossRef] [PubMed]
- Colson, A.; Sonveaux, P.; Debieve, F.; Sferruzzi-Perri, A.N. Adaptations of the human placenta to hypoxia: Opportunities for interventions in fetal growth restriction. Hum. Reprod. Update 2021, 27, 531–569. [Google Scholar] [CrossRef] [PubMed]
- James, J.L.; Stone, P.R.; Chamley, L.W. The regulation of trophoblast differentiation by oxygen in the first trimester of pregnancy. Hum. Reprod. Update 2006, 12, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Pascarella, F.; Pini, A.; Cammalleri, M.; Bagnoli, P.; Morganti, R.; Innocenti, F.; Castagnini, N.; Melosi, A.; Scaramuzzo, R.T. Fetal Oxygenation from the 23rd to the 36th Week of Gestation Evaluated through the Umbilical Cord Blood Gas Analysis. Int. J. Mol. Sci. 2023, 24, 12487. [Google Scholar] [CrossRef]
- Burton, G.J.; Cindrova-Davies, T.; Yung, H.w.; Jauniaux, E. Hypoxia and reproductive health: Oxygen and development of the human placenta. Reproduction 2021, 161, F53–F65. [Google Scholar] [CrossRef]
- Rodesch, F.; Simon, P.; Donner, C.; Jauniaux, E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 1992, 80, 283–285. [Google Scholar]
- Mohyeldin, A.; Garzón-Muvdi, T.; Quiñones-Hinojosa, A. Oxygen in stem cell biology: A critical component of the stem cell niche. Cell Stem Cell 2010, 7, 150–161. [Google Scholar] [CrossRef]
- Zhao, H.; Wong, R.J.; Stevenson, D.K. The Impact of Hypoxia in Early Pregnancy on Placental Cells. Int. J. Mol. Sci. 2021, 22, 9675. [Google Scholar] [CrossRef]
- Morin, S.J. Oxygen tension in embryo culture: Does a shift to 2% O(2) in extended culture represent the most physiologic system? J. Assist. Reprod. Genet. 2017, 34, 309–314. [Google Scholar] [CrossRef]
- Genbacev, O.; Zhou, Y.; Ludlow, J.W.; Fisher, S.J. Regulation of Human Placental Development by Oxygen Tension. Science 1997, 277, 1669–1672. [Google Scholar] [CrossRef]
- Freeman, B.A.; Crapo, J.D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J. Biol. Chem. 1981, 256, 10986–10992. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J. Oxygen, the Janus gas; its effects on human placental development and function. J. Anat. 2009, 215, 27–35. [Google Scholar] [CrossRef]
- Chang, R.Q.; Zhou, W.J.; Li, D.J.; Li, M.Q. Innate Lymphoid Cells at the Maternal-Fetal Interface in Human Pregnancy. Int. J. Biol. Sci. 2020, 16, 957–969. [Google Scholar] [CrossRef]
- Ander, S.E.; Diamond, M.S.; Coyne, C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019, 4, eaat6114. [Google Scholar] [CrossRef] [PubMed]
- Houser, B.L. Decidual macrophages and their roles at the maternal-fetal interface. Yale J. Biol. Med. 2012, 85, 105–118. [Google Scholar]
- Weisser, S.B.; McLarren, K.W.; Kuroda, E.; Sly, L.M. Generation and Characterization of Murine Alternatively Activated Macrophages. In Basic Cell Culture Protocols; Helgason, C.D., Miller, C.L., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 225–239. [Google Scholar]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; von Chamier, M.; Allam, A.B.; Reyes, L. M1/M2 Macrophage Polarity in Normal and Complicated Pregnancy. Front. Immunol. 2014, 5, 606. [Google Scholar] [CrossRef]
- Chang, R.; Dai, J.; Wang, L.; Liu, H.; Jiang, H.; Liu, X.; Jiang, L.; He, F.; Hu, L. PlGF/FLT-1 deficiency leads to reduced STAT3-C/EBPβ signaling and aberrant polarization in decidual macrophages during early spontaneous abortion. Front. Immunol. 2023, 14, 1061949. [Google Scholar] [CrossRef]
- Jiang, X.; Du, M.R.; Li, M.; Wang, H. Three macrophage subsets are identified in the uterus during early human pregnancy. Cell Mol. Immunol. 2018, 15, 1027–1037. [Google Scholar] [CrossRef]
- Houser, B.L.; Tilburgs, T.; Hill, J.; Nicotra, M.L.; Strominger, J.L. Two unique human decidual macrophage populations. J. Immunol. 2011, 186, 2633–2642. [Google Scholar] [CrossRef]
- Sun, F.; Wang, S.; Du, M. Functional regulation of decidual macrophages during pregnancy. J. Reprod. Immunol. 2021, 143, 103264. [Google Scholar] [CrossRef]
- Helige, C.; Ahammer, H.; Moser, G.; Hammer, A.; Dohr, G.; Huppertz, B.; Sedlmayr, P. Distribution of decidual natural killer cells and macrophages in the neighbourhood of the trophoblast invasion front: A quantitative evaluation. Hum. Reprod. 2014, 29, 8–17. [Google Scholar] [CrossRef]
- Hazan, A.D.; Smith, S.D.; Jones, R.L.; Whittle, W.; Lye, S.J.; Dunk, C.E. Vascular-Leukocyte Interactions: Mechanisms of Human Decidual Spiral Artery Remodeling in Vitro. Am. J. Pathol. 2010, 177, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yang, C.; Zhang, Y.; Wang, J.; Zhang, S.; Guo, D.; Yin, T.; Yang, J. M2 macrophage-derived G-CSF promotes trophoblasts EMT, invasion and migration via activating PI3K/Akt/Erk1/2 pathway to mediate normal pregnancy. J. Cell Mol. Med. 2021, 25, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Vrekoussis, T.; Heublein, S.; Bayer, B.; Anz, D.; Knabl, J.; Navrozoglou, I.; Dian, D.; Friese, K.; Makrigiannakis, A.; et al. Decidual macrophages are significantly increased in spontaneous miscarriages and over-express FasL: A potential role for macrophages in trophoblast apoptosis. Int. J. Mol. Sci. 2012, 13, 9069–9080. [Google Scholar] [CrossRef] [PubMed]
- Brindle, N.P.; Saharinen, P.; Alitalo, K. Signaling and functions of angiopoietin-1 in vascular protection. Circ. Res. 2006, 98, 1014–1023. [Google Scholar] [CrossRef]
- Schröcksnadel, H.; Baier-Bitterlich, G.; Dapunt, O.; Wachter, H.; Fuchs, D. Decreased plasma tryptophan in pregnancy. Obstet. Gynecol. 1996, 88, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Sayama, S.; Nagamatsu, T.; Schust, D.J.; Itaoka, N.; Ichikawa, M.; Kawana, K.; Yamashita, T.; Kozuma, S.; Fujii, T. Human decidual macrophages suppress IFN-γ production by T cells through costimulatory B7-H1:PD-1 signaling in early pregnancy. J. Reprod. Immunol. 2013, 100, 109–117. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, W.; Niu, M.; Tian, J.; Xu, K. Hypoxia-active nanoparticles used in tumor theranostic. Int. J. Nanomed. 2019, 14, 3705–3722. [Google Scholar] [CrossRef]
- Rahat, M.; Bitterman, H.; Lahat, N. Molecular Mechanisms Regulating Macrophage Response to Hypoxia. Front. Immunol. 2011, 2, 45. [Google Scholar] [CrossRef]
- Chang, Q.; Bournazou, E.; Sansone, P.; Berishaj, M.; Gao, S.P.; Daly, L.; Wels, J.; Theilen, T.; Granitto, S.; Zhang, X.; et al. The IL-6/JAK/Stat3 Feed-Forward Loop Drives Tumorigenesis and Metastasis. Neoplasia 2013, 15, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.; Giannoudis, A.; Lewis, C.E. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 2004, 104, 2224–2234. [Google Scholar] [CrossRef]
- Lewis, C.; Murdoch, C. Macrophage responses to hypoxia: Implications for tumor progression and anti-cancer therapies. Am. J. Pathol. 2005, 167, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, C.; Tewari, B.N.; Kanchan, R.K.; Baghel, K.S.; Nautiyal, N.; Shrivastava, R.; Kaur, H.; Bhatt, M.L.; Bhadauria, S. Macrophages are recruited to hypoxic tumor areas and acquire a pro-angiogenic M2-polarized phenotype via hypoxic cancer cell derived cytokines Oncostatin M and Eotaxin. Oncotarget 2014, 5, 5350–5368. [Google Scholar] [CrossRef] [PubMed]
- Du, M.R.; Wang, S.C.; Li, D.J. The integrative roles of chemokines at the maternal-fetal interface in early pregnancy. Cell Mol. Immunol. 2014, 11, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-Y.; Shen, H.-H.; Zhang, X.-Y.; Zhang, X.; Xie, F.; Wang, W.-J.; Xiong, Y.; Mei, J.; Li, M.-Q. Hypoxia-mediated chemotaxis and residence of macrophage in decidua by secreting VEGFA and CCL2 during normal pregnancy. Reproduction 2023, 165, 543–555. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Li, M.-Q.; Zhu, X.-Y.; Li, D.-J. Immune status of decidual macrophages is dependent on the CCL2/CCR2/JAK2 pathway during early pregnancy. Am. J. Reprod. Immunol. 2021, 86, e13480. [Google Scholar] [CrossRef]
- She, S.; Ren, L.; Chen, P.; Wang, M.; Chen, D.; Wang, Y.; Chen, H. Functional Roles of Chemokine Receptor CCR2 and Its Ligands in Liver Disease. Front. Immunol. 2022, 13, 812431. [Google Scholar] [CrossRef]
- Chen, X.; Song, Q.L.; Ji, R.; Wang, J.Y.; Li, Z.H.; Xiao, Z.N.; Guo, D.Y.; Yang, J. Hypoxia-induced polarization of M2 macrophages and C-C motif chemokine ligand 5 secretion promotes the migration and invasion of trophoblasts. Biol. Reprod. 2022, 107, 834–845. [Google Scholar] [CrossRef]
- Lewis, J.S.; Landers, R.J.; Underwood, J.C.; Harris, A.L.; Lewis, C.E. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J. Pathol. 2000, 192, 150–158. [Google Scholar] [CrossRef]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.-D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PloS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef]
- Meng, Y.-H.; Zhou, W.-J.; Jin, L.-P.; Liu, L.-B.; Chang, K.-K.; Mei, J.; Li, H.; Wang, J.; Li, D.-J.; Li, M.-Q. RANKL-mediated harmonious dialogue between fetus and mother guarantees smooth gestation by inducing decidual M2 macrophage polarization. Cell Death Dis. 2017, 8, e3105. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.H.; Li, H.; Chen, X.; Liu, L.B.; Shao, J.; Chang, K.K.; Du, M.R.; Jin, L.P.; Li, M.Q.; Li, D.J. RANKL promotes the growth of decidual stromal cells in an autocrine manner via CCL2/CCR2 interaction in human early pregnancy. Placenta 2013, 34, 663–671. [Google Scholar] [CrossRef]
- Liao, H.Q.; Han, M.T.; Cheng, W.; Zhang, C.; Li, H.; Li, M.Q.; Zhu, R. Decidual-derived RANKL facilitates macrophages accumulation and residence at the maternal-fetal interface in human early pregnancy. Am. J. Reprod. Immunol. 2021, 86, e13406. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-N.; Zhang, F.; Tang, P.; Qi, X.-W.; Jiang, J. Hypoxia induces RANK and RANKL expression by activating HIF-1α in breast cancer cells. Biochem. Biophys. Res. Commun. 2011, 408, 411–416. [Google Scholar] [CrossRef]
- Lu, Z.; Kim, D.H.; Fan, J.; Lu, Q.; Verbanac, K.; Ding, L.; Renegar, R.; Chen, Y.-H. A non-tight junction function of claudin-7—Interaction with integrin signaling in suppressing lung cancer cell proliferation and detachment. Mol. Cancer 2015, 14, 120. [Google Scholar] [CrossRef]
- Zhang, T.; Shen, H.H.; Qin, X.Y.; Li, M.Q. The metabolic characteristic of decidual immune cells and their unique properties in pregnancy loss. Immunol. Rev. 2022, 308, 168–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Lai, Z.Z.; Shi, J.W.; Zhou, W.J.; Mei, J.; Ye, J.F.; Zhang, T.; Wang, J.; Zhao, J.Y.; Li, D.J.; et al. A defective lysophosphatidic acid-autophagy axis increases miscarriage risk by restricting decidual macrophage residence. Autophagy 2022, 18, 2459–2480. [Google Scholar] [CrossRef]
- Krop, J.; van der Zwan, A.; Ijsselsteijn, M.E.; Kapsenberg, H.; Luk, S.J.; Hendriks, S.H.; van der Keur, C.; Verleng, L.J.; Somarakis, A.; van der Meeren, L.; et al. Imaging mass cytometry reveals the prominent role of myeloid cells at the maternal-fetal interface. iScience 2022, 25, 104648. [Google Scholar] [CrossRef]
- Bai, R.; Li, Y.; Jian, L.; Yang, Y.; Zhao, L.; Wei, M. The hypoxia-driven crosstalk between tumor and tumor-associated macrophages: Mechanisms and clinical treatment strategies. Mol. Cancer 2022, 21, 177. [Google Scholar] [CrossRef]
- Ma, L.N.; Huang, X.B.; Muyayalo, K.P.; Mor, G.; Liao, A.H. Lactic Acid: A Novel Signaling Molecule in Early Pregnancy? Front. Immunol. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, Q.H.; Ma, L.N.; Luo, J.; Muyayalo, K.P.; Wang, L.L.; Huang, D.H.; Xiao, X.J.; Cheng, S.B.; Mor, G.; et al. Trophoblast-derived Lactic Acid Orchestrates Decidual Macrophage Differentiation via SRC/LDHA Signaling in Early Pregnancy. Int. J. Biol. Sci. 2022, 18, 599–616. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Li, Q.H.; Fu, Y.Y.; Ren, C.E.; Jiang, A.F.; Meng, Y.H. Decidual macrophages in recurrent spontaneous abortion. Front. Immunol. 2022, 13, 994888. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, Q.L.; Wang, J.Y.; Ji, R.; Cao, M.L.; Guo, D.Y.; Zhang, Y.; Yang, J. FKBP5 regulates trophoblast-macrophage crosstalk in recurrent spontaneous abortion through PI3K/AKT and NF-kappaB signaling pathways. Free Radic. Biol. Med. 2023, 209, 55–69. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Li, Y.; Zhang, Y.; Bian, Y.; Zeng, Y.; Yao, X.; Wan, J.; Chen, X.; Li, J.; et al. S100A4 enhances protumor macrophage polarization by control of PPAR-gamma-dependent induction of fatty acid oxidation. J. Immunother. Cancer 2021, 9, e002548. [Google Scholar] [CrossRef]
- Kolben, T.M.; Rogatsch, E.; Vattai, A.; Hester, A.; Kuhn, C.; Schmoeckel, E.; Mahner, S.; Jeschke, U.; Kolben, T. PPARgamma Expression Is Diminished in Macrophages of Recurrent Miscarriage Placentas. Int. J. Mol. Sci. 2018, 19, 1872. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Ding, J.; Wang, Z.; Zhang, F.; Li, J.; Zhang, Y.; Wu, S.; Yang, L.; Pang, X.; Zhang, Y.; et al. CTRP6 regulates M1 macrophage polarization via the PPAR-gamma/NF-kappaB pathway and reprogramming glycolysis in recurrent spontaneous abortion. Int. Immunopharmacol. 2023, 124, 110840. [Google Scholar] [CrossRef]
- Ding, J.; Yang, C.; Cheng, Y.; Wang, J.; Zhang, S.; Yan, S.; He, F.; Yin, T.; Yang, J. Trophoblast-derived IL-6 serves as an important factor for normal pregnancy by activating Stat3-mediated M2 macrophages polarization. Int. Immunopharmacol. 2021, 90, 106788. [Google Scholar] [CrossRef]
- Arbildi, P.; Rodriguez-Camejo, C.; Perelmuter, K.; Bollati-Fogolin, M.; Sonora, C.; Hernandez, A. Hypoxia and inflammation conditions differentially affect the expression of tissue transglutaminase spliced variants and functional properties of extravillous trophoblast cells. Am. J. Reprod. Immunol. 2022, 87, e13534. [Google Scholar] [CrossRef]
- Seno, K.; Tanikawa, N.; Takahashi, H.; Ohkuchi, A.; Suzuki, H.; Matsubara, S.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Oxygen concentration modulates cellular senescence and autophagy in human trophoblast cells. Am. J. Reprod. Immunol. 2018, 79, e12826. [Google Scholar] [CrossRef]
- Sagrillo-Fagundes, L.; Assuncao Salustiano, E.M.; Ruano, R.; Markus, R.P.; Vaillancourt, C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pineal Res. 2018, 65, e12520. [Google Scholar] [CrossRef]
- Bae, S.; Kim, K.; Kang, K.; Kim, H.; Lee, M.; Oh, B.; Kaneko, K.; Ma, S.; Choi, J.H.; Kwak, H.; et al. RANKL-responsive epigenetic mechanism reprograms macrophages into bone-resorbing osteoclasts. Cell Mol. Immunol. 2023, 20, 94–109. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Qu, Z.; Zhang, B.; Gao, X.; Huang, W.; Feng, M.; Gong, Y.; Kong, L.; Wang, Y.; et al. Hypoxia-inducible factor 1alpha enhances RANKL-induced osteoclast differentiation by upregulating the MAPK pathway. Ann. Transl. Med. 2022, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lin, Z.; Cao, S.; Janowska, I.; Sonomoto, K.; Andreev, D.; Katharina, K.; Wen, J.; Knaup, K.X.; Wiesener, M.S.; et al. Estrogen-mediated downregulation of HIF-1alpha signaling in B lymphocytes influences postmenopausal bone loss. Bone Res. 2022, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Pham, J.; Arul Nambi Rajan, K.; Li, P.; Parast, M.M. The role of Sirtuin1-PPARgamma axis in placental development and function. J. Mol. Endocrinol. 2018, 60, R201–R212. [Google Scholar] [CrossRef] [PubMed]
- Straszewski-Chavez, S.L.; Abrahams, V.M.; Mor, G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocr. Rev. 2005, 26, 877–897. [Google Scholar] [CrossRef]
- Doran, A.C.; Yurdagul, A., Jr.; Tabas, I. Efferocytosis in health and disease. Nat. Rev. Immunol. 2020, 20, 254–267. [Google Scholar] [CrossRef]
- Sheng, Y.R.; Hu, W.T.; Wei, C.Y.; Tang, L.L.; Liu, Y.K.; Liu, Y.Y.; Qiu, J.P.; Li, D.J.; Zhu, X.Y. Insights of efferocytosis in normal and pathological pregnancy. Am. J. Reprod. Immunol. 2019, 82, e13088. [Google Scholar] [CrossRef]
- Kourtzelis, I.; Hajishengallis, G.; Chavakis, T. Phagocytosis of Apoptotic Cells in Resolution of Inflammation. Front. Immunol. 2020, 11, 553. [Google Scholar] [CrossRef]
- Wang, Y.T.; Trzeciak, A.J.; Rojas, W.S.; Saavedra, P.; Chen, Y.T.; Chirayil, R.; Etchegaray, J.I.; Lucas, C.D.; Puleston, D.J.; Keshari, K.R.; et al. Metabolic adaptation supports enhanced macrophage efferocytosis in limited-oxygen environments. Cell Metab. 2023, 35, 316–331.e6. [Google Scholar] [CrossRef]
- Sheng, Y.-R.; Hu, W.-T.; Wei, C.-Y.; Tang, L.-L.; Liu, Y.-K.; Liu, Y.-Y.; Qiu, J.-P.; Li, D.-J.; Zhu, X.-Y. IL-33/ST2 axis affects the polarization and efferocytosis of decidual macrophages in early pregnancy. Am. J. Reprod. Immunol. 2018, 79, e12836. [Google Scholar] [CrossRef] [PubMed]
- Granne, I.; Southcombe, J.H.; Snider, J.V.; Tannetta, D.S.; Child, T.; Redman, C.W.; Sargent, I.L. ST2 and IL-33 in pregnancy and pre-eclampsia. PLoS ONE 2011, 6, e24463. [Google Scholar] [CrossRef]
- Paparini, D.E.; Grasso, E.; Fernandez, L.D.C.; Merech, F.; Weingrill-Barbano, R.; Correa-Silva, S.; Izbizky, G.; Abasolo, J.I.; Hauk, V.; Ramhorst, R.; et al. Decidual factors and vasoactive intestinal peptide guide monocytes to higher migration, efferocytosis and wound healing in term human pregnancy. Acta Physiol. 2021, 232, e13579. [Google Scholar] [CrossRef] [PubMed]
- Paparini, D.E.; Choudhury, R.H.; Vota, D.M.; Karolczak-Bayatti, M.; Finn-Sell, S.; Grasso, E.N.; Hauk, V.C.; Ramhorst, R.; Perez Leiros, C.; Aplin, J.D. Vasoactive intestinal peptide shapes first-trimester placenta trophoblast, vascular, and immune cell cooperation. Br. J. Pharmacol. 2019, 176, 964–980. [Google Scholar] [CrossRef]
- Gallino, L.; Calo, G.; Hauk, V.; Fraccaroli, L.; Grasso, E.; Vermeulen, M.; Leirós, C.P.; Ramhorst, R. VIP treatment prevents embryo resorption by modulating efferocytosis and activation profile of maternal macrophages in the CBAxDBA resorption prone model. Sci. Rep. 2016, 6, 18633. [Google Scholar] [CrossRef] [PubMed]
- Proto, J.D.; Doran, A.C.; Gusarova, G.; Yurdagul, A., Jr.; Sozen, E.; Subramanian, M.; Islam, M.N.; Rymond, C.C.; Du, J.; Hook, J.; et al. Regulatory T Cells Promote Macrophage Efferocytosis during Inflammation Resolution. Immunity 2018, 49, 666–677.e6. [Google Scholar] [CrossRef]
- Zhang, S.; Weinberg, S.; DeBerge, M.; Gainullina, A.; Schipma, M.; Kinchen, J.M.; Ben-Sahra, I.; Gius, D.R.; Yvan-Charvet, L.; Chandel, N.S.; et al. Efferocytosis Fuels Requirements of Fatty Acid Oxidation and the Electron Transport Chain to Polarize Macrophages for Tissue Repair. Cell Metab. 2019, 29, 443–456. [Google Scholar] [CrossRef]
- Hauk, F.; Gori, S.; Calo, G.; Hauk, V.; Paparini, D.; Rios, D.; Lara, B.; D’Eramo, L.; Squassi, A.; Ramhorst, R.; et al. Monocyte immunometabolic reprogramming in human pregnancy contribution of trophoblast cells. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E215–E225. [Google Scholar] [CrossRef]
- Norris, P.C.; Libreros, S.; Serhan, C.N. Serhan Resolution metabolomes activated by hypoxic environment. Sci Adv 2019, 5, eaax4895. [Google Scholar] [CrossRef]
- Jauniaux, E.; Poston, L.; Burton, G.J. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum. Reprod. Update 2006, 12, 747–755. [Google Scholar] [CrossRef]
- Tian, P.; Xu, Z.; Guo, J.; Zhao, J.; Chen, W.; Huang, W.; Wang, M.; Mi, C.; Zhang, Y.; Yang, Y.; et al. Hypoxia causes trophoblast cell ferroptosis to induce miscarriage through lnc-HZ06/HIF1alpha-SUMO/NCOA4 axis. Redox Biol. 2024, 70, 103073. [Google Scholar] [CrossRef]
- Namlı Kalem, M.; Akgun, N.; Kalem, Z.; Bakirarar, B.; Celik, T. Chemokine (C-C motif) ligand-2 (CCL2) and oxidative stress markers in recurrent pregnancy loss and repeated implantation failure. J. Assist. Reprod. Genet. 2017, 34, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Spathakis, M.; Filidou, E.; Pappa, C.; Arzou, B.C.; Georgiadis, A.; Kontomanolis, E.N.; Nikolettos, N.; Kolios, G.; Galazios, G.; Arvanitidis, K. Spontaneous abortion is associated with differentially expressed angiogenic chemokines in placenta and decidua. Arch. Gynecol. Obstet. 2023, 308, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Li, Y.; Xu, L.; Chen, J.; Li, D.; Du, M. Dysfunction of CCR1(+) decidual macrophages is a potential risk factor in the occurrence of unexplained recurrent pregnancy loss. Front. Immunol. 2022, 13, 1045532. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Luo, J.; Xie, T.; Mor, G.; Liao, A. Decorin promotes decidual M1-like macrophage polarization via mitochondrial dysfunction resulting in recurrent pregnancy loss. Theranostics 2022, 12, 7216–7236. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, Q.L.; Ji, R.; Wang, J.Y.; Cao, M.L.; Guo, D.Y.; Zhang, Y.; Yang, J. JPT2 Affects Trophoblast Functions and Macrophage Polarization and Metabolism, and Acts as a Potential Therapeutic Target for Recurrent Spontaneous Abortion. Adv. Sci. 2024, 11, e2306359. [Google Scholar] [CrossRef]
- Sheng, Y.R.; Hu, W.T.; Shen, H.H.; Wei, C.Y.; Liu, Y.K.; Ma, X.Q.; Li, M.Q.; Zhu, X.Y. An imbalance of the IL-33/ST2-AXL-efferocytosis axis induces pregnancy loss through metabolic reprogramming of decidual macrophages. Cell Mol. Life Sci. 2022, 79, 173. [Google Scholar] [CrossRef]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef]

| Molecule | Polarization | Mechanism | Correlation with Oxygen | References |
|---|---|---|---|---|
| Lactate | M2 M1 | Activating SRC-LDHA-VEGF Activating HIF-1α-SRC-LDHA | Inducing M2 polarization in normoxia and M1 polarization in hypoxia conditions | [63] |
| ROS | M1 | Activating NF-kB pathways | Heaps up in the deficiency of oxygen | [65] |
| PPARγ | M2 | Inhibiting NF-κB, STAT, AP-1 pathways | Impaired under placental hypoxia; Sirt1 can reverse this effect | [76] |
| IL-6 | M2 | Activating STAT3 pathways | Low oxygen (≤5%) suppresses IL-6; hypoxia/reoxygenation (0.5% to 8%) increases IL-6 | [70,72] |
| RANKL | M2 | Intervening in Akt/STAT6 signaling; increasing Jmjd3/IRF4 expression | [53,74] |
| Molecule | Influence on Phagocytosis | Mechanism | Impact of Oxygen Level on Phagocytosis | References |
|---|---|---|---|---|
| NADPH | Enhancing phagocytosis | A noncanonical pentose phosphate pathway (PPP) during chronic hypoxia | Strengthened under hypoxia | [81] |
| IL-33/ST2 | Regulating phagocytosis to avoid excessive activity | Controlling phagocytosis via PI3K/AKT and ERK1/2 pathways, inhibiting AXL | Inhibited by sST2 under hypoxia/reoxygenation | [79,82] |
| VIP | Enhancing phagocytosis | Promoting IL-10 release from decidua, potentially enhancing phagocytosis | Strengthened under hypoxia via FAO | [88,89] |
| SPM | Enhancing phagocytosis | Potential benefits for phagocytosis | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Lin, Z.; Zheng, Z.-M.; Shi, J.-L.; Lu, K.-Y.; Wang, J.-R.; Li, M.-Q.; Shao, J. A Hypoxia–Decidual Macrophage Regulatory Axis in Normal Pregnancy and Spontaneous Miscarriage. Int. J. Mol. Sci. 2024, 25, 9710. https://doi.org/10.3390/ijms25179710
Huang X, Lin Z, Zheng Z-M, Shi J-L, Lu K-Y, Wang J-R, Li M-Q, Shao J. A Hypoxia–Decidual Macrophage Regulatory Axis in Normal Pregnancy and Spontaneous Miscarriage. International Journal of Molecular Sciences. 2024; 25(17):9710. https://doi.org/10.3390/ijms25179710
Chicago/Turabian StyleHuang, Xu, Zhi Lin, Zi-Meng Zheng, Jia-Lu Shi, Ke-Yu Lu, Jia-Rui Wang, Ming-Qing Li, and Jun Shao. 2024. "A Hypoxia–Decidual Macrophage Regulatory Axis in Normal Pregnancy and Spontaneous Miscarriage" International Journal of Molecular Sciences 25, no. 17: 9710. https://doi.org/10.3390/ijms25179710
APA StyleHuang, X., Lin, Z., Zheng, Z.-M., Shi, J.-L., Lu, K.-Y., Wang, J.-R., Li, M.-Q., & Shao, J. (2024). A Hypoxia–Decidual Macrophage Regulatory Axis in Normal Pregnancy and Spontaneous Miscarriage. International Journal of Molecular Sciences, 25(17), 9710. https://doi.org/10.3390/ijms25179710






