Gene Therapeutic Drug pCMV-VEGF165 Plasmid (‘Neovasculgen’) Promotes Gingiva Soft Tissue Augmentation in Rabbits
Abstract
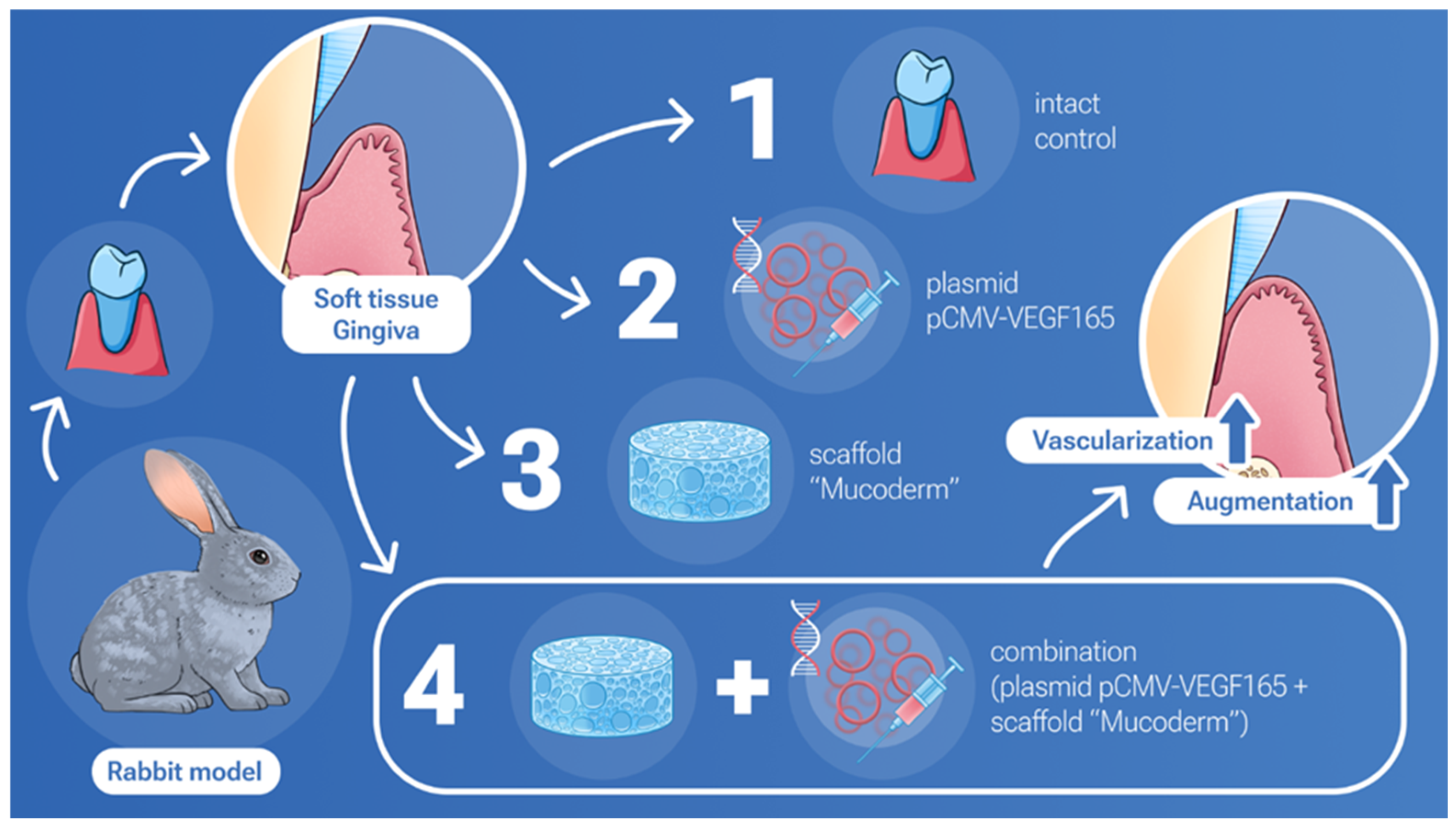
:1. Introduction
2. Results
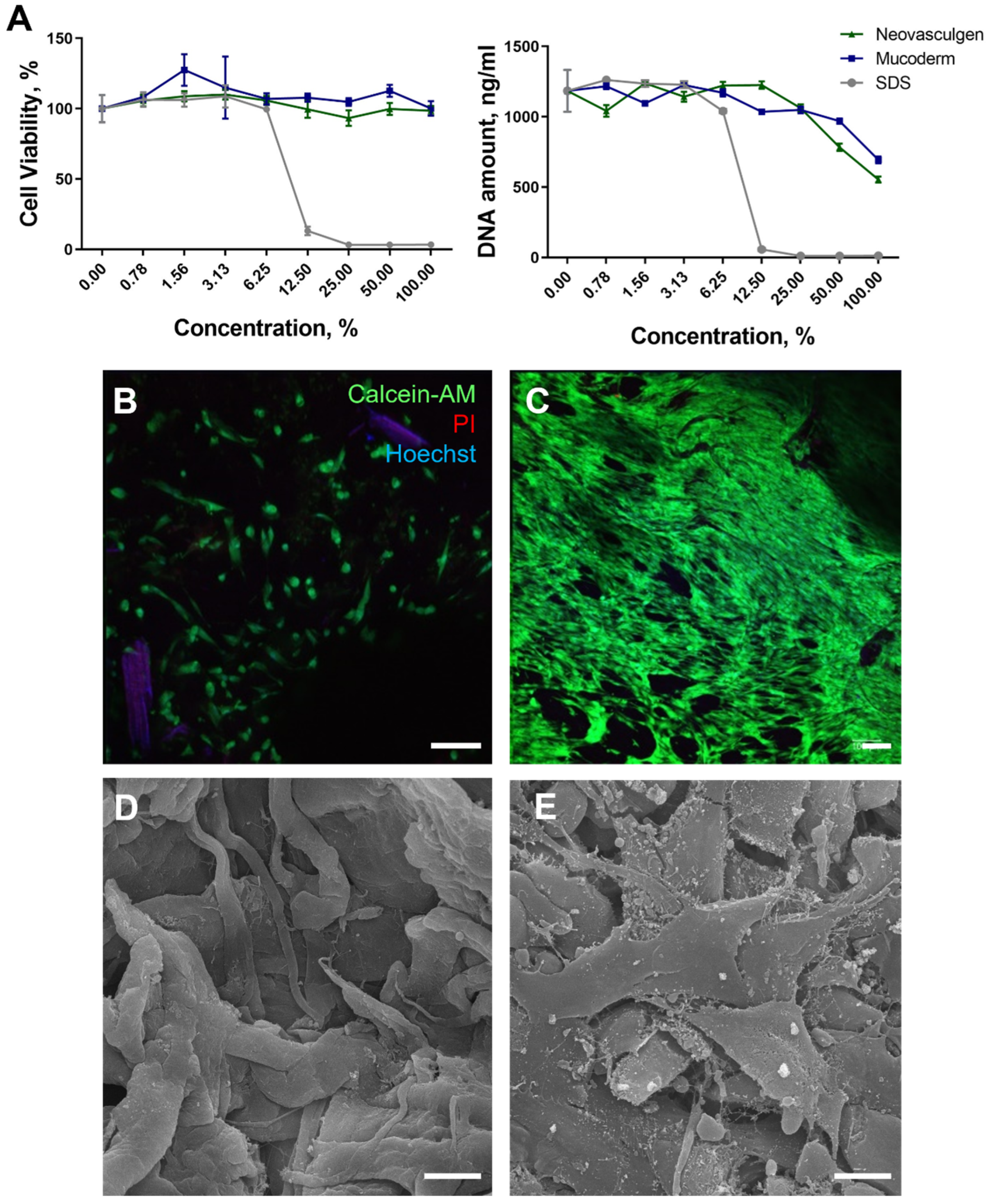
2.1. Biocompatibility of Mucoderm and pCMV-VEGF165 Plasmid
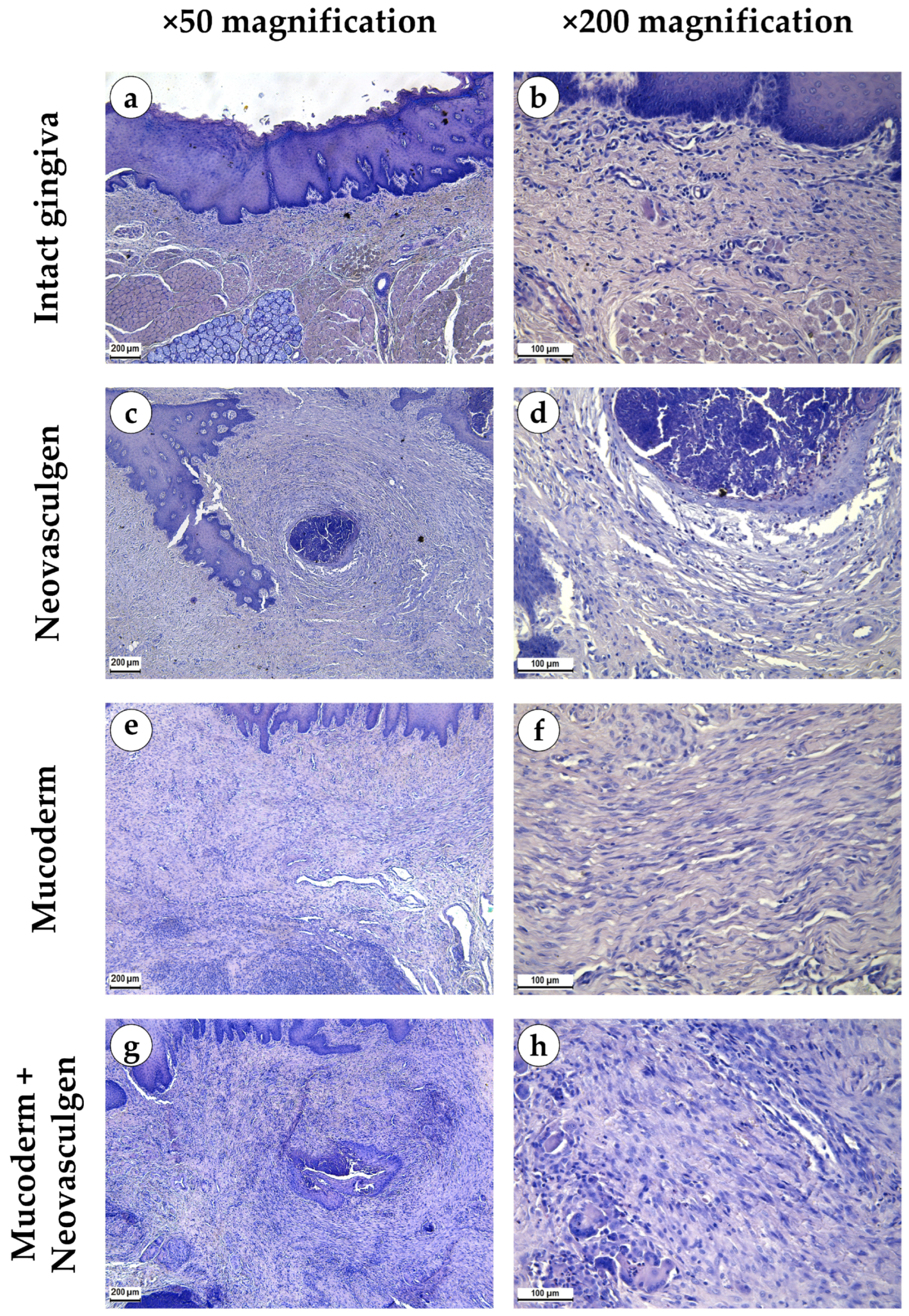
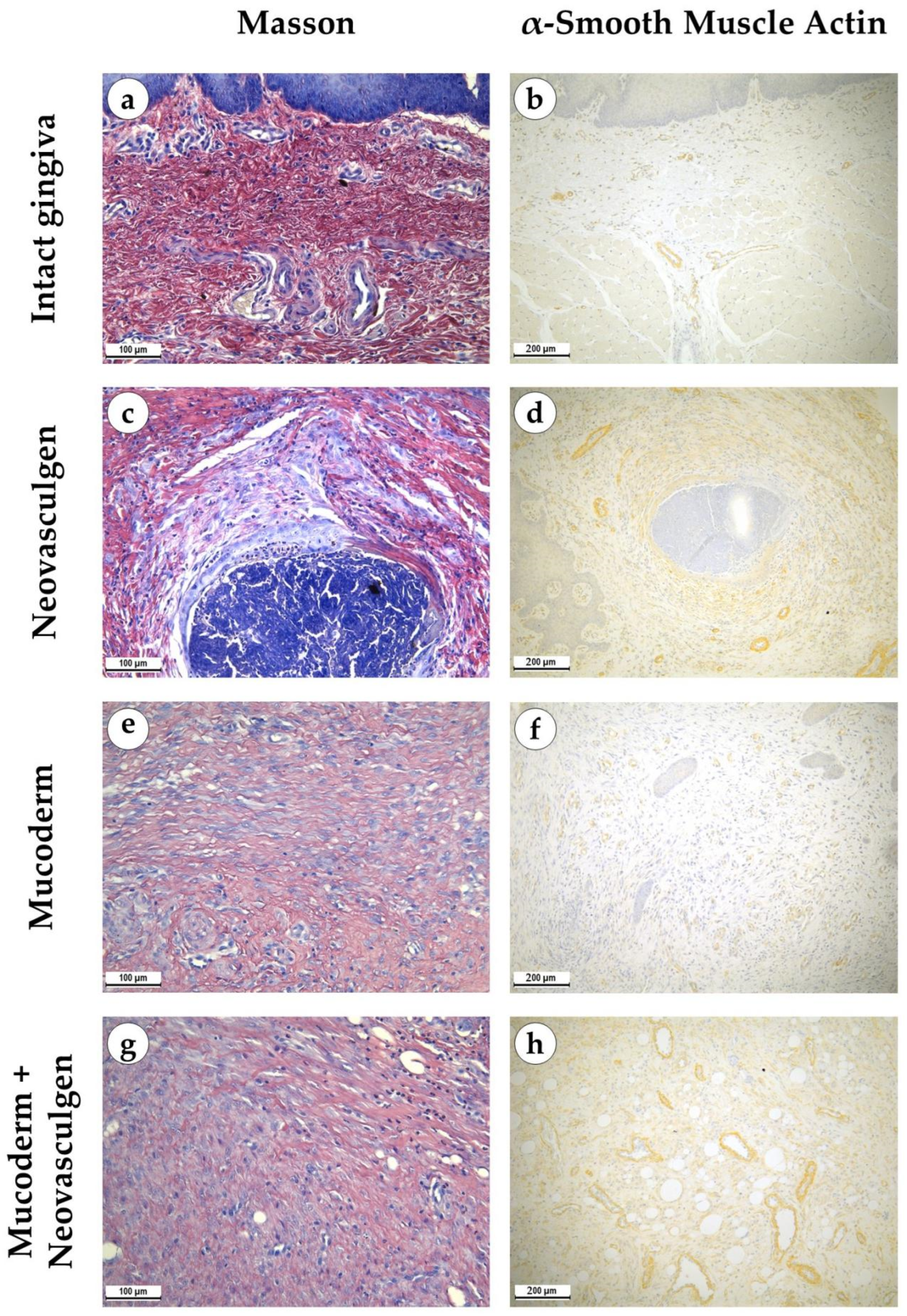
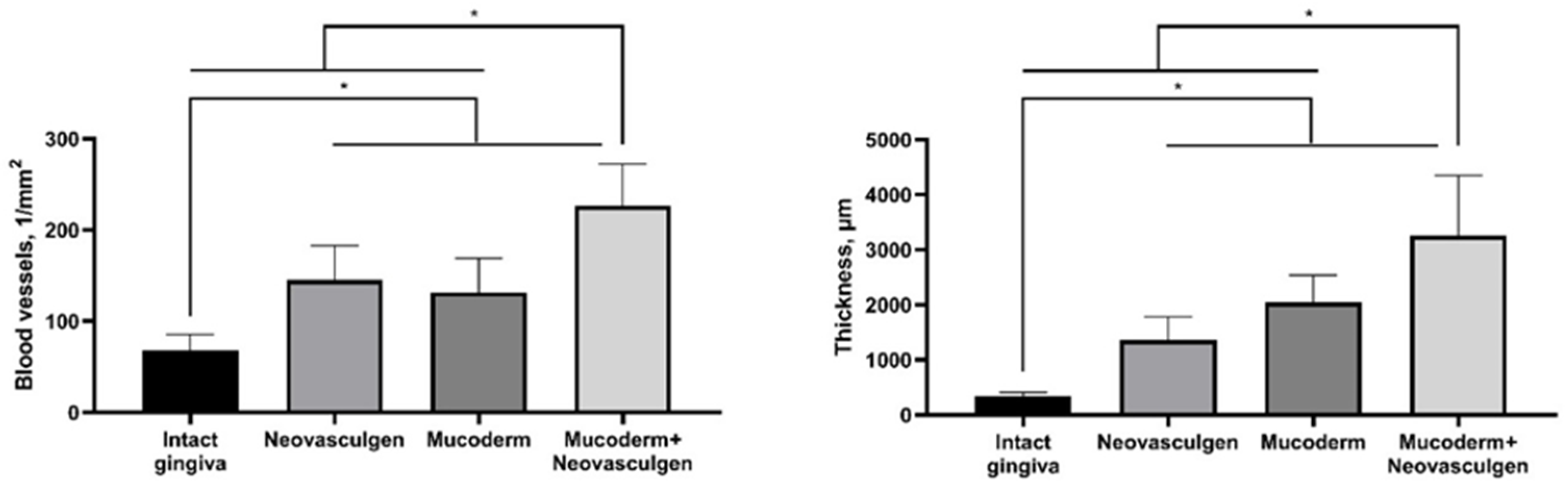
2.2. In Vivo Studies of Mucoderm Material and pCMV-VEGF165 Plasmid (‘Neovasculgen’) Vascularization Properties
3. Discussion
4. Materials and Methods
4.1. Mucoderm and ‘Neovasculgen’ Materials
4.2. Cell Culturing
4.3. Biocompatibility
4.4. Extract Cytotoxicity
4.5. Contact Culturing (Live/Dead Assay)
4.6. Scanning Electron Microscopy
4.7. Animal Experiments
4.8. Histological Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esfahrood, Z.R.; Kadkhodazadeh, M.; Talebi Ardakani, M.R. Gingival biotype: A review. Gen. Dent. 2013, 61, 14–17. [Google Scholar] [PubMed]
- Morris, J.W.; Campbell, P.M.; Tadlock, L.P.; Boley, J.; Buschang, P.H. Prevalence of gingival recession after orthodontic tooth movements. Am. J. Orthod. Dentofac. Orthop. 2017, 151, 851–859. [Google Scholar] [CrossRef]
- Mythri, S.; Arunkumar, S.M.; Hegde, S.; Rajesh, S.K.; Munaz, M.; Ashwin, D. Etiology and occurrence of gingival recession—An epidemiological study. J. Indian Soc. Periodontol. 2015, 19, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Rasperini, G.; Acunzo, R.; Cannalire, P.; Farronato, G. Influence of Periodontal Biotype on Root Surface Exposure during Orthodontic Treatment: A Preliminary Study. Int. J. Periodontics Restor. Dent. 2015, 35, 665–675. [Google Scholar] [CrossRef]
- Huang, H.; Dong, Z.; Ren, X.; Jia, B.; Li, G.; Zhou, S.; Zhao, X.; Wang, W. High-strength hydrogels: Fabrication, reinforcement mechanisms, and applications. Nano Res. 2023, 16, 3475–3515. [Google Scholar] [CrossRef]
- Shpichka, A.; Osipova, D.; Efremov, Y.; Bikmulina, P.; Kosheleva, N.; Lipina, M.; Bezrukov, E.A.; Sukhanov, R.B.; Solovieva, A.B.; Vosough, M. Fibrin-Based Bioinks: New Tricks from an Old Dog. Int. J. Bioprint. 2020, 6, 269. [Google Scholar]
- Jia, B.; Huang, H.; Dong, Z.; Ren, X.; Lu, Y.; Wang, W.; Zhou, S.; Zhao, X.; Guo, B. Degradable biomedical elastomers: Paving the future of tissue repair and regenerative medicine. Chem. Soc. Rev. 2024, 53, 4086–4153. [Google Scholar] [CrossRef]
- De Annuntiis, C.; Testarelli, L.; Guarnieri, R. Use of Xenogenic Collagen Matrices in Peri-Implant Soft Tissue Volume Augmentation: A Critical Review on the Current Evidence and New Technique Presentation. Materials 2022, 15, 3937. [Google Scholar] [CrossRef]
- Antoshin, A.; Dubinin, O.; Miao, L.; Istranova, E.; Bikmulina, P.; Fayzullin, A.; Magdanov, A.; Kravchik, M.; Kosheleva, N.; Solovieva, A.; et al. Semipermeable barrier-assisted electrophoretic deposition of robust collagen membranes. J. Mater. Sci. 2023, 58, 9675–9697. [Google Scholar] [CrossRef]
- Zuhr, O.; Bäumer, D.; Hürzeler, M. The addition of soft tissue replacement grafts in plastic periodontal and implant surgery: Critical elements in design and execution. J. Clin. Periodontol. 2014, 41 (Suppl. 15), S123–S142. [Google Scholar] [CrossRef]
- Huang, N.; Khan, A.; Ashrafpour, H.; Neligan, P.C.; Forrest, C.R.; Kontos, C.D.; Pang, C.Y. Efficacy and mechanism of adenovirus-mediated VEGF-165 gene therapy for augmentation of skin flap viability. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H127–H137. [Google Scholar] [CrossRef] [PubMed]
- Barć, P.; Antkiewicz, M.; Śliwa, B.; Frączkowska, K.; Guziński, M.; Dawiskiba, T.; Małodobra-Mazur, M.; Witkiewicz, W.; Kupczyńska, D.; Strzelec, B.; et al. Double VEGF/HGF Gene Therapy in Critical Limb Ischemia Complicated by Diabetes Mellitus. J. Cardiovasc. Transl. Res. 2021, 14, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Stafeev, I.I.; Boldyreva, M.A.; Michurina, S.S.; Agareva, M.Y.; Radnaeva, A.V.; Menshikov, M.Y.; Hu, Y.C.; Makarevich, P.I.; Parfyonova, Y.V. The Efficacy of HGF/VEGF Gene Therapy for Limb Ischemia in Mice with Impaired Glucose Tolerance: Shift from Angiogenesis to Axonal Growth and Oxidative Potential in Skeletal Muscle. Cells 2022, 11, 3824. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, R.T.; Eibel, B.; Markoski, M.M.; Rodrigues, C.G.; de Salles, F.B.; Giusti, I.I.; Nesralla, I.A.; Nardi, N.B.; Kalil, R.A.K. Gene therapy for refractory angina and cell therapy for heart failure: Experience of a Brazilian research group. Gene Ther. 2020, 27, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.; Hüsler, J.; Jung, R.E. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef]
- Cosyn, J.; Eeckhout, C.; Christiaens, V.; Eghbali, A.; Vervaeke, S.; Younes, F.; de Bruyckere, T. A multi-centre randomized controlled trial comparing connective tissue graft with collagen matrix to increase soft tissue thickness at the buccal aspect of single implants: 3-month results. J. Clin. Periodontol. 2021, 48, 1502–1515. [Google Scholar] [CrossRef]
- Vallecillo, C.; Toledano-Osorio, M.; Vallecillo-Rivas, M.; Toledano, M.; Rodriguez-Archilla, A.; Osorio, R. Collagen Matrix vs. Autogenous Connective Tissue Graft for Soft Tissue Augmentation: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 1810. [Google Scholar] [CrossRef]
- Maiorana, C.; Pivetti, L.; Signorino, F.; Grossi, G.B.; Herford, A.S.; Beretta, M. The efficacy of a porcine collagen matrix in keratinized tissue augmentation: A 5-year follow-up study. Int. J. Implant Dent. 2018, 4, 1. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Zhang, S.; Ferrantino, L.; Simion, M.; Chappuis, V.; Bosshardt, D.D. Tissue Response to a Porous Collagen Matrix Used for Soft Tissue Augmentation. Materials 2019, 12, 3721. [Google Scholar] [CrossRef]
- Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.A.; Benítez-García, J.A.; Osorio, R. Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration. Polymers 2020, 12, 1290. [Google Scholar] [CrossRef]
- Gatina, D.Z.; Garanina, E.E.; Zhuravleva, M.N.; Synbulatova, G.E.; Mullakhmetova, A.F.; Solovyeva, V.V.; Kiyasov, A.P.; Rutland, C.S.; Rizvanov, A.A.; Salafutdinov, I.I. Proangiogenic Effect of 2A-Peptide Based Multicistronic Recombinant Constructs Encoding VEGF and FGF2 Growth Factors. Int. J. Mol. Sci. 2021, 22, 5922. [Google Scholar] [CrossRef] [PubMed]
- Makarevich, P.I.; Dergilev, K.V.; Tsokolaeva, Z.I.; Boldyreva, M.A.; Shevchenko, E.K.; Gluhanyuk, E.V.; Gallinger, J.O.; Menshikov, M.Y.; Parfyonova, Y.V. Angiogenic and pleiotropic effects of VEGF165 and HGF combined gene therapy in a rat model of myocardial infarction. PLoS ONE 2018, 13, e0197566. [Google Scholar] [CrossRef]
- Pan, P.; Li, J.; Liu, X.; Hu, C.; Wang, M.; Zhang, W.; Li, M.; Liu, Y. Plasmid containing VEGF-165 and ANG-1 dual genes packaged with fibroin-modified PEI to promote the regeneration of vascular network and dermal tissue. Coll. Surf. B Biointerfaces 2023, 224, 113210. [Google Scholar] [CrossRef]
- Skóra, J.P.; Antkiewicz, M.; Kupczyńska, D.; Kulikowska, K.; Strzelec, B.; Janczak, D.; Barć, P. Local intramuscular administration of ANG1 and VEGF genes using plasmid vectors mobilizes CD34+ cells to peripheral tissues and promotes angiogenesis in an animal model. Biomed. Pharmacother. 2021, 143, 112186. [Google Scholar] [CrossRef]
- Solovyeva, V.V.; Chulpanova, D.S.; Tazetdinova, L.G.; Salafutdinov, I.I.; Bozo, I.Y.; Isaev, A.A.; Deev, R.V.; Rizvanov, A.A. In Vitro Angiogenic Properties of Plasmid DNA Encoding SDF-1α and VEGF165 Genes. Appl. Biochem. Biotechnol. 2020, 190, 773–788. [Google Scholar] [CrossRef]
- Keeney, M.; van den Beucken, J.J.; van der Kraan, P.M.; Jansen, J.A.; Pandit, A. The ability of a collagen/calcium phosphate scaffold to act as its own vector for gene delivery and to promote bone formation via transfection with VEGF(165). Biomaterials 2010, 31, 2893–2902. [Google Scholar] [CrossRef]
- Klabukov, I.; Balyasin, M.; Krasilnikova, O.; Tenchurin, T.; Titov, A.; Krasheninnikov, M.; Mudryak, D.; Sulina, Y.; Shepelev, A.; Chvalun, S.; et al. Angiogenic Modification of Microfibrous Polycaprolactone by pCMV-VEGF165 Plasmid Promotes Local Vascular Growth after Implantation in Rats. Int. J. Mol. Sci. 2023, 24, 1399. [Google Scholar] [CrossRef]
- Guo, R.; Xu, S.; Ma, L.; Huang, A.; Gao, C. The healing of full-thickness burns treated by using plasmid DNA encoding VEGF-165 activated collagen-chitosan dermal equivalents. Biomaterials 2011, 32, 1019–1031. [Google Scholar] [CrossRef]
- Luo, Z.; Li, J.; Qu, J.; Sheng, W.; Yang, J.; Li, M. Cationized Bombyx mori silk fibroin as a delivery carrier of the VEGF165-Ang-1 coexpression plasmid for dermal tissue regeneration. J. Mater. Chem. B 2019, 7, 80–94. [Google Scholar] [CrossRef]
- D’Mello, S.R.; Elangovan, S.; Hong, L.; Ross, R.D.; Sumner, D.R.; Salem, A.K. A Pilot Study Evaluating Combinatorial and Simultaneous Delivery of Polyethylenimine-Plasmid DNA Complexes Encoding for VEGF and PDGF for Bone Regeneration in Calvarial Bone Defects. Curr. Pharm. Biotechnol. 2015, 16, 655–660. [Google Scholar] [CrossRef]
- Chakka, J.L.; Acri, T.; Laird, N.Z.; Zhong, L.; Shin, K.; Elangovan, S.; Salem, A.K. Polydopamine functionalized VEGF gene-activated 3D printed scaffolds for bone regeneration. RSC Adv. 2021, 11, 13282–13291. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.N.; Wu, Y.J.; Lee, H.I.; Wang, H.H.; Chang, C.Y.; Tien, T.Y.; Lin, C.F.; Su, C.H.; Yeh, H.I. Ultrasonic microbubble VEGF gene delivery improves angiogenesis of senescent endothelial progenitor cells. Sci. Rep. 2021, 11, 13449. [Google Scholar] [CrossRef]
- Barć, P.; Antkiewicz, M.; Śliwa, B.; Baczyńska, D.; Witkiewicz, W.; Skóra, J.P. Treatment of Critical Limb Ischemia by pIRES/VEGF165/HGF Administration. Ann. Vasc. Surg. 2019, 60, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Slobodkina, E.; Boldyreva, M.; Karagyaur, M.; Eremichev, R.; Alexandrushkina, N.; Balabanyan, V.; Akopyan, Z.; Parfyonova, Y.; Tkachuk, V.; Makarevich, P. Therapeutic Angiogenesis by a “Dynamic Duo”: Simultaneous Expression of HGF and VEGF165 by Novel Bicistronic Plasmid Restores Blood Flow in Ischemic Skeletal Muscle. Pharmaceutics 2020, 12, 1231. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Gai, W.; Ji, F.; Zhao, J.; Sun, D.; Yang, F.; Jiang, H.; Feng, Y. Bovine serum albumin-based biomimetic gene complexes with specificity facilitate rapid re-endothelialization for anti-restenosis. Acta Biomater. 2022, 142, 221–241. [Google Scholar] [CrossRef]
- Bozo, I.Y.; Deev, R.V.; Drobyshev, A.Y.; Isaev, A.A.; Eremin, I.I. World’s First Clinical Case of Gene-Activated Bone Substitute Application. Case Rep. Dent. 2016, 2016, 8648949. [Google Scholar] [CrossRef]
- Kalinin, R.E.; Suchkov, I.A.; Deev, R.V.; Mzhavanadze, N.D.; Krylov, A.A. Gene-mediated induction of angiogenesis in inoperable patients with atherosclerosis and diabetes mellitus. Angiol. Sosud. Khir. 2018, 24, 33–40. [Google Scholar]
- Chervyakov, Y.V.; Staroverov, I.N.; Vlasenko, O.N.; Bozo, I.Y.; Isaev, A.A.; Deev, R.V. Five-year results of treating patients with chronic lower limb ischaemia by means of gene engineering. Angiol. Sosud. Khir. 2016, 22, 38–44. [Google Scholar]
- Krivoshchekov, E.P.; El’shin, E.B.; Romanov, V.E.; Aliapyshev, G.S.; Rodnianskiĭ, D.V. Ways of limbs salvage in postoperative period of treatment of complications of diabetic foot syndrome. Angiol. Sosud. Khir. 2020, 26, 33–41. [Google Scholar] [CrossRef]
- Gavrilenko, A.V.; Oleĭnik, E.M. Comprehensive treatment of a patient with Buerger’s disease using genetically engineered complexes VEGF-165. Angiol. Sosud. Khir. 2019, 25, 177–180. [Google Scholar] [CrossRef]
- Preidl, R.H.M.; Reichert, S.; Coronel, T.V.; Kesting, M.; Wehrhan, F.; Schmitt, C.M. Free Gingival Graft and Collagen Matrix Revascularization in an Enoral Open Wound Situation. J. Oral Maxillofac. Surg. 2021, 79, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Bienz, S.P.; Gadzo, N.; Zuercher, A.N.; Wiedemeier, D.; Jung, R.E.; Thoma, D.S. Clinical and histological wound healing patterns of collagen-based substitutes: An experimental randomized controlled trial in standardized palatal defects in humans. J. Clin. Periodontol. 2023, 51, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Fathiazar, A.; Shariatmadar Ahmadi, R.; Sayar, F. A Comparison between Mucoderm® and Connective Tissue Graft for Root Coverage. J. Dent. 2022, 23, 402–409. [Google Scholar] [CrossRef]
- Saburina, I.N.; Kosheleva, N.V.; Kopylov, A.T.; Lipina, T.V.; Krasina, M.E.; Zurina, I.M.; Gorkun, A.A.; Girina, S.S.; Pulin, A.A.; Kaysheva, A.L.; et al. Proteomic and electron microscopy study of myogenic differentiation of alveolar mucosa multipotent mesenchymal stromal cells in three-dimensional culture. Proteomics 2022, 22, e2000304. [Google Scholar] [CrossRef] [PubMed]
- Kosheleva, N.V.; Efremov, Y.M.; Koteneva, P.I.; Ilina, I.V.; Zurina, I.M.; Bikmulina, P.Y.; Shpichka, A.I.; Timashev, P.S. Building a tissue: Mesenchymal and epithelial cell spheroids mechanical properties at micro- and nanoscale. Acta Biomater. 2023, 165, 140–152. [Google Scholar] [CrossRef]
- Peshkova, M.; Korneev, A.; Suleimanov, S.; Vlasova, I.I.; Svistunov, A.; Kosheleva, N.; Timashev, P. MSCs’ conditioned media cytokine and growth factor profiles and their impact on macrophage polarization. Stem Cell Res. Ther. 2023, 14, 142. [Google Scholar] [CrossRef]
- Peshkova, M.; Korneev, A.; Revokatova, D.; Smirnova, O.; Klyucherev, T.; Shender, V.; Arapidi, G.; Kosheleva, N.; Timashev, P. Four sides to the story: A proteomic comparison of liquid-phase and matrix-bound extracellular vesicles in 2D and 3D cell cultures. Proteomics 2024, 24, 2300375. [Google Scholar] [CrossRef]
- Bardakova, K.N.; Faletrov, Y.V.; Epifanov, E.O.; Minaev, N.V.; Kaplin, V.S.; Piskun, Y.A.; Koteneva, P.I.; Shkumatov, V.M.; Aksenova, N.A.; Shpichka, A.I. A hydrophobic derivative of ciprofloxacin as a new photoinitiator of two-photon polymerization: Synthesis and usage for the formation of biocompatible polylactide-based 3d scaffolds. Polymers 2021, 13, 3385. [Google Scholar] [CrossRef]
- Bikmulina, P.; Kosheleva, N.; Efremov, Y.; Bakulina, A.; Kuryanova, A.; Aksenova, N.; Shavkuta, B.; Kotova, S.; Shpichka, A.; Timashev, P. Building a tissue: Gingiva-and adipose-derived mesenchymal cell spheroids’ survivability and functionality after 3D extrusion bioprinting. Bioprinting 2023, 32, e00279. [Google Scholar]
- Giacca, M.; Zacchigna, S. VEGF gene therapy: Therapeutic angiogenesis in the clinic and beyond. Gene Ther. 2012, 19, 622–629. [Google Scholar] [CrossRef]
- Peng, L.H.; Wei, W.; Shan, Y.H.; Zhang, T.Y.; Zhang, C.Z.; Wu, J.H.; Yu, L.; Lin, J.; Liang, W.Q.; Khang, G.; et al. β-Cyclodextrin-Linked Polyethylenimine Nanoparticles Facilitate Gene Transfer and Enhance the Angiogenic Capacity of Mesenchymal Stem Cells for Wound Repair and Regeneration. J. Biomed. Nanotechnol. 2015, 11, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.; Yang, M.; Zhang, W.; Jin, R.; Xia, S.; Zhang, M.; Wu, P.; He, X.; Han, C.; Zhao, X.; et al. Dual gene-activated dermal scaffolds regulate angiogenesis and wound healing by mediating the coexpression of VEGF and angiopoietin-1. Bioeng. Transl. Med. 2023, 8, e10562. [Google Scholar] [CrossRef]
- Lou, D.; Luo, Y.; Pang, Q.; Tan, W.Q.; Ma, L. Gene-activated dermal equivalents to accelerate healing of diabetic chronic wounds by regulating inflammation and promoting angiogenesis. Bioact. Mater. 2020, 5, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Geiger, F.; Bertram, H.; Berger, I.; Lorenz, H.; Wall, O.; Eckhardt, C.; Simank, H.G.; Richter, W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J. Bone Miner. Res. 2005, 20, 2028–2035. [Google Scholar] [CrossRef]
- Naeini, A.T.; Oryan, A.; Dehghani, S.; Nikahval, B. Experimental cutaneous wound healing in rabbits: Using continuous microamperage low-voltage electrical stimulation. Comp. Clin. Pathol. 2008, 17, 203–210. [Google Scholar]
- Shpichka, A.; Koroleva, A.; Kuznetsova, D.; Dmitriev, R.I.; Timashev, P. Fabrication and Handling of 3D Scaffolds Based on Polymers and Decellularized Tissues. Adv. Exp. Med. Biol. 2017, 1035, 71–81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koteneva, P.; Kosheleva, N.; Fayzullin, A.; Khristidis, Y.; Rasulov, T.; Kulova, A.; Rozhkov, S.; Vedyaeva, A.; Brailovskaya, T.; Timashev, P. Gene Therapeutic Drug pCMV-VEGF165 Plasmid (‘Neovasculgen’) Promotes Gingiva Soft Tissue Augmentation in Rabbits. Int. J. Mol. Sci. 2024, 25, 10013. https://doi.org/10.3390/ijms251810013
Koteneva P, Kosheleva N, Fayzullin A, Khristidis Y, Rasulov T, Kulova A, Rozhkov S, Vedyaeva A, Brailovskaya T, Timashev P. Gene Therapeutic Drug pCMV-VEGF165 Plasmid (‘Neovasculgen’) Promotes Gingiva Soft Tissue Augmentation in Rabbits. International Journal of Molecular Sciences. 2024; 25(18):10013. https://doi.org/10.3390/ijms251810013
Chicago/Turabian StyleKoteneva, Polina, Nastasia Kosheleva, Alexey Fayzullin, Yana Khristidis, Timur Rasulov, Aida Kulova, Sergey Rozhkov, Anna Vedyaeva, Tatiana Brailovskaya, and Peter Timashev. 2024. "Gene Therapeutic Drug pCMV-VEGF165 Plasmid (‘Neovasculgen’) Promotes Gingiva Soft Tissue Augmentation in Rabbits" International Journal of Molecular Sciences 25, no. 18: 10013. https://doi.org/10.3390/ijms251810013
APA StyleKoteneva, P., Kosheleva, N., Fayzullin, A., Khristidis, Y., Rasulov, T., Kulova, A., Rozhkov, S., Vedyaeva, A., Brailovskaya, T., & Timashev, P. (2024). Gene Therapeutic Drug pCMV-VEGF165 Plasmid (‘Neovasculgen’) Promotes Gingiva Soft Tissue Augmentation in Rabbits. International Journal of Molecular Sciences, 25(18), 10013. https://doi.org/10.3390/ijms251810013








