LncRNAs Are Key Regulators of Transcription Factor-Mediated Endothelial Stress Responses
Abstract
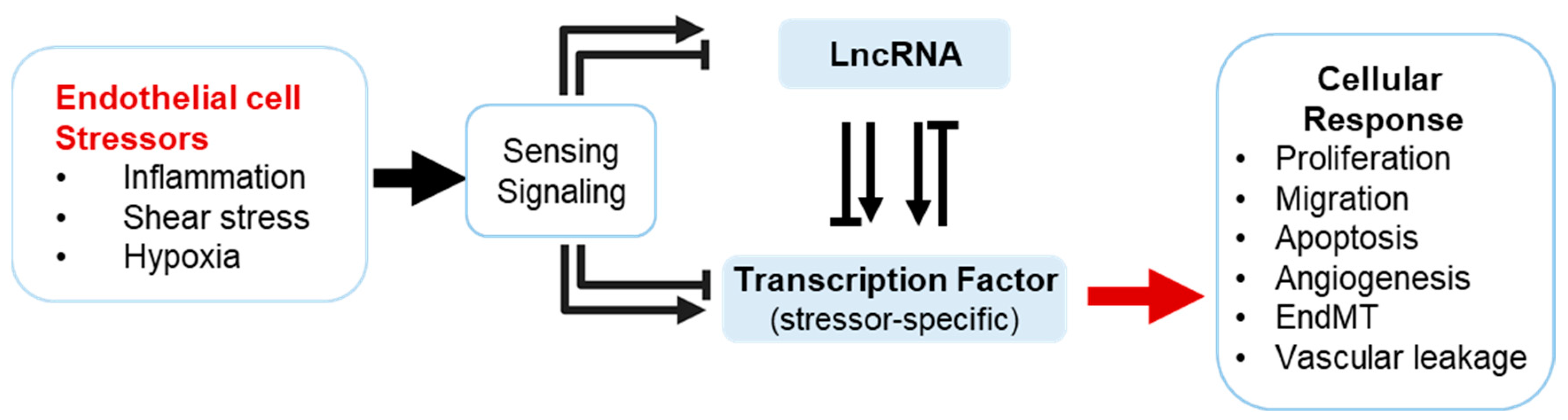
1. Introduction
2. Key Endothelial Transcription Factors and Their Regulatory lncRNA Partners
2.1. Ets Family Transcription Factors
2.2. Krüppel-like Factors—KLF2 and KLF4
2.3. Nuclear Factor-κB (NF-κB)
2.4. GATA Transcription Factors
2.5. Hypoxia-Inducible Factor (HIF)
2.6. Sma- and Mad-Related Proteins (SMADs) and Forkhead Box (FOX) Proteins
2.7. Signal Transducer and Activator of Transcription (STAT) Proteins
2.8. SRY (Sex Determining Region Y)-Related HMG Box of DNA Binding Proteins—SOX
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deanfield, J.E.; Halcox, J.P.; Rabelink, T.J. Endothelial function and dysfunction: Testing and clinical relevance. Circulation 2007, 115, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Ricard, N.; Bailly, S.; Guignabert, C.; Simons, M. The quiescent endothelium: Signalling pathways regulating organ-specific endothelial normalcy. Nat. Rev. Cardiol. 2021, 18, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Mäkinen, T. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 477–494. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, S. Angio-LncRs: LncRNAs that regulate angiogenesis and vascular disease. Theranostics 2018, 8, 3654–3675. [Google Scholar] [CrossRef]
- Jayasuriya, R.; Ganesan, K.; Xu, B.; Ramkumar, K.M. Emerging role of long non-coding RNAs in endothelial dysfunction and their molecular mechanisms. Biomed. Pharmacother. 2022, 145, 112421. [Google Scholar] [CrossRef]
- Uchida, S.; Dimmeler, S. Long noncoding RNAs in cardiovascular diseases. Circ. Res. 2015, 116, 737–750. [Google Scholar] [CrossRef]
- Oo, J.A.; Brandes, R.P.; Leisegang, M.S. Long non-coding RNAs: Novel regulators of cellular physiology and function. Pflug. Arch. 2022, 474, 191–204. [Google Scholar] [CrossRef]
- Das, S.; Shah, R.; Dimmeler, S.; Freedman, J.E.; Holley, C.; Lee, J.-M.; Moore, K.; Musunuru, K.; Wang, D.-Z.; Xiao, J.; et al. Noncoding RNAs in Cardiovascular Disease: Current Knowledge, Tools and Technologies for Investigation, and Future Directions: A Scientific Statement from the American Heart Association. Circ. Genom. Precis. Med. 2020, 13, e000062. [Google Scholar] [CrossRef]
- Lambert, S.A.; Jolma, A.; Campitelli, L.F.; Das, P.K.; Yin, Y.; Albu, M.; Chen, X.; Taipale, J.; Hughes, T.R.; Weirauch, M.T. The Human Transcription Factors. Cell 2018, 172, 650–665. [Google Scholar] [CrossRef]
- Hofmann, M.; Heineke, J. The Impact of Endothelial Transcription Factors in Sprouting Angiogenesis. In Tumor Angiogenesis; Marmé, D., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 73–90. ISBN 978-3-319-33671-8. [Google Scholar]
- De Val, S.; Black, B.L. Transcriptional control of endothelial cell development. Dev. Cell 2009, 16, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Melé, M.; Mattioli, K.; Mallard, W.; Shechner, D.M.; Gerhardinger, C.; Rinn, J.L. Chromatin environment, transcriptional regulation, and splicing distinguish lincRNAs and mRNAs. Genome Res. 2017, 27, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.-H.; Badis, G.; Berger, M.F.; Kivioja, T.; Palin, K.; Enge, M.; Bonke, M.; Jolma, A.; Varjosalo, M.; Gehrke, A.R.; et al. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 2010, 29, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Kulakovskiy, I.V.; Vorontsov, I.E.; Yevshin, I.S.; Sharipov, R.N.; Fedorova, A.D.; Rumynskiy, E.I.; Medvedeva, Y.A.; Magana-Mora, A.; Bajic, V.B.; Papatsenko, D.A.; et al. HOCOMOCO: Towards a complete collection of transcription factor binding models for human and mouse via large-scale ChIP-Seq analysis. Nucleic Acids Res. 2018, 46, D252–D259. [Google Scholar] [CrossRef] [PubMed]
- Randi, A.M.; Sperone, A.; Dryden, N.H.; Birdsey, G.M. Regulation of angiogenesis by ETS transcription factors. Biochem. Soc. Trans. 2009, 37, 1248–1253. [Google Scholar] [CrossRef]
- Kalna, V.; Yang, Y.; Peghaire, C.R.; Frudd, K.; Hannah, R.; Shah, A.V.; Osuna Almagro, L.; Boyle, J.J.; Göttgens, B.; Ferrer, J.; et al. The Transcription Factor ERG Regulates Super-Enhancers Associated with an Endothelial-Specific Gene Expression Program. Circ. Res. 2019, 124, 1337–1349. [Google Scholar] [CrossRef]
- Boos, F.; Oo, J.A.; Warwick, T.; Günther, S.; Izquierdo Ponce, J.; Lopez, M.; Rafii, D.; Buchmann, G.; Pham, M.D.; Msheik, Z.S.; et al. The endothelial-enriched lncRNA LINC00607 mediates angiogenic function. Basic Res. Cardiol. 2023, 118, 5. [Google Scholar] [CrossRef]
- Zhao, W.; Qin, P.; Zhang, D.; Cui, X.; Gao, J.; Yu, Z.; Chai, Y.; Wang, J.; Li, J. Long non-coding RNA PVT1 encapsulated in bone marrow mesenchymal stem cell-derived exosomes promotes osteosarcoma growth and metastasis by stabilizing ERG and sponging miR-183-5p. Aging 2019, 11, 9581–9596. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, H.; Cui, G.; Guo, Q.; Si, L.; Yan, H.; Fang, D.; Jiang, L.; Jiang, Z.; Zhou, J. ERG-Associated lncRNA (ERGAL) Promotes the Stability and Integrity of Vascular Endothelial Barrier during Dengue Viral Infection via Interaction with miR-183-5p. Front. Cell. Infect. Microbiol. 2020, 10, 477. [Google Scholar] [CrossRef]
- Doddaballapur, A.; Michalik, K.M.; Manavski, Y.; Lucas, T.; Houtkooper, R.H.; You, X.; Chen, W.; Zeiher, A.M.; Potente, M.; Dimmeler, S.; et al. Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Dekker, R.J.; van Soest, S.; Fontijn, R.D.; Salamanca, S.; de Groot, P.G.; VanBavel, E.; Pannekoek, H.; Horrevoets, A.J.G. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Krüppel-like factor (KLF2). Blood 2002, 100, 1689–1698. [Google Scholar] [CrossRef]
- Sangwung, P.; Zhou, G.; Nayak, L.; Chan, E.R.; Kumar, S.; Kang, D.-W.; Zhang, R.; Liao, X.; Lu, Y.; Sugi, K.; et al. KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight 2017, 2, e91700. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, G.; Zhang, Y.; Larman, H.B.; Gracia-Sancho, J.; Koo, A.; García-Cardeña, G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem. Biophys. Res. Commun. 2010, 391, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Fledderus, J.O.; Boon, R.A.; Volger, O.L.; Hurttila, H.; Ylä-Herttuala, S.; Pannekoek, H.; Levonen, A.-L.; Horrevoets, A.J.G. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Parmar, K.M.; Larman, H.B.; Dai, G.; Zhang, Y.; Wang, E.T.; Moorthy, S.N.; Kratz, J.R.; Lin, Z.; Jain, M.K.; Gimbrone, M.A.; et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J. Clin. Investig. 2006, 116, 49–58. [Google Scholar] [CrossRef]
- Moonen, J.-R.; Chappell, J.; Shi, M.; Shinohara, T.; Li, D.; Mumbach, M.R.; Zhang, F.; Nair, R.V.; Nasser, J.; Mai, D.H.; et al. KLF4 recruits SWI/SNF to increase chromatin accessibility and reprogram the endothelial enhancer landscape under laminar shear stress. Nat. Commun. 2022, 13, 4941. [Google Scholar] [CrossRef]
- Lu, Q.; Meng, Q.; Qi, M.; Li, F.; Liu, B. Shear-Sensitive lncRNA AF131217.1 Inhibits Inflammation in HUVECs via Regulation of KLF4. Hypertension 2019, 73, e25–e34. [Google Scholar] [CrossRef]
- Stanicek, L.; Lozano-Vidal, N.; Bink, D.I.; Hooglugt, A.; Yao, W.; Wittig, I.; van Rijssel, J.; van Buul, J.D.; van Bergen, A.; Klems, A.; et al. Long non-coding RNA LASSIE regulates shear stress sensing and endothelial barrier function. Commun. Biol. 2020, 3, 265. [Google Scholar] [CrossRef]
- Leisegang, M.S.; Bibli, S.-I.; Günther, S.; Pflüger-Müller, B.; Oo, J.A.; Höper, C.; Seredinski, S.; Yekelchyk, M.; Schmitz-Rixen, T.; Schürmann, C.; et al. Pleiotropic effects of laminar flow and statins depend on the Krüppel-like factor-induced lncRNA MANTIS. Eur. Heart J. 2019, 40, 2523–2533. [Google Scholar] [CrossRef]
- Josipovic, I.; Pflüger, B.; Fork, C.; Vasconez, A.E.; Oo, J.A.; Hitzel, J.; Seredinski, S.; Gamen, E.; Zu Heringdorf, D.M.; Chen, W.; et al. Long noncoding RNA LISPR1 is required for S1P signaling and endothelial cell function. J. Mol. Cell. Cardiol. 2018, 116, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Du, J.; Yu, J.; Guo, R.; Feng, Y.; Qiao, L.; Xu, Z.; Yang, F.; Zhong, G.; Liu, F.; et al. LncRNA NKILA regulates endothelium inflammation by controlling a NF-κB/KLF4 positive feedback loop. J. Mol. Cell. Cardiol. 2019, 126, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Piva, R.; Belardo, G.; Santoro, M.G. NF-kappaB: A stress-regulated switch for cell survival. Antioxid. Redox Signal. 2006, 8, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Yu, J.; Du, J.; Guo, R.; Feng, Y.; Zhong, G.; Jiang, Y.; Lin, J. LncRNA HOXA-AS2 represses endothelium inflammation by regulating the activity of NF-κB signaling. Atherosclerosis 2019, 281, 38–46. [Google Scholar] [CrossRef]
- Liu, L.; Yan, L.-N.; Sui, Z. MicroRNA-150 affects endoplasmic reticulum stress via MALAT1-miR-150 axis-mediated NF-κB pathway in LPS-challenged HUVECs and septic mice. Life Sci. 2021, 265, 118744. [Google Scholar] [CrossRef]
- Huang, S.; Lu, W.; Ge, D.; Meng, N.; Li, Y.; Le, S.; Zhang, S.; Zhang, Y.; Zhao, B.; Miao, J. A new microRNA signal pathway regulated by long noncoding RNA TGFB2-OT1 in autophagy and inflammation of vascular endothelial cells. Autophagy 2015, 11, 2172–2183. [Google Scholar] [CrossRef]
- Lentjes, M.H.F.M.; Niessen, H.E.C.; Akiyama, Y.; de Bruïne, A.P.; Melotte, V.; van Engeland, M. The emerging role of GATA transcription factors in development and disease. Expert Rev. Mol. Med. 2016, 18, e3. [Google Scholar] [CrossRef]
- Umetani, M.; Mataki, C.; Minegishi, N.; Yamamoto, M.; Hamakubo, T.; Kodama, T. Function of GATA transcription factors in induction of endothelial vascular cell adhesion molecule-1 by tumor necrosis factor-alpha. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 917–922. [Google Scholar] [CrossRef]
- Song, H.; Suehiro, J.; Kanki, Y.; Kawai, Y.; Inoue, K.; Daida, H.; Yano, K.; Ohhashi, T.; Oettgen, P.; Aird, W.C.; et al. Critical role for GATA3 in mediating Tie2 expression and function in large vessel endothelial cells. J. Biol. Chem. 2009, 284, 29109–29124. [Google Scholar] [CrossRef] [PubMed]
- Froese, N.; Kattih, B.; Breitbart, A.; Grund, A.; Geffers, R.; Molkentin, J.D.; Kispert, A.; Wollert, K.C.; Drexler, H.; Heineke, J. GATA6 promotes angiogenic function and survival in endothelial cells by suppression of autocrine transforming growth factor beta/activin receptor-like kinase 5 signaling. J. Biol. Chem. 2011, 286, 5680–5690. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, J.; Breckwoldt, K.; Remmele, C.W.; Hartmann, D.; Dittrich, M.; Pfanne, A.; Just, A.; Xiao, K.; Kunz, M.; Müller, T.; et al. Development of Long Noncoding RNA-Based Strategies to Modulate Tissue Vascularization. J. Am. Coll. Cardiol. 2015, 66, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.P.; Rodor, J.; Caudrillier, A.; Scanlon, J.P.; Spiroski, A.-M.; Dudnakova, T.; Pflüger-Müller, B.; Shmakova, A.; von Kriegsheim, A.; Deng, L.; et al. MIR503HG Loss Promotes Endothelial-to-Mesenchymal Transition in Vascular Disease. Circ. Res. 2021, 128, 1173–1190. [Google Scholar] [CrossRef] [PubMed]
- Froese, N.; Szaroszyk, M.; Korf-Klingebiel, M.; Koch, K.; Schmitto, J.D.; Geffers, R.; Hilfiker-Kleiner, D.; Riehle, C.; Wollert, K.C.; Bauersachs, J.; et al. Endothelial Cell GATA2 Modulates the Cardiomyocyte Stress Response through the Regulation of Two Long Non-Coding RNAs. Biology 2022, 11, 1736. [Google Scholar] [CrossRef]
- Guo, C.; Hua, Y.; Qian, Z. Differentially expressed genes, lncRNAs, and competing endogenous RNAs in Kawasaki disease. PeerJ 2021, 9, e11169. [Google Scholar] [CrossRef]
- Neumann, P.; Jaé, N.; Knau, A.; Glaser, S.F.; Fouani, Y.; Rossbach, O.; Krüger, M.; John, D.; Bindereif, A.; Grote, P.; et al. The lncRNA GATA6-AS epigenetically regulates endothelial gene expression via interaction with LOXL2. Nat. Commun. 2018, 9, 237. [Google Scholar] [CrossRef]
- Downes, N.; Niskanen, H.; Tomas Bosch, V.; Taipale, M.; Godiwala, M.; Väänänen, M.-A.; Turunen, T.A.; Aavik, E.; Laham-Karam, N.; Ylä-Herttuala, S.; et al. Hypoxic regulation of hypoxia inducible factor 1 alpha via antisense transcription. J. Biol. Chem. 2023, 299, 105291. [Google Scholar] [CrossRef]
- Downes, N.L.; Laham-Karam, N.; Kaikkonen, M.U.; Ylä-Herttuala, S. Differential but Complementary HIF1α and HIF2α Transcriptional Regulation. Mol. Ther. 2018, 26, 1735–1745. [Google Scholar] [CrossRef]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Coulon, C.; Georgiadou, M.; Roncal, C.; de Bock, K.; Langenberg, T.; Carmeliet, P. From vessel sprouting to normalization: Role of the prolyl hydroxylase domain protein/hypoxia-inducible factor oxygen-sensing machinery. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, M.; Mei, Z.; Cao, W.; Yang, Y.; Wang, Y.; Wen, A. lncRNAs HIF1A-AS2 facilitates the up-regulation of HIF-1α by sponging to miR-153-3p, whereby promoting angiogenesis in HUVECs in hypoxia. Biomed. Pharmacother. 2017, 96, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xing, J.; Zhang, J.; Jiang, J.; Liu, X.; Zhao, D.; Zhang, Y. Inhibition of long noncoding RNA HIF1A-AS2 confers protection against atherosclerosis via ATF2 downregulation. J. Adv. Res. 2020, 26, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Leisegang, M.S.; Bains, J.K.; Seredinski, S.; Oo, J.A.; Krause, N.M.; Kuo, C.-C.; Günther, S.; Sentürk Cetin, N.; Warwick, T.; Cao, C.; et al. HIF1α-AS1 is a DNA:DNA:RNA triplex-forming lncRNA interacting with the HUSH complex. Nat. Commun. 2022, 13, 6563. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, G.; Wang, Y.; Yue, Y.; Zhao, W. Regulation of apoptosis by long non-coding RNA HIF1A-AS1 in VSMCs: Implications for TAA pathogenesis. Int. J. Clin. Exp. Pathol. 2014, 7, 7643–7652. [Google Scholar]
- Zhao, D.; Zhao, Y.; Wang, J.; Wu, L.; Liu, Y.; Zhao, S.; Guo, F.; Ma, X.; Zhang, H.; Li, Z.; et al. Long noncoding RNA Hotair facilitates retinal endothelial cell dysfunction in diabetic retinopathy. Clin. Sci. 2020, 134, 2419–2434. [Google Scholar] [CrossRef]
- Liu, H.; Shi, C.; Deng, Y. MALAT1 affects hypoxia-induced vascular endothelial cell injury and autophagy by regulating miR-19b-3p/HIF-1α axis. Mol. Cell. Biochem. 2020, 466, 25–34. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Cong, L.; Jiang, Y.; Du, N.; Zhang, H. Implication of long non-coding RNA NEAT1 in the pathogenesis of bacterial meningitis-induced blood-brain barrier damage. Microvasc. Res. 2021, 138, 104225. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, X.; Chen, F.; Yuan, W.; Xiao, X.; Zhang, X.; Dong, Y.; Zhang, Y.; Liu, Y. LncRNA SNHG1 regulates cerebrovascular pathologies as a competing endogenous RNA through HIF-1α/VEGF signaling in ischemic stroke. J. Cell. Biochem. 2018, 119, 5460–5472. [Google Scholar] [CrossRef]
- Liang, S.; Ren, K.; Li, B.; Li, F.; Liang, Z.; Hu, J.; Xu, B.; Zhang, A. LncRNA SNHG1 alleviates hypoxia-reoxygenation-induced vascular endothelial cell injury as a competing endogenous RNA through the HIF-1α/VEGF signal pathway. Mol. Cell. Biochem. 2020, 465, 1–11. [Google Scholar] [CrossRef]
- Man, H.S.J.; Subramaniam, N.; Downs, T.; Sukumar, A.N.; Saha, A.D.; Nair, R.; Chen, L.; Teitelbaum, D.; Turgeon, P.J.; Ku, K.H.; et al. Long noncoding RNA GATA2-AS1 augments endothelial hypoxia inducible factor 1-α induction and regulates hypoxic signaling. J. Biol. Chem. 2023, 299, 103029. [Google Scholar] [CrossRef]
- Kölling, M.; Genschel, C.; Kaucsar, T.; Hübner, A.; Rong, S.; Schmitt, R.; Sörensen-Zender, I.; Haddad, G.; Kistler, A.; Seeger, H.; et al. Hypoxia-induced long non-coding RNA Malat1 is dispensable for renal ischemia/reperfusion-injury. Sci. Rep. 2018, 8, 3438. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Qiu, C.; Wang, X.; Xiang, Y.; Lu, T.; He, Y.; Shang, T.; Zhu, Q.; Wang, X.; et al. Long non-coding RNA GAPLINC promotes angiogenesis by regulating miR-211 under hypoxia in human umbilical vein endothelial cells. J. Cell. Mol. Med. 2019, 23, 8090–8100. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Bai, X.; Liu, C.; Hu, Z. Long noncoding RNA XIST participates hypoxia-induced angiogenesis in human brain microvascular endothelial cells through regulating miR-485/SOX7 axis. Am. J. Transl. Res. 2019, 11, 6487–6497. [Google Scholar] [PubMed]
- Voellenkle, C.; Garcia-Manteiga, J.M.; Pedrotti, S.; Perfetti, A.; de Toma, I.; Da Silva, D.; Maimone, B.; Greco, S.; Fasanaro, P.; Creo, P.; et al. Implication of Long noncoding RNAs in the endothelial cell response to hypoxia revealed by RNA-sequencing. Sci. Rep. 2016, 6, 24141. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, F.C.; Werner, A.; John, D.; Boeckel, J.-N.; Melissari, M.-T.; Grote, P.; Glaser, S.F.; Demolli, S.; Uchida, S.; Michalik, K.M.; et al. Identification and Functional Characterization of Hypoxia-Induced Endoplasmic Reticulum Stress Regulating lncRNA (HypERlnc) in Pericytes. Circ. Res. 2017, 121, 368–375. [Google Scholar] [CrossRef]
- Choudhry, H.; Harris, A.L.; McIntyre, A. The tumour hypoxia induced non-coding transcriptome. Mol. Aspects Med. 2016, 47–48, 35–53. [Google Scholar] [CrossRef]
- Li, W.; Han, S.; Hu, P.; Chen, D.; Zeng, Z.; Hu, Y.; Xu, F.; Tang, J.; Wang, F.; Zhao, Y.; et al. LncRNA ZNFTR functions as an inhibitor in pancreatic cancer by modulating ATF3/ZNF24/VEGFA pathway. Cell Death Dis. 2021, 12, 830. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Tian, Y.F.; Wu, H.; Ouyang, S.Y.; Kuang, W.L. LncRNA H19 promotes glioma angiogenesis through miR-138/HIF-1α/VEGF axis. Neoplasma 2020, 67, 111–118. [Google Scholar] [CrossRef]
- Wei, H.; Xu, Z.; Chen, L.; Wei, Q.; Huang, Z.; Liu, G.; Li, W.; Wang, J.; Tang, Q.; Pu, J. Long non-coding RNA PAARH promotes hepatocellular carcinoma progression and angiogenesis via upregulating HOTTIP and activating HIF-1α/VEGF signaling. Cell Death Dis. 2022, 13, 102. [Google Scholar] [CrossRef]
- Hata, A.; Chen, Y.-G. TGF-β Signaling from Receptors to Smads. Cold Spring Harb. Perspect. Biol. 2016, 8, a022061. [Google Scholar] [CrossRef] [PubMed]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef]
- Ma, J.; Sanchez-Duffhues, G.; Goumans, M.-J.; Ten Dijke, P. TGF-β-Induced Endothelial to Mesenchymal Transition in Disease and Tissue Engineering. Front. Cell Dev. Biol. 2020, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.S. Transcriptional Control by the SMADs. Cold Spring Harb. Perspect. Biol. 2016, 8, a022079. [Google Scholar] [CrossRef] [PubMed]
- Lou, C.; Li, T. Long non-coding RNA SENCR alleviates endothelial-to-mesenchymal transition via targeting miR-126a. Arch. Med. Sci. 2023, 19, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, S.; Yang, Y.; Sun, Y.; Yang, Q.; Gu, N.; Li, J.; Huang, T.; Liu, Y.; Dong, H.; et al. The lncRNA ANRIL regulates endothelial dysfunction by targeting the let-7b/TGF-βR1 signalling pathway. J. Cell. Physiol. 2021, 236, 2058–2069. [Google Scholar] [CrossRef]
- Meng, Y.; Hao, Z.; Zhang, H.; Bai, P.; Guo, W.; Tian, X.; Xu, J. lncRNA NEAT1/miR-495-3p regulates angiogenesis in burn sepsis through the TGF-β1 and SMAD signaling pathways. Immun. Inflamm. Dis. 2023, 11, e758. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Zheng, Y.; Wang, G.; Jiang, M. Exosomes-Mediated LncRNA ZEB1-AS1 Facilitates Cell Injuries by miR-590-5p/ETS1 Axis Through the TGF-β/Smad Pathway in Oxidized Low-density Lipoprotein-induced Human Umbilical Vein Endothelial Cells. J. Cardiovasc. Pharmacol. 2021, 77, 480–490. [Google Scholar] [CrossRef]
- Leisegang, M.S.; Fork, C.; Josipovic, I.; Richter, F.M.; Preussner, J.; Hu, J.; Miller, M.J.; Epah, J.; Hofmann, P.; Günther, S.; et al. Long Noncoding RNA MANTIS Facilitates Endothelial Angiogenic Function. Circulation 2017, 136, 65–79. [Google Scholar] [CrossRef]
- Kelleher, F.C.; O’Sullivan, H. FOXM1 in sarcoma: Role in cell cycle, pluripotency genes and stem cell pathways. Oncotarget 2016, 7, 42792–42804. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Zhao, Z.-W.; Li, W.-M.; Yang, G.; Jing, P.-Y.; Li, P.; Dang, H.-Z.; Chen, Z.; Zhou, Y.-A.; Li, X.-F. Knockdown of MALAT1 expression inhibits HUVEC proliferation by upregulation of miR-320a and downregulation of FOXM1 expression. Oncotarget 2017, 8, 61499–61509. [Google Scholar] [CrossRef]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Niu, N.; Wei, T.; Tozawa, H.; Chen, X.; Zhang, C.; Zhang, J.; Wada, Y.; Kapron, C.M.; Liu, J. The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis. Oncotarget 2017, 8, 69139–69161. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Du, P.; Cui, P.; Qin, Y.; Hu, C.; Wu, J.; Zhou, Z.; Zhang, W.; Qin, L.; Huang, G. LncRNA PVT1 promotes angiogenesis via activating the STAT3/VEGFA axis in gastric cancer. Oncogene 2018, 37, 4094–4109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dong, B.; Hao, J.; Yi, S.; Cai, W.; Luo, Z. LncRNA Snhg3 contributes to dysfunction of cerebral microvascular cells in intracerebral hemorrhage rats by activating the TWEAK/Fn14/STAT3 pathway. Life Sci. 2019, 237, 116929. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fu, H.; Zhang, S.; Sun, S.; Liu, Y. LncRNA SNHG16 drives proliferation, migration, and invasion of hemangioma endothelial cell through modulation of miR-520d-3p/STAT3 axis. Cancer Med. 2018, 7, 3311–3320. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, P.; Sommer, J.; Theodorou, K.; Kirchhof, L.; Fischer, A.; Li, Y.; Perisic, L.; Hedin, U.; Maegdefessel, L.; Dimmeler, S.; et al. Long non-coding RNA H19 regulates endothelial cell aging via inhibition of STAT3 signalling. Cardiovasc. Res. 2019, 115, 230–242. [Google Scholar] [CrossRef]
- Bowles, J.; Schepers, G.; Koopman, P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000, 227, 239–255. [Google Scholar] [CrossRef]
- Yao, Y.; Yao, J.; Boström, K.I. SOX Transcription Factors in Endothelial Differentiation and Endothelial-Mesenchymal Transitions. Front. Cardiovasc. Med. 2019, 6, 30. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Y.; Zhong, Y.; Zhao, M.; Yao, W.; Ren, X.; Wang, Q.; Guo, X.; Zhang, Q.-Q.; Dai, J. The long noncoding RNA HCG18 participates in PM2.5-mediated vascular endothelial barrier dysfunction. Aging 2020, 12, 23960–23973. [Google Scholar] [CrossRef] [PubMed]
- Natarelli, L.; Geißler, C.; Csaba, G.; Wei, Y.; Zhu, M.; Di Francesco, A.; Hartmann, P.; Zimmer, R.; Schober, A. miR-103 promotes endothelial maladaptation by targeting lncWDR59. Nat. Commun. 2018, 9, 2645. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zheng, D.; Li, Y.; Liu, G.; Zhou, H.; Liu, Y. Long noncoding RNA MANTIS relieved the protein-bound uremic toxin-induced injury on human umbilical vein endothelial cells in chronic kidney disease and end-stage renal disease. Int. J. Clin. Exp. Pathol. 2018, 11, 3236–3246. [Google Scholar] [PubMed]
- Kong, C.; Lyu, D.; He, C.; Li, R.; Lu, Q. Dioscin elevates lncRNA MANTIS in therapeutic angiogenesis for heart diseases. Aging Cell 2021, 20, e13392. [Google Scholar] [CrossRef]
- Kurian, L.; Aguirre, A.; Sancho-Martinez, I.; Benner, C.; Hishida, T.; Nguyen, T.B.; Reddy, P.; Nivet, E.; Krause, M.N.; Nelles, D.A.; et al. Identification of novel long noncoding RNAs underlying vertebrate cardiovascular development. Circulation 2015, 131, 1278–1290. [Google Scholar] [CrossRef]
- Trembinski, D.J.; Bink, D.I.; Theodorou, K.; Sommer, J.; Fischer, A.; van Bergen, A.; Kuo, C.-C.; Costa, I.G.; Schürmann, C.; Leisegang, M.S.; et al. Aging-regulated anti-apoptotic long non-coding RNA Sarrah augments recovery from acute myocardial infarction. Nat. Commun. 2020, 11, 2039. [Google Scholar] [CrossRef]
- Oo, J.A.; Pálfi, K.; Warwick, T.; Wittig, I.; Prieto-Garcia, C.; Matkovic, V.; Tomašković, I.; Boos, F.; Izquierdo Ponce, J.; Teichmann, T.; et al. Long non-coding RNA PCAT19 safeguards DNA in quiescent endothelial cells by preventing uncontrolled phosphorylation of RPA2. Cell Rep. 2022, 41, 111670. [Google Scholar] [CrossRef]
- Dunn-Davies, H.; Dudnakova, T.; Nogara, A.; Rodor, J.; Thomas, A.C.; Parish, E.; Gautier, P.; Meynert, A.; Ulitsky, I.; Madeddu, P.; et al. Control of endothelial cell function and arteriogenesis by MEG3:EZH2 epigenetic regulation of integrin expression. Mol. Ther. Nucleic Acids 2024, 35, 102173. [Google Scholar] [CrossRef]
- Leisegang, M.S.; Warwick, T.; Stötzel, J.; Brandes, R.P. RNA-DNA triplexes: Molecular mechanisms and functional relevance. Trends Biochem. Sci. 2024, 49, 532–544. [Google Scholar] [CrossRef]
- Seredinski, S.; Boos, F.; Günther, S.; Oo, J.A.; Warwick, T.; Izquierdo Ponce, J.; Lillich, F.F.; Proschak, E.; Knapp, S.; Gilsbach, R.; et al. DNA topoisomerase inhibition with the HIF inhibitor acriflavine promotes transcription of lncRNAs in endothelial cells. Mol. Ther. Nucleic Acids 2022, 27, 1023–1035. [Google Scholar] [CrossRef]
- Mattioli, K.; Volders, P.-J.; Gerhardinger, C.; Lee, J.C.; Maass, P.G.; Melé, M.; Rinn, J.L. High-throughput functional analysis of lncRNA core promoters elucidates rules governing tissue specificity. Genome Res. 2019, 29, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
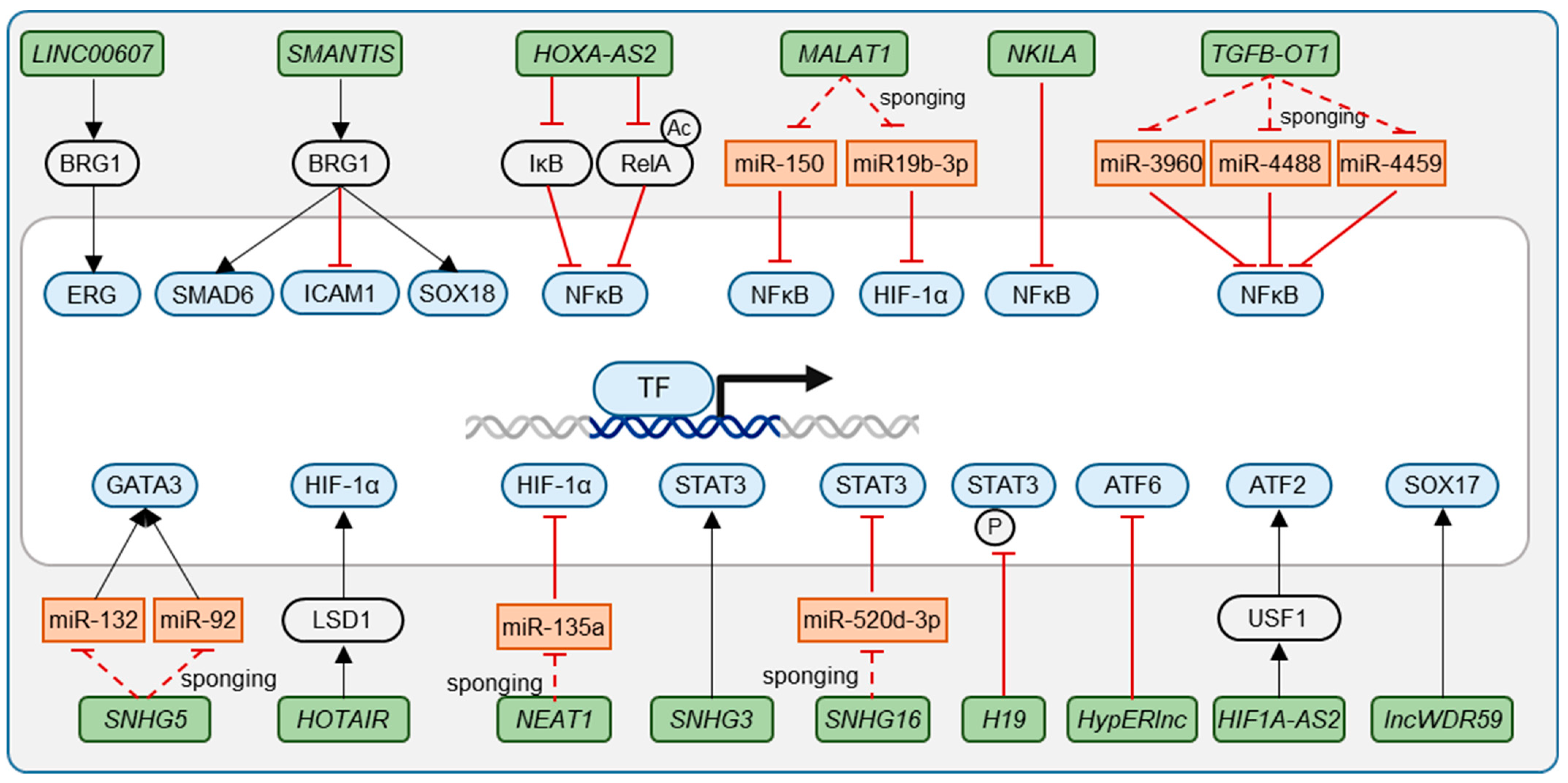
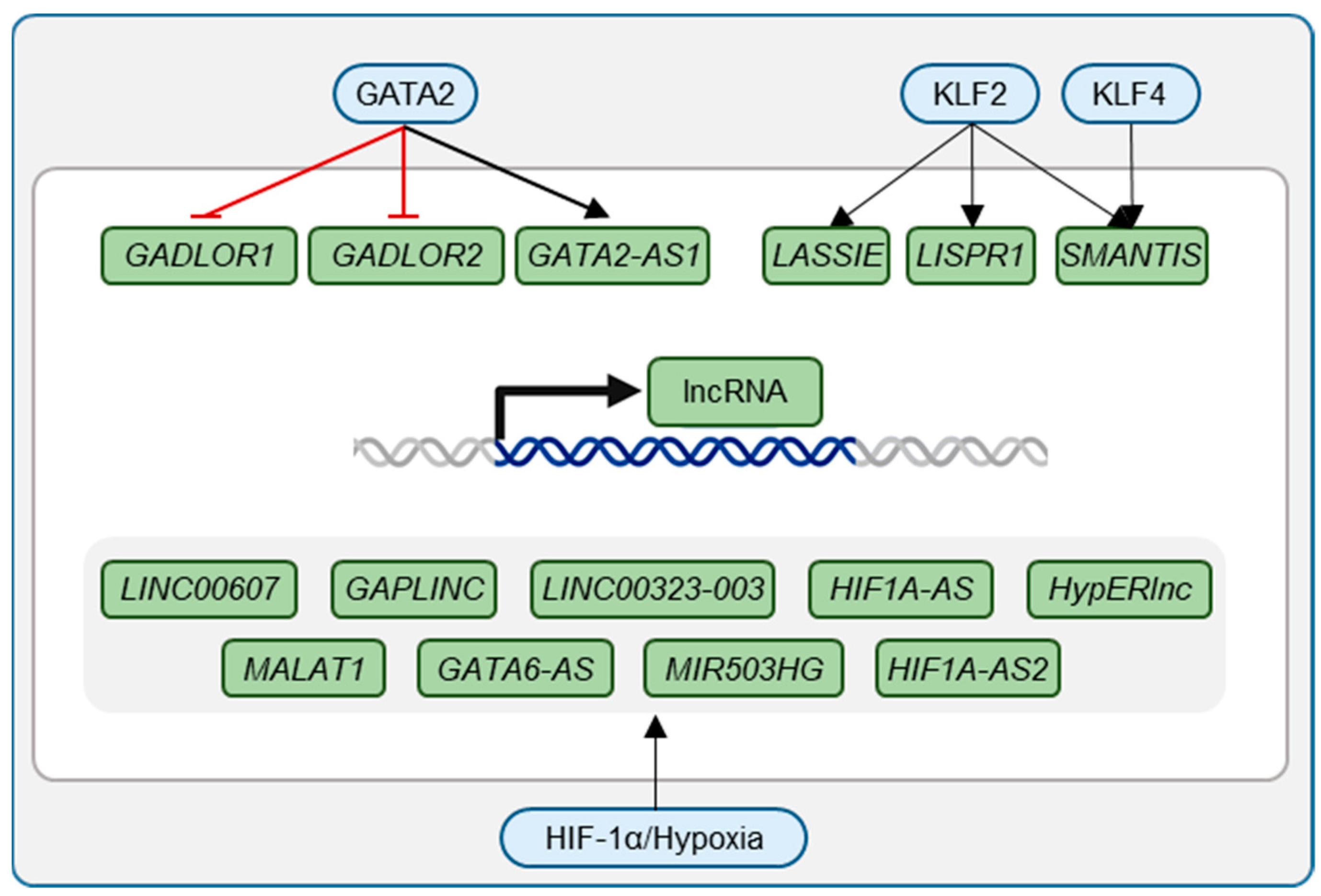

| Transcription Factor | lncRNA | Mechanism | Relevance | Source |
|---|---|---|---|---|
| ERG | LINC00607 | BRG1-mediated ERG DNA motif accessibility | [19] | |
| ERGAL | ceRNA for miR-183-5p, preventing degradation of ERG | Dengue Virus infection | [21] | |
| KLF2/KLF4 | SMANTIS | Scaffold for chromatin remodeling protein BRG1 | Laminar shear stress, statin treatment | [31] |
| KLF2 | LASSIE | Regulation of barrier function by connecting adherens junctions to the cytoskeleton | Laminar shear stress | [30] |
| LISPR1 | Needed for migration and sprouting; upregulated under laminar flow and statins | Reduced in patients with COPD, CTEPH, and IPAH | [32] | |
| KLF4 | AF131217.1 | Promoting anti-inflammatory phenotype. ceRNA for miR-128-3p, preventing mRNA degradation of KLF4 | Laminar shear stress | [29] |
| NFκB | HOXA-AS2 | Repressing NFκB activation by controlling IκBα degradation and RelA acetylation | Carotid artery atherosclerosis | [37] |
| MALAT1 | Enhancing NFκB activity by sponging miR-150, a NFκB repressor | LPS-induced inflammation, sepsis | [38] | |
| TGFB2-OT1 | Sponging miR-3960, miR-4488 and miR-4459 | LPS and oxLDL induced inflammation and autophagy | [39] | |
| NKILA | Represses NFκB activity | Released upon inflammation | [33] | |
| GATA2 | LINC00323-003 | Enhancing GATA2 abundance by potential binding translation initiator elF4A3 | Regulation of tissue vascularization after hypoxic event | [44] |
| MIR503HG | Repression of expression of miR-424 | Regulation of tissue Vascularization after hypoxic event | [44] | |
| GADLOR1 and 2 | GATA2 repressed lncRNAs | Mechanical overload of the heart | [46] | |
| GATA3 | SNHG5 | Potential GATA3 repression by sponging miR-132 and miR-92. | Kawasaki disease | [47] |
| HIF-1α | HIF1A-AS2 | Increasing HIF-1α expression by sponging miR-153-3p | Hypoxia | [53] |
| HIF1A-AS2 | Inducing ATF2 expression through recruitment of USF1 | Atherosclerosis | [54] | |
| HOTAIR | Scaffolding interaction of LSD1 and HIF-1α | Diabetic retinopathy | [57] | |
| MALAT1 | Increasing HIF-1α signaling by sponging miR-19b-3p | Hypoxia | [58] | |
| NEAT1 | Securing BBB integrity by sponging HIF-1α-activating miR-135a | Hypoxia | [59] | |
| SNHG1 | Securing BBB integrity by sponging miR-18a | Ischemic stroke | [60] | |
| Sponging miR-140-3p | Myocardial ischemia/reperfusion injury | [61] | ||
| HypERlnc | Reduction results in enhanced ER stress through induction of ATF6 activity | Heart failure | [67] | |
| GATA2-AS1 | Increasing HIF-1α stability and translation under acute hypoxia | Chronic and acute hypoxia | [62] | |
| SMAD6 | SMANTIS | Securing SMAD6 expression by enabling BRG1-mediated chromatin remodeling | EC homeostasis | [80] |
| FOXM1 | MALAT1 | Promoting stability of FOXM1 by sponging miR-320a | EC proliferation | [82] |
| STAT3 | PVT1 | Preventing STAT3 proteasomal degradation, driving VEGF-driven proangiogenic signaling | Tumor angiogenesis in gastric cancer | [86] |
| SNHG3 | Activating the TWEAK/Fn14/STAT3 pathway and enhancing MMP-2 and MMP-9 expression. Worsening of BBB integrity | Intracerebral hemorrhage | [87] | |
| SNHG16 | Enhancing STAT3-mediated proliferation, migration, and invasion by sponging miR-520d-3p | Hemangioma | [88] | |
| H19 | Inhibiting STAT3 phosphorylation. Repressing EC senescence, proliferation, inflammatory activation and angiogenic sprouting. | Repressed during aging | [89] | |
| SOX7 | XIST | Increasing SOX7 abundance by sponging the translational repressor miR-485-3p. Induction of VEGF signaling | Ischemic stroke | [65] |
| HCG18 | Increasing SOX7 abundance by sponging the translational repressor miR-21. Secure barrier integrity by VE-cadherin induction. | Vascular barrier breakdown through ambient particulate matter with an aerodynamic diameter < 2.5 μm | [92] | |
| SOX17 | lncWDR59 | Inducing SOX17 expression to prevent oxidative stress-induced DNA damage | Hyperlipidemia and oxLDL induced maladapted phenotype under disturbed flow | [93] |
| SOX18 | SMANTIS | Securing SOX18 expression by enabling BRG1-mediated chromatin remodeling | CKD; mode of action of Dioscin in treatment of CAD | [80,94,95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, F.; Leisegang, M.S.; Brandes, R.P. LncRNAs Are Key Regulators of Transcription Factor-Mediated Endothelial Stress Responses. Int. J. Mol. Sci. 2024, 25, 9726. https://doi.org/10.3390/ijms25179726
Lam F, Leisegang MS, Brandes RP. LncRNAs Are Key Regulators of Transcription Factor-Mediated Endothelial Stress Responses. International Journal of Molecular Sciences. 2024; 25(17):9726. https://doi.org/10.3390/ijms25179726
Chicago/Turabian StyleLam, Frederike, Matthias S. Leisegang, and Ralf P. Brandes. 2024. "LncRNAs Are Key Regulators of Transcription Factor-Mediated Endothelial Stress Responses" International Journal of Molecular Sciences 25, no. 17: 9726. https://doi.org/10.3390/ijms25179726
APA StyleLam, F., Leisegang, M. S., & Brandes, R. P. (2024). LncRNAs Are Key Regulators of Transcription Factor-Mediated Endothelial Stress Responses. International Journal of Molecular Sciences, 25(17), 9726. https://doi.org/10.3390/ijms25179726






