Chondroitin Sulfate for Cartilage Regeneration, Administered Topically Using a Nanostructured Formulation
Abstract
1. Introduction
2. Results and Discussion
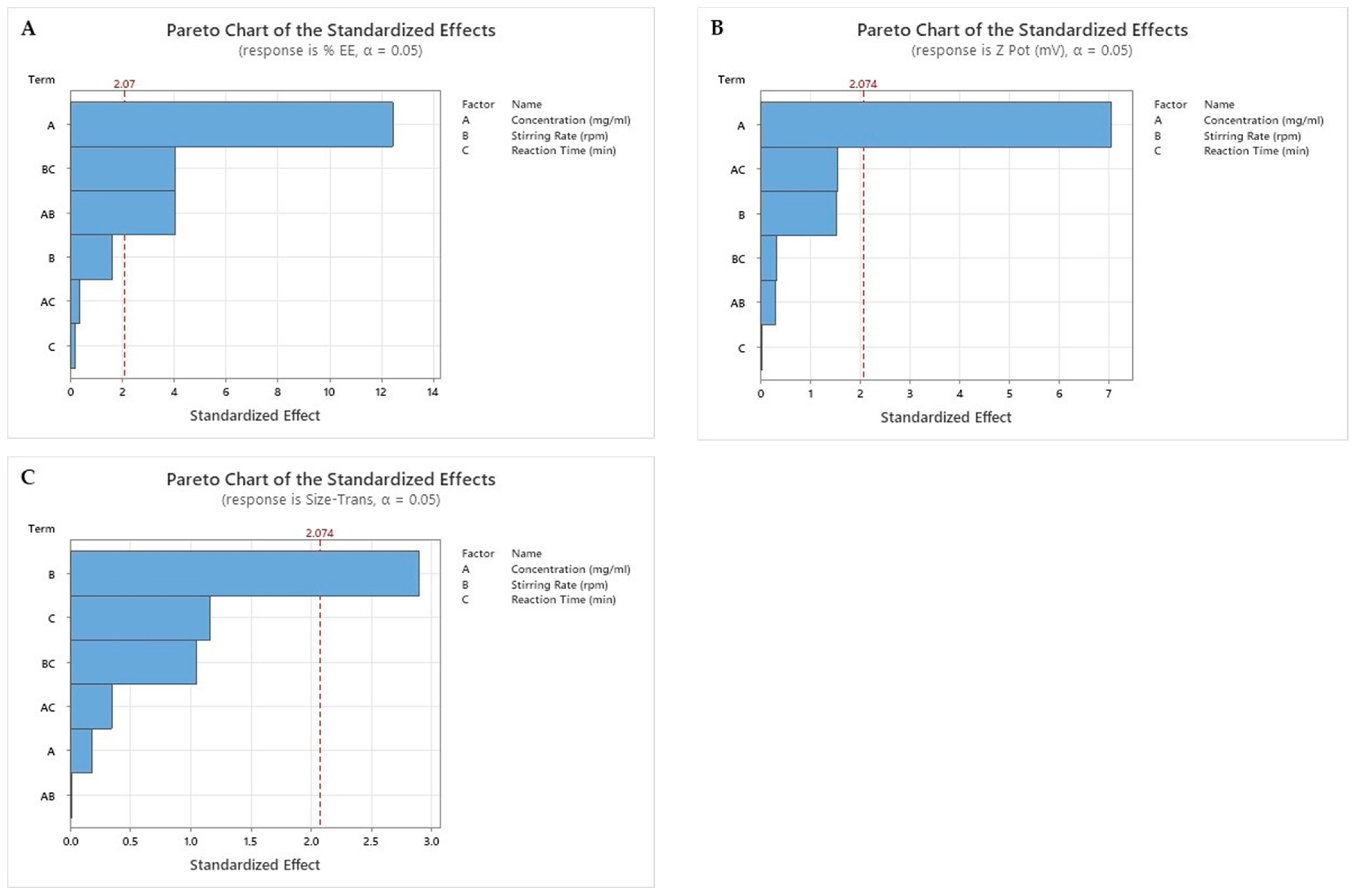
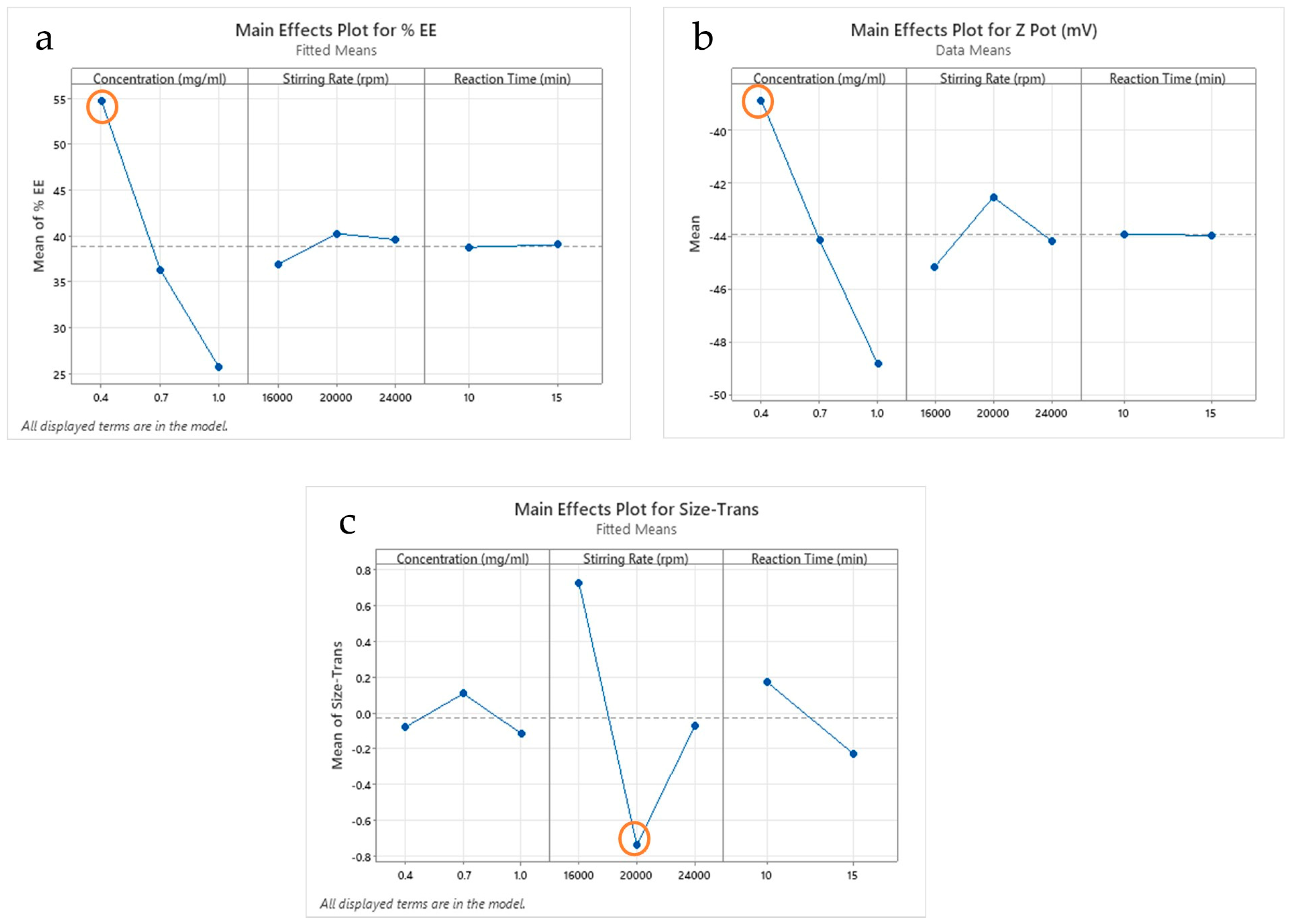
2.1. Results of Factorial Design
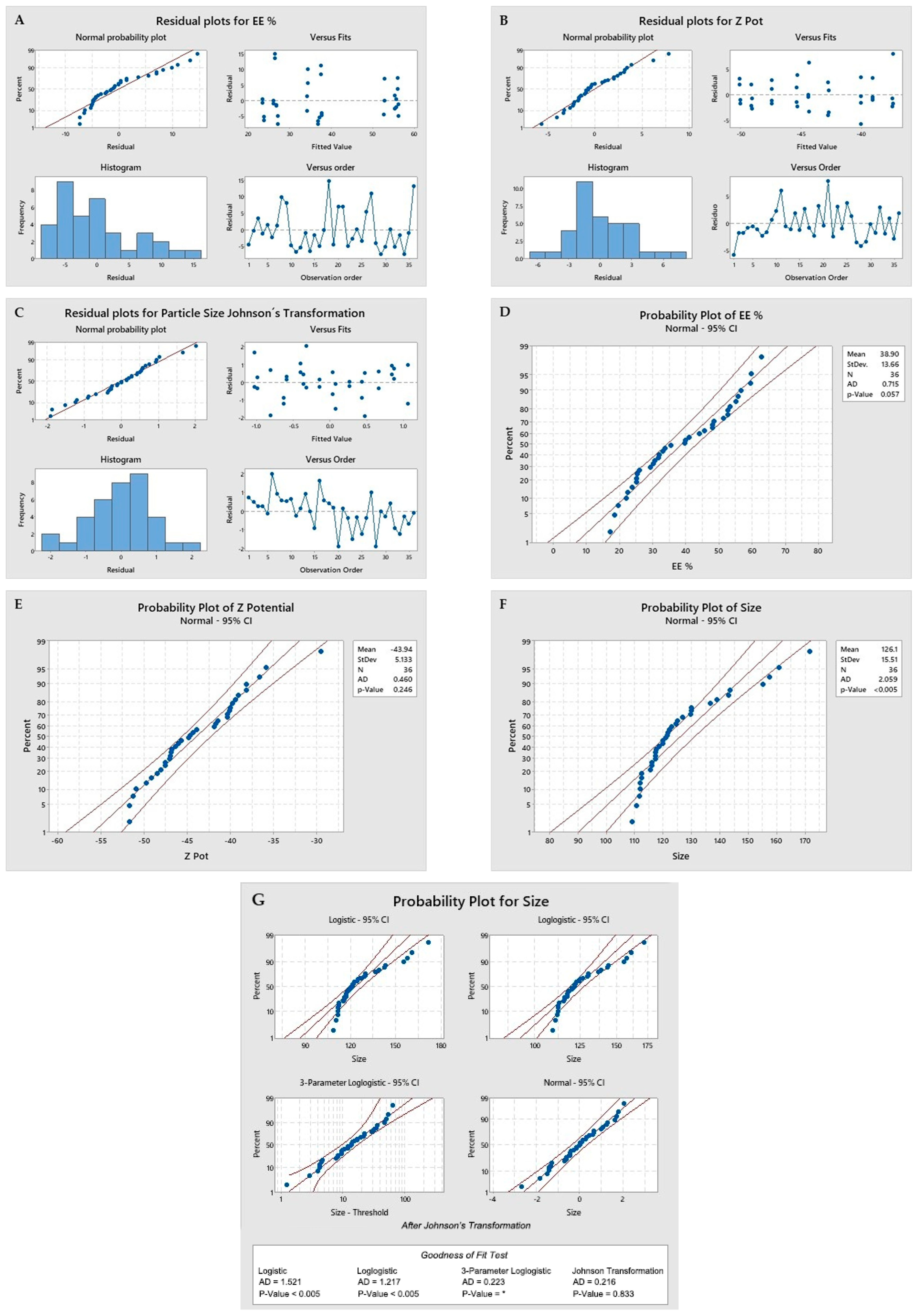
- Normality of residuals and linearity: It is shown when the residuals are aligned to the red line in the upper left box of images A, B, and C. Images D, E, and F show a p-value greater than 0.05, indicating a normal distribution. In the case of the evaluation of the size values, a Johnson transformation was required. Images F and G, for which the transformed data were used in the statistical results, are reported in Table 1;
- Homoscedasticity: The fitted value of the residuals shows values above and below zero, clearly seen in the second box at the top right of images A, B, and C;
- Independence: When evaluating the residuals versus order of observation, in the boxes at the bottom right of images A, B, and C. As no patterns are observed in the distribution of the residuals either above or below zero, there is independence.
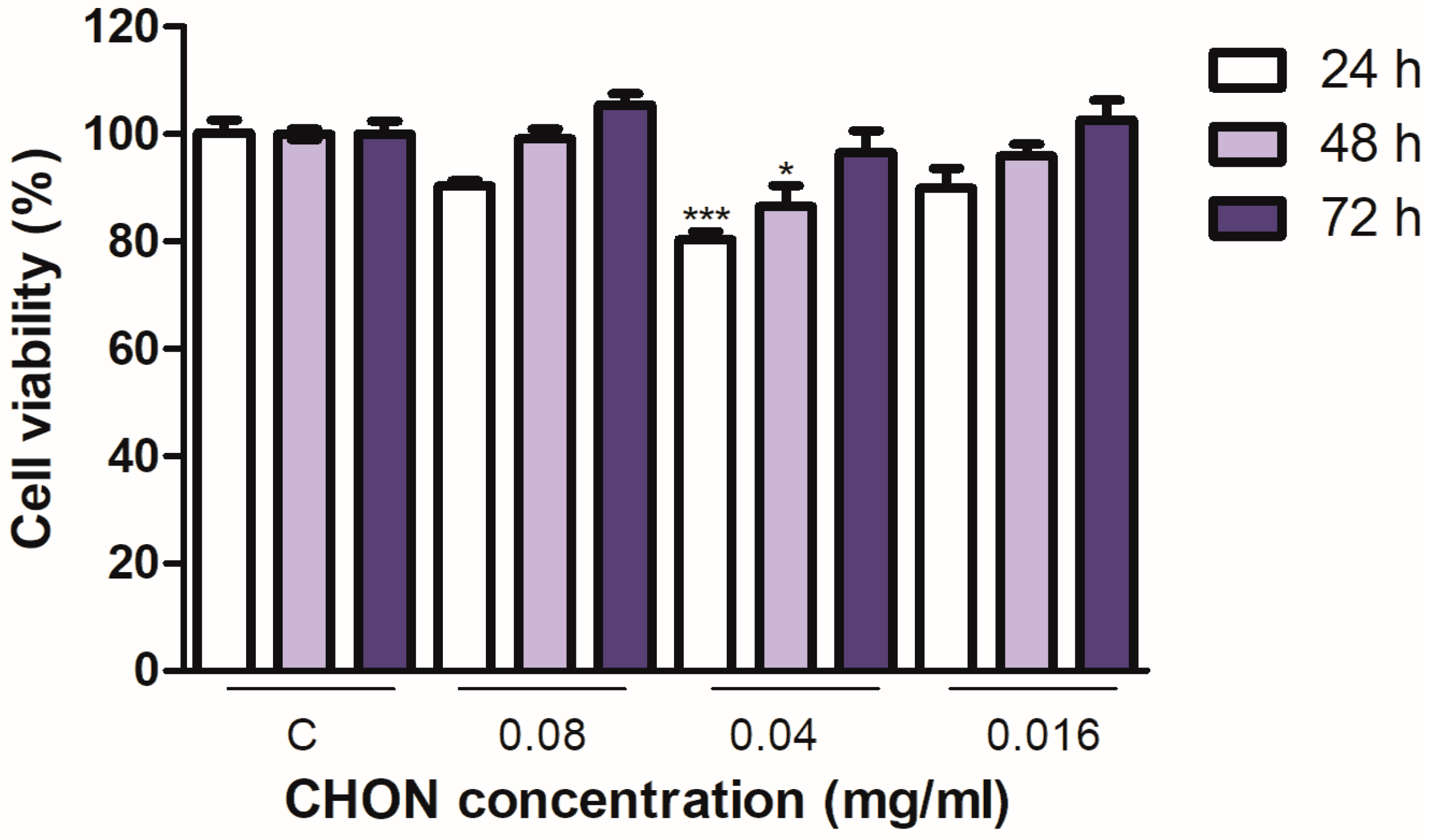
2.2. Cell Viability; Cytotoxicity
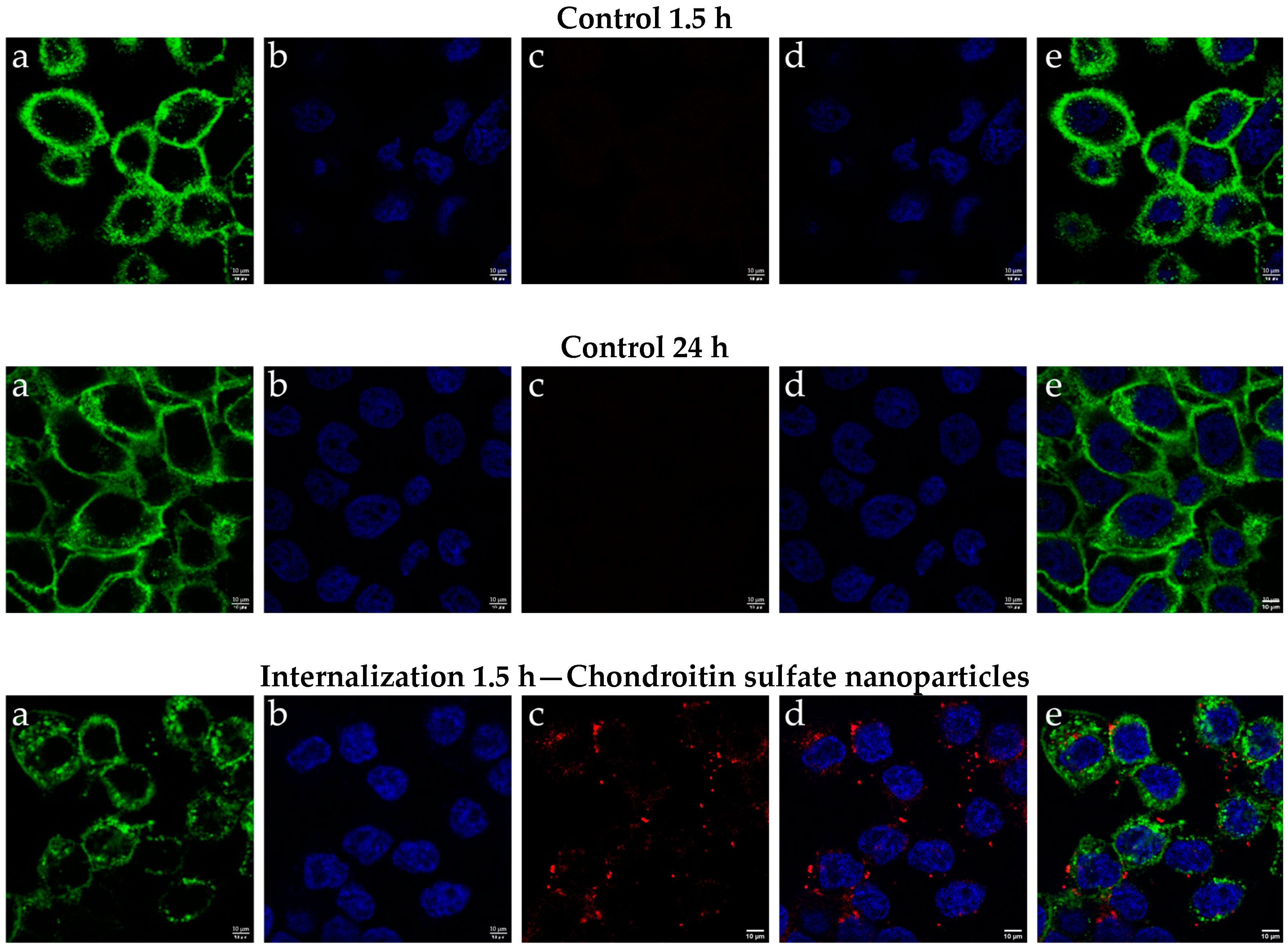
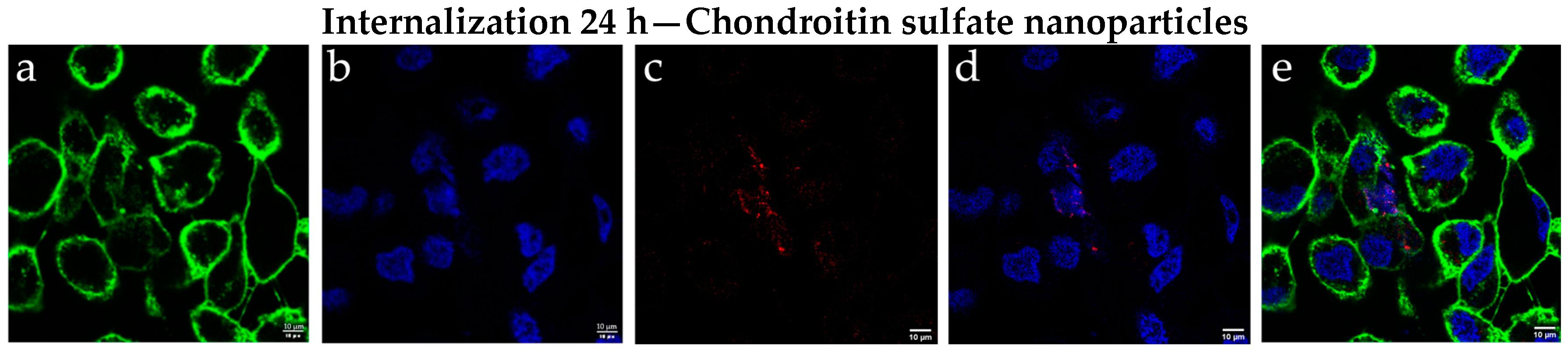
2.3. Results of Cellular Internalization of Chondroitin Sulfate Nanoparticles
2.4. Results of Biopharmaceutical Studies
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Nanoparticles Manufacturing
3.2.2. Factorial Design
3.2.3. Physicochemical Analysis
3.2.4. Cell Lines
3.2.5. Cytotoxicity Assay
3.2.6. Cellular Internalization of Chondroitin Sulfate Nanoparticles
3.2.7. Biopharmaceutical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C 2016, 68, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Pund, S.; Joshi, A. Nanoarchitectures for Neglected Tropical Protozoal Diseases: Challenges and State of the Art. In Nano- and Microscale Drug Delivery Systems: Design and Fabrication, 1st ed.; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; p. 449. [Google Scholar]
- Gasco, M.R. Method for producing solid lipid microspheres having a narrow size distribution. U.S. Patent No. 5,250,236, 5 October 1993. [Google Scholar]
- Villafuerte, L.; García, B.; de Lourdes Garzón, M.; Hernández, A.; Vázquez, M.L. Nanopartículas lipídicas sólidas. Rev. Mex. Cienc. Farm. [En Linea] 2008, 39, 38–52. Available online: https://www.redalyc.org/pdf/579/57939107.pdf (accessed on 23 November 2023).
- Mukherjee, S.; Ray, S.; Thakur, R. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349. [Google Scholar] [CrossRef] [PubMed]
- Lingayat, V.J.; Zarekar, N.S.; Shendge, R.S. Solid Lipid Nanoparticles: A Review. Nanosci. Nanotechnol. Res. 2017, 4, 67–72. [Google Scholar] [CrossRef]
- Garcês, A.; Amaral, M.; Lobo, J.S.; Silva, A. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef]
- Alcantara, K.P.; Malabanan, J.W.T.; Nalinratana, N.; Thitikornpong, W.; Rojsitthisak, P.; Rojsitthisak, P. Cannabidiol-Loaded Solid Lipid Nanoparticles Ameliorate the Inhibition of Proinflammatory Cytokines and Free Radicals in an In Vitro Inflammation-Induced Cell Model. Int. J. Mol. Sci. 2024, 25, 4744. [Google Scholar] [CrossRef]
- Muller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Bahari, L.A.S.; Hamishehkar, H. The Impact of Variables on Particle Size of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers; A Comparative Literature Review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef]
- Akanda, M.; Mithu, S.H.; Douroumis, D. Solid lipid nanoparticles: An effective lipid-based technology for cancer treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; Hamishehkar, H.; Valizadeh, H. Development of dry powder inhaler formulation loaded with alendronate solid lipid nanoparticles: Solid-state characterization and aerosol dispersion performance. Drug Dev. Ind. Pharm. 2014, 41, 1431–1437. [Google Scholar] [CrossRef]
- Padhye, S.G.; Nagarsenker, M.S. Simvastatin Solid Lipid Nanoparticles for Oral Delivery: Formulation Development and In vivo Evaluation Padhye and Nagarsenker: Solid Lipid Nanoparticles of Simvastatin for Improved Oral Delivery. Available online: www.ijpsonline.com (accessed on 23 November 2023).
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles Production, characterization and applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Eldridge, D.; Palombo, E.; Harding, I. Lipid Nanoparticles: Production, Characterization and Stability; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Berenbaum, F. Osteoarthritis year in review 2019: Epidemiology and therapy. Osteoarthr. Cartil. 2020, 28, 242–248. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858, Erratum in: Lancet 2019, 393, e44. [Google Scholar] [CrossRef]
- De Mori, A.; Heyraud, A.; Tallia, F.; Blunn, G.; Jones, J.R.; Roncada, T.; Cobb, J.; Al-Jabri, T. Ovine Mesenchymal Stem Cell Chondrogenesis on a Novel 3D-Printed Hybrid Scaffold In Vitro. Bioengineering 2024, 11, 112. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2021, 9, 603408. [Google Scholar] [CrossRef]
- De Mori, A.; Fernández, M.P.; Blunn, G.; Tozzi, G.; Roldo, M. 3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef]
- Belluzzi, E.; Todros, S.; Pozzuoli, A.; Ruggieri, P.; Carniel, E.L.; Berardo, A. Human Cartilage Biomechanics: Experimental and Theoretical Approaches towards the Identification of Mechanical Properties in Healthy and Osteoarthritic Conditions. Processes 2023, 11, 1014. [Google Scholar] [CrossRef]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp. Rheumatol. 2019, 37, 3–6. [Google Scholar] [PubMed]
- MedlinePlus. “Chondroitin Sulfate,” National Library of Medicine (NLM). Available online: https://medlineplus.gov/druginfo/natural/744.html (accessed on 23 November 2023).
- Li, L.; Li, Y.; Feng, D.; Xu, L.; Yin, F.; Zang, H.; Liu, C.; Wang, F. Preparation of Low Molecular Weight Chondroitin Sulfates, Screening of a High Anti-Complement Capacity of Low Molecular Weight Chondroitin Sulfate and Its Biological Activity Studies in Attenuating Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1685. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.S.; Mancera, R.L. The Structure of Glycosaminoglycans and their Interactions with Proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Calvo, R. Sulfato de glucosamina y condroitín sulfato, fármacos para el tratamiento de la artrosis, acusados de no presentar eficacia clínica. Culpables 2012, 109, 158–164. [Google Scholar] [CrossRef]
- Dorta, L.F. Glucosamina y sulfato de condroitina en el tratamiento de la osteoartritis. Rev. CENIC Cienc. Biológicas 2016, 47, 93–99. [Google Scholar]
- Leite, C.B.S.; Coelho, J.M.; Muehlmann, L.A.; de Azevedo, R.B.; Sousa, M.H. Skin Delivery of Glucosamine and Chondroitin Sulphates—A Perspective on the Conservative Treatment for Osteoarthritis of the Knee. J. Biosci. Med. 2017, 05, 11–20. [Google Scholar] [CrossRef]
- Aydin, B.S.; Sagiroglu, A.A.; Civelek, D.O.; Gokce, M.; Bahadori, F. Development of Curcumin and Turmerone Loaded Solid Lipid Nanoparticle for Topical Delivery: Optimization, Characterization and Skin Irritation Evaluation with 3D Tissue Model. Int. J. Nanomed. 2024, 19, 1951–1966. [Google Scholar] [CrossRef]
- Limeres, M.J.; Suñé-Pou, M.; Prieto-Sánchez, S.; Moreno-Castro, C.; Nusblat, A.D.; Hernández-Munain, C.; Castro, G.R.; Suñé, C.; Suñé-Negre, J.M.; Cuestas, M.L. Development and characterization of an improved formulation of cholesteryl oleate-loaded cationic solid-lipid nanoparticles as an efficient non-viral gene delivery system. Colloids Surf. B Biointerfaces 2019, 184, 110533. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Miralles, E.; Kamma-Lorger, C.S.; Domènech, Ò.; Sosa, L.; Casals, I.; Calpena, A.C.; Silva-Abreu, M. Assessment of Efficacy and Safety Using PPAR-γ Agonist-Loaded Nanocarriers for Inflammatory Eye Diseases. Int. J. Mol. Sci. 2022, 23, 11184. [Google Scholar] [CrossRef]
- Alvarado, H.-L.; Limón, D.; Calpena-Campmany, A.-C.; Mallandrich, M.; Rodríguez-Cid, L.; Aliaga-Alcalde, N.; González-Campo, A.; Pérez-García, L. Intrinsic Permeation and Anti-Inflammatory Evaluation of Curcumin, Bisdemethoxycurcumin and Bisdemethylcurcumin by a Validated HPLC-UV Method. Int. J. Mol. Sci. 2023, 24, 6640. [Google Scholar] [CrossRef] [PubMed]
- El Moussaoui, S.; Mallandrich, M.; Garrós, N.; Calpena, A.C.; Lagunas, M.J.R.; Fernández-Campos, F. HPV Lesions and Other Issues in the Oral Cavity Treatment and Removal without Pain. Int. J. Mol. Sci. 2021, 22, 11158. [Google Scholar] [CrossRef] [PubMed]
- Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Kosakai, M.; Yosizawa, Z. Study on the Factors Yielding High Color in the Carbazole Reaction with Hexuronic Acid-Containinig Substances. 1978. Available online: https://www.jstage.jst.go.jp/article/biochemistry1922/84/4/84_4_779/_pdf (accessed on 23 November 2023).
- Somashekar, P.L.; Sathish, K.P.; Javali, C.; Tripathy, A.S.; Kotanal, R.B. Development and Validation of Spectrophotometric Method for the Estimation of Chondroitin Sulfate in Bulk Drug and Pharmaceutical Formulations. Chem. Sci. Trans. 2013, 2, 1427–1433. [Google Scholar] [CrossRef][Green Version]
- Vighi, E.; Montanari, M.; Ruozi, B.; Tosi, G.; Magli, A.; Leo, E. Nuclear localization of cationic solid lipid nanoparticles containing Protamine as transfection promoter. Eur. J. Pharm. Biopharm. 2010, 76, 384–393. [Google Scholar] [CrossRef]
- Pérez-González, N.; Febrer, N.B.-D.; Calpena-Campmany, A.C.; Nardi-Ricart, A.; Rodríguez-Lagunas, M.J.; Morales-Molina, J.A.; Soriano-Ruiz, J.L.; Fernández-Campos, F.; Clares-Naveros, B. New Formulations Loading Caspofungin for Topical Therapy of Vulvovaginal Candidiasis. Gels 2021, 7, 259. [Google Scholar] [CrossRef]
- Mohammadi-Meyabadi, R.; Beirampour, N.; Garrós, N.; Alvarado, H.L.; Limón, D.; Silva-Abreu, M.; Calpena, A.C.; Mallandrich, M. Assessing the Solubility of Baricitinib and Drug Uptake in Different Tissues Using Absorption and Fluorescence Spectroscopies. Pharmaceutics 2022, 14, 2714. [Google Scholar] [CrossRef]
- AOAC International Officers and Committees. AOAC International Officers and Committees: 1993. J. AOAC Int. 1993, 76, 223–250. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text And Methodology Q2(R1). 2005. Available online: https://somatek.com/wp-content/uploads/2014/06/sk140605h.pdf (accessed on 23 November 2023).
- Du Plessis, J.; Stefaniak, A.; Eloff, F.; John, S.; Agner, T.; Chou, T.-C.; Nixon, R.; Steiner, M.; Franken, A.; Kudla, I.; et al. International guidelines for the in vivo assessment of skin properties in non-clinical settings: Part 2. transepidermal water loss and skin hydration. Skin Res. Technol. 2013, 19, 265–278. [Google Scholar] [CrossRef]

| Experiment | Concentration (mg/mL) | Stirring Rate (rpm) | Reaction Time (min) | Entrapment Efficiency (EE %) | Z Potential (mV) | Particle Size (nm) | Particle Size | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Johnson’s Transformation (1) | ||||
| 1 | 0.4 | 16,000 | 10 | 48.24 | 1.21 | −45.67 | 0.98 | 155.33 | 2.08 | 1.62853 |
| 2 | 0.4 | 16,000 | 15 | 52.77 | 1.48 | −41.67 | 0.72 | 136.67 | 0.58 | 0.97257 |
| 3 | 0.4 | 20,000 | 10 | 59.57 | 1.27 | −39.10 | 1.61 | 118.50 | 0.71 | −0.30277 |
| 4 | 0.4 | 20,000 | 15 | 55.16 | 0.55 | −38.17 | 1.29 | 115.50 | 0.71 | −0.71042 |
| 5 | 0.4 | 24,000 | 10 | 56.80 | 0.00 | −39.43 | 0.23 | 121.00 | 2.00 | −0.03776 |
| 6 | 0.4 | 24,000 | 15 | 53.37 | 0.02 | −40.13 | 1.70 | 180.00 | 11.31 | 1.69187 |
| 7 | 0.7 | 16,000 | 10 | 35.54 | 1.29 | −47.53 | 1.10 | 147.50 | 0.71 | 2.01961 |
| 8 | 0.7 | 16,000 | 15 | 44.27 | 0.00 | −46.83 | 0.97 | 143.00 | 1.41 | 1.23266 |
| 9 | 0.7 | 20,000 | 10 | 45.74 | 0.97 | −41.90 | 0.44 | 123.00 | 0.00 | 0.14203 |
| 10 | 0.7 | 20,000 | 15 | 33.15 | 0.05 | −40.33 | 0.15 | 120.00 | 1.73 | −0.13758 |
| 11 | 0.7 | 24,000 | 10 | 30.34 | 0.06 | −38.13 | 0.61 | 121.67 | 12.50 | 0.02488 |
| 12 | 0.7 | 24,000 | 15 | 31.86 | 0.00 | −44.83 | 0.40 | 121.50 | 2.52 | 0.00949 |
| 13 | 1.0 | 16,000 | 10 | 22.67 | 0.62 | −50.97 | 0.64 | 161.00 | 4.36 | 1.77747 |
| 14 | 1.0 | 16,000 | 15 | 17.41 | 0.62 | −48.03 | 1.40 | 127.00 | 8.66 | 0.44232 |
| 15 | 1.0 | 20,000 | 10 | 25.44 | 0.00 | −48.47 | 0.61 | 111.67 | 0.58 | −1.52134 |
| 16 | 1.0 | 20,000 | 15 | 22.23 | 0.00 | −44.67 | 0.45 | 130.00 | 8.19 | 0.63032 |
| 17 | 1.0 | 24,000 | 10 | 26.18 | 0.44 | −49.73 | 0.90 | 129.67 | 7.51 | 0.61069 |
| 18 | 1.0 | 24,000 | 15 | 41.17 | 0.03 | −51.23 | 0.67 | 122.33 | 0.58 | 0.08473 |
| 19 | 0.4 | 16,000 | 10 | 48.25 | 0.00 | −36.63 | 0.31 | 139.00 | 2.83 | 1.07435 |
| 20 | 0.4 | 16,000 | 15 | 59.85 | 1.49 | −40.37 | 0.81 | 112.00 | 0.00 | −1.42783 |
| 21 | 0.4 | 20,000 | 10 | 63.01 | 0.00 | −29.53 | 0.51 | 117.33 | 3.79 | −0.44696 |
| 22 | 0.4 | 20,000 | 15 | 51.38 | 0.60 | −39.75 | 0.35 | 112.33 | 2.31 | −1.34042 |
| 23 | 0.4 | 24,000 | 10 | 52.77 | 1.05 | −35.87 | 0.95 | 112.00 | 2.00 | −1.42783 |
| 24 | 0.4 | 24,000 | 15 | 55.91 | 0.00 | −40.00 | 0.53 | 116.00 | 3.46 | −0.63329 |
| 25 | 0.7 | 16,000 | 10 | 30.80 | 0.00 | −41.47 | 1.53 | 120.00 | 0.00 | −0.13758 |
| 26 | 0.7 | 16,000 | 15 | 39.99 | 0.44 | −43.93 | 1.56 | 125.00 | 2.65 | 0.30055 |
| 27 | 0.7 | 20,000 | 10 | 48.56 | 0.02 | −46.03 | 0.86 | 130.00 | 1.41 | 0.63032 |
| 28 | 0.7 | 20,000 | 15 | 33.85 | 0.36 | −46.77 | 0.40 | 109.00 | 2.83 | −2.67674 |
| 29 | 0.7 | 24,000 | 10 | 29.48 | 0.72 | −47.57 | 1.43 | 124.67 | 0.58 | 0.27540 |
| 30 | 0.7 | 24,000 | 15 | 32.00 | 0.00 | −44.37 | 0.15 | 117.67 | 3.21 | −0.40416 |
| 31 | 1.0 | 16,000 | 10 | 23.83 | 0.30 | −51.70 | 0.50 | 143.67 | 0.58 | 1.25732 |
| 32 | 1.0 | 16,000 | 15 | 18.73 | 0.00 | −46.93 | 1.90 | 117.33 | 3.06 | −0.44696 |
| 33 | 1.0 | 20,000 | 10 | 25.27 | 0.29 | −49.13 | 0.78 | 110.67 | 0.58 | −1.84872 |
| 34 | 1.0 | 20,000 | 15 | 19.83 | 0.85 | −46.33 | 0.87 | 112.50 | 0.50 | −1.29876 |
| 35 | 1.0 | 24,000 | 10 | 25.31 | 0.00 | −51.70 | 0.36 | 116.00 | 0.00 | −0.63329 |
| 36 | 1.0 | 24,000 | 15 | 39.77 | 0.88 | −47.07 | 0.71 | 117.33 | 2.31 | −0.44696 |
| CHON Concentration (mg/mL) | Stirring Rate (rpm) | Reaction Time (min) | EE % | Pot Z (mV) | Size (nm) | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| 0.4 | 20,000 | 10 | 61.29 | 2.05 | −34.32 | 5.35 | 117.80 | 2.77 |
| Sample | CHON Cumulative Permeate Amount (mg) | Percentage Permeates of CHON (%) | Median and Range (%) |
|---|---|---|---|
| Control (CHON 0.4 mg/mL in aqueous solution) | 0.0060 | 1.47 | 2.44 (1.47–3.41) |
| 0.0140 | 3.41 | ||
| 0.0070 | 1.82 | ||
| 0.0120 | 3.06 | ||
| 0.0070 | 1.66 | ||
| 0.0130 | 3.22 | ||
| SLN | 0.2119 | 86.98 | 77.03 (58.4–86.98) |
| 0.1968 | 84.96 | ||
| 0.1784 | 77.03 | ||
| 0.1353 | 58.40 | ||
| 0.1661 | 71.70 |
| Sample | Amount Retained in Skin (Qr, µg/g/cm2) | Median and Range |
|---|---|---|
| Control (CHON 0.4 mg/mL in aqueous solution) | 4.51 | 6.67 (4.49–8.83) |
| 8.83 | ||
| 4.49 | ||
| 8.82 | ||
| SLN-1 | 173.20 | 499.80 (173.20–567.01) |
| 435.46 | ||
| 567.01 | ||
| 564.05 |
| Factors | Levels |
|---|---|
| Concentration (mg/mL) | 0.4–0.7–1.0 |
| Stirring rate (rpm) | 16,000–20,000–24,000 |
| Reaction time (min) | 10–15 |
| Experiment | Concentration (mg/mL) | Stirring Speed (rpm) | Reaction Time (min) |
|---|---|---|---|
| 1 | 0.4 | 16,000 | 10 |
| 2 | 0.4 | 16,000 | 15 |
| 3 | 0.4 | 20,000 | 10 |
| 4 | 0.4 | 20,000 | 15 |
| 5 | 0.4 | 24,000 | 10 |
| 6 | 0.4 | 24,000 | 15 |
| 7 | 0.7 | 16,000 | 10 |
| 8 | 0.7 | 16,000 | 15 |
| 9 | 0.7 | 20,000 | 10 |
| 10 | 0.7 | 20,000 | 15 |
| 11 | 0.7 | 24,000 | 10 |
| 12 | 0.7 | 24,000 | 15 |
| 13 | 1.0 | 16,000 | 10 |
| 14 | 1.0 | 16,000 | 15 |
| 15 | 1.0 | 20,000 | 10 |
| 16 | 1.0 | 20,000 | 15 |
| 17 | 1.0 | 24,000 | 10 |
| 18 | 1.0 | 24,000 | 15 |
| Property | Criteria |
|---|---|
| Entrapment efficiency of CHON (EE%) | Highest % EE possible |
| Zeta potential (mV) | <−25 and >25 |
| Particle size (nm) | Minor size possible |
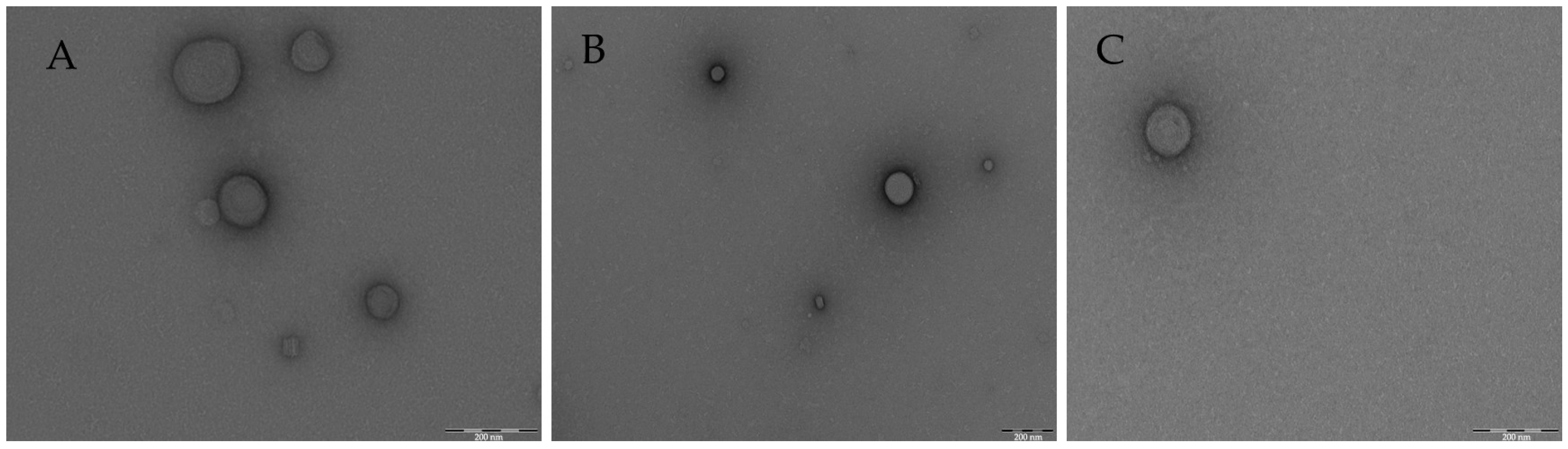
| Morphology | TEM technique to characterize SLN |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustos Araya, M.E.; Nardi-Ricart, A.; Calpena Capmany, A.C.; Miñarro Carmona, M. Chondroitin Sulfate for Cartilage Regeneration, Administered Topically Using a Nanostructured Formulation. Int. J. Mol. Sci. 2024, 25, 10023. https://doi.org/10.3390/ijms251810023
Bustos Araya ME, Nardi-Ricart A, Calpena Capmany AC, Miñarro Carmona M. Chondroitin Sulfate for Cartilage Regeneration, Administered Topically Using a Nanostructured Formulation. International Journal of Molecular Sciences. 2024; 25(18):10023. https://doi.org/10.3390/ijms251810023
Chicago/Turabian StyleBustos Araya, Marta E., Anna Nardi-Ricart, Ana C. Calpena Capmany, and Montserrat Miñarro Carmona. 2024. "Chondroitin Sulfate for Cartilage Regeneration, Administered Topically Using a Nanostructured Formulation" International Journal of Molecular Sciences 25, no. 18: 10023. https://doi.org/10.3390/ijms251810023
APA StyleBustos Araya, M. E., Nardi-Ricart, A., Calpena Capmany, A. C., & Miñarro Carmona, M. (2024). Chondroitin Sulfate for Cartilage Regeneration, Administered Topically Using a Nanostructured Formulation. International Journal of Molecular Sciences, 25(18), 10023. https://doi.org/10.3390/ijms251810023








