Mobilization of Endogenous CD34+/CD133+ Endothelial Progenitor Cells by Enhanced External Counter Pulsation for Treatment of Refractory Angina
Abstract
1. Introduction
2. Results
2.1. Subject Characteristics
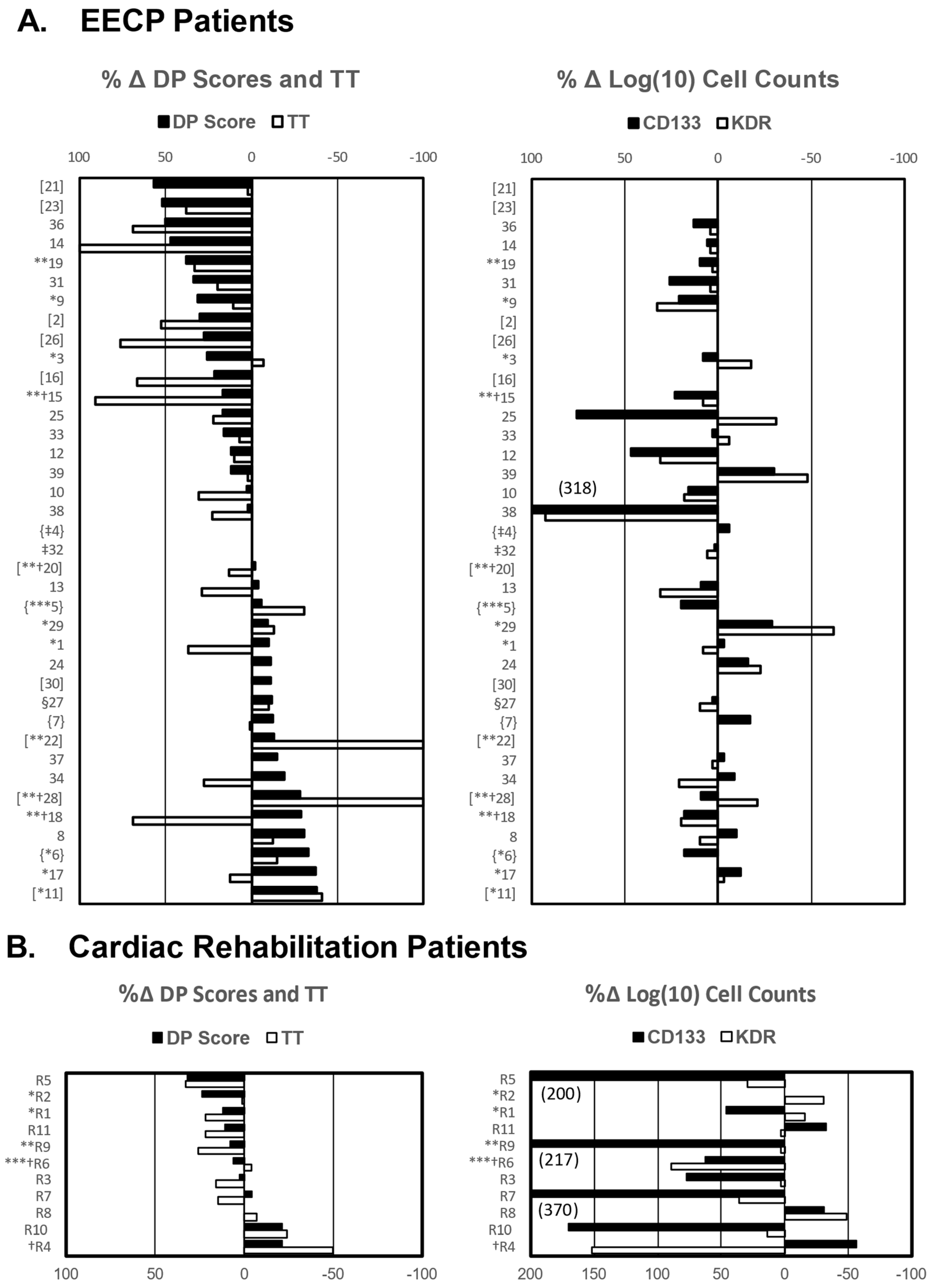
2.2. EECP-Induced Improvement in Exercise Capacity Is Associated with Mobilization of CD34+/CD133+ Stem Cells
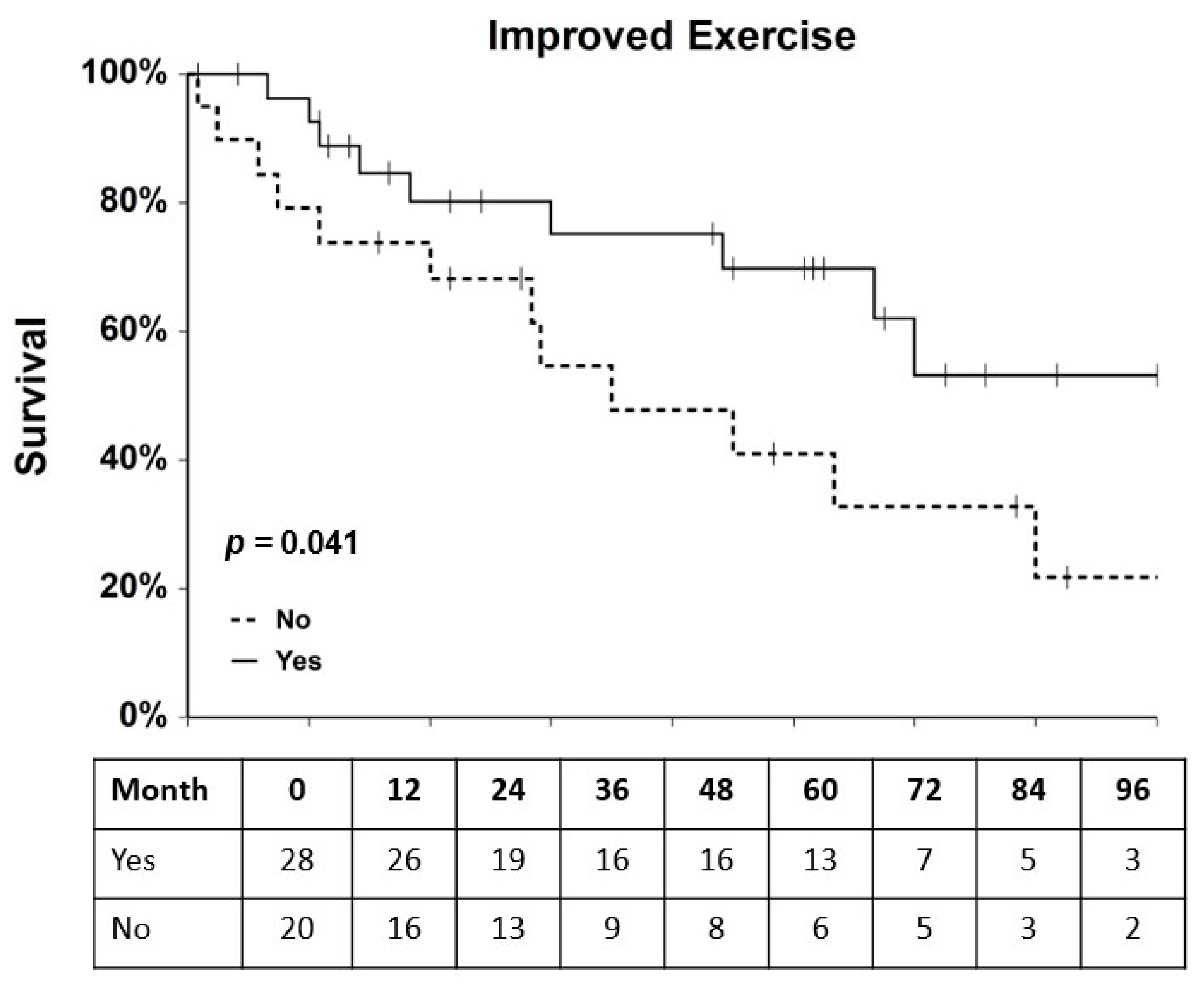
2.3. Association of Clinical and Experimental Variables with MACE Hazard
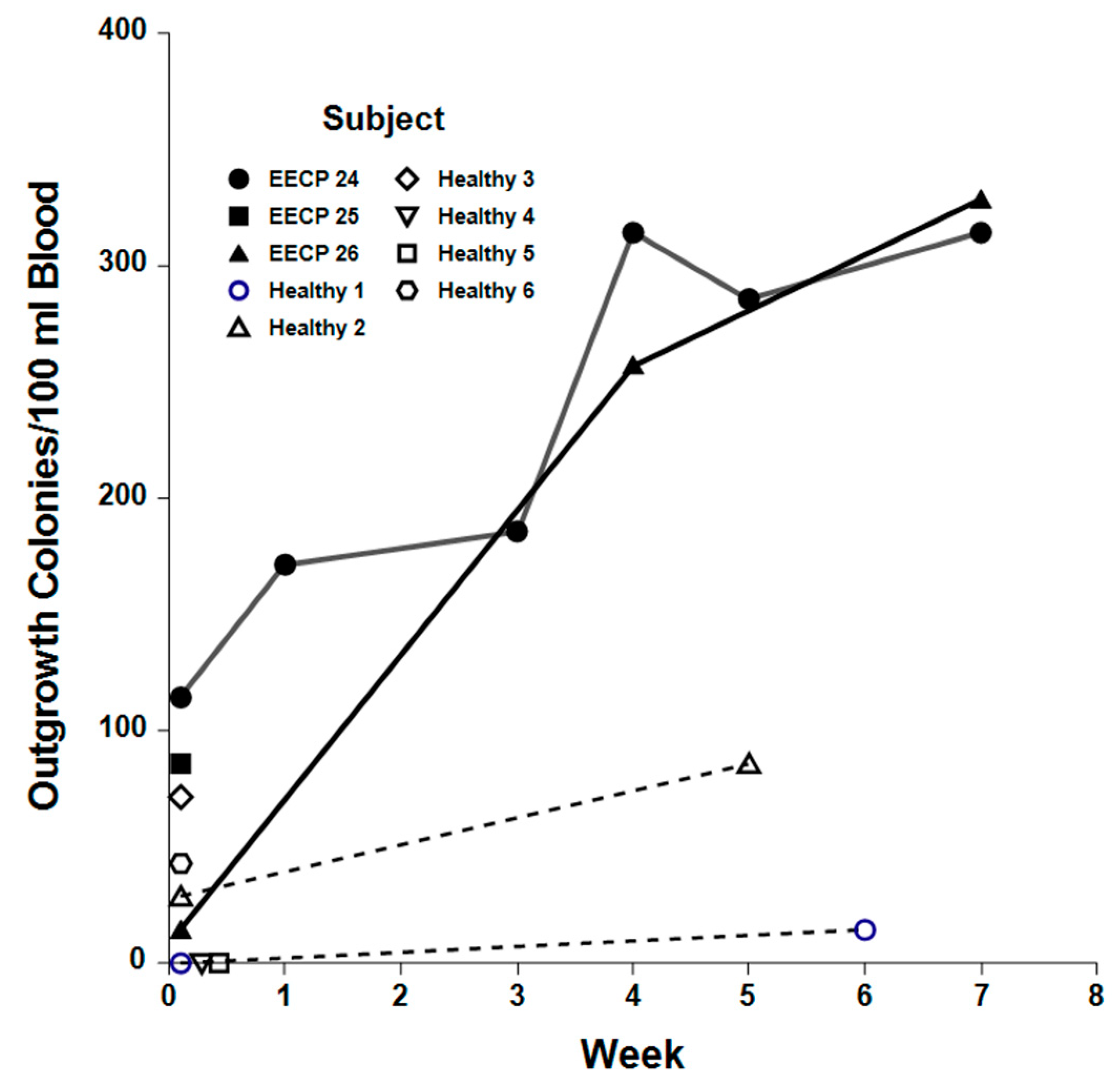
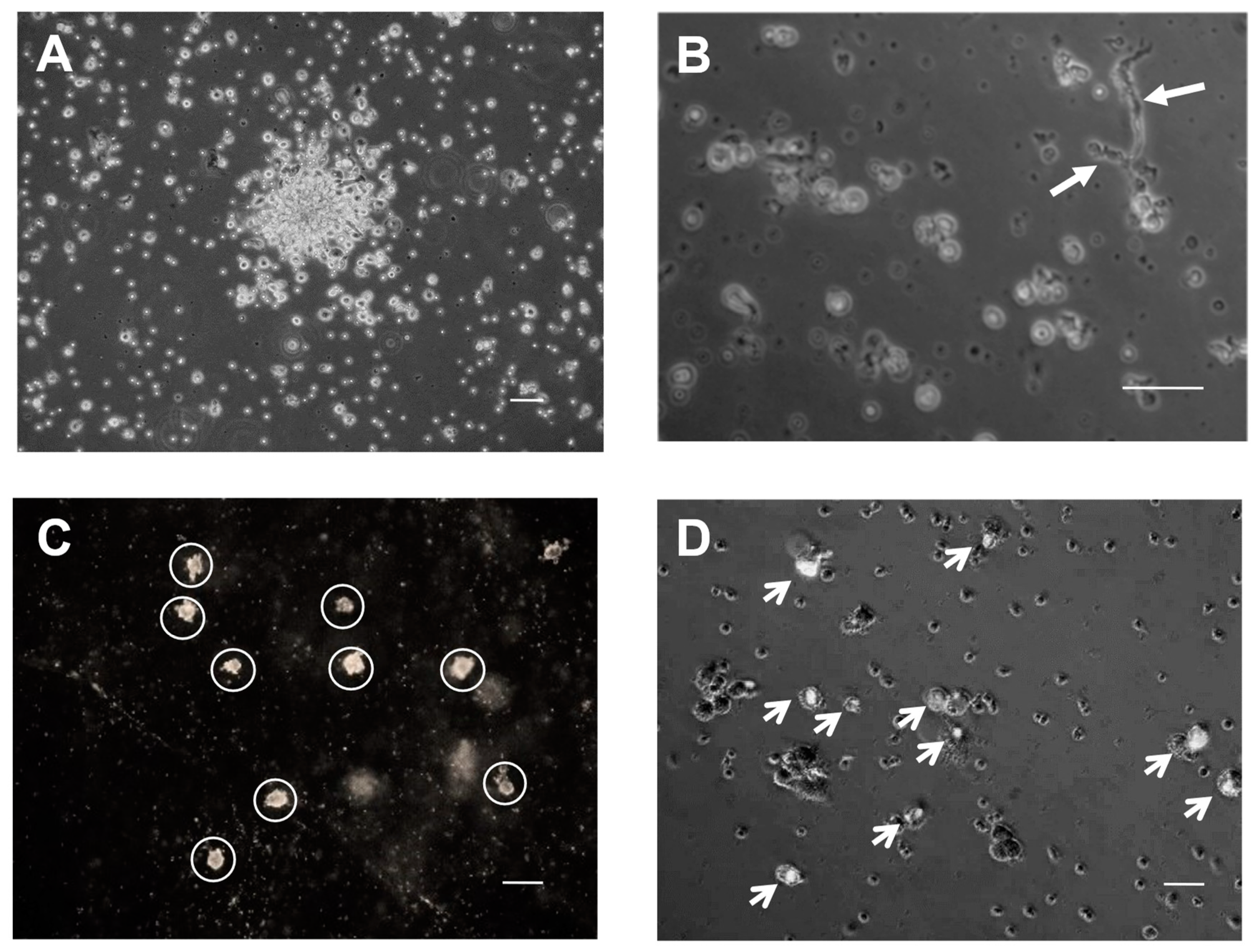
2.4. Clonogenic Potential of EPCs
3. Discussion
4. Materials and Methods
4.1. Subject Characteristics
4.2. EECP Treatments
4.3. Cardiac Rehabilitation Therapy
4.4. Treadmill Exercise Stress Tests
4.5. Multicolor Flow Cytometry
4.6. In Vitro Clonogenic Potential of EPCs
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Sainsbury, P.; Fisher, M.; de Silva, R. Management of refractory angina pectoris. Eur. Cardiol. Rev. 2016, 11, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Salybekov, A.A.; Kobayashi, S.; Asahara, T. CD34 positive cells as endothelial progenitor cells in biology and medicine. Front. Cell Dev. Biol. 2023, 11, 1128134. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.D.; Losordo, D.W.; Traverse, J.H.; Schatz, R.A.; Jolicoeur, E.M.; Schaer, G.L.; Clare, R.; Chiswell, K.; White, C.J.; Fortuin, F.D.; et al. Autologous CD34+ cell therapy improves exercise capacity, angina frequency and reduces mortality in no-option refractory angina: A patient-level pooled analysis of randomized double-blinded trials. Eur. Heart J. 2018, 39, 2208–2216. [Google Scholar] [CrossRef]
- Sainsbury, P.A.; Fisher, M.; de Silva, R. Alternative interventions for refractory angina. Heart 2017, 103, 1911–1922. [Google Scholar] [CrossRef]
- Arora, R.R.; Chou, T.M.; Jain, D.; Fleishman, B.; Crawford, L.; McKiernan, T.; Nesto, R.W. The multicenter study of enhanced external counterpulsation (MUST-EECP. J. Am. Coll. Cardiol. 1999, 33, 1833–1840. [Google Scholar] [CrossRef]
- Fraker, T.D., Jr.; Fihn, S.D.; Gibbons, R.J.; Abrams, J.; Chatterjee, K.; Daley, J.; Deedwania, P.C.; Douglas, J.S.; Ferguson, T.B., Jr.; Gardin, J.M.; et al. Chronic angina focused update of the ACC/AHA 2002 guidelines for the management of patients with chronic stable angina: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to develop the focused update of the 2002 guidelines for the management of patients with chronic stable angina. J. Am. Coll. Cardiol. 2007, 50, 2264–2274. [Google Scholar]
- Michaels, A.D.; McCullough, P.A.; Soran, O.Z.; Lawson, W.E.; Barsness, G.W.; Henry, T.D.; Linnemeier, G.; Ochoa, A.; Kelsey, S.F.; Kennard, E.D. Primer: Practical approach to the selection of patients for and application of EECP. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 623–632. [Google Scholar] [CrossRef]
- Lawson, W.E.; Hui, J.C.; Lang, G. Treatment benefit in the enhanced external counterpulsation consortium. Cardiology 2000, 94, 31–35. [Google Scholar] [CrossRef]
- McKenna, C.; Hawkins, N.; Claxton, K.; McDaid, C.; Suekarran, S.; Light, K.; Chester, M.; Cleland, J.G.; Woolacott, N.; Sculpher, M. Cost-effectiveness of enhanced external counterpulsation (EECP) for the treatment of stable angina in the United Kingdom. Int. J. Technol. Assess. Health Care 2010, 26, 175–182. [Google Scholar] [CrossRef]
- Lawson, W.; Hui, J.C.; Cohen, P. Long-term prognosis of patients with angina treated with enhanced external counterpulsation: Five-year follow-up study. Clin. Cardiol. 2000, 23, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Caplice, N.M.; Yoder, M.C. Unresolved questions, changing definitions, and novel paradims for defining endothelial progenitor cells. Blood 2005, 106, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Rafii, S.; Wu, M.H. Evidence for circulating bone marrow-derived endothelial cells. Blood 1998, 92, 362–367. [Google Scholar] [CrossRef]
- Lawson, W.E.; Hui, J.C.; Zheng, Z.S.; Burger, L.; Jiang, L.; Lillis, O.; Soroff, H.S.; Cohn, P.F. Can angiographic findings predict which coronary patients will benefit from enhanced external counterpulsation? Am. J. Cardiol. 1996, 77, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Barsheshet, A.; Hod, H.; Shechter, M.; Sharabani-Yosef, O.; Rosenthal, E.; Barbarsh, I.M.; Matetzky, S.; Tal, R.; Bentancur, A.G.; Sela, B.A.; et al. The effects of external counterpulsation therapy on circulating endothelial progenitor cells in patients with angina pectoris. Circulation 2008, 110, 160–166. [Google Scholar]
- Kiernan, T.J.; Boilson, B.A.; Tesmer, L.; Harbuzariu, A.; Simari, R.D.; Barsness, G.W. Effect of enhanced external counterpulsation on circulating CD34+ progenitor cell subsets. Int. J. Cardiol. 2011, 153, 202–206. [Google Scholar] [CrossRef]
- Murohara, T.; Witzenbichler, B.; Spyridopoulos, I.; Asahara, T.; Ding, B.; Sullivan, A.; Losordo, D.; Isner, J. Role of endothelial nitric oxide synthase in endothelial cell migration. Arter. Thromb. Vasc. Biol. 1999, 19, 1156–1161. [Google Scholar] [CrossRef]
- Masuda, D.; Nohara, R.; Kataoka, K. Enhanced external counterpulsation promotes angiogenesis factors in patients with chronic stable angina. Circulation 2001, 104 (Suppl. II), 444. [Google Scholar]
- Li, S.; Huang, N.F.; Hsu, S. Mechanotransduction in endothelial cell migration. J. Cell. Biochem. 2005, 96, 1110–1126. [Google Scholar] [CrossRef]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Chen, X.; Ma, H.; Liu, D.; Luo, J.; Du, Z.; Jin, Y.; Xiong, Y.; He, J.; et al. Enhanced counterpulsation inhibits intimal hyperplasia by modifying shear stress responsive gene expression in hypercholesterolemic pigs. Circulation 2007, 116, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.Y.; Wu, G.E.; Xiong, Y.; Chen, G.W.; Xie, Q.; Yang, D.Y.; He, X.H.; Zhang, Y.; Liu, D.H.; Wang, K.J.; et al. Enhanced external counterpulsation promotes growth cytokines-mediated myocardial angiogenesis in a porcine model of hypercholesterolemia. Chin. Med. J. 2009, 122, 1188–1194. [Google Scholar] [PubMed]
- Gloekler, S.; Meier, P.; de Marchi, S.F.; Rutz, T.; Traupe, T.; Rimoldi, S.F.; Wustmann, K.; Steck, H.; Cook, S.; Vogel, R.; et al. Coronary collateral growth by external counterpulsation: A randomized controlled trial. Heart 2010, 96, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Lawson, W.E.; Hui, J.C.K.; Zheng, Z.S.; Burger, L.; Jiang, L.; Lillis, O.; Osier, Z.; Soroff, H.; Cohn, P.F. Improved exercise tolerance following enhanced external counterpulsation:cardiac or peripheral effect? Cardiology 1996, 87, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, J.; Stenerson, J.; Charney, R.; Ramasamy, S.; Fleishman, B.L.; Gerardi, P.; Hui, J.C.K. Exercise capability and myocardial perfusion in chronic angina patients treated with enhanced external counterpulsation. Clin. Cardiol. 2003, 26, 287–290. [Google Scholar] [CrossRef]
- Guerin, C.L.; Guyonnet, L.; Goudot, G.; Revets, D.; Konstantinou, M.; Chipont, A.; Chocron, R.; Blandinieres, A.; Khider, L.; Rancic, J.; et al. Multidimensional Proteomic Approach of Endothelial Progenitors Demonstrate Expression of KDR Restricted to CD19 Cells. Stem Cell Rev. Rep. 2021, 17, 639–651. [Google Scholar] [CrossRef]
- Pillarisetti, K.; Gupta, S.K. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation 2001, 25, 293–300. [Google Scholar] [CrossRef]
- Shantsila, E.; Watson, T.; Tse, H.; Lip, G. New insights on endothelial progenitor cell subpopulations and their angiogenic properties. J. Am. Coll. Cardiol. 2008, 51, 669–671. [Google Scholar] [CrossRef]
- Pourmoghadas, M.; Nourmohamamadi, H.; Tabesh, F.; Haghjoo, S.; Tabesh, E. Effect of enhanced external counterpulsation on plasma level of nitric oxide and vascular endothelial growth factor. Atheroscler J. 2009, 5, 59–63. [Google Scholar]
- Lee, S.H.; Wolf, P.L.; Escudero, R. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef]
- Stys, T.; Lawson, W.; Hui, J.C.K.; Fleishman, B.; Manzo, K.; Strobeck, J.E.; Tartaglia, J.; Ramasamy, S.; Suwita, R.; Zheng, Z.S.; et al. Effects of enhanced counterpulsation on stress radionuclide coronary perfusion and exercise capacity in chronic stable angina pectoris. Am. J. Cardiol. 2002, 89, 822–824. [Google Scholar] [CrossRef] [PubMed]
- Scapin, G.; Cillis, J.; Patch, T.; Dharampuriya, P.R.; Hagedorn, E.J.; Lundin, V.; Tamplin, O.J.; Andeson, H.; Zon, L.I.; Daley, G.Q.; et al. Piezo1-senstivie biomechanical pulsation stimulates long-term hematopoietic stem cell formation. Blood 2018, 132 (Suppl. S1). [Google Scholar] [CrossRef]
- Pathak, M.M.; Nourse, J.L.; Tran, T.; Hwe, J.; Arulmoli, J.; Dai Trang, T.L.; Bernardis, E.; Flanagan, L.A.; Tombola, F. Stretch-activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc. Natl. Am. Soc. USA 2014, 111, 16148–16153. [Google Scholar] [CrossRef] [PubMed]
- Challen, G.A.; Sun, D.; Mayle, A.; Jeong, M.; Luo, M.; Rodriguez, B.; Mallaney, C.; Celik, H.; Yang, L.; Xia, Z.; et al. Dnmt3a and Dnmt3b have overlapping and distinct functions in hematopoietic stem cells. Cell 2014, 15, 350–364. [Google Scholar] [CrossRef]
- Mansour, S.; Roy, D.C.; Bouchard, V.; Stevens, L.M.; Gobeil, F.; Rivard, A.; Leclerc, G.; Reeves, F.; Noiseux, N. One-Year Safety Analysis of the COMPARE-AMI Trial: Comparison of Intracoronary Injection of CD133+ Bone Marrow Stem Cells to Placebo in Patients after Acute Myocardial Infarction and Left Ventricular Dysfunction. Bone Marrow Res. 2011, 2011, 385124. [Google Scholar] [CrossRef] [PubMed]
- Losordo, D.W.; Henry, T.D.; Davidson, C.; Sup Lee, J.; Costa, M.A.; Bass, T.; Mendelsohn, F.; Fortuin, F.D.; Pepine, C.J.; Traverse, J.H.; et al. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ. Res. 2011, 109, 428–436. [Google Scholar] [CrossRef]
- May, O.; Lynggaard, V.; Mortensen, J.C.; Malczynski, J. Enhanced external counterpulsation—Effect on angina pectoris, QoL and exercise capacity after 1 year. Scand. Cardiovasc. J. 2015, 49, 1–6. [Google Scholar] [CrossRef]
- Losordo, D.W.; Schatz, R.A.; White, C.J.; Udelson, J.E.; Veereshwarayya, V.; Durgin, M.; Poh, K.K.; Weinstein, R.; Kearney, M.; Chaudhry, M.; et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: A phase I/IIa double-blind, randomized controlled trial. Circulation 2007, 115, 3165–3172. [Google Scholar] [CrossRef]
- Povsic, T.J.; Henry, T.D.; Traverse, J.H.; Fortuin, F.D.; Schaer, G.L.; Kereiakes, D.J.; Schatz, R.A.; Zeiher, A.M.; White, C.J.; Stewart, D.J.; et al. RENEW Investigators. The RENEW trial: Efficacy and safety of intramyocardial autologous CD34(+) cell administration in patients with refractory angina. JACC Cardiovasc. Interv. 2016, 9, 1576–1585. [Google Scholar] [CrossRef]
- Povsic, T.J.; Broderick, S.; Anstrom, K.J.; Shaw, L.J.; Ohman, E.M.; Eisenstein, E.L.; Smith, P.K.; Alexander, J.H. Predictors of long-term clinical endpoints in patients with refractory angina. J. Am. Heart Assoc. 2015, 4, e001287. [Google Scholar] [CrossRef]
- Gordon, P.R.; Leimig, T.; Babarin-Dorner, A.; Houston, J.; Holladay, M.; Mueller, I.; Geiger, T.; Handgretinger, R. Large-scale isolation of CD133+ progenitor cells from G-CSF mobilized peripheral blood stem cells. Bone Marrow Transpl. 2003, 31, 17–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lawson, W.E.; Hui, J.C.; Kennard, E.D.; Linnemeier, G.; IEPR-II Investigators. Enhanced External Counterpulsation is cost-effective in reducing hospital costs in refractory angina patients. Clin. Cardiol. 2015, 38, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Zalos, G.; Halcox, J.P.J.; Schenke, W.H.; Quyyumi, A.A.; Finkel, T. Circulation endothelial progenitor cell, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Dye, J.F.; Caiado, F.; Di, Y.; Dunn, S.; Edwards, F.C.; Clayton, E.A.; Dias, S.J. Could fibrin-E from degradation of fibrin based scaffolds recruit EPC? Eur. Cells Mater. 2008, 16 (Suppl. S3), 50. [Google Scholar]
- Eisenberg, L.M.; Kaur, K.; Castillo, J.M.; Edwars, J.G.; Eisenberg, C.A. Dexamthasone treatment preserves the structure of adult cardiac explants and supports their long-term contractility in vitro. Int. J. Tansl. Med. 2023, 3, 360–373. [Google Scholar] [CrossRef]


| Therapy | Circulating Cell Population (Counts/100,000 CD45+ Mononuclear Cells) | |||||
|---|---|---|---|---|---|---|
| CD45+/CD34+ | CD34+/CD133+ | CD34+/KDR+ | ||||
| Start | Finish | Start | Finish | Start | Finish | |
| EECP | 2817 ± 1513 | 2544 ± 1356 | 50 ± 9 | 81 ± 16 | 98 ± 26 | 136 ± 33 |
| Cardiac Rehab | 113.3 ± 1.7 | 112.8 ± 13.2 | 5.6 ± 6.6 | 8.2 ± 6.4 | 8.3 ± 2.2 | 8.1 ± 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tartaglia, J.T.; Eisenberg, C.A.; DeMarco, J.C.; Puccio, G.; Tartaglia, C.E.; Hamby, C.V. Mobilization of Endogenous CD34+/CD133+ Endothelial Progenitor Cells by Enhanced External Counter Pulsation for Treatment of Refractory Angina. Int. J. Mol. Sci. 2024, 25, 10030. https://doi.org/10.3390/ijms251810030
Tartaglia JT, Eisenberg CA, DeMarco JC, Puccio G, Tartaglia CE, Hamby CV. Mobilization of Endogenous CD34+/CD133+ Endothelial Progenitor Cells by Enhanced External Counter Pulsation for Treatment of Refractory Angina. International Journal of Molecular Sciences. 2024; 25(18):10030. https://doi.org/10.3390/ijms251810030
Chicago/Turabian StyleTartaglia, Joseph T., Carol A. Eisenberg, Joseph C. DeMarco, Gregory Puccio, Christina E. Tartaglia, and Carl V. Hamby. 2024. "Mobilization of Endogenous CD34+/CD133+ Endothelial Progenitor Cells by Enhanced External Counter Pulsation for Treatment of Refractory Angina" International Journal of Molecular Sciences 25, no. 18: 10030. https://doi.org/10.3390/ijms251810030
APA StyleTartaglia, J. T., Eisenberg, C. A., DeMarco, J. C., Puccio, G., Tartaglia, C. E., & Hamby, C. V. (2024). Mobilization of Endogenous CD34+/CD133+ Endothelial Progenitor Cells by Enhanced External Counter Pulsation for Treatment of Refractory Angina. International Journal of Molecular Sciences, 25(18), 10030. https://doi.org/10.3390/ijms251810030






