A New Approach for CRISPR/Cas9 Editing and Selection of Pathogen-Resistant Plant Cells of Wine Grape cv. ‘Merlot’
Abstract
:1. Introduction
1.1. Molecular Mechanisms of Plant Defense against Pathogens
1.2. CRISPR/Cas Editing of Grapes
2. Results
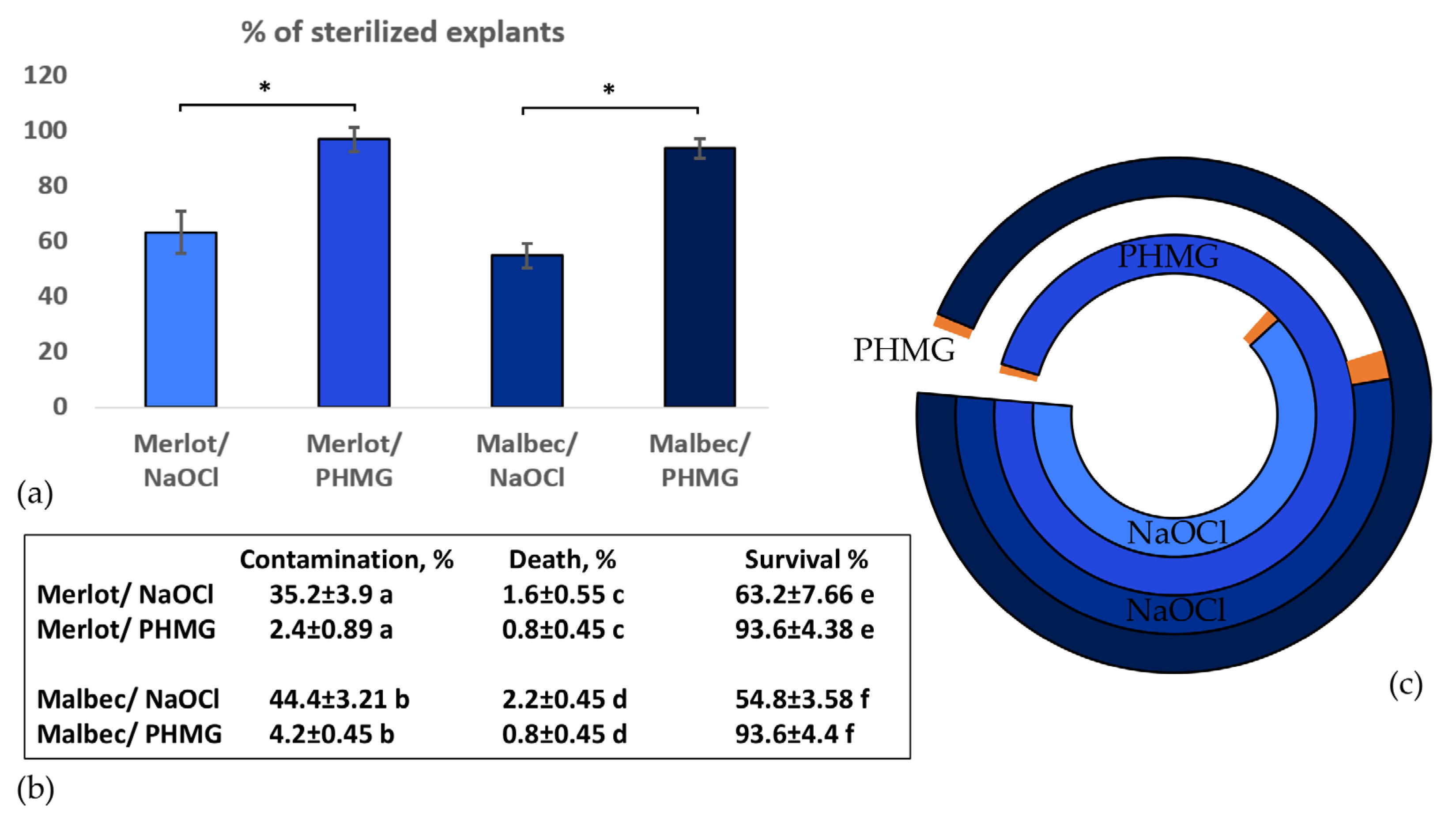
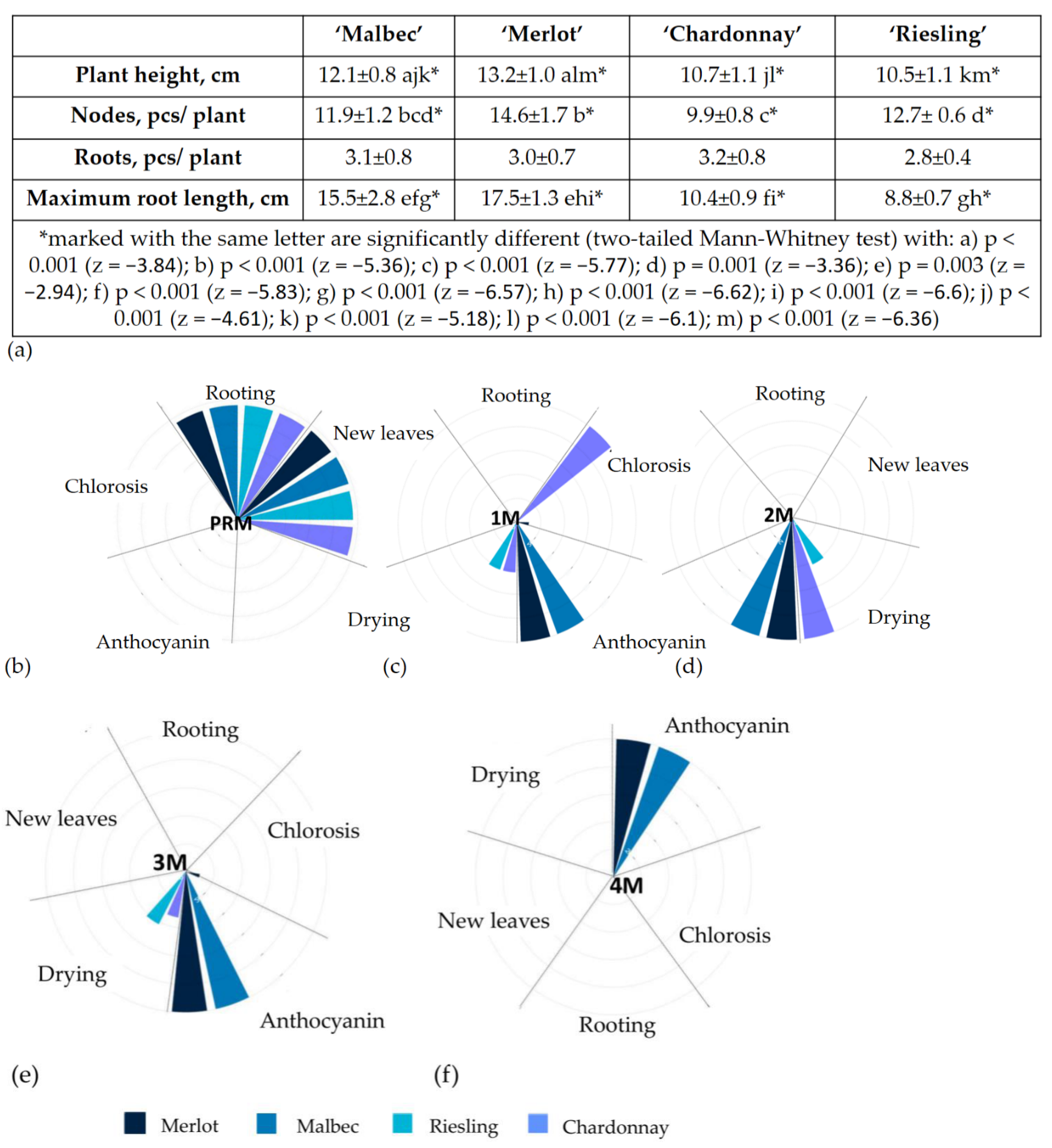
2.1. Micropropagation
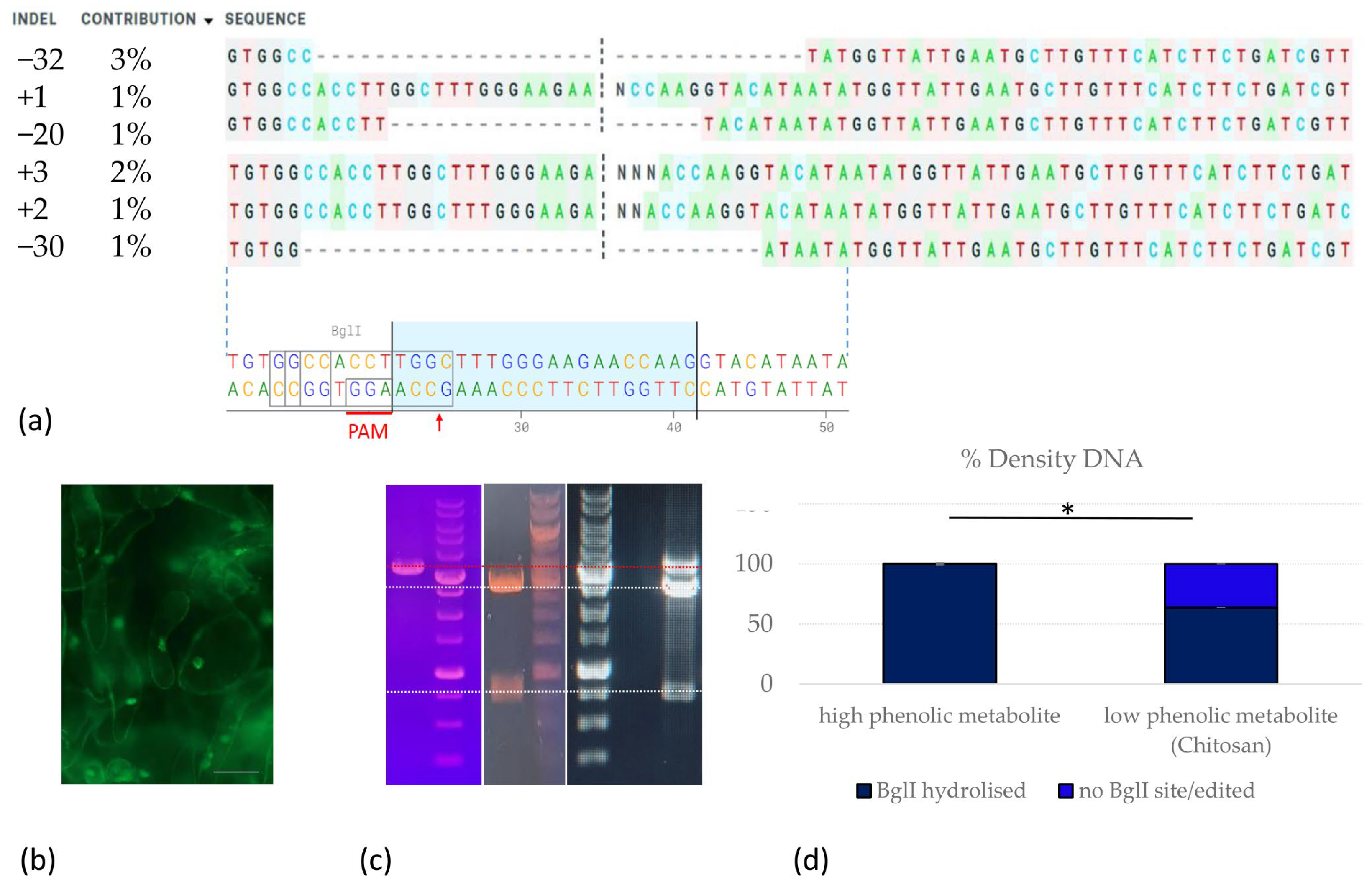
2.2. Evaluation of Phenolic Metabolite and Peroxide Content in ‘Merlot’ Grapevine Cells upon Induction of Plant Immune Response (PTI) by Chitin
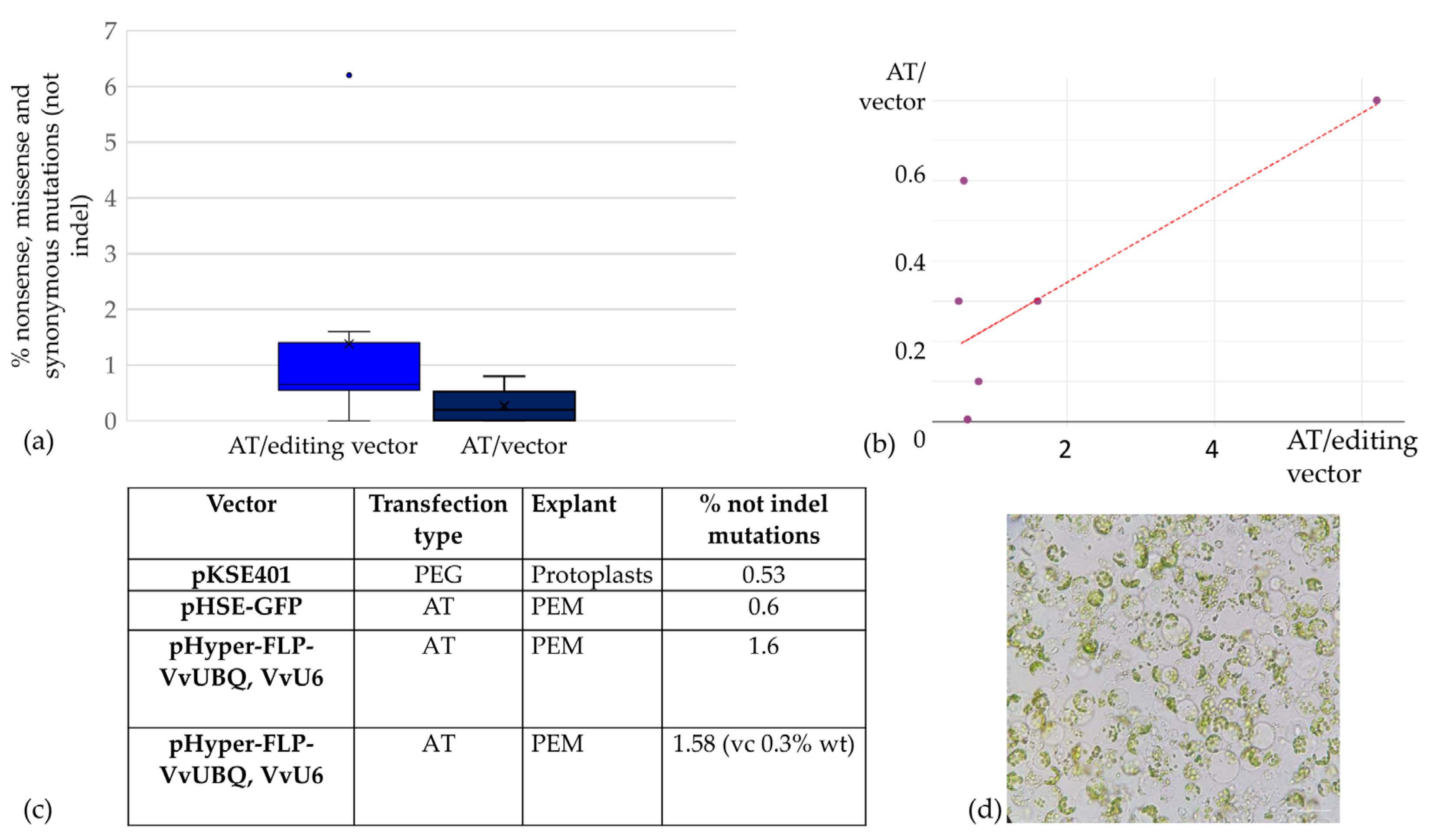
2.3. CRISPR/Cas9 Genome Editing of ‘Merlot’ Callus and Proembryogenic Mass
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Bacterial Strains
4.1.2. Plant Materials
4.1.3. Editing Vectors
4.1.4. Primers
4.1.5. Enzymes and Kits
4.1.6. Agarose Gels
4.2. Methods
4.2.1. Decontamination and Cultivation of Plants
4.2.2. Micropropagation of Plants
4.2.3. Callus Induction
4.2.4. Agrobacterium Transformation and Transfection
4.2.5. Plasmid Construction
4.2.6. Editing Efficiency and Genotyping
4.2.7. Sequencing
4.2.8. Isolation of Protoplasts
4.2.9. Measurement of Phenolic Content
4.2.10. Assessment of Intracellular Peroxide Compound Levels
4.2.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGovern, P. Ancient Wine: The Search for the Origins of Viniculture; Princeton University Press: Princeton, NJ, USA, 2003. [Google Scholar]
- Barbalho, S.; Ottoboni, A.; Fiorini, A.; Landgraf Guiguer, E.; Nicolau, C.; Goulart, R.; Flato, U. Grape Juice or Wine: Which Is the Best Option? Crit. Rev. Food Sci. Nutr. 2020, 60, 3876–3889. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.; Labrador, M.; Gutiérrez, A.; Hornedo Ortega, R.; Troncoso, A.; Garcia-Parrilla, M. Anti-VEGF Signalling Mechanism in HUVECs by Melatonin, Serotonin, Hydroxytyrosol and Other Bioactive Compounds. Nutrients 2019, 11, 2421. [Google Scholar] [CrossRef] [PubMed]
- Copetti, C.; Franco, F.W.; Machado, E.D.R.; Soquetta, M.B.; Quatrin, A.; Ramos, V.M. Acute Consumption of Bordo Grape Juice and Wine Improves Serum Antioxidant Status in Healthy Individuals and Inhibits Reactive Oxygen Species Production in Human Neuron-Like Cells. J. Nutr. Metab. 2018, 2018, 4384012. [Google Scholar] [CrossRef] [PubMed]
- Toaldo, I.M.; Cruz, F.A.; da Silva, E.L.; Bordignon-Luiz, M.T. Acute Consumption of Organic and Conventional Tropical Grape Juices (Vitis labrusca L.) Increases Antioxidants in Plasma and Erythrocytes, but Not Glucose and Uric Acid Levels, in Healthy Individuals. Nutr. Res. 2016, 36, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Bae, H. Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef] [PubMed]
- Schwager, J.; Richard, N.; Widmer, F.; Raederstorff, D. Resveratrol Distinctively Modulates the Inflammatory Profiles of Immune and Endothelial Cells. BMC Complement. Altern. Med. 2017, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Munir, I.; Yen, H.-W.; Baruth, T.; Tarkowski, R.; Azziz, R.; Magoffin, D.A.; Jakimiuk, A.J. Resistin Stimulation of 17α-Hydroxylase Activity in Ovarian Theca Cells in Vitro: Relevance to Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 4852–4857. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Bacilieri, R.; Ramos-Madrigal, J.; Privman, E.; Boaretto, E.; Weber, A.; Fuks, D.; Weiss, E.; Erickson-Gini, T.; Bucking, S.; et al. Ancient DNA from a Lost Negev Highlands Desert Grape Reveals a Late Antiquity Wine Lineage. Proc. Natl. Acad. Sci. USA 2023, 120, e2213563120. [Google Scholar] [CrossRef] [PubMed]
- Pessina, S.; Lenzi, L.; Perazzolli, M.; Campa, M.; Dalla Costa, L.; Urso, S.; Valè, G.; Salamini, F.; Velasco, R.; Malnoy, M. Knockdown of MLO Genes Reduces Susceptibility to Powdery Mildew in Grapevine. Hortic. Res. 2016, 3, 16016. [Google Scholar] [CrossRef]
- Dries, L.; Hendgen, M.; Schnell, S.; Löhnertz, O.; Vortkamp, A. Rhizosphere Engineering: Leading towards a Sustainable Viticulture? OENO One 2021, 55, 353–363. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Troggio, M.; Cartwright, D.A.; Cestaro, A.; Pruss, D.; Pindo, M.; FitzGerald, L.M.; Vezzulli, S.; Reid, J.; et al. A High Quality Draft Consensus Sequence of the Genome of a Heterozygous Grapevine Variety. PLoS ONE 2007, 2, e1326. [Google Scholar] [CrossRef]
- Ramos, M.J.N.; Coito, J.L.; Faísca-Silva, D.; Cunha, J.; Costa, M.M.R.; Amâncio, S.; Rocheta, M. Portuguese Wild Grapevine Genome Re-Sequencing (Vitis vinifera sylvestris). Sci. Rep. 2020, 10, 18993. [Google Scholar] [CrossRef]
- Bavaresco, L. Impact of Grapevine Breeding for Disease Resistance on the Global Wine Industry. Acta Hortic. 2019, 1248, 7–14. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ding, P.; Jones, J.D.G. Thirty Years of Resistance: Zig-Zag through the Plant Immune System. Plant Cell 2022, 34, 1447–1478. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cheng, Y. Advances in Fungal Elicitor-Triggered Plant Immunity. Int. J. Mol. Sci. 2022, 23, 12003. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Zurbriggen, M.; Carrillo, N.; Hajirezaei, M. ROS Signaling in the Hypersensitive Response: When, Where and What For? Plant Signal Behav. 2010, 5, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Rkhaila, A.; Chtouki, T.; Erguig, H.; El Haloui, N.; Ounine, K. Chemical Proprieties of Biopolymers (Chitin/Chitosan) and Their Synergic Effects with Endophytic Bacillus Species: Unlimited Applications in Agriculture. Molecules 2021, 26, 1117. [Google Scholar] [CrossRef]
- Melotto, M.; Underwood, W.; Sheng, Y.H. Role of Stomata in Plant Innate Immunity and Foliar Bacterial Diseases. Annu. Rev. Phytopathol. 2008, 46, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Sawinski, K.; Mersmann, S.; Robatzek, S.; Böhmer, M. Guarding the Green: Pathways to Stomatal Immunity. Mol. Plant Microbe Interact. 2013, 26, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Q.; Hou, B.-H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.-Q.; Guo, W.-J.; Kim, J.-G.; Underwood, W.; Chaudhuri, B.; et al. Sugar Transporters for Intercellular Exchange and Nutrition of Pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Senthil-Kumar, M.; Ryu, C.M.; Kang, L.; Mysore, K.S. Phytosterols Play a Key Role in Plant Innate Immunity against Bacterial Pathogens by Regulating Nutrient Efflux into the Apoplast. Plant Physiol. 2012, 158, 1789–1802. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.J.; Kenton, P.; Lloyd, A.J.; Ougham, H.; Prats, E. The Hypersensitive Response; The Centenary Is upon Us but How Much Do We Know? J. Exp. Bot. 2008, 59, 501–520. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.A.; Benková, E. Cytokinin Cross-Talking during Biotic and Abiotic Stress Responses. Front. Plant Sci. 2013, 4, 451. [Google Scholar] [CrossRef]
- Murphy, C.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of Inducible Defense-Related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Qiu, W.; Feechan, A.; Dry, I. Current Understanding of Grapevine Defense Mechanisms against the Biotrophic Fungus (Erysiphe necator), the Causal Agent of Powdery Mildew Disease. Hortic. Res. 2015, 2, 15020. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Schiavulli, A.; Appiano, M.; Marcotrigiano, A.; Cillo, F.; Visser, R.; Bai, Y.; Lotti, C.; Ricciardi, L. Pea Powdery Mildew Er1 Resistance Is Associated to Loss-of-Function Mutations at a MLO Homologous Locus. Theor. Appl. Genet. 2011, 123, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Jacobsen, E.; Visser, R.G.F.; Bai, Y. Loss of Susceptibility as a Novel Breeding Strategy for Durable and Broad-Spectrum Resistance. Mol. Breed. 2010, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, A.D.; Goritschnig, S.; Krasileva, K.V.; Schreiber, K.J.; Staskawicz, B.J. Effector Recognition and Activation of the Arabidopsis Thaliana NLR Innate Immune Receptors. Cold Spring Harb. Symp. Quant. Biol. 2012, 77, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, J.H. Discovery, Characterization and Exploitation of Mlo Powdery Mildew Resistance in Barley. Euphytica 2004, 63, 141–152. [Google Scholar] [CrossRef]
- Consonni, C.; Humphry, M.E.; Hartmann, H.A.; Livaja, M.; Durner, J.; Westphal, L.; Vogel, J.; Lipka, V.; Kemmerling, B.; Schulze-Lefert, P.; et al. Conserved Requirement for a Plant Host Cell Protein in Powdery Mildew Pathogenesis. Nat. Genet. 2006, 38, 716–720. [Google Scholar] [CrossRef]
- Bai, Y.; Pavan, S.; Zheng, Z.; Zappel, N.F.; Reinstädler, A.; Lotti, C.; De Giovanni, C.; Ricciardi, L.; Lindhout, P.; Visser, R.; et al. Naturally Occurring Broad-Spectrum Powdery Mildew Resistance in a Central American Tomato Accession Is Caused by Loss of Mlo Function. Mol. Plant Microbe Interact. 2007, 21, 30–39. [Google Scholar] [CrossRef]
- Zheng, Z.; Nonomura, T.; Appiano, M.; Pavan, S.; Matsuda, Y.; Toyoda, H.; Wolters, A.M.A.; Visser, R.G.F.; Bai, Y. Loss of Function in Mlo Orthologs Reduces Susceptibility of Pepper and Tomato to Powdery Mildew Disease Caused by Leveillula taurica. PLoS ONE 2013, 8, e70723. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Cadle-Davidson, L.A.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine Powdery Mildew (Erysiphe necator): A Fascinating System for the Study of the Biology, Ecology and Epidemiology of an Obligate Biotroph. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef]
- Dufour, M.-C.; Fontaine, S.; Montarry, J.; Corio-Costet, M.-F. Assessment of Fungicide Resistance and Pathogen Diversity in Erysiphe Necator Using Quantitative Real-Time PCR Assays. Pest. Manag. Sci. 2011, 67, 60–69. [Google Scholar] [CrossRef]
- Feechan, A.; Jermakow, A.; Torregrosa, L.; Panstruga, R.; Dry, I. Identification of Grapevine MLO Gene Candidates Involved in Susceptibility to Powdery Mildew. Funct. Plant Biol. 2009, 35, 1255–1266. [Google Scholar] [CrossRef]
- Winterhagen, P.; Howard, S.; Qiu, W.; Kovacs, L. Transcriptional Up-Regulation of Grapevine MLO Genes in Response to Powdery Mildew Infection. Am. J. Enol. Vitic. 2008, 59, 159–168. [Google Scholar] [CrossRef]
- Chen, Z.; Kloek, A.P.; Boch, J.; Katagiri, F.; Kunkel, B.N. The Pseudomonas Syringae AvrRpt2 Gene Product Promotes Pathogen Virulence from Inside Plant Cells. Mol. Plant Microbe Interact. 2000, 13, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Massonnet, M.; Cochetel, N.; Minio, A.; Vondras, A.M.; Lin, J.; Muyle, A.; Garcia, J.F.; Zhou, Y.; Delledonne, M.; Riaz, S.; et al. The Genetic Basis of Sex Determination in Grapes. Nat. Commun. 2020, 11, 2902. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, R.; Trapp, O. A Cool Climate Perspective on Grapevine Breeding: Climate Change and Sustainability Are Driving Forces for Changing Varieties in a Traditional Market. Theor. Appl. Genet. 2022, 135, 3947–3960. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.; De Lorenzis, G. Back to the Origins: Background and Perspectives of Grapevine Domestication. Int. J. Mol. Sci. 2021, 22, 4518. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR–Cas in Agriculture and Plant Biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Fizikova, A.; Tikhonova, N.; Ukhatova, Y.; Ivanov, R.; Khlestkina, E. Applications of CRISPR/Cas9 System in Vegetatively Propagated Fruit and Berry Crops. Agronomy 2021, 11, 1849. [Google Scholar] [CrossRef]
- Jogam, P.; Sandhya, D.; Alok, A.; Peddaboina, V.; Allini, V.R.; Zhang, B. A Review on CRISPR/Cas-Based Epigenetic Regulation in Plants. Int. J. Biol. Macromol. 2022, 219, 1261–1271. [Google Scholar] [CrossRef]
- Qi, Q.; Hu, B.; Jiang, W.; Wang, Y.; Yan, J.; Ma, F.; Guan, Q.; Xu, J. Advances in Plant Epigenome Editing Research and Its Application in Plants. Int. J. Mol. Sci. 2023, 24, 3442. [Google Scholar] [CrossRef]
- Ren, C.; Liu, X.; Zhang, Z.; Wang, Y.; Duan, W.; Li, S.; Liang, Z. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis in Chardonnay (Vitis vinifera L.). Sci. Rep. 2016, 6, 32289. [Google Scholar] [CrossRef]
- Malnoy, M.; Viola, R.; Jung, M.-H.; Koo, O.; Kim, S.; Kim, J.-S.; Velasco, R.; Kanchiswamy, C. DNA-Free Genetically Edited Grapevine and Apple Protoplast Using CRISPR/Cas9 Ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef]
- Nakajima, I.; Ban, Y.; Azuma, A.; Onoue, N.; Moriguchi, T.; Yamamoto, T.; Toki, S.; Endo, M. CRISPR/Cas9-Mediated Targeted Mutagenesis in Grape. PLoS ONE 2017, 12, e0177966. [Google Scholar] [CrossRef]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis in Grape in the First Generation. Plant Biotechnol. J. 2018, 16, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Liang, Z.; Ren, C.; Nishitani, C.; Osakabe, K.; Wada, M.; Komori, S.; Malnoy, M.; Velasco, R.; Poli, M.; et al. CRISPR–Cas9-Mediated Genome Editing in Apple and Grapevine. Nat. Protoc. 2018, 13, 2844–2863. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, S.; Li, X.; Zhang, X.; Wang, X.; Wang, L.; Li, Z.; Wang, X. A MADS-Box Transcription Factor from Grapevine, VvMADS45, Influences Seed Development. Plant Cell Tissue Organ. Cult. 2020, 141, 105–118. [Google Scholar] [CrossRef]
- Sunitha, S.; Rock, C.D. CRISPR/Cas9-Mediated Targeted Mutagenesis of TAS4 and MYBA7 Loci in Grapevine Rootstock 101-14. Transgenic Res. 2020, 29, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Jiao, Y.T.; Wang, Y.T.; Zhang, N.; Wang, B.B.; Liu, R.Q.; Yin, X.; Xu, Y.; Liu, G.T. CRISPR/Cas9-Mediated VvPR4b Editing Decreases Downy Mildew Resistance in Grapevine (Vitis vinifera L.). Hortic. Res. 2020, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Guo, Y.; Kong, J.; Lecourieux, F.; Dai, Z.; Li, S.; Liang, Z. Knockout of VvCCD8 Gene in Grapevine Affects Shoot Branching. BMC Plant Biol. 2020, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.Y.; Guo, Y.; Cheng, Y.; Hu, Y.; Xiao, S.; Wang, Y.; Wen, Y.Q. CRISPR/Cas9-Mediated Mutagenesis of VvMLO3 Results in Enhanced Resistance to Powdery Mildew in Grapevine (Vitis vinifera). Hortic. Res. 2020, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Liu, Y.; Guo, Y.; Duan, W.; Fan, P.; Li, S.; Liang, Z. Optimizing the CRISPR/Cas9 System for Genome Editing in Grape by Using Grape Promoters. Hortic. Res. 2021, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Olivares, F.; Loyola, R.; Olmedo, B.; Miccono, M.D.; Aguirre, C.; Vergara, R.; Riquelme, D.; Madrid, G.; Plantat, P.; Mora, R.; et al. CRISPR/Cas9 Targeted Editing of Genes Associated with Fungal Susceptibility in Vitis vinifera L. cv. Thompson Seedless Using Geminivirus-Derived Replicons. Front. Plant Sci. 2021, 12, 791030. [Google Scholar] [CrossRef]
- Scintilla, S.; Salvagnin, U.; Giacomelli, L.; Zeilmaker, T.; Malnoy, M.A.; van der Voort, J.; Moser, C. Regeneration of Non-Chimeric Plants from DNA-Free Edited Grapevine Protoplasts. Front. Plant Sci. 2022, 13, 1078931. [Google Scholar] [CrossRef]
- Ren, C.; Gathunga, E.K.; Li, X.; Li, H.; Kong, J.; Dai, Z.; Liang, Z. Efficient Genome Editing in Grapevine Using CRISPR/LbCas12a System. Mol. Hortic. 2023, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Villette, J.; Lecourieux, F.; Bastiancig, E.; Héloir, M.-C.; Poinssot, B. New Improvements in Grapevine Genome Editing: High Efficiency Biallelic Homozygous Knock-out from Regenerated Plantlets by Using an Optimized ZCas9i. Plant Methods 2024, 20, 45. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Nuzzo, F.; Moine, A.; Chitarra, W.; Pagliarani, C.; Petrelli, A.; Boccacci, P.; Delliri, A.; Velasco, R.; Nerva, L.; et al. Genome Editing of a Recalcitrant Wine Grape Genotype by Lipofectamine-Mediated Delivery of CRISPR/Cas9 Ribonucleoproteins to Protoplasts. Plant J. 2024, 119, 404–412. [Google Scholar] [CrossRef]
- Yang, Y.; Wheatley, M.; Meakem, V.; Galarneau, E.; Gutierrez, B.; Zhong, G.-Y. Editing VvDXS1 for the Creation of Muscat Flavour in Vitis vinifera cv. Scarlet Royal. Plant Biotechnol. J. 2024, 22, 1610–1621. [Google Scholar] [CrossRef]
- Cui, Z.-H.; Bi, W.-L.; Hao, X.-Y.; Li, P.-M.; Duan, Y.; Walker, M.A.; Xu, Y.; Wang, Q.-C. Drought Stress Enhances Up-Regulation of Anthocyanin Biosynthesis in Grapevine Leafroll-Associated Virus 3-Infected in Vitro Grapevine (Vitis vinifera) Leaves. Plant Dis. 2017, 101, 1606–1615. [Google Scholar] [CrossRef]
- Meggio, F.; Zarco-Tejada, P.J.; Núñez, L.C.; Sepulcre-Cantó, G.; González, M.R.; Martín, P. Grape Quality Assessment in Vineyards Affected by Iron Deficiency Chlorosis Using Narrow-Band Physiological Remote Sensing Indices. Remote Sens. Environ. 2010, 114, 1968–1986. [Google Scholar] [CrossRef]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically Encoded Fluorescent Indicator for Intracellular Hydrogen Peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.-L.; Dong, L.; Wang, Z.-P.; Zhang, H.-Y.; Han, C.-Y.; Liu, B.; Wang, X.-C.; Chen, Q.-J. A CRISPR/Cas9 Toolkit for Multiplex Genome Editing in Plants. BMC Plant Biol. 2014, 14, 327. [Google Scholar] [CrossRef] [PubMed]
- Pompili, V.; Dalla Costa, L.; Piazza, S.; Pindo, M.; Malnoy, M. Reduced Fire Blight Susceptibility in Apple Cultivars Using a High-Efficiency CRISPR/Cas9-FLP/FRT-Based Gene Editing System. Plant Biotechnol. J. 2020, 18, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Beza, K.; Feyissa, T.; Bedada, G. In Vitro Micropropagation of Grape Vine (Vitis vinifera L.) from Nodal Culture. Afr. J. Biotechnol. 2017, 16, 2083–2091. [Google Scholar] [CrossRef]
- Mostafa, F.; Shaaban, M.; Elazab, D.; Kamel, M. In Vitro Propagation of Four Grape Cultivars. Assiut J. Agric. Sci. 2015, 46, 65–76. [Google Scholar]
- Li, G.; Xianwei, Z.; Zhong, C.; Mo, J.; Quan, R.; Yang, J.; Liu, D.; Li, Z.; Yang, H.; Wu, Z. Small Molecules Enhance CRISPR/Cas9-Mediated Homology-Directed Genome Editing in Primary Cells. Sci. Rep. 2017, 7, 8943. [Google Scholar] [CrossRef]
- Ma, F.; Ma, Y.; Liu, K.; Gao, J.; Li, S.; Sun, X.; Li, G. Resveratrol Induces DNA Damage-Mediated Cancer Cell Senescence through the DLC1–DYRK1A–EGFR Axis. Food Funct. 2023, 14, 1484–1497. [Google Scholar] [CrossRef]
- Demeyer, A.; Benhelli-Mokrani, H.; Chenais, B.; Weigel, P.; Fleury, F. Inhibiting Homologous Recombination by Targeting RAD51 Protein. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 2021, 1876, 188597. [Google Scholar] [CrossRef]
- Yang, Y.; Ke, J.; Han, X.; Wuddineh, W.A.; Song, G.Q.; Zhong, G.Y. Removal of a 10-Kb Gret1 Transposon from VvMybA1 of Vitis vinifera cv. Chardonnay. Hortic. Res. 2022, 9, uhac201. [Google Scholar] [CrossRef] [PubMed]
- Gharari, Z.; Bagheri, K.; Danafar, H.; Sharafi, A. Enhanced Flavonoid Production in Hairy Root Cultures of Scutellaria Bornmuelleri by Elicitor Induced Over-Expression of MYB7 and FNSП2 Genes. Plant Physiol. Biochem. 2020, 148, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Tariq, H.; Asif, S.; Andleeb, A.; Hano, C.; Abbasi, B.H. Flavonoid Production: Current Trends in Plant Metabolic Engineering and De Novo Microbial Production. Metabolites 2023, 13, 124. [Google Scholar] [CrossRef]
- Davuluri, G.R.; Van Tuinen, A.; Fraser, P.D.; Manfredonia, A.; Newman, R.; Burgess, D.; Brummell, D.A.; King, S.R.; Palys, J.; Uhlig, J.; et al. Fruit-Specific RNAi-Mediated Suppression of DET1 Enhances Carotenoid and Flavonoid Content in Tomatoes. Nat. Biotechnol. 2005, 23, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yu, S.; Zeng, D.; Liu, H.; Wang, H.; Yang, Z.; Xie, X.; Shen, R.; Tan, J.; Li, H.; et al. Development of “Purple Endosperm Rice” by Engineering Anthocyanin Biosynthesis in the Endosperm with a High-Efficiency Transgene Stacking System. Mol. Plant 2017, 10, 918–929. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhu, Z.; Cao, P.; Chen, H.; Chen, C.; Zhou, X.; Mao, Y.; Lei, J.; Jiang, Y.; Meng, W.; et al. Purple Foliage Coloration in Tea (Camellia sinensis L.) Arises from Activation of the R2R3-MYB Transcription Factor CsAN1. Sci. Rep. 2016, 6, 32534. [Google Scholar] [CrossRef]
- Wang, N.; Xu, H.; Jiang, S.; Zhang, Z.; Lu, N.; Qiu, H.; Qu, C.; Wang, Y.; Wu, S.; Chen, X. MYB12 and MYB22 Play Essential Roles in Proanthocyanidin and Flavonol Synthesis in Red-Fleshed Apple (Malus sieversii f. Niedzwetzkyana). Plant J. 2017, 90, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Fatihah, H.N.N.; Moñino López, D.; van Arkel, G.; Schaart, J.G.; Visser, R.G.F.; Krens, F.A. The ROSEA1 and DELILA Transcription Factors Control Anthocyanin Biosynthesis in Nicotiana Benthamiana and Lilium Flowers. Sci. Hortic. 2019, 243, 327–337. [Google Scholar] [CrossRef]
- Schaart, J.G.; Dubos, C.; Romero De La Fuente, I.; van Houwelingen, A.M.M.L.; de Vos, R.C.H.; Jonker, H.H.; Xu, W.; Routaboul, J.-M.; Lepiniec, L.; Bovy, A.G. Identification and Characterization of MYB-BHLH-WD40 Regulatory Complexes Controlling Proanthocyanidin Biosynthesis in Strawberry (Fragaria × Ananassa) Fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Terrier, N.; Torregrosa, L.; Ageorges, A.; Vialet, S.; Verriès, C.; Cheynier, V.; Romieu, C. Ectopic Expression of VvMybPA2 Promotes Proanthocyanidin Biosynthesis in Grapevine and Suggests Additional Targets in the Pathway. Plant Physiol. 2009, 149, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Vialet, S.; Guiraud, J.-L.; Torregrosa, L.; Bertrand, Y.; Cheynier, V.; This, P.; Terrier, N. A Negative MYB Regulator of Proanthocyanidin Accumulation, Identified through Expression Quantitative Locus Mapping in the Grape Berry. New Phytol. 2014, 201, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Guo, D.; Li, G.; Yang, Y.; Zhang, G.; Li, S.; Liang, Z. The Grapevine R2R3-Type MYB Transcription Factor VdMYB1 Positively Regulates Defense Responses by Activating the Stilbene Synthase Gene 2 (VdSTS2). BMC Plant Biol. 2019, 19, 478. [Google Scholar] [CrossRef]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.M.; Robinson, S.P.; Barrieu, F. The Transcription Factor VvMYB5b Contributes to the Regulation of Anthocyanin and Proanthocyanidin Biosynthesis in Developing Grape Berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef]
- Akagi, T.; Ikegami, A.; Tsujimoto, T.; Kobayashi, S.; Sato, A.; Kono, A.; Yonemori, K. DkMyb4 Is a Myb Transcription Factor Involved in Proanthocyanidin Biosynthesis in Persimmon Fruit. Plant Physiol. 2009, 151, 2028–2045. [Google Scholar] [CrossRef]
- Anupa, T.; Sahijram, T.; Samarth, L. In Vitro Shoot Induction of Three Grape (Vitis vinifera L.) Varieties Using Nodal and Axillary Explants. BioScan 2016, 11, 201–204. [Google Scholar]
- Fizikova, A.Y. Microclonal Propagation of Elite Industrial Grape Cultivars (Vitis vinifera L.). Proc. Appl. Bot. Genet. Breed. 2023, 184, 222–231. [Google Scholar] [CrossRef]
- Weigel, D.; Glazebrook, J. Transformation of Agrobacterium Using the Freeze-Thaw Method. CSH Protoc. 2006, 2006, pdb.prot4666. [Google Scholar] [CrossRef]
- Konagaya, K.I.; Nanasato, Y.; Taniguchi, T. A Protocol for Agrobacterium-Mediated Transformation of Japanese Cedar, Sugi (Cryptomeria japonica D. Don) Using Embryogenic Tissue Explants. Plant Biotechnol. 2020, 37, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Conant, D.; Hsiau, T.; Rossi, N.; Oki, J.; Maures, T.; Waite, K.; Yang, J.; Joshi, S.; Kelso, R.; Holden, K.; et al. Inference of CRISPR Edits from Sanger Trace Data. CRISPR J. 2022, 5, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lim, K.; Kim, J.S.; Bae, S. Cas-Analyzer: An Online Tool for Assessing Genome Editing Results Using NGS Data. Bioinformatics 2017, 33, 286–288. [Google Scholar] [CrossRef]
- Kamlesh, R.P.; Narpat, S.S.; Graeme, P.B.; Trevor, A.T. Isolation and Culture of Protoplasts 388 from Cytoledons of Pinus Coulteri D. Don. Plant Cell Tissue Organ Cult. 1984, 3, 85–90. [Google Scholar]
- Nikolaeva, T.N.; Lapshin, P.V.; Zagoskina, N.V. Method for Determining the Total Content of Phenolic Compounds in Plant Extracts with Folin-Denis Reagent and Folin-Chocalteu Reagent: Modification and Comparison. Khimiya Rastit. Syr’ya 2021, 2, 291–299. [Google Scholar] [CrossRef]
- Altman, D.G.; Machi, D.; Bryan, T.N.; Gardn, M.J. Statistics with Confidence, 2nd ed.; BMJ: London, UK, 2000. [Google Scholar]
- Richardson, J.T. The Analysis of 2 × 2 Contingency Tables—Yet Again. Stat. Med. 2011, 30, 890. [Google Scholar] [CrossRef]
- Campbell, I. Chi-Squared and Fisher–Irwin Tests of Two-by-Two Tables with Small Sample Recommendations. Stat. Med. 2007, 26, 3661–3675. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fizikova, A.; Tukhuzheva, Z.; Zhokhova, L.; Tvorogova, V.; Lutova, L. A New Approach for CRISPR/Cas9 Editing and Selection of Pathogen-Resistant Plant Cells of Wine Grape cv. ‘Merlot’. Int. J. Mol. Sci. 2024, 25, 10011. https://doi.org/10.3390/ijms251810011
Fizikova A, Tukhuzheva Z, Zhokhova L, Tvorogova V, Lutova L. A New Approach for CRISPR/Cas9 Editing and Selection of Pathogen-Resistant Plant Cells of Wine Grape cv. ‘Merlot’. International Journal of Molecular Sciences. 2024; 25(18):10011. https://doi.org/10.3390/ijms251810011
Chicago/Turabian StyleFizikova, Anastasia, Zhanneta Tukhuzheva, Lada Zhokhova, Varvara Tvorogova, and Ludmila Lutova. 2024. "A New Approach for CRISPR/Cas9 Editing and Selection of Pathogen-Resistant Plant Cells of Wine Grape cv. ‘Merlot’" International Journal of Molecular Sciences 25, no. 18: 10011. https://doi.org/10.3390/ijms251810011
APA StyleFizikova, A., Tukhuzheva, Z., Zhokhova, L., Tvorogova, V., & Lutova, L. (2024). A New Approach for CRISPR/Cas9 Editing and Selection of Pathogen-Resistant Plant Cells of Wine Grape cv. ‘Merlot’. International Journal of Molecular Sciences, 25(18), 10011. https://doi.org/10.3390/ijms251810011





