Establishing Treatment Effectiveness in Fabry Disease: Observation-Based Recommendations for Improvement
Abstract
:1. Introduction
2. Fabry Disease-Specific Therapies
2.1. Enzyme Replacement Therapy
2.2. Chaperone Therapy
2.3. Second Generation Enzyme Replacement Therapy
3. Meta-Analyses of FD-Specific Therapy
3.1. Meta-Analyses on the Effect of Enzyme Replacement Therapy
3.2. Evaluation of Meta-Analyses on the Effect of Enzyme Replacement Therapy
4. Recent Evaluations of FD-Specific Therapies
4.1. Recent Studies on the Effect of ERT (Agalsidase Alfa and Agalsidase Beta)
4.2. Evaluation of Recent Studies on the Effect of ERT
4.3. Phase 3 Studies on the Effect of Migalastat
4.4. Evaluation of Phase 3 Studies on the Effect of Migalastat
4.5. Phase 3 Study on the Effect of Pegunigalsidase Alfa
| Study | Follow-Up | Analyzed FD Patients (% Male, if Combined) | Pegunigalsidase-α or Migalastat | Agalsidase-α or Agalsidase-β Treated | Outcomes | Results (IMP vs. Control) Mean ± SD/Mean (95% CI)/ %/Median [95% CI] | ||
|---|---|---|---|---|---|---|---|---|
| Treated | Age | Untreated | Age | |||||
| Germain 2016 [35] Migalastat vs. untreated | 6 mos | Combined analysis of males and females | n = 28 (32.1%) | 41.5 ± 13 y/o | n = 22 (40.9%) | 45.1 ± 8 y/o | - LVMI † (g/m2) | −0.4 ± 8.2 vs. 6.3 ± 15.3 |
| - eGFR ‡ (mL/min/1.73 m2) | 1.8 ± 1.5 vs. −0.3 ± 1.4 | |||||||
| - mGFR # (mL/min/1.73 m2) | −1.19 ± 3.4 vs. 0.41 ± 2.0 | |||||||
| - Urinary protein excretion (mg/24 h) | 2.2 ± 252 vs. −12.9 ± 224 | |||||||
| Hughes 2017 [36] Migalastat vs. agalsidase alfa/ agalsidase beta | 18 mos | Combined analysis of males and females | n = 36 (44.4%) | 50.5 ± 2.3 y/o | n = 21 (42.9%) | 46.3 ± 3.3 y/o | - eGFR ‡ (mL/min/1.73 m2) | −0.40 (−2.27, 1.48) vs. −1.03 (−3.64, 1.58) |
| - eGFR ¶ (mL/min/1.73 m2) | −1.51 (−3.43, 0.40) vs. −1.53 (−4.20, 1.13) | |||||||
| - mGFR # (mL/min/1.73 m2) | −4.35 (−7.65, −1.06) vs. −3.24 (−7.81, 1.33) | |||||||
| - LVPWT † (mm) | −0.35 (−0.77, 0.07) vs. 0.029 (−0.37, 0.94) | |||||||
| - IVSWT † (mm) | 0.58 (−2.00, 1.40) vs. 0.37 (−0.51, 1.24) | |||||||
| - LVEF † (%) | −1.07 ± 0.53 vs. −0.49 ± 1.1 ‡‡ | |||||||
| - LVMI † (g/m2) | −6.6 (−11.0, −2.2) vs. −2.0 [−11.0. 7.0] | |||||||
| - Clinical events †† | 29% vs. 44% | |||||||
| Subgroup analysis: | - with LVH | n = 13 (30.8%) | NS | n = 5 (80%) | NS | - LVMI † (g/m2) | −8.4 (−15.7, 2.6) vs. 4.5 (−20.9, 30.0) | |
| Wallace 2023 [39] Pegunigalsidase alfa vs. agalsidase beta | 24 mos | Combined analysis of males and females | n = 52 (55.8%) | 43.9 ± 10.2 y/o | n = 25 (72%) | 44.3 ± 10 y/o | - eGFR ‡ (mL/min/1.73 m2) | −2.51 [−3.79, −1.24] vs. −2.16 [−3.81, −0.51] |
| Subgroup analysis: | - males | n = 29 | 42.6 ± 11.5 y/o | n = 18 | 46.5 ± 6.9 y/o | - eGFR ‡ (mL/min/1.73 m2) | −3.44 [−5.38, −1.50] vs. −2.01 [−3.98, −0.04] | |
| - females | n = 23 | 45.6 ± 8.3 y/o | n = 7 | 41.7 ± 14.5 y/o | - eGFR ‡ (mL/min/1.73 m2) | −1.15 [−3.11, −0.81] vs. −2.79 [−6.28, 0.70] | ||
4.6. Evaluation of Phase 3 Study on the Effect of Pegunigalsidase Alfa
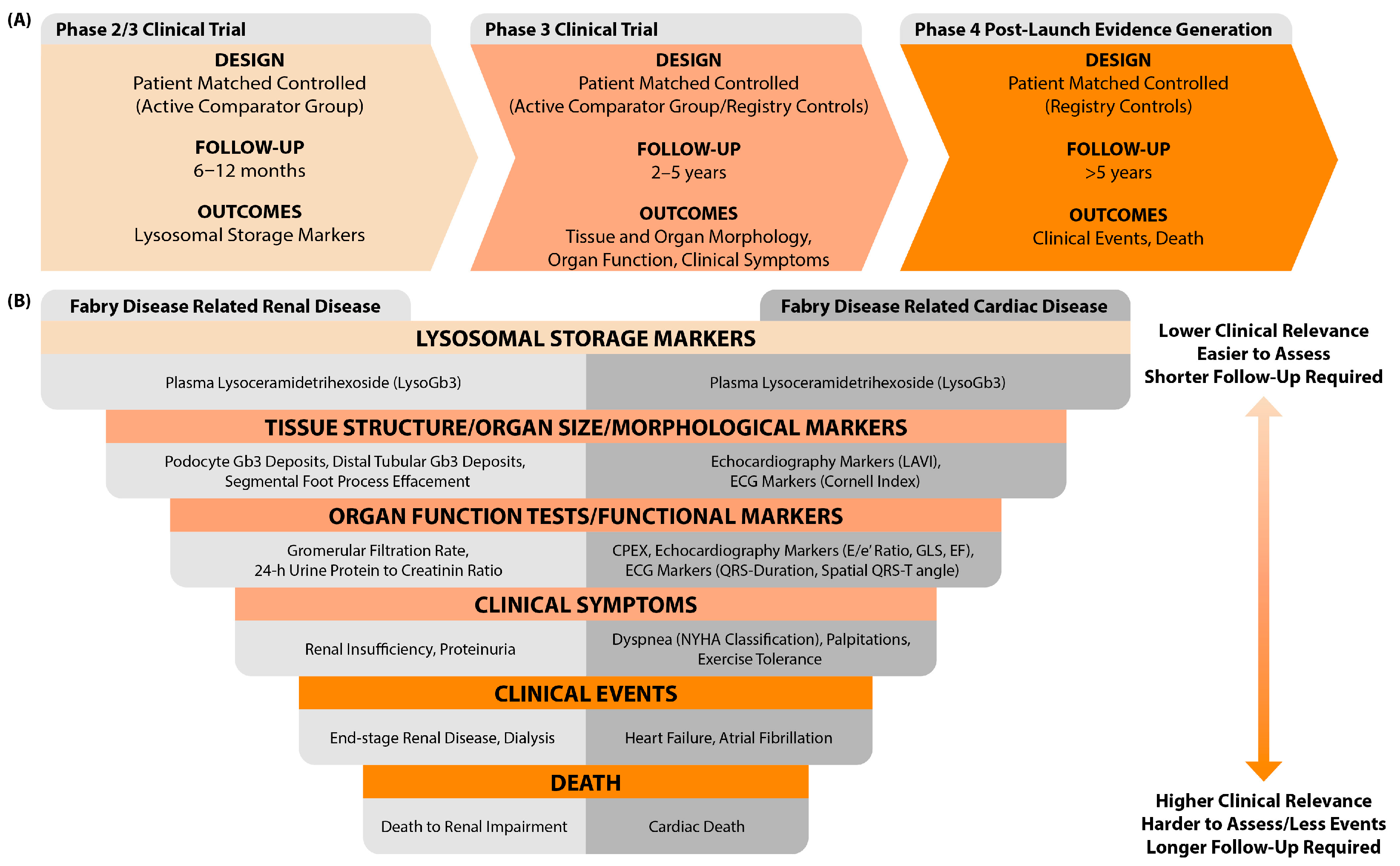
5. Interpretation of the Results
6. Identified Problems and Recommendations for Future Studies
6.1. Problems: Comparisons of Dissimilar Groups, Lack of Accounting for Prognostic Factors, Inadequate Follow-Up, and Inappropriate Choice of Study Outcomes
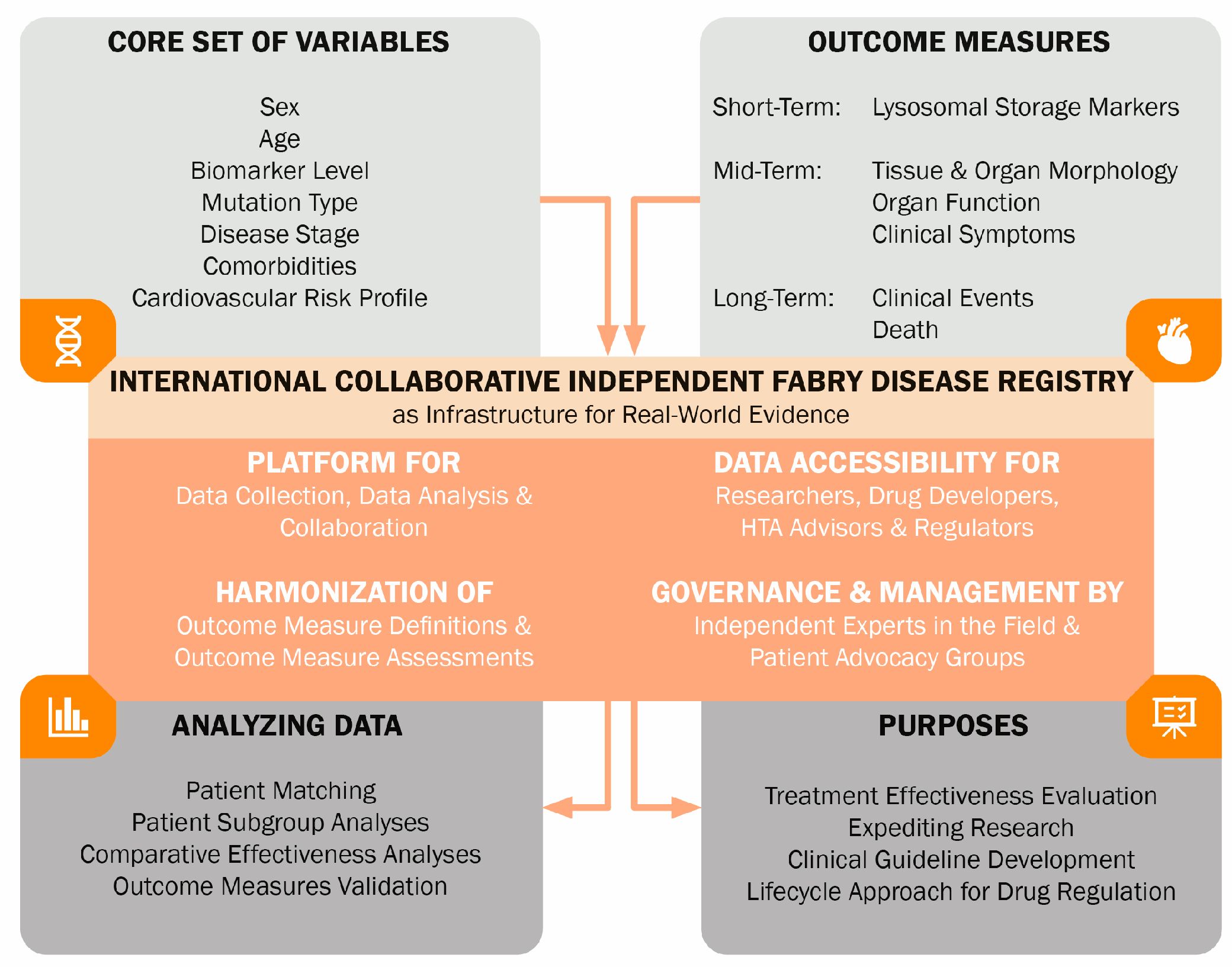
6.2. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Search Strategy Meta-Analyses
Appendix B
Appendix B.1. Search Strategy Recent Studies
References
- Germain, D.P.; Fouilhoux, A.; Decramer, S.; Tardieu, M.; Pillet, P.; Fila, M.; Rivera, S.; Deschenes, G.; Lacombe, D. Consensus recommendations for diagnosis, management and treatment of Fabry disease in paediatric patients. Clin. Genet. 2019, 96, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Metha, A.; Hughes, D.A. Fabry Disease. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1292/ (accessed on 1 June 2024).
- Echevarria, L.; Benistan, K.; Toussaint, A.; Dubourg, O.; Hagege, A.A.; Eladari, D.; Jabbour, F.; Beldjord, C.; De Mazancourt, P.; Germain, D.P. X-chromosome inactivation in female patients with Fabry disease. Clin. Genet. 2016, 89, 44–54. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, S.J.; Sayed, M.E.; Hollak, C.E.M.; Brands, M.M.; Snelder, C.K.S.; Boekholdt, S.M.; Vogt, L.; Goorden, S.M.I.; van Kuilenburg, A.B.P.; Langeveld, M. Early Risk Stratification for Natural Disease Course in Fabry Patients Using Plasma Globotriaosylsphingosine Levels. Clin. J. Am. Soc. Nephrol. 2023, 18, 1272–1282. [Google Scholar] [CrossRef]
- Guo, W.; Xie, Y.; Ji, P.; Li, S.; Cai, G.; Chen, X. The evolution of the initial manifestations and renal involvement of Chinese patients with classical and late-onset Fabry disease at different sexes and ages. BMC Nephrol. 2023, 24, 90. [Google Scholar] [CrossRef]
- Korver, S.; Vergouwe, M.; Hollak, C.E.M.; van Schaik, I.N.; Langeveld, M. Development and clinical consequences of white matter lesions in Fabry disease: A systematic review. Mol. Genet. Metab. 2018, 125, 205–216. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, M.; Hirsch, A.; Boekholdt, M.; van Dussen, L.; Datema, M.; Hollak, C.; Langeveld, M. Influence of sex and phenotype on cardiac outcomes in patients with Fabry disease. Heart 2021, 107, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Houge, G.; Langeveld, M.; Oliveira, J.P. GLA insufficiency should not be called Fabry disease. Eur. J. Hum. Genet. 2024. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Ferreira, S. Multiple phenotypic domains of Fabry disease and their relevance for establishing genotype-phenotype correlations. Appl. Clin. Genet. 2019, 12, 35–50. [Google Scholar] [CrossRef]
- Ferreira, S.; Ortiz, A.; Germain, D.P.; Viana-Baptista, M.; Caldeira-Gomes, A.; Camprecios, M.; Fenollar-Cortes, M.; Gallegos-Villalobos, A.; Garcia, D.; Garcia-Robles, J.A.; et al. The alpha-galactosidase A p.Arg118Cys variant does not cause a Fabry disease phenotype: Data from individual patients and family studies. Mol. Genet. Metab. 2015, 114, 248–258. [Google Scholar] [CrossRef]
- Nakamura, K.; Sekijima, Y.; Nakamura, K.; Hattori, K.; Nagamatsu, K.; Shimizu, Y.; Yazaki, M.; Sakurai, A.; Endo, F.; Fukushima, Y.; et al. p.E66Q mutation in the GLA gene is associated with a high risk of cerebral small-vessel occlusion in elderly Japanese males. Eur. J. Neurol. 2014, 21, 49–56. [Google Scholar] [CrossRef]
- Schiffmann, R.; Fuller, M.; Clarke, L.A.; Aerts, J.M. Is it Fabry disease? Genet. Med. 2016, 18, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, S.J.; Hollak, C.E.M.; van Kuilenburg, A.B.P.; Langeveld, M. Developments in the treatment of Fabry disease. J. Inherit. Metab. Dis. 2020, 43, 908–921. [Google Scholar] [CrossRef]
- El Dib, R.; Gomaa, H.; Carvalho, R.P.; Camargo, S.E.; Bazan, R.; Barretti, P.; Barreto, F.C. Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst. Rev. 2016, 7, CD006663. [Google Scholar] [CrossRef]
- El Dib, R.; Gomaa, H.; Ortiz, A.; Politei, J.; Kapoor, A.; Barreto, F. Enzyme replacement therapy for Anderson-Fabry disease: A complementary overview of a Cochrane publication through a linear regression and a pooled analysis of proportions from cohort studies. PLoS ONE 2017, 12, e0173358. [Google Scholar] [CrossRef] [PubMed]
- Orsborne, C.; Black, N.; Naish, J.H.; Woolfson, P.; Reid, A.B.; Schmitt, M.; Jovanovic, A.; Miller, C.A. Disease-specific therapy for the treatment of the cardiovascular manifestations of Fabry disease: A systematic review. Heart 2023, 110, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.R.; Della Valle, M.C.; Wu, X.; Katz, E.; Pruthi, F.; Bond, S.; Bronfin, B.; Williams, H.; Yu, J.; Bichet, D.G.; et al. The validation of pharmacogenetics for the identification of Fabry patients to be treated with migalastat. Genet. Med. 2017, 19, 430–438. [Google Scholar] [CrossRef]
- Schiffmann, R.; Goker-Alpan, O.; Holida, M.; Giraldo, P.; Barisoni, L.; Colvin, R.B.; Jennette, C.J.; Maegawa, G.; Boyadjiev, S.A.; Gonzalez, D.; et al. Pegunigalsidase alfa, a novel PEGylated enzyme replacement therapy for Fabry disease, provides sustained plasma concentrations and favorable pharmacodynamics: A 1-year Phase 1/2 clinical trial. J. Inherit. Metab. Dis. 2019, 42, 534–544. [Google Scholar] [CrossRef]
- Burlina, A.; Brand, E.; Hughes, D.; Kantola, I.; Krämer, J.; Nowak, A.; Tondel, C.; Wanner, C.; Spada, M. An expert consensus on the recommendations for the use of biomarkers in Fabry disease. Mol. Genet. Metab. 2023, 139, 107585. [Google Scholar] [CrossRef] [PubMed]
- Alegra, T.; Vairo, F.; de Souza, M.V.; Krug, B.C.; Schwartz, I.V. Enzyme replacement therapy for Fabry disease: A systematic review and meta-analysis. Genet. Mol. Biol. 2012, 35, 947–954. [Google Scholar] [CrossRef]
- Rombach, S.M.; Smid, B.E.; Linthorst, G.E.; Dijkgraaf, M.G.; Hollak, C.E. Natural course of Fabry disease and the effectiveness of enzyme replacement therapy: A systematic review and meta-analysis: Effectiveness of ERT in different disease stages. J. Inherit. Metab. Dis. 2014, 37, 341–352. [Google Scholar] [CrossRef]
- Sheng, S.; Wu, L.; Nalleballe, K.; Sharma, R.; Brown, A.; Ranabothu, S.; Kapoor, N.; Onteddu, S. Fabry’s disease and stroke: Effectiveness of enzyme replacement therapy (ERT) in stroke prevention, a review with meta-analysis. J. Clin. Neurosci. 2019, 65, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Kanters, S.; Hamed, A.; DasMahapatra, P.; Poggio, E.; Maski, M.; Aguiar, M.; Ponce, E.; Jansen, J.P.; Ayers, D.; et al. Agalsidase beta treatment slows estimated glomerular filtration rate loss in classic Fabry disease patients: Results from an individual patient data meta-analysis. Clin. Kidney J. 2021, 14, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Lin, S.P.; Niu, D.M.; Lin, H.Y. Fabry Disease and the Effectiveness of Enzyme Replacement Therapy (ERT) in Left Ventricular Hypertrophy (LVH) Improvement: A Review and Meta-Analysis. Int. J. Med. Sci. 2022, 19, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Arends, M.; Biegstraaten, M.; Hughes, D.A.; Mehta, A.; Elliott, P.M.; Oder, D.; Watkinson, O.T.; Vaz, F.M.; van Kuilenburg, A.B.P.; Wanner, C.; et al. Retrospective study of long-term outcomes of enzyme replacement therapy in Fabry disease: Analysis of prognostic factors. PLoS ONE 2017, 12, e0182379. [Google Scholar] [CrossRef] [PubMed]
- Arends, M.; Wanner, C.; Hughes, D.; Mehta, A.; Oder, D.; Watkinson, O.T.; Elliott, P.M.; Linthorst, G.E.; Wijburg, F.A.; Biegstraaten, M.; et al. Characterization of Classical and Nonclassical Fabry Disease: A Multicenter Study. J. Am. Soc. Nephrol. 2017, 28, 1631–1641. [Google Scholar] [CrossRef]
- Hanneman, K.; Karur, G.R.; Wasim, S.; Wald, R.M.; Iwanochko, R.M.; Morel, C.F. Left Ventricular Hypertrophy and Late Gadolinium Enhancement at Cardiac MRI Are Associated with Adverse Cardiac Events in Fabry Disease. Radiology 2020, 294, 42–49. [Google Scholar] [CrossRef]
- Nowak, A.; Beuschlein, F.; Sivasubramaniam, V.; Kasper, D.; Warnock, D.G. Lyso-Gb3 associates with adverse long-term outcome in patients with Fabry disease. J. Med. Genet. 2022, 59, 287–293. [Google Scholar] [CrossRef]
- Hongo, K.; Ito, K.; Date, T.; Anan, I.; Inoue, Y.; Morimoto, S.; Ogawa, K.; Kawai, M.; Kobayashi, H.; Kobayashi, M.; et al. The beneficial effects of long-term enzyme replacement therapy on cardiac involvement in Japanese Fabry patients. Mol. Genet. Metab. 2018, 124, 143–151. [Google Scholar] [CrossRef]
- Nordin, S.; Kozor, R.; Vijapurapu, R.; Augusto, J.B.; Knott, K.D.; Captur, G.; Treibel, T.A.; Ramaswami, U.; Tchan, M.; Geberhiwot, T.; et al. Myocardial Storage, Inflammation, and Cardiac Phenotype in Fabry Disease After One Year of Enzyme Replacement Therapy. Circ. Cardiovasc. Imaging 2019, 12, e009430. [Google Scholar] [CrossRef]
- van der Veen, S.J.; Korver, S.; Hirsch, A.; Hollak, C.E.M.; Wijburg, F.A.; Brands, M.M.; Tondel, C.; van Kuilenburg, A.B.P.; Langeveld, M. Early start of enzyme replacement therapy in pediatric male patients with classical Fabry disease is associated with attenuated disease progression. Mol. Genet. Metab. 2022, 135, 163–169. [Google Scholar] [CrossRef]
- Pogoda, C.; Brand, S.M.; Duning, T.; Schmidt-Pogoda, A.; Sindermann, J.; Lenders, M.; Brand, E. Impact of enzyme replacement therapy and migalastat on left atrial strain and cardiomyopathy in patients with Fabry disease. Front. Cardiovasc. Med. 2023, 10, 1223635. [Google Scholar] [CrossRef] [PubMed]
- Smid, B.E.; Hollak, C.E.; Poorthuis, B.J.; van den Bergh Weerman, M.A.; Florquin, S.; Kok, W.E.; Lekanne Deprez, R.H.; Timmermans, J.; Linthorst, G.E. Diagnostic dilemmas in Fabry disease: A case series study on GLA mutations of unknown clinical significance. Clin. Genet. 2015, 88, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Terryn, W.; Vanholder, R.; Hemelsoet, D.; Leroy, B.P.; Van Biesen, W.; De Schoenmakere, G.; Wuyts, B.; Claes, K.; De Backer, J.; De Paepe, G.; et al. Questioning the Pathogenic Role of the GLA p.Ala143Thr “Mutation” in Fabry Disease: Implications for Screening Studies and ERT. JIMD Rep. 2013, 8, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P.; Hughes, D.A.; Nicholls, K.; Bichet, D.G.; Giugliani, R.; Wilcox, W.R.; Feliciani, C.; Shankar, S.P.; Ezgu, F.; Amartino, H.; et al. Treatment of Fabry’s Disease with the Pharmacologic Chaperone Migalastat. N. Engl. J. Med. 2016, 375, 545–555. [Google Scholar] [CrossRef]
- Hughes, D.A.; Nicholls, K.; Shankar, S.P.; Sunder-Plassmann, G.; Koeller, D.; Nedd, K.; Vockley, G.; Hamazaki, T.; Lachmann, R.; Ohashi, T.; et al. Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study. J. Med. Genet. 2017, 54, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Lenders, M.; Stappers, F.; Brand, E. In Vitro and In Vivo Amenability to Migalastat in Fabry Disease. Mol. Ther. Methods Clin. Dev. 2020, 19, 24–34. [Google Scholar] [CrossRef]
- Savostyanov, K.; Pushkov, A.; Zhanin, I.; Mazanova, N.; Pakhomov, A.; Trufanova, E.; Alexeeva, A.; Sladkov, D.; Kuzenkova, L.; Asanov, A.; et al. Genotype-Phenotype Correlations in 293 Russian Patients with Causal Fabry Disease Variants. Genes 2023, 14, 2016. [Google Scholar] [CrossRef]
- Wallace, E.L.; Goker-Alpan, O.; Wilcox, W.R.; Holida, M.; Bernat, J.; Longo, N.; Linhart, A.; Hughes, D.A.; Hopkin, R.J.; Tondel, C.; et al. Head-to-head trial of pegunigalsidase alfa versus agalsidase beta in patients with Fabry disease and deteriorating renal function: Results from the 2-year randomised phase III BALANCE study. J. Med. Genet. 2023, 61, 520–530. [Google Scholar] [CrossRef]
- Lavalle, L.; Thomas, A.S.; Beaton, B.; Ebrahim, H.; Reed, M.; Ramaswami, U.; Elliott, P.; Mehta, A.B.; Hughes, D.A. Phenotype and biochemical heterogeneity in late onset Fabry disease defined by N215S mutation. PLoS ONE 2018, 13, e0193550. [Google Scholar] [CrossRef]
- Braunholtz, D.A.; Edwards, S.J.; Lilford, R.J. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J. Clin. Epidemiol. 2001, 54, 217–224. [Google Scholar] [CrossRef]
- van Breemen, M.J.; Rombach, S.M.; Dekker, N.; Poorthuis, B.J.; Linthorst, G.E.; Zwinderman, A.H.; Breunig, F.; Wanner, C.; Aerts, J.M.; Hollak, C.E. Reduction of elevated plasma globotriaosylsphingosine in patients with classic Fabry disease following enzyme replacement therapy. Biochim. Biophys. Acta 2011, 1812, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.M.; Guffon, N.; Wilcox, W.R.; Germain, D.P.; Lee, P.; Waldek, S.; Caplan, L.; Linthorst, G.E.; Desnick, R.J.; International Collaborative Fabry Disease Study, G. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N. Engl. J. Med. 2001, 345, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hajioff, D.; Enever, Y.; Quiney, R.; Zuckerman, J.; Mackermot, K.; Mehta, A. Hearing loss in Fabry disease: The effect of agalsidase alfa replacement therapy. J. Inherit. Metab. Dis. 2003, 26, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Elliott, P.M.; Shah, J.; Zuckerman, J.; Coghlan, G.; Brookes, J.; Mehta, A.B. Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: A randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart 2008, 94, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.F.; Altarescu, G.; Herscovitch, P.; Schiffmann, R. Enzyme replacement reverses abnormal cerebrovascular responses in Fabry disease. BMC Neurol. 2002, 2, 4. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, R.; Kopp, J.B.; Austin, H.A., III; Sabnis, S.; Moore, D.F.; Weibel, T.; Balow, J.E.; Brady, R.O. Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 2001, 285, 2743–2749. [Google Scholar] [CrossRef]
- Branton, M.H.; Schiffmann, R.; Sabnis, S.G.; Murray, G.J.; Quirk, J.M.; Altarescu, G.; Goldfarb, L.; Brady, R.O.; Balow, J.E.; Austin Iii, H.A.; et al. Natural history of Fabry renal disease: Influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine 2002, 81, 122–138. [Google Scholar] [CrossRef] [PubMed]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J. Med. Genet. 2001, 38, 769–775. [Google Scholar] [CrossRef]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J. Med. Genet. 2001, 38, 750–760. [Google Scholar] [CrossRef]
- Schiffmann, R.; Warnock, D.G.; Banikazemi, M.; Bultas, J.; Linthorst, G.E.; Packman, S.; Sorensen, S.A.; Wilcox, W.R.; Desnick, R.J. Fabry disease: Progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol. Dial. Transplant. 2009, 24, 2102–2111. [Google Scholar] [CrossRef]
- Kampmann, C.; Linhart, A.; Baehner, F.; Palecek, T.; Wiethoff, C.M.; Miebach, E.; Whybra, C.; Gal, A.; Bultas, J.; Beck, M. Onset and progression of the Anderson-Fabry disease related cardiomyopathy. Int. J. Cardiol. 2008, 130, 367–373. [Google Scholar] [CrossRef]
- Warnock, D.G.; Thomas, C.P.; Vujkovac, B.; Campbell, R.C.; Charrow, J.; Laney, D.A.; Jackson, L.L.; Wilcox, W.R.; Wanner, C. Antiproteinuric therapy and Fabry nephropathy: Factors associated with preserved kidney function during agalsidase-beta therapy. J. Med. Genet. 2015, 52, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Gakidou, E.; Lopez, A.D. Evolution of the global smoking epidemic over the past half century: Strengthening the evidence base for policy action. Tob. Control 2022, 31, 129–137. [Google Scholar] [CrossRef]
- Riccio, E.; Zanfardino, M.; Ferreri, L.; Santoro, C.; Cocozza, S.; Capuano, I.; Imbriaco, M.; Feriozzi, S.; Pisani, A.; Group, A. Switch from enzyme replacement therapy to oral chaperone migalastat for treating fabry disease: Real-life data. Eur. J. Hum. Genet. 2020, 28, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Franzen, D.; Haile, S.R.; Kasper, D.C.; Mechtler, T.P.; Flammer, A.J.; Krayenbuhl, P.A.; Nowak, A. Pulmonary involvement in Fabry disease: Effect of plasma globotriaosylsphingosine and time to initiation of enzyme replacement therapy. BMJ Open Respir. Res. 2018, 5, e000277. [Google Scholar] [CrossRef] [PubMed]
- Pietila-Effati, P.; Saarinen, J.T.; Loyttyniemi, E.; Saarenhovi, M.; Autio, R.; Kantola, I. Long-term effectiveness of enzyme replacement therapy in Fabry disease with the p.Arg227Ter variant: Fabry disease in Ostrobothnia (FAST) study. Am. J. Med. Genet. A 2023, 191, 1858–1869. [Google Scholar] [CrossRef]
- Pietila-Effati, P.; Soderstrom, J.; Saarinen, J.T.; Loyttyniemi, E.; Kantola, I. Pulmonary manifestations and the effectiveness of enzyme replacement therapy in Fabry Disease with the p. Arg227Ter (p.R227*) mutation. Mol. Genet. Genom. Med. 2022, 10, e1915. [Google Scholar] [CrossRef]
- Arends, M.; Wijburg, F.A.; Wanner, C.; Vaz, F.M.; van Kuilenburg, A.B.P.; Hughes, D.A.; Biegstraaten, M.; Mehta, A.; Hollak, C.E.M.; Langeveld, M. Favourable effect of early versus late start of enzyme replacement therapy on plasma globotriaosylsphingosine levels in men with classical Fabry disease. Mol. Genet. Metab. 2017, 121, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Banikazemi, M.; Bultas, J.; Waldek, S.; Wilcox, W.R.; Whitley, C.B.; McDonald, M.; Finkel, R.; Packman, S.; Bichet, D.G.; Warnock, D.G.; et al. Agalsidase-beta therapy for advanced Fabry disease: A randomized trial. Ann. Intern. Med. 2007, 146, 77–86. [Google Scholar] [CrossRef]
- Rombach, S.M.; Smid, B.E.; Bouwman, M.G.; Linthorst, G.E.; Dijkgraaf, M.G.; Hollak, C.E. Long term enzyme replacement therapy for Fabry disease: Effectiveness on kidney, heart and brain. Orphanet J. Rare Dis. 2013, 8, 47. [Google Scholar] [CrossRef]
- Weidemann, F.; Niemann, M.; Stork, S.; Breunig, F.; Beer, M.; Sommer, C.; Herrmann, S.; Ertl, G.; Wanner, C. Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: Evidence for disease progression towards serious complications. J. Intern. Med. 2013, 274, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Spada, M.; Baron, R.; Elliott, P.M.; Falissard, B.; Hilz, M.J.; Monserrat, L.; Tondel, C.; Tylki-Szymanska, A.; Wanner, C.; Germain, D.P. The effect of enzyme replacement therapy on clinical outcomes in paediatric patients with Fabry disease—A systematic literature review by a European panel of experts. Mol. Genet. Metab. 2019, 126, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Tondel, C.; Bostad, L.; Larsen, K.K.; Hirth, A.; Vikse, B.E.; Houge, G.; Svarstad, E. Agalsidase benefits renal histology in young patients with Fabry disease. J. Am. Soc. Nephrol. 2013, 24, 137–148. [Google Scholar] [CrossRef]
- Moreno-Martinez, D.; Aguiar, P.; Auray-Blais, C.; Beck, M.; Bichet, D.G.; Burlina, A.; Cole, D.; Elliott, P.; Feldt-Rasmussen, U.; Feriozzi, S.; et al. Standardising clinical outcomes measures for adult clinical trials in Fabry disease: A global Delphi consensus. Mol. Genet. Metab. 2021, 132, 234–243. [Google Scholar] [CrossRef]
- Armstrong, A.C.; Gidding, S.; Gjesdal, O.; Wu, C.; Bluemke, D.A.; Lima, J.A. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc. Imaging 2012, 5, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Hazari, H.; Belenkie, I.; Kryski, A.; White, J.A.; Oudit, G.Y.; Thompson, R.; Fung, T.; Dehar, N.; Khan, A. Comparison of Cardiac Magnetic Resonance Imaging and Echocardiography in Assessment of Left Ventricular Hypertrophy in Fabry Disease. Can. J. Cardiol. 2018, 34, 1041–1047. [Google Scholar] [CrossRef]
- O’Brien, C.; Britton, I.; Karur, G.R.; Iwanochko, R.M.; Morel, C.F.; Nguyen, E.T.; Thavendiranathan, P.; Woo, A.; Hanneman, K. Left Ventricular Mass and Wall Thickness Measurements Using Echocardiography and Cardiac MRI in Patients with Fabry Disease: Clinical Significance of Discrepant Findings. Radiol. Cardiothorac. Imaging 2020, 2, e190149. [Google Scholar] [CrossRef]
- Niemann, M.; Herrmann, S.; Hu, K.; Breunig, F.; Strotmann, J.; Beer, M.; Machann, W.; Voelker, W.; Ertl, G.; Wanner, C.; et al. Differences in Fabry cardiomyopathy between female and male patients: Consequences for diagnostic assessment. JACC Cardiovasc. Imaging 2011, 4, 592–601. [Google Scholar] [CrossRef]
- Faro, D.C.; Losi, V.; Rodolico, M.S.; Licciardi, S.; Monte, I.P. Speckle tracking echocardiography-derived parameters as new prognostic markers in hypertrophic cardiomyopathies. Eur. Heart J. Open 2023, 3, oead014. [Google Scholar] [CrossRef] [PubMed]
- Effraimidis, G.; Rasmussen, A.K.; Dunoe, M.; Hasholt, L.F.; Wibrand, F.; Sorensen, S.S.; Lund, A.M.; Kober, L.; Bundgaard, H.; Yazdanfard, P.D.W.; et al. Systematic cascade screening in the Danish Fabry Disease Centre: 20 years of a national single-centre experience. PLoS ONE 2022, 17, e0277767. [Google Scholar] [CrossRef]
- Moiseev, S.; Tao, E.; Moiseev, A.; Bulanov, N.; Filatova, E.; Fomin, V.; Germain, D.P. The Benefits of Family Screening in Rare Diseases: Genetic Testing Reveals 165 New Cases of Fabry Disease among At-Risk Family Members of 83 Index Patients. Genes 2022, 13, 1619. [Google Scholar] [CrossRef]
- Harun, N.; Gupta, N.; McCormack, F.X.; Macaluso, M. Dynamic use of historical controls in clinical trials for rare disease research: A re-evaluation of the MILES trial. Clin. Trials 2023, 20, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Schuller, Y.; Arends, M.; Korver, S.; Langeveld, M.; Hollak, C.E.M. Adaptive pathway development for Fabry disease: A clinical approach. Drug Discov. Today 2018, 23, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.M.; Groener, J.E.; Kuiper, S.; Donker-Koopman, W.E.; Strijland, A.; Ottenhoff, R.; van Roomen, C.; Mirzaian, M.; Wijburg, F.A.; Linthorst, G.E.; et al. Elevated globotriaosylsphingosine is a hallmark of Fabry disease. Proc. Natl. Acad. Sci. USA 2008, 105, 2812–2817. [Google Scholar] [CrossRef]
- van der Veen, S.J.; van Kuilenburg, A.B.P.; Hollak, C.E.M.; Kaijen, P.H.P.; Voorberg, J.; Langeveld, M. Antibodies against recombinant alpha-galactosidase A in Fabry disease: Subclass analysis and impact on response to treatment. Mol. Genet. Metab. 2019, 126, 162–168. [Google Scholar] [CrossRef]
- Najafian, B.; Tondel, C.; Svarstad, E.; Sokolovkiy, A.; Smith, K.; Mauer, M. One Year of Enzyme Replacement Therapy Reduces Globotriaosylceramide Inclusions in Podocytes in Male Adult Patients with Fabry Disease. PLoS ONE 2016, 11, e0152812. [Google Scholar] [CrossRef]
- Tondel, C.; Thurberg, B.L.; DasMahapatra, P.; Lyn, N.; Maski, M.; Batista, J.L.; George, K.; Patel, H.; Hariri, A. Clinical relevance of globotriaosylceramide accumulation in Fabry disease and the effect of agalsidase beta in affected tissues. Mol. Genet. Metab. 2022, 137, 328–341. [Google Scholar] [CrossRef]
- Schoenmakers, D.H.; Van den Berg, S.; Timmers, L.; Adang, L.A.; Bäumer, T.; Bosch, A.; Van de Casteele, M.; Datema, M.R.; Dekker, H.; Donnelly, C.; et al. Framework for Multistakeholder Patient Registries in the Field of Rare Diseases—Focus on Neurogenetic Diseases. Neurology 2024, 103, e209743. [Google Scholar] [CrossRef]
| Meta-Analysis | Included Studies (Follow-Up) | Analyzed FD Patients | FD Patients (% Male) and Number of Studies Reporting the Outcome | Outcomes | Results (Treated vs. Untreated) Mean (95% CI)/% (95% CI)/Median [IQR] | ||
|---|---|---|---|---|---|---|---|
| Treated | Untreated | ||||||
| Alegra 2012 [20] Agalsidase alfa/agalsidase beta vs. untreated | 10 RCTs (NS) | Combined analysis of males and females | n = 50 (96%) 8 studies | n = 49 (100%) 8 studies | - QRS-complex duration (ms) | No statistical difference | |
| Rombach 2014 [21] Agalsidase alfa/agalsidase beta vs. untreated | 2 pooled analyses, 4 cohort studies (5 yrs) | Separate analysis of males † | |||||
| - with baseline GFR >60 mL/min/1.73 m2 | n = 90 2 studies | n = 117 1 study | - GFR ‡ (mL/min/1.73 m2) | −2.57 (−3.21, −1.93) vs. −3.00 (−3.20, −2.80) | |||
| - with baseline GFR <60 mL/min/1.73 m2 | n = 23 2 studies | n = 28 1 study | - GFR ‡ (mL/min/1.73 m2) | −3.04 (−4.99, −1.09) vs. −6.80 (−9.74, −3.86) * | |||
| - with LVH at baseline | n = 20 2 studies | n = 18 1 study | - LVM § (g/m2.7) | 0.34 (−2.19, 2.88) vs. 6.59 (2.67, 10.51) * | |||
| - w/o LVH at baseline (vs. untreated patients with and w/o LVH) | n = 41 2 studies | n = 39 1 study | - LVM § (g/m2.7) | 0.94 (0.41, 1.47) vs. 4.07 (3.76, 4.38) * | |||
| Separate analysis of females † | |||||||
| - with baseline GFR >60 mL/min/1.73 m2 | n = 52 2 studies | n = 42 1 study | - GFR ‡ (mL/min/1.73 m2) | −0.48 (−1.32, 0.36) vs. −0.90 (−2.66, 0.86) | |||
| - with baseline GFR <60 mL/min/1.73 m2 | n = 6 2 studies | n = 13 1 study | - GFR ‡ (mL/min/1.73 m2) | −1.37 (−2.33, −0.41) vs. −2.10 (−5.24, 1.04) | |||
| - with LVH at baseline | n = 38 2 studies | n = 15 1 study | - LVM § (g/m2.7) | −1.81 (−3.41, −0.21) vs. 3.77 (−0.13, 7.67) * | |||
| - w/o LVH at baseline (vs. untreated patients with and w/o LVH) | n = 23 2 studies | n = 39 1 study | - LVM § (g/m2.7) | −0.57 (−1.37, 0.23) vs. 2.31 (2.06, 2.56) * | |||
| Sheng 2019 [22] Agalsidase alfa/agalsidase beta vs. untreated | 2 RCTs, 7 cohort studies (4.5 yrs) | Combined analysis of males and females | n = 1471 (62.5%) 8 studies | n = 6042 (49.1%) 5 studies | - Ischemic stroke recurrence rate | 8.2% (3.8%, 12.6%) vs. 16% (10.2%, 21.7%) * | |
| Ortiz 2020 [23] Agalsidase beta vs. untreated | 2 RCTs, 8 cohort studies (2.9 ± 1.4 yrs) | Combined analysis of males and females | n = 133 (68.3%) 8 studies | n = 182 (92.9%) 3 studies | - eGFR ¶ (mL/min/1.73 m2) | −1.01 [−3.64, 1.10] vs. −3.47 [−7.74, 0.17] * | |
| Lee 2022 [24] Agalsidase alfa/agalsidase beta vs. untreated | 2 RCTs, 5 cohort studies (4.1 yrs) | Combined analysis of males and females | n = 267 (55.1%) 7 studies | n = 285 (44.6%) 7 studies | - LVMI § (g/m2) | −0.149 (−0.431, 0.132) # | |
| Meta-Analyses | Included Studies (Follow-Up) | Analyzed FD Patients | FD Patients and Number of Studies Reporting the Outcome | Outcomes | Results (Agal-α vs. Agal-β vs. Untreated) % (95% CI) | ||
| Agal-α | Agal-β | Untreated | |||||
| El Dib 2017 [15] Agalsidase alfa vs. agalsidase beta vs. untreated | 39 cohort studies (NS) | Combined analysis of males and females | n = 344 6 studies | n = 1053 2 studies | n = 812 6 studies | - All-cause mortality | 9% (3%, 16%) vs. 4.4% (0.2%, 20.1%) vs. 10.8% (2.05%, 25.2%) |
| n = 168 6 studies | n = 1044 1 study | n = 1698 11 studies | - Renal complications †† | 15.3% (4.8%, 30.3%) vs. 6% (4%, 7%) * vs. 21.4% (15.2%, 28.4%) | |||
| n = 524 4 studies | n = 1096 3 studies | n = 5854 14 studies | - Cardiovascular complications †† | 28% (7%, 55%) vs. 7% (5%, 8%) * vs. 26.2% (14.9%, 39.4%) | |||
| n = 461 7 studies | n = 1062 3 studies | n = 5544 15 studies | - Cerebrovascular complications †† | 11.1% (5.8%, 17.9%) vs. 3.5% (2.4%, 4.6%) ** vs. 17.8% (12.3%, 24%) | |||
| Sensitivity analysis: | Excluding children | n = 309 4 studies | n = 1053 2 studies | n = 812 6 studies | - All-cause mortality | 12% (6%, 20%) vs. 4.4% (0.2%, 20.1%) vs. 10.8% (2.05%, 25.2%) | |
| n = 152 5 studies | n = 1044 1 study | n = 1698 11 studies | - Renal complications †† | 16.8% (4.1%, 35.6%) vs. 6% (4%, 7%) * vs. 21.4% (15.2%, 28.4%) | |||
| n = 426 3 studies | n = 1053 4 studies | n = 5854 14 studies | - Cardiovascular complications †† | 35% (11%, 65%) vs. 7% (5%, 8%) * vs. 26.2% (14.9%, 39.4%) | |||
| n = 339 5 studies | n = 1062 3 studies | n = 5544 15 studies | - Cerebrovascular complications †† | 10.5% (4.3%, 19%) vs. 3.5% (2.4%, 4.6%) ** vs. 17.8% (12.3%, 24%) | |||
| Study | Follow-Up | Analyzed FD Patients (% Male, If Combined) | Treated | Age | Untreated | Age | Outcomes | Results (Treated vs. Untreated) Mean ± SD/ Median [Range]/% |
|---|---|---|---|---|---|---|---|---|
| Hongo 2018 [29] Agalsidase alfa/agalsidase beta vs. untreated | 11.6 ± 2.4 yrs | Separate analysis of males | n = 17 | 30.7 ± 9.5 y/o | n = 39 | 31.3 ± 11.9 y/o | - LVM † indexed to height (g/ht2.7/year) | 1.25 ± 1.39 vs. 4.07 ± 1.03 * |
| Subgroup analysis: | - with LVH | n = 6 | NS | n = 18 | NS | - LVM † indexed to height (g/ht2.7/year) | 1.51 ± 1.32 vs. 6.59 ± 8.5 | |
| - w/o extensive LVH | n = 14 | 27.8 ± 8.1 y/o | n = 39 | 31.3 ± 11.9 y/o | - LVM † indexed to height (g/ht2.7/year) | 1.00 ± 1.36 vs. 4.07 ± 1.03 * | ||
| - matched on LVM | n = 8 | 36.3 ± 7.6 y/o | n = 39 | 31.3 ± 11.9 y/o | - LVM † indexed to height (g/ht2.7/year) | 1.59 ± 1.23 vs. 4.07 ± 1.03 * | ||
| 7.4 ± 3.0 yrs | Separate analysis of females | n = 25 | 40.6 ± 14.2 y/o | n = 39 | 36.3 ± 17.4 y/o | - LVM † indexed to height (g/ht2.7/year) | 0.78 ± 1.23 vs. 2.31 ± 0.81 * | |
| Subgroup analysis: | - with LVH | n = 7 | NS | n = 15 | NS | - LVM † indexed to height (g/ht2.7/year) | 1.54 ± 1.37 vs. 3.77 ± 7.7 | |
| - w/o extensive LVH | n = 22 | 39.1 ± 14.4 y/o | n = 39 | 36.3 ± 17.4 y/o | - LVM † indexed to height (g/ht2.7/year) | 0.70 ± 1.17 vs. 2.31 ± 0.81 * | ||
| - matched on LVM | n = 16 | 46.5 ± 14.1 y/o | n = 39 | 36.3 ± 17.4 y/o | - LVM † indexed to height (g/ht2.7/year) | 1.00 ± 1.25 vs. 2.31 ± 0.81 * | ||
| Nordin 2019 [30] Agalsidase alfa/agalsidase beta vs. untreated | 1.1 ± 0.2 yrs | Combined analysis of males and females | n = 20 (35%) | 49 ± 10 y/o | n = 18 (16.7%) | 41 ± 12 y/o | - LVMI ‡ (g/m2) | Unchanged vs. Increased |
| - Native T1 (ms) | Increased vs. Decreased | |||||||
| - T2 (ms) | Unchanged vs. Unchanged | |||||||
| - Troponin T (ng/L) | Unchanged vs. Increased | |||||||
| Van der Veen 2022 [31] Agalsidase beta vs. untreated | Cross-sectional | Separate analysis of males | n = 7 | 24 [14, 26] y/o | n = 23 | 22 [13, 27] y/o | - LVM † (g/m2) | 80 [67, 84] vs. 94 [59, 149] * |
| - LVM ‡ (g/m2) | 53 [46, 59] vs. 68 [53, 99] * | |||||||
| - uACR (mg/mmol) | 0,4 [0, 8.8] vs. 3,7 [0, 248] * | |||||||
| - eGFR § (mL/min/1.73 m2) | 116 [92, 132] vs. 116 [46, 165] | |||||||
| - LGE | 0% vs. 0% | |||||||
| - IRBBB | 0% vs. 31% | |||||||
| - Sinus bradycardia | 14% vs. 52% | |||||||
| - WML | 14% vs. 27% | |||||||
| Pogoda 2023 [32] Agalsidase alfa/agalsidase beta vs. untreated | 6.3 ± 3.3 yrs | Separate analysis of females | n = 24 | 48 [25, 71] y/o | n = 30 | 28 [16, 68] y/o | 20 morphological and function echocardiography parameters (see Table 3 in original article) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veldman, B.C.F.; Schoenmakers, D.H.; van Dussen, L.; Datema, M.R.; Langeveld, M. Establishing Treatment Effectiveness in Fabry Disease: Observation-Based Recommendations for Improvement. Int. J. Mol. Sci. 2024, 25, 9752. https://doi.org/10.3390/ijms25179752
Veldman BCF, Schoenmakers DH, van Dussen L, Datema MR, Langeveld M. Establishing Treatment Effectiveness in Fabry Disease: Observation-Based Recommendations for Improvement. International Journal of Molecular Sciences. 2024; 25(17):9752. https://doi.org/10.3390/ijms25179752
Chicago/Turabian StyleVeldman, Bram C. F., Daphne H. Schoenmakers, Laura van Dussen, Mareen R. Datema, and Mirjam Langeveld. 2024. "Establishing Treatment Effectiveness in Fabry Disease: Observation-Based Recommendations for Improvement" International Journal of Molecular Sciences 25, no. 17: 9752. https://doi.org/10.3390/ijms25179752
APA StyleVeldman, B. C. F., Schoenmakers, D. H., van Dussen, L., Datema, M. R., & Langeveld, M. (2024). Establishing Treatment Effectiveness in Fabry Disease: Observation-Based Recommendations for Improvement. International Journal of Molecular Sciences, 25(17), 9752. https://doi.org/10.3390/ijms25179752






