The Mechanism of Zinc Oxide in Alleviating Diarrhea in Piglets after Weaning: A Review from the Perspective of Intestinal Barrier Function
Abstract
1. Introduction
2. Important Role of Zinc in Piglet Health
3. Possible Mechanism of ZnO to Prevent Diarrhea in Piglets
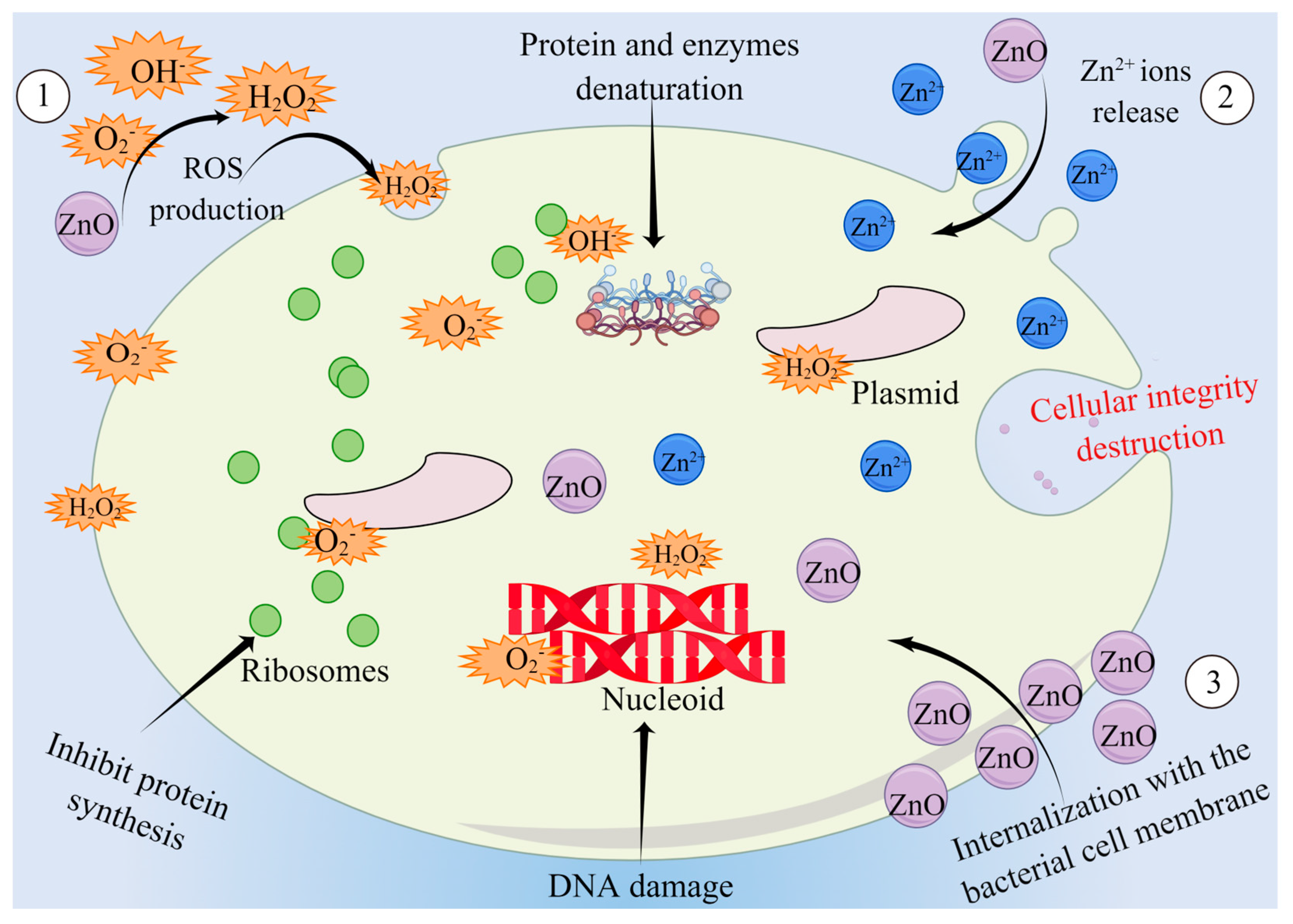
3.1. Antibacterial Effect of ZnO
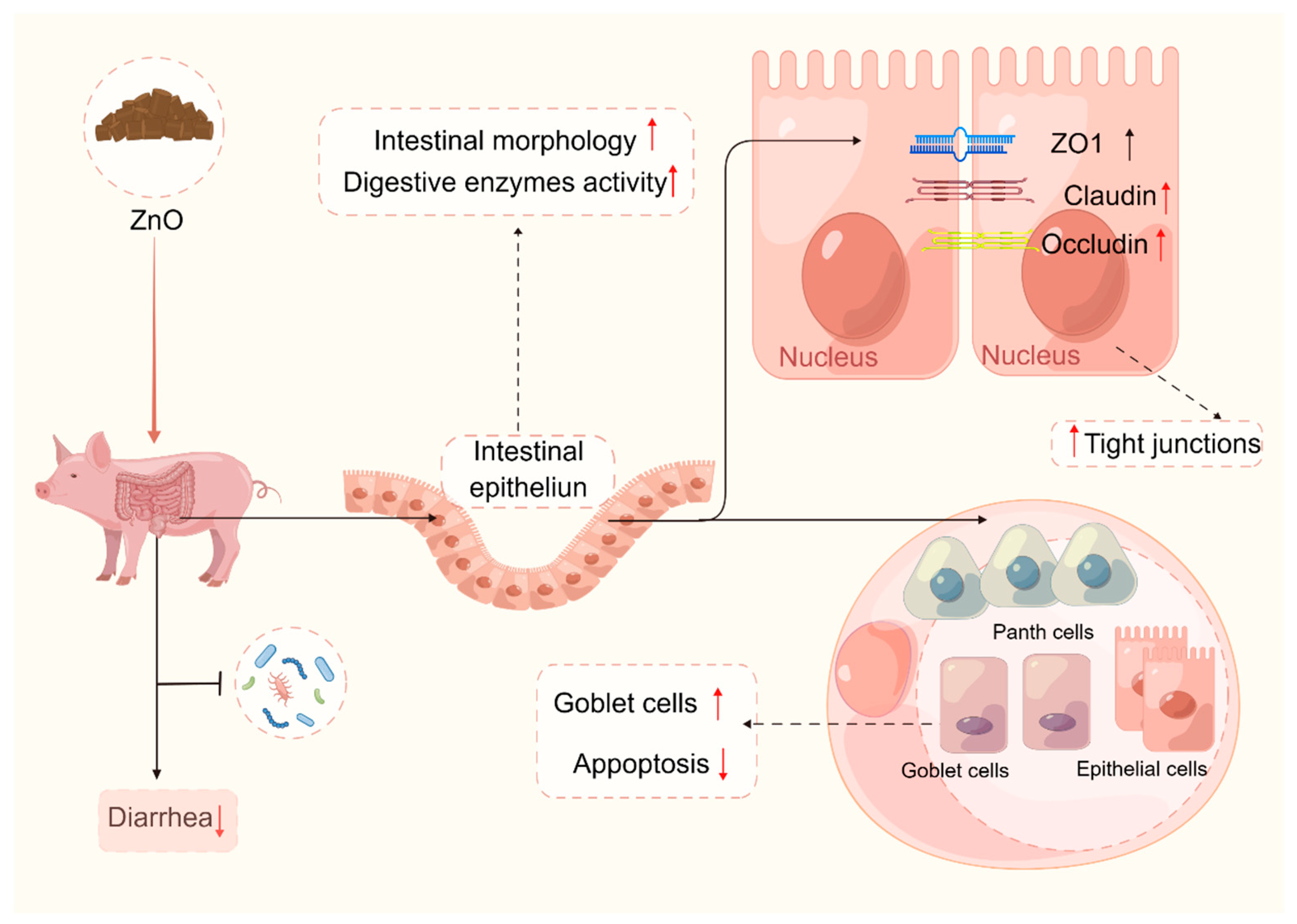
3.2. ZnO and Intestinal Physical Barrier of Piglets
- (i)
- Pharmacological doses of ZnO (about 3000 mg/kg) can promote intestinal development in piglets, resulting in increased villus height (VH), decreased crypt depth (CD), and increased villus height/crypt depth (VCR) [33,40,111,112,113]. Intestinal morphology is an intuitive reaction to intestinal function. Weaning may lead to intestinal mucosal atrophy and affect intestinal absorption function [114], while pharmacological doses of ZnO can restore intestinal mucosal atrophy caused by weaning stress. For example, Pei et al. [115] showed that dietary ZnO (3000 mg/kg) supplementation could improve the duodenum and jejunum VCR of weaned piglets; Yi et al. [110] showed that adding 1500 mg/kg of ZnO could increase the ileum VH of weaned piglets.
- (ii)
- Pharmacological doses of ZnO can enhance the quantity of goblet cells in the small intestine of piglets [116,117,118], and also enhance the activities of intestinal digestive enzymes in piglets [119,120,121]. Goblet cells are secretory cells that secrete mucins and are important for animal gut health. It has been observed that weaning stress hinders the proliferation and differentiation of goblet cells [122,123,124]. Nevertheless, the supplementation of ZnO in the diet has been found to notably enhance the population of goblet cells in the villus and crypt of the small intestine of weaned piglets [116,117,118]. Intestinal digestive enzymes play a crucial role in the development and digestive capacity of weaned piglets [3]. However, weaning triggers a notable reduction in intestinal digestive enzyme activities, which is considered a significant contributor to the incidence of diarrhea in weaned piglets [125,126]. ZnO could enhance the digestion and absorption of nutrients in piglets by promoting the activities of intestinal digestive enzymes. It has been demonstrated that dietary supplementation with ZnO at an effective dose of 2500 mg/kg increased the chymotrypsin activity in the small intestinal contents of weaned piglets [119]. A study by Hu et al. [120] found that a 2000 mg/kg supplementation increased intestinal protease, lipase, and amylase activities in weaned piglets. Liu et al. [121] showed that piglets fed high doses of ZnO had a higher lipase activity in the jejunum, as well as a higher lipase and trypsin activity in the ileum than piglets fed non-ZnO diets.
- (iii)
- Pharmacological doses of ZnO can up-regulate the expression of proteins associated with the intestinal physical barrier function of the intestine, such as zonula occludens-1 (ZO-1), Claudin 1, and occludin, while simultaneously reducing intestinal permeability in piglets [38,53,110,127,128,129]. The levels of mRNA and protein expression of TJ proteins (ZO-1, occludin, and Claudin 1), and the concentrations of diamine oxidase (DAO) and D-lactic acid in the blood, serve as reliable indicators of the extent of the physical barrier function. Previous research has demonstrated that the intestinal physical barrier function of piglets may be impaired during the weaning stage, as evidenced by the disordered distribution of TJ structures and increased intestinal permeability [7,124,130]. The dietary addition of ZnO could reduce the content of DAO and D-lactic acid in the blood, up-regulate TJ protein (ZO-1 Claudin 1, occludin) expression, and reduce intestinal permeability to alleviate diarrhea in piglets to a certain degree [38,53,110,112,127,131]. Therefore, the administration of a pharmacological dosage of ZnO has the potential to enhance the integrity of the intestinal physical barrier in weaned piglets. This is achieved through improvements in intestinal morphology, increased intestinal digestive enzyme activity, and the up regulation of the expression of intestinal TJ proteins. Consequently, this intervention effectively mitigates the risk of pathogenic microorganism invasion and successfully alleviates episodes of diarrhea (Figure 2).
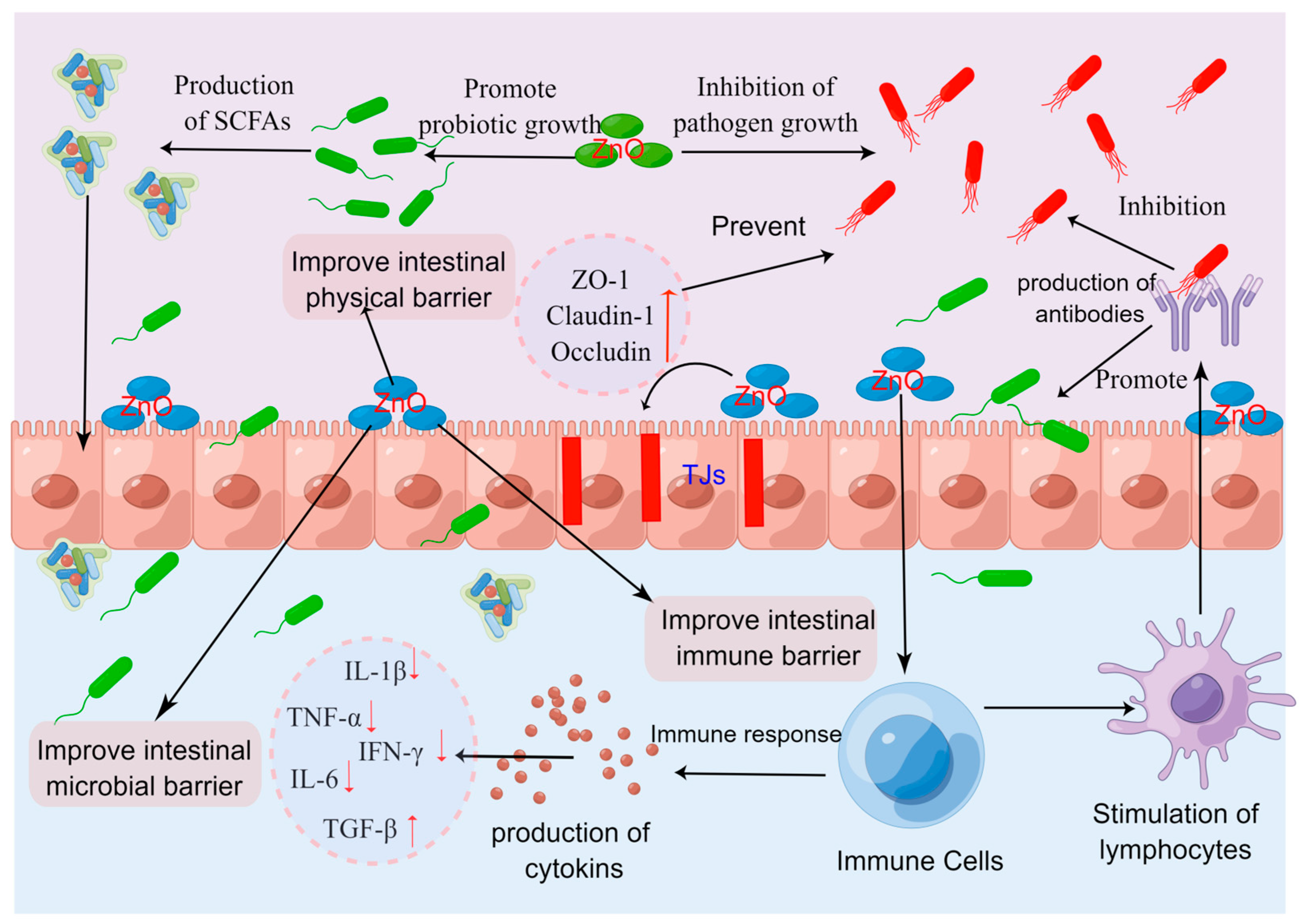
3.3. ZnO and Intestinal Immune Barrier of Piglets
- (i)
- ZnO may promote the intestinal innate immunity and adaptive immunity of piglets. In their study, Kloubert et al. [139] conducted an analysis of the impact of ZnO on the innate and adaptive immune cells of weaned piglets. The findings revealed that the inclusion of 2500 mg/kg of ZnO in the diet resulted in enhanced innate immunity in pigs, as evidenced by heightened phagocytic activity and a slight increase in oxidative burst within the cells. Moreover, ZnO supplementation also demonstrated improvements in the adaptive immunity of piglets, characterized by an increase in T cells (CD3+) and Treg cells (CD3+CD4+Foxp3+) in the peripheral blood of porcine subjects. However, on the contrary, Oh et al. [137] conducted a study which demonstrated that the introduction of dietary supplementation containing 2500 mg/kg of ZnO resulted in a slight reduction in CD4+ T cell subsets (specifically T-bet+, RORgt+, GATA3+, FoxP3+ T cells) within the gut lymph node of piglets. Conversely, the low dose of chelated ZnO (200 mg/kg) group exhibited an increased number of T-bet+CD4+T cells (Th1) and FoxP3+CD4+ T cells (Treg), while, the low dose of nano-ZnO group (200 mg/kg) displayed elevated levels of GATA3+CD4+T cells (Th2), and RORγt+CD4+ (Th17) T cells compared to the high dose of ZnO group. These findings suggest that the immunomodulatory effects of ZnO may vary depending on the form and dosage, with lower doses of chelated or nano-ZnO potentially enhancing specific T cell subsets more effectively than higher doses of standard ZnO.
- (ii)
- High doses of ZnO may enhance intestinal health by increasing the secretion of immunoglobulin A (sIgA), a critical component of the mucosal immune response that helps neutralize pathogens and prevent their adhesion to the intestinal epithelium [132]. For example, Han et al. [140] demonstrated an increase in sIgA concentration in the ileal mucus of the dietary group that received a dosage of 3000 mg/kg of ZnO. Similarly, Shen et al. [141] found that dietary supplementation with 2250 mg/kg of ZnO resulted in an improvement in sIgA concentration in the jejunal mucosa of piglets. These findings collectively suggest that high doses of ZnO have the potential to enhance intestinal health by stimulating the secretion of sIgA in the intestinal mucosa.
- (iii)
- ZnO may enhance the function of the intestinal immune barrier by suppressing the expression of pro-inflammatory cytokines, such as interferon γ (IFN-γ), IL-1β, IL-6, and tumor necrosis factor (TNF-α) [33,45,142]. Weaning stress usually induces intestinal immune cells to secrete IFN-γ, IL-1β, IL-6, and TNF-α, which ultimately mediate local inflammatory response [134,135]. Using transcriptomic technology, Sargeant et al. [143] found a suppression of gene expression linked to inflammatory response in the small intestinal epithelial tissue of piglets treated with ZnO at high doses (3100 mg/kg). According to Zhu et al. [33], pharmacological doses of ZnO (3000 mg/kg) decreased the expression of genes associated with inflammation (IL-1β, and IFN-γ), and increased the expression of genes associated with anti-inflammation [transforming growth factor-β (TGF-β)] in the jejunum mucosa of piglets. Additionally, it has been demonstrated in certain studies that by inhibiting the toll-like receptor 4-myeloid differentiation factor 88 (TLR4-MyD88) signaling pathway, pharmacological doses of ZnO (2200 mg/kg) can effectively reduce the expression of inflammation-related genes, namely, TNF-α and IFN-γ [142,144]. Moreover, ZnO also induces the activation of mitogen-activated protein kinase (MAPK)/extracellular regulated protein kinase 1/2 (ERK1/2), rather than the c-Jun N-terminal kinase (JNK) and p38 signaling pathways, resulting in an increased intestinal TGF-β1 expression [145,146]. As a result, this protective mechanism helps maintain the intestinal integrity of piglets. Consequently, pharmacological doses of ZnO help maintain the intestinal health of weaned piglets by strengthening the intestinal immune barrier, which in turn helps alleviate PWD (Figure 3).
3.4. ZnO and Intestinal Microbial Barrier of Piglets
4. Alternatives to ZnO
4.1. Zinc Oxide Nanoparticles
4.2. Organic Zinc
4.3. Coated Zinc Oxide
4.4. Porous Zinc Oxide
4.5. Other Forms of Zinc Oxide
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, Y.; Xu, T.; Qian, M.; Yang, Z.; Zhan, X.; Han, X. Early-life intervention using exogenous fecal microbiota alleviates gut injury and reduce inflammation caused by weaning stress in piglets. Front. Microbiol. 2021, 12, 671683. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. The impact of weaning stress on gut health and the mechanistic aspects of several feed additives contributing to improved gut health function in weanling piglets—A review. Animals 2021, 11, 2418. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, H.; Peng, Z.; Ge, Y.; Liu, J.; Wang, T.; Wang, H.; Dong, L. Early weaning affects live antioxidant function in piglets. Animals 2021, 11, 2679. [Google Scholar] [CrossRef] [PubMed]
- Dudink, S.; Simonse, H.; Marks, I.; de Jonge, F.H.; Spruijt, B.M. Announcing the arrival of enrichment increases play behaviour and reduces weaning-stress-induced behaviours of piglets directly after weaning. Appl. Anim. Behavi. Sci. 2006, 101, 86–101. [Google Scholar] [CrossRef]
- Smith, F.; Clark, J.E.; Overman, B.L.; Tozel, C.C.; Huang, J.H.; Rivier, J.E.; Blikslager, A.T.; Moeser, A.J. Early weaning stress impairs development of mucosal barrier function in the porcine intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G352–G363. [Google Scholar] [CrossRef]
- Peace, R.M.; Campbell, J.; Polo, J.; Crenshaw, J.; Russell, L.; Moeser, A. Spray-dried porcine plasma influences intestinal barrier function, inflammation, and diarrhea in weaned pigs. J. Nutr. 2011, 141, 1312–1317. [Google Scholar] [CrossRef]
- Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Pluske, J.R. Nutrition and pathology of weaner pigs: Nutritional strategies to support barrier function in the gastrointestinal tract. Anim. Feed Sci. Technol. 2012, 173, 3–16. [Google Scholar] [CrossRef]
- Su, W.; Gong, T.; Jiang, Z.; Lu, Z.; Wang, Y. The role of probiotics in alleviating postweaning diarrhea in piglets from the perspective of intestinal barriers. Front. Cell Infect. Microbiol. 2022, 12, 883107. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef]
- Van Kerschaver, C.; Turpin, D.; Michiels, J.; Pluske, J. Reducing Weaning Stress in Piglets by Pre-Weaning Socialization and Gradual Separation from the Sow: A Review. Animals 2023, 13, 1644. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Bin, P.; Tang, Z.; Liu, S.; Chen, S.; Xia, Y.; Liu, J.; Wu, H.; Zhu, G. Intestinal microbiota mediates Enterotoxigenic Escherichia coli-induced diarrhea in piglets. BMC Vet. Res. 2018, 14, 385. [Google Scholar] [CrossRef] [PubMed]
- Zhen, R.; Feng, J.; He, D.; Chen, Y.; Chen, T.; Cai, W.; Xiong, Y.; Qiu, Y.; Jiang, Z.; Wang, L.; et al. Effects of Niacin on Resistance to Enterotoxigenic Escherichia coli Infection in Weaned Piglets. Front. Nutr. 2022, 9, 865311. [Google Scholar] [CrossRef]
- Fairbrother, J.M.; Nadeau, E.; Gyles, C.L. Escherichia coli in postweaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef]
- Dubreuil, J.D. Enterotoxigenic Escherichia coli and probiotics in swine: What the bleep do we know? Biosci. Microbiota. Food Health 2017, 36, 75–90. [Google Scholar] [CrossRef]
- Pupa, P.; Apiwatsiri, P.; Sirichokchatchawan, W.; Pirarat, N.; Nedumpun, T.; Hampson, D.J.; Muangsin, N.; Prapasarakul, N. Microencapsulated probiotic Lactiplantibacillus plantarum and/or Pediococcus acidilactici strains ameliorate diarrhoea in piglets challenged with enterotoxigenic Escherichia coli. Sci. Rep. 2022, 12, 7210. [Google Scholar] [CrossRef]
- Diana, A.; Manzanilla, E.G.; Calderón Díaz, J.A.; Leonard, F.C.; Boyle, L.A. Do weaner pigs need in-feed antibiotics to ensure good health and welfare? PLoS ONE 2017, 12, e0185622. [Google Scholar] [CrossRef] [PubMed]
- Kyung-Hyo, D.; Jae-Won, B.; Wan-Kyu, L. Antimicrobial resistance profiles of Escherichia coli from diarrheic weaned piglets after the ban on antibiotic growth promoters in feed. Antibiotics 2020, 9, 755. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, Y.; Niu, K.; Liang, X.; Wang, H.; Shan, J.; Wu, X. Enteromorpha polysaccharide-zinc replacing prophylactic antibiotics contributes to improving gut health of weaned piglets. Anim. Nutr. 2021, 7, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Kim, J.C.; Hansen, C.F.; Mullan, B.P.; Hampson, D.J.; Pluske, J.R. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhoea and performance after weaning. Arch. Anim. Nutr. 2008, 62, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Lynegaard, J.C.; Kjeldsen, N.J.; Hansen, C.F.; Williams, A.R.; Nielsen, J.P.; Amdi, C. Reduction in diarrhoea and modulation of intestinal gene expression in pigs allocated a low protein diet without medicinal zinc oxide post-weaning. Animals 2022, 12, 989. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, R.; Faeti, V.; Gallo, M.; Pindo, M.; Bochicchio, D.; Buttazzoni, L.; Della Casa, G. Protein content in the diet influences growth and diarrhea in weaning piglets. Animals 2023, 13, 795. [Google Scholar] [CrossRef]
- Hayakawa, T.; Masuda, T.; Kurosawa, D.; Tsukahara, T. Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets. Anim. Sci. J. 2016, 87, 1501–1510. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Li, J.; Li, Y.; Wang, L.; Wang, Q.; Fang, L.; Ding, X.; Huang, P.; Yin, J.; et al. Protective effect of chicken egg yolk immunoglobulins (IgY) against enterotoxigenic Escherichia coli K88 adhesion in weaned piglets. BMC Vet. Res. 2019, 15, 234. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Xie, Y.; Chen, Y.; Yi, H.; He, D. Effects of antimicrobial peptide cathelicidin-BF on diarrhea controlling, immune responses, intestinal inflammation and intestinal barrier function in piglets with postweaning diarrhea. Int. Immunopharmacol. 2020, 85, 106658. [Google Scholar] [CrossRef]
- Xiang, X.D.; Deng, Z.C.; Wang, Y.W.; Sun, H.; Wang, L.; Han, Y.M.; Wu, Y.Y.; Liu, J.G.; Sun, L.H. Organic Acids Improve Growth Performance with Potential Regulation of Redox Homeostasis, Immunity, and Microflora in Intestines of Weaned Piglets. Antioxidants 2021, 10, 1665. [Google Scholar] [CrossRef]
- Pi, G.; Wang, J.; Song, W.; Li, Y.; Yang, H. Effects of isomalto-oligosaccharides and herbal extracts on growth performance, serum biochemical profiles and intestinal bacterial populations in early-weaned piglets. J. Anim. Physiol. Anim. Nutr. 2022, 106, 671–681. [Google Scholar] [CrossRef]
- Zha, A.; Tan, B.; Wang, J.; Qi, M.; Deng, Y.; Liao, P.; Yin, Y. The nanocomposites of modified attapulgite with vitamin E and mannan oligosaccharide regulated the intestinal epithelial barrier and improved intestinal microbiota composition to prevent diarrhea in weaned piglets. J. Sci. Food Agric. 2023, 103, 5569–5577. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Yin, Y.; Zhang, S.; Li, X.; Sun, Q.; Wan, D. Effects of Zinc Oxide/Zeolite on intestinal morphology, intestinal microflora, and diarrhea rates in weaned piglets. Biol. Trace Elem. Res. 2021, 199, 1405–1413. [Google Scholar] [CrossRef]
- Su, W.; Li, Z.; Gong, T.; Wang, F.; Jin, M.; Wang, Y.; Lu, Z. An alternative ZnO with large specific surface area: Preparation, physicochemical characterization and effects on growth performance, diarrhea, zinc metabolism and gut barrier function of weaning piglets. Sci. Total Environ. 2023, 15, 163558. [Google Scholar] [CrossRef]
- Zhu, C.; Lv, H.; Chen, Z.; Wang, L.; Wu, X.; Chen, Z.; Zhang, W.; Liang, R.; Jiang, Z. Dietary zinc oxide modulates antioxidant capacity, small intestine development, and jejunal gene expression in weaned piglets. Biol. Trace Elem. Res. 2017, 175, 331–338. [Google Scholar] [CrossRef]
- Hansen, S.V.; Nørskov, N.P.; Nørgaard, J.V.; Woyengo, T.A.; Poulsen, H.D.; Nielsen, T.S. Determination of the Optimal Level of Dietary Zinc for Newly Weaned Pigs: A Dose-Response Study. Animals 2022, 12, 1552. [Google Scholar] [CrossRef] [PubMed]
- Pejsak, Z.; Kaźmierczak, P.; Butkiewicz, A.F.; Wojciechowski, J.; Woźniakowski, G. Alternatives to zinc oxide in pig production. Pol. J. Vet. Sci. 2023, 26, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.M.; Liu, H.N.; Xu, K.; Wen, C.Y.; Yu, R.; Deng, J.P.; Yin, Y.L. Use of coated nano zinc oxide as an additive to improve the zinc excretion and intestinal morphology of growing pigs. J. Anim. Sci. 2019, 97, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards zero zinc oxide: Feeding strategies to manage post-weaning diarrhea in piglets. Animals 2021, 11, 642. [Google Scholar] [CrossRef]
- Peng, P.; Deng, D.; Chen, S.; Li, C.; Luo, J.; Romeo, A.; Li, T.; Tang, X.; Fang, R. The Effects of dietary porous zinc oxide supplementation on growth performance, inflammatory cytokines and tight junction’s gene expression in early-weaned piglets. J. Nutr. Sci. Vitaminol. 2020, 66, 311–318. [Google Scholar] [CrossRef]
- Yu, L.; Lu, M.; Zhang, W.; Alarfaj, A.A.; Hirad, A.H.; Zhang, H. Ameliorative effect of Albizia chinensis synthesized ZnO-NPs on Mycoplasma pneumoniae infected pneumonia mice model. Microb. Pathog. 2020, 141, 103960. [Google Scholar] [CrossRef]
- Peng, P.; Chen, J.; Yao, K.; Yin, Y.; Long, L.; Fang, R. The effects of dietary supplementation with porous zinc oxide on growth performance, intestinal microbiota, morphology, and permeability in weaned piglets. Anim. Sci. J. 2019, 90, 1220–1228. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Andreini, C.; Bertini, I. A bioinformatics view of zinc enzymes. J. Inorg. Biochem. 2012, 111, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Romeo, A.; Vacchina, V.; Legros, S.; Doelsch, E. Zinc fate in animal husbandry systems. Metallomics 2014, 6, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Dadi, T.H.; Pieper, L.; Vahjen, W.; Franke, A.; Reinert, K.; Zentek, J. Concentration and chemical form of dietary zinc shape the porcine colon microbiome, its functional capacity and antibiotic resistance gene repertoire. ISME J. 2020, 14, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; He, Y.; Yan, G.; Hou, S.; Xie, Z.; Liao, C. Zinc enzymes in medicinal chemistry. Eur. J. Med. Chem. 2021, 226, 113877. [Google Scholar] [CrossRef]
- Gamsjaeger, R.; Liew, C.K.; Loughlin, F.E.; Crossley, M.; Mackay, J.P. Sticky fingers: Zinc-fingers as protein-recognition motifs. Trends Biochem. Sci. 2007, 32, 63–70. [Google Scholar] [CrossRef]
- Abbehausen, C. Zinc finger domains as therapeutic targets for metal-based compounds—An update. Metallomics 2019, 11, 15–28. [Google Scholar] [CrossRef]
- Iyer, A.S.; Shaik, M.R.; Raufman, J.P.; Xie, G. The Roles of Zinc Finger Proteins in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 10249. [Google Scholar] [CrossRef]
- Davin, R.; Manzanilla, E.G.; Klasing, K.C.; Pérez, J.F. Effect of weaning and in-feed high doses of zinc oxide on zinc levels in different body compartments of piglets. J. Anim. Physiol. Anim. Nutr. 2013, 97, 6–12. [Google Scholar] [CrossRef]
- Brugger, D.; Windisch, W.M. Subclinical zinc deficiency impairs pancreatic digestive enzyme activity and digestive capacity of weaned piglets. Br. J. Nutr. 2016, 116, 425–433. [Google Scholar] [CrossRef]
- Medida, R.L.; Sharma, A.K.; Guo, Y.; Johnston, L.; Urriola, P.E.; Gomez, A.; Saqui-Salces, M. Dietary zinc restriction affects the expression of genes related to immunity and stress response in the small intestine of pigs. J. Nutr. Sci. 2022, 11, e104. [Google Scholar] [CrossRef] [PubMed]
- Stensland, I.; Kim, J.C.; Bowring, B.; Collins, A.M.; Mansfield, J.P.; Pluske, J.R. A comparison of diets supplemented with a feed additive containing organic acids, cinnamaldehyde and a permeabilizing complex, or zinc oxide, on post-weaning diarrhoea, selected bacterial populations, blood measures and performance in weaned pigs experimentally infected with Enterotoxigenic E. coli. Animals 2015, 5, 1147–1168. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Ying, Z.; He, J.; Zhou, L.; Zhang, L.; Zhong, X.; Wang, T. Effects of dietary zinc oxide nanoparticles on growth, diarrhea, mineral deposition, intestinal morphology, and barrier of weaned piglets. Biol. Trace Elem. Res. 2018, 185, 364–374. [Google Scholar] [CrossRef]
- Lokman, M.; Ashraf, E.; Kassab, R.B.; Abdel Moneim, A.E.; El-Yamany, N.A. Aluminum chloride-induced reproductive toxicity in rats: The protective role of zinc oxide nanoparticles. Biol. Trace Elem. Res. 2022, 200, 4035–4044. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Ramesh, T.; Seshadri, V.D.; Zhu, L. Zinc oxide nanoparticles from Corydalis yanhusuo attenuated the mycoplasmal pneumonia in mice through inhibiting the MAPKs signaling pathway. Microb. Pathog. 2020, 147, 104270. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, H.; Yang, K.; Tang, X.; Chen, S.; Ajuwon, K.M.; Degen, A.; Fang, R. Effect of the level and source of supplementary dietary zinc on egg production, quality, and zinc content and on serum antioxidant parameters and zinc concentration in laying hens. Poult. Sci. 2020, 99, 6233–6238. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wareth, A.A.A.; Amer, S.A.; Mobashar, M.; El-Sayed, H.G.M. Use of zinc oxide nanoparticles in the growing rabbit diets to mitigate hot environmental conditions for sustainable production and improved meat quality. BMC Vet. Res. 2022, 18, 354. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wareth, A.A.A.; Raslan, M.A.H.; Ismail, Z.S.H.; Salem, W.; Lohakare, J. Effects of Zinc Oxide Nanoparticle Supplementation on Performance, Digestibility, and Blood Biochemistry of Californian Male Rabbits Under Hot Climatic Conditions. Biol. Trace Elem. Res. 2023, 201, 3418–3427. [Google Scholar] [CrossRef]
- Hassan, F.A.; Elkassas, N.E.M.; El-Bltagy, E.A.; Mohamed, M.S.; Mobarez, S.; Salim, I.H.; Abdel-Aal, M.M. Dietary zinc-chitosan nanoparticles addition influences on growth performance, apparent total tract digestibility, carcass indices, and immune function in weaned rabbits. Anim. Biotechnol. 2023, 13, 1–9. [Google Scholar] [CrossRef]
- Swain, P.S.; Rao, S.B.N.; Rajendran, D.; Krishnamoorthy, P.; Mondal, S.; Pal, D.; Selvaraju, S. Nano zinc supplementation in goat (Capra hircus) ration improves immunity, serum zinc profile and IGF-1 hormones without affecting thyroid hormones. J. Anim. Physiol. Anim. Nutr. 2021, 105, 621–629. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, J.; Fazaeli, H. Performance, rumen fermentation, blood minerals, leukocyte and antioxidant capacity of young Holstein calves receiving high-surface ZnO instead of common ZnO. Arch. Anim. Nutr. 2020, 74, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Mei, J.; Yang, T.; Zhang, Z.; Liu, G.; Zhao, H.; Chen, X.; Tian, G.; Cai, J.; Wu, B.; et al. Effect of dietary zinc methionine supplementation on growth performance, immune function and intestinal health of cherry valley ducks challenged with avian pathogenic Escherichia coli. Front. Microbiol. 2022, 13, 849067. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wareth, A.A.A.; Hussein, K.R.A.; Ismail, Z.S.H.; Lohakare, J. Effects of Zinc Oxide Nanoparticles on the Performance of Broiler Chickens Under Hot Climatic Conditions. Biol. Trace Elem. Res. 2022, 200, 5218–5225. [Google Scholar] [CrossRef]
- Hatab, M.H.; Rashad, E.; Saleh, H.M.; El-Sayed, E.R.; Taleb, A.M.A. Effects of dietary supplementation of myco-fabricated zinc oxide nanoparticles on performance, histological changes, and tissues Zn concentration in broiler chicks. Sci. Rep. 2022, 12, 18791. [Google Scholar] [CrossRef]
- Wang, Z.C.; Yu, H.M.; Xie, J.J.; Cui, H.; Nie, H.; Zhang, T.; Gao, X.H. Effect of dietary zinc pectin oligosaccharides chelate on growth performance.; enzyme activities, Zn accumulation, metallothionein concentration, and gene expression of Zn transporters in broiler chickens. J. Anim. Sci. 2019, 97, 2114–2124. [Google Scholar] [CrossRef]
- Zhu, X.; Shang, X.; Lin, G.; Li, H.; Feng, X.; Zhang, H. Effects of zinc glycinate on growth performance, serum biochemical indexes, and intestinal morphology of yellow feather broilers. Biol. Trace Elem. Res. 2022, 200, 4089–4097. [Google Scholar] [CrossRef] [PubMed]
- Radi, A.M.; Abdel Azeem, N.M.; El-Nahass, E.S. Comparative effects of zinc oxide and zinc oxide nanoparticle as feed additives on growth, feed choice test, tissue residues, and histopathological changes in broiler chickens. Environ. Sci. Pollut. Res. Int. 2021, 28, 5158–5167. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; Zhou, W.; Feng, J.; Zou, X. Effects of zinc methionine supplementation on laying performance, zinc status, intestinal morphology, and expressions of zinc transporters’ mRNA in laying hens. J. Sci. Food Agric. 2019, 99, 6582–6588. [Google Scholar] [CrossRef]
- Li, L.; Li, P.; Chen, Y.; Wen, C.; Zhuang, S.; Zhou, Y. Zinc-bearing zeolite clinoptilolite improves tissue zinc accumulation in laying hens by enhancing zinc transporter gene mRNA abundance. Anim. Sci. J. 2015, 86, 782–789. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Chaudhry, M.T.; Saeed, M.; Ahmad, E.A.M.; El-Sayed, S.A.A. Does the gradual increase in dietary zinc oxide supplementation can affect egg quality, serum indices, and productive performance of laying hens? Trop. Anim. Health Prod. 2020, 52, 525–531. [Google Scholar] [CrossRef]
- Diao, H.; Yan, J.; Li, S.; Kuang, S.; Wei, X.; Zhou, M.; Zhang, J.; Huang, C.; He, P.; Tang, W. Effects of dietary zinc sources on growth performance and gut health of weaned piglets. Front. Microbiol. 2021, 12, 771617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hu, G.; Yang, Z.; Zhao, J. Effects of Tetrabasic Zinc Chloride on Growth Performance, Nutrient Digestibility and Fecal Microbial Community in Weaned Piglets. Front. Vet. Sci. 2022, 9, 905242. [Google Scholar] [CrossRef]
- Lee, J.; Hosseindoust, A.; Kim, K.; Kim, T.; Mun, J.; Chae, B.; Kim, M. Improved growth performance, antioxidant status, digestive enzymes, nutrient digestibility and zinc bioavailability of broiler chickens with nano-sized hot-melt extruded zinc sulfate. Biol. Trace Elem. Res. 2022, 200, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.M.; Kim, M.J.; Hosseindoust, A.; Kim, K.Y.; Choi, Y.H.; Ham, H.B.; Hwang, S.J.; Lee, J.H.; Cho, H.J.; Kang, W.S.; et al. Hot melt extruded-based nano zinc as an alternative to the pharmacological dose of ZnO in weanling piglets. Asian Australas. J. Anim. Sci. 2020, 33, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kim, S.W. Intestinal challenge with enterotoxigenic Escherichia coli in pigs, and nutritional intervention to prevent postweaning diarrhea. Anim. Nutr. 2017, 3, 322–330. [Google Scholar] [CrossRef]
- Kachoei, M.; Nourian, A.; Divband, B.; Kachoei, Z.; Shirazi, S. Zinc-oxide nanocoating for improvement of the antibacterial and frictional behavior of nickel-titanium alloy. Nanomedicine 2016, 11, 2511–2527. [Google Scholar] [CrossRef]
- Wei, X.; Tsai, T.; Knapp, J.; Bottoms, K.; Deng, F.; Story, R.; Maxwell, C.; Zhao, J. ZnO modulates swine gut microbiota and improves growth performance of nursery pigs when combined with peptide cocktail. Microorganisms 2020, 8, 146. [Google Scholar] [CrossRef]
- Zeidan, N.K.; Enany, N.M.; Mohamed, G.G.; Marzouk, E.S. The antibacterial effect of silver, zinc-oxide and combination of silver/ zinc oxide nanoparticles coating of orthodontic brackets (an in vitro study). BMC Oral Health 2022, 22, 230. [Google Scholar] [CrossRef]
- Liu, Y.; He, L.; Mustapha, A.; Li, H.; Hu, Z.Q.; Lin, M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 1193–1201. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef]
- Bratz, K.; Gölz, G.; Riedel, C.; Janczyk, P.; Nöckler, K.; Alter, T. Inhibitory effect of high-dosage zinc oxide dietary supplementation on Campylobacter coli excretion in weaned piglets. J. Appl. Microbiol. 2013, 115, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lallo da Silva, B.; Abuçafy, M.P.; Berbel Manaia, E.; Oshiro Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liao, C.; Tjong, S.C. Recent advances in zinc oxide nanostructures with antimicrobial activities. Int. J. Mol. Sci. 2020, 21, 8836. [Google Scholar] [CrossRef]
- Calabrese, G.; De Luca, G.; Franco, D.; Morganti, D.; Rizzo, M.G.; Bonavita, A.; Neri, G.; Fazio, E.; Neri, F.; Fazio, B.; et al. Structural and antibacterial studies of novel ZnO and ZnxMn(1-x)O nanostructured titanium scaffolds for biomedical applications. Biomater. Adv. 2023, 145, 213193. [Google Scholar] [CrossRef]
- Khan, M.M.; Harunsani, M.H.; Tan, A.L.; Hojamberdiev, M.; Azamay, S.; Ahmad, N. Antibacterial activities of zinc oxide and Mn-doped zinc oxide synthesized using Melastoma malabathricum (L.) leaf extract. Bioprocess Biosyst. Eng. 2020, 43, 1499–1508. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Guo, B.L.; Han, P.; Guo, L.C.; Cao, Y.Q.; Li, A.D.; Kong, J.Z.; Zhai, H.F.; Wu, D. The Antibacterial Activity of Ta-doped ZnO Nanoparticles. Nanoscale Res. Lett. 2015, 10, 1047. [Google Scholar] [CrossRef]
- Biswas, A.; Kar, U.; Jana, N.R. Cytotoxicity of ZnO nanoparticles under dark conditions via oxygen vacancy dependent reactive oxygen species generation. Phys. Chem. Chem. Phys. 2022, 24, 13965–13975. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.K.; Nenavathu, B.P.; Gangishetty, M.K.; Reddy, A.V. Studies on antibacterial activity of ZnO nanoparticles by ROS induced lipid peroxidation. Colloids Surf. B Biointerfaces 2012, 94, 143–150. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Perkas, N.; Nitzan, Y.; Gedanken, A.; Banin, E. ZnO nanoparticle-coated surfaces inhibit bacterial biofilm formation and increase antibiotic susceptibility. RSC Adv. 2012, 2, 2314–2321. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, S.; Lim, S.; Sim, J.; Rhie, H.G.; Lee, S.J. Oxidative stress response of Deinococcus geothermalis via a cystine importer. J. Microbiol. 2017, 55, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Cheng, S.; Singh, S. Oxidative stress-mediated genotoxic effect of zinc oxide nanoparticles on Deinococcus radiodurans. 3 Biotech 2020, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- do Carmo Neto, J.R.; Guerra, R.O.; Machado, J.R.; Silva, A.C.A.; da Silva, M.V. Antiprotozoal and anthelmintic activity of zinc oxide nanoparticles. Curr. Med. Chem. 2022, 29, 2127–2141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, L.; Wen, D.; Ding, Y. Role of physical and chemical interactions in the antibacterial behavior of ZnO nanoparticles against E. coli. Mater. Sci. Eng. C 2016, 69, 1361–1366. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO nanoparticles to Escherichia coli: Mechanism and the influence of medium components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
- Dimapilis, E.A.; Hsu, C.S.; Mendoza, R.M.; Lu, M.C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Joe, A.; Park, S.H.; Shim, K.D.; Kim, D.J.; Jhee, K.H.; Lee, H.W.; Heo, C.H.; Kim, H.M.; Jang, E.S. Antibacterial mechanism of ZnO nanoparticles under dark conditions. J. Ind. Eng. Chem. 2017, 45, 430–439. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Puspasari, V.; Ridhova, A.; Hermawan, A.; Amal, M.I.; Khan, M.M. ZnO-based antimicrobial coatings for biomedical applications. Bioprocess Biosyst. Eng. 2022, 45, 1421–1445. [Google Scholar] [CrossRef] [PubMed]
- Karli, G.; Buford, S.; Mark, K.; Gaharwar, A.K. Antimicrobial activity of metal and metal-oxide based nanoparticles. Adv. Ther. 2018, 1, 1700033. [Google Scholar]
- Ahmed, B.; Solanki, B.; Zaidi, A.; Khan, M.S.; Musarrat, J. Bacterial toxicity of biomimetic green zinc oxide nanoantibiotic: Insights into ZnONP uptake and nanocolloid-bacteria interface. Toxicol. Res. 2018, 8, 246–261. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, B.; Negm, W.A.; Elekhnawy, E.; El-Masry, T.A.; Elharty, M.E.; Saleh, A.; Abdelkader, D.H.; Mokhtar, F.A. Antibacterial activity of nano zinc oxide green-synthesised from Gardenia thailandica triveng. Leaves against Pseudomonas aeruginosa clinical isolates: In vitro and in vivo study. Artif. Cells Nanomed. Biotechnol. 2022, 50, 96–106. [Google Scholar]
- Schoultz, I.; Keita, A.V. The Intestinal barrier and current techniques for the assessment of gut permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Kang, X.; Liu, J.; Li, M. Lipopolysaccharide promotes apoptosis and oxidative injury of porcine small intestinal epithelial cells by down-regulating the expression of glutamine transporter ASCT2. J. Anim. Sci. 2023, 101, skad229. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zeng, Y.; Xiong, K.; Li, M. The inflammatory injury of porcine small intestinal epithelial cells induced by deoxynivalenol is related to the decrease in glucose transport. J. Anim. Sci. 2024, 102, skae107. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, N.; Qi, Z.; Han, M.; Ma, X. Coated Zinc Oxide Improves Growth Performance of Weaned Piglets via Gut Microbiota. Front. Nutr. 2022, 9, 819722. [Google Scholar] [CrossRef]
- Yi, H.; Wang, Z.; Yang, B.; Yang, X.; Gao, K.; Xiong, Y.; Wu, Q.; Qiu, Y.; Hu, S.; Wang, L.; et al. Effects of zinc oxide and condensed tannins on the growth performance and intestinal health of weaned piglets in ETEC-challenged environment. Front. Microbiol. 2023, 14, 1181519. [Google Scholar] [CrossRef]
- Li, X.; Yin, J.; Li, D.; Chen, X.; Zang, J.; Zhou, X. Dietary supplementation with zinc oxide increases IGF-I and IGF-I receptor gene expression in the small intestine of weanling piglets. J. Nutr. 2006, 136, 1786–1791. [Google Scholar] [CrossRef]
- Hu, C.; Song, J.; Li, Y.; Luan, Z.; Zhu, K. Diosmectite-zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br. J. Nutr. 2013, 110, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Grilli, E.; Tugnoli, B.; Vitari, F.; Domeneghini, C.; Morlacchini, M.; Piva, A.; Prandini, A. Low doses of microencapsulated zinc oxide improve performance and modulate the ileum architecture, inflammatory cytokines and tight junctions expression of weaned pigs. Animal 2015, 9, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Ji, W.; Wang, J.; Li, B.; Hu, J.; Wu, X. Effects of dietary supplementation with yeast glycoprotein on growth performance, intestinal mucosal morphology, immune response and colonic microbiota in weaned piglets. Food Funct. 2019, 10, 2359–2571. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Xiao, Z.; Liu, L.; Wang, G.; Tao, W.; Wang, M.; Zou, J.; Leng, D. Effects of dietary zinc oxide nanoparticles supplementation on growth performance, zinc status, intestinal morphology, microflora population.; and immune response in weaned pigs. J. Sci. Food Agric. 2019, 99, 1366–1374. [Google Scholar] [CrossRef]
- Kwon, C.H.; Lee, C.Y.; Han, S.J.; Kim, S.J.; Park, B.C.; Jang, I.; Han, J.H. Effects of dietary supplementation of lipid-encapsulated zinc oxide on colibacillosis, growth and intestinal morphology in weaned piglets challenged with enterotoxigenic Escherichia coli. Anim. Sci. J. 2014, 85, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kwon, C.H.; Park, B.C.; Lee, C.Y.; Han, J.H. Effects of a lipid-encapsulated zinc oxide dietary supplement.; on growth parameters and intestinal morphology in weanling pigs artificially infected with enterotoxigenic Escherichia coli. J. Anim. Sci. Technol. 2015, 57, 4. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Mao, J.; Peng, Z.; Zhong, Z.; Wang, H.; Dong, L. Dietary palygorskite clay-adsorbed nano-ZnO supplementation improves the intestinal barrier function of weanling pigs. Front. Nutr. 2022, 9, 857898. [Google Scholar] [CrossRef]
- Hedemann, M.S.; Jensen, B.B.; Poulsen, H.D. Influence of dietary zinc and copper on digestive enzyme activity and intestinal morphology in weaned pigs. J. Anim. Sci. 2006, 84, 3310–3320. [Google Scholar] [CrossRef]
- Hu, C.; Song, J.; You, Z.; Luan, Z.; Li, W. Zinc oxide-montmorillonite hybrid influences diarrhea, intestinal mucosal integrity, and digestive enzyme activity in weaned pigs. Biol. Trace Elem. Res. 2012, 149, 190–196. [Google Scholar] [CrossRef]
- Liu, H.; Hu, J.; Mahfuz, S.; Piao, X. Effects of Hydrolysable Tannins as Zinc Oxide Substitutes on Antioxidant Status, Immune Function, Intestinal Morphology, and Digestive Enzyme Activities in Weaned Piglets. Animals 2020, 10, 757. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, X.; Wang, X.; Tan, B.; Li, T.; Yin, Y. Effects of weaning on intestinal upper villus epithelial cells of piglets. PLoS ONE 2016, 113, e0150216. [Google Scholar] [CrossRef]
- Wang, L.X.; Zhu, F.; Li, J.Z.; Li, Y.L.; Ding, X.Q.; Yin, J.; Xiong, X.; Yang, H.S. Epidermal growth factor promotes intestinal secretory cell differentiation in weaning piglets via wnt/bcatenin signalling. Animal 2020, 14, 790–798. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Tan, X.; Wang, L.; Xiong, X.; Wang, Y.; Wang, Q.; Yang, H.; Yin, Y. The developmental changes in intestinal epithelial cell proliferation, differentiation, and shedding in weaning piglets. Anim. Nutr. 2022, 9, 214–222. [Google Scholar] [CrossRef]
- Marion, J.; Petersen, Y.M.; Romé, V.; Thomas, F.; Sangild, P.T.; Le Dividich, J.; Le Huërou-Luron, I. Early weaning stimulates intestinal brush border enzyme activities in piglets, mainly at the posttranscriptional level. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 401–410. [Google Scholar] [CrossRef]
- Tsukahara, T.; Kishino, E.; Inoue, R.; Nakanishi, N.; Nakayama, K.; Ito, T.; Ushida, K. Correlation between villous height and the disaccharidase activity in the small intestine of piglets from nursing to growing. Anim. Sci. J. 2013, 84, 54–59. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Brit. J. Nutr. 2009, 102, 687–693. [Google Scholar] [CrossRef]
- Liu, H.; Bai, M.; Xu, K.; Zhou, J.; Zhang, X.; Yu, R.; Huang, R.; Yin, Y. Effects of different concentrations of coated nano zinc oxide material on fecal bacterial composition and intestinal barrier in weaned piglets. J. Sci. Food Agric. 2021, 101, 735–745. [Google Scholar] [CrossRef]
- Christensen, B.; Zhu, C.; Mohammadigheisar, M.; Schulze, H.; Huber, L.A.; Kiarie, E.G. Growth performance, immune status, gastrointestinal tract ecology, and function in nursery pigs fed enzymatically treated yeast without or with pharmacological levels of zinc. J. Anim. Sci. 2022, 100, skac094. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H. Tight Junction Proteins in the Weaned Piglet Intestine: Roles and Regulation. Curr. Protein Pept. Sci. 2019, 20, 652–660. [Google Scholar] [CrossRef]
- Xia, T.; Lai, W.; Han, M.; Han, M.; Ma, X.; Zhang, L. Dietary ZnO nanoparticles alters intestinal microbiota and inflammation response in weaned piglets. Oncotarget 2017, 8, 64878–64891. [Google Scholar] [CrossRef]
- Tang, X.; Liu, X.; Zhong, J.; Fang, R. Potential Application of Lonicera japonica Extracts in Animal Production: From the Perspective of Intestinal Health. Front. Microbiol. 2021, 12, 719877. [Google Scholar] [CrossRef]
- Tang, X. Probiotic Roles of Clostridium butyricum in Piglets: Considering Aspects of Intestinal Barrier Function. Animals 2024, 14, 1069. [Google Scholar] [CrossRef]
- Deng, Q.; Tan, X.; Wang, H.; Wang, Q.; Huang, P.; Li, Y.; Li, J.; Huang, J.; Yang, H.; Yin, Y. Changes in cecal morphology, cell proliferation, antioxidant enzyme, volatile fatty acids, lipopolysaccharide, and cytokines in piglets during the postweaning period. J. Anim. Sci. 2020, 98, skaa046. [Google Scholar] [CrossRef]
- de Groot, N.; Fariñas, F.; Cabrera-Gómez, C.G.; Pallares, F.J.; Ramis, G. Weaning causes a prolonged but transient change in immune gene expression in the intestine of piglets. J. Anim. Sci. 2021, 99, skab065. [Google Scholar] [CrossRef]
- Cao, S.; Hou, L.; Sun, L.; Gao, J.; Gao, K.; Yang, X.; Jiang, Z.; Wang, L. Intestinal morphology and immune profiles are altered in piglets by early-weaning. Int. Immunopharmacol. 2022, 105, 108520. [Google Scholar] [CrossRef]
- Oh, H.J.; Park, Y.J.; Cho, J.H.; Song, M.H.; Gu, B.H.; Yun, W.; Lee, J.H.; An, J.S.; Kim, Y.J.; Lee, J.S.; et al. Changes in Diarrhea score, nutrient digestibility, zinc utilization, intestinal immune profiles, and fecal microbiome in weaned piglets by different forms of zinc. Animals 2021, 11, 1356. [Google Scholar] [CrossRef]
- Long, S.; He, T.; Kim, S.W.; Shang, Q.; Kiros, T.; Mahfuz, S.U.; Wang, C.; Piao, X. Live Yeast or Live Yeast Combined with Zinc Oxide Enhanced Growth Performance, Antioxidative Capacity, Immunoglobulins and Gut Health in Nursery Pigs. Animals 2021, 11, 1626. [Google Scholar] [CrossRef]
- Kloubert, V.; Blaabjerg, K.; Dalgaard, T.S.; Poulsen, H.D.; Rink, L.; Wessels, I. Influence of zinc supplementation on immune parameters in weaned pigs. J. Trace Elem. Med. Biol. 2018, 49, 231–240. [Google Scholar] [CrossRef]
- Han, X.; Ma, Y.; Lv, M.; Wu, Z.; Qian, L. Chitosan-zinc chelate improves intestinal structure and mucosal function and decreases apoptosis in ileal mucosal epithelial cells in weaned pigs. Brit. J. Nutr. 2014, 111, 1405–1411. [Google Scholar] [CrossRef]
- Shen, J.; Chen, Y.; Wang, Z.; Zhou, A.; He, M.; Mao, L.; Zou, H.; Peng, Q.; Xue, B.; Wang, L.; et al. Coated zinc oxide improves intestinal immunity function and regulates microbiota composition in weaned piglets. Br. J. Nutr. 2014, 111, 2123–2134. [Google Scholar] [CrossRef]
- Hu, C.H.; Song, Z.H.; Xiao, K.; Song, J.; Jiao, L.F.; Ke, Y.L. Zinc oxide influences intestinal integrity, the expressions of genes associated with inflammation and TLR4-myeloid differentiation factor 88 signaling pathways in weanling pigs. Innate Immun. 2014, 20, 478–486. [Google Scholar] [CrossRef]
- Sargeant, H.R.; McDowall, K.J.; Miller, H.M.; Shaw, M.A. Dietary zinc oxide affects the expression of genes associated with inflammation, Transcriptome analysis in piglets challenged with ETEC K88. Vet. Immunol. Immunopathol. 2010, 137, 120–129. [Google Scholar] [CrossRef]
- Hou, G.; Zhang, M.; Wang, J.; Zhu, W. Chitosan-chelated zinc modulates ileal microbiota, ileal microbial metabolites, and intestinal function in weaned piglets challenged with Escherichia coli K88. Appl. Microbiol. Biotechnol. 2021, 105, 7529–7544. [Google Scholar] [CrossRef]
- Song, Z.H.; Ke, Y.L.; Xiao, K.; Jiao, L.F.; Hong, Q.H.; Hu, C.H. Diosmectite-zinc oxide composite improves intestinal barrier restoration and modulates TGF-β1, ERK1/2, and Akt in piglets after acetic acid challenge. J. Anim. Sci. 2015, 93, 1599–1607. [Google Scholar] [CrossRef]
- Song, Z.H.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H. Zinc oxide influences mitogen-activated protein kinase and TGF-β1 signaling pathways, and enhances intestinal barrier integrity in weaned pigs. Innate Immun. 2015, 21, 341–348. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Zhou, B.; Yuan, Y.; Zhang, S.; Guo, C.; Li, X.; Li, G.; Xiong, W.; Zeng, Z. Intestinal flora and disease mutually shape the regional immune system in the intestinal tract. Front. Immunol. 2020, 11, 575. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and metabolic disorders and the microbiome: The intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef]
- Juhász, Á.; Molnár-Nagy, V.; Bata, Z.; Tso, K.H.; Mayer, Z.; Posta, K. Alternative to ZnO to establish balanced intestinal microbiota for weaning piglets. PLoS ONE 2022, 17, e0265573. [Google Scholar] [CrossRef]
- Johanns, V.C.; Ghazisaeedi, F.; Epping, L.; Semmler, T.; Lübke-Becker, A.; Pfeifer, Y.; Bethe, A.; Eichhorn, I.; Merle, R.; Walther, B.; et al. Effects of a Four-Week High-Dosage Zinc Oxide Supplemented Diet on Commensal Escherichia coli of Weaned Pigs. Front. Microbiol. 2019, 10, 2734. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Song, M.; Liu, Y.; Ji, P. Enterotoxigenic Escherichia coli infection of weaned pigs: Intestinal challenges and nutritional intervention to enhance disease resistance. Front. Immunol. 2022, 13, 885253. [Google Scholar] [CrossRef]
- Reynolds, F.H.; Forbes, J.M.; Miller, H.M. Does the newly weaned piglet select a zinc oxide supplemented feed, when given the choice? Animal 2010, 4, 1359–1367. [Google Scholar] [CrossRef]
- Wang, W.; Van Noten, N.; Degroote, J.; Romeo, A.; Vermeir, P.; Michiels, J. Effect of zinc oxide sources and dosages on gut microbiota and integrity of weaned piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 231–241. [Google Scholar] [CrossRef]
- Papadomichelakis, G.; Palamidi, I.; Paraskeuas, V.V.; Giamouri, E.; Mountzouris, K.C. Evaluation of a Natural Phytogenic Formulation as an Alternative to Pharmaceutical Zinc Oxide in the Diet of Weaned Piglets. Animals 2023, 13, 431. [Google Scholar] [CrossRef]
- Starke, I.C.; Pieper, R.; Neumann, K.; Zentek, J.; Vahjen, W. The impact of high dietary zinc oxide on the development of the intestinal microbiota in weaned piglets. FEMS Microbiol. Ecol. 2014, 87, 416–427. [Google Scholar] [CrossRef]
- Yu, T.; Zhu, C.; Chen, S.; Gao, L.; Lv, H.; Feng, R.; Zhu, Q.; Xu, J.; Chen, Z.; Jiang, Z. Dietary high zinc oxide modulates the microbiome of ileum and colon in weaned piglets. Front. Microbiol. 2017, 8, 825. [Google Scholar] [CrossRef]
- Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J. Anim. Sci. 2020, 98, skaa086. [Google Scholar] [CrossRef]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Tang, Z.; Sun, Z. Regulatory functions of fatty acids with different chain lengths on the intestinal health in pigs and relative signaling pathways. Curr. Protein Pept. Sci. 2019, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, H.D. Zinc oxide for weanling piglets. Acta Agric. Scand. A Anim. Sci. 1995, 45, 159–167. [Google Scholar] [CrossRef]
- Burrough, E.R.; De Mille, C.; Gabler, N.K. Zinc overload in weaned pigs, tissue accumulation, pathology, and growth impacts. J. Vet. Diagn. Investig. 2019, 31, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.J.; Liu, Z.Z.; Park, J.H.; Kim, I.H. Novel zinc sources as antimicrobial growth promoters for monogastric animals: A review. J. Anim. Sci. Technol. 2022, 64, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Bednorz, C.; Oelgeschläger, K.; Kinnemann, B.; Hartmann, S.; Neumann, K.; Pieper, R.; Bethe, A.; Semmler, T.; Tedin, K.; Schierack, P.; et al. The broader context of antibiotic resistance, zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. Int. J. Med. Microbiol. 2013, 303, 396–403. [Google Scholar] [CrossRef]
- Vahjen, W.; Pietruszyńska, D.; Starke, I.C.; Zentek, J. High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs. Gut Pathog. 2015, 7, 23. [Google Scholar] [CrossRef]
- Ciesinski, L.; Guenther, S.; Pieper, R.; Kalisch, M.; Bednorz, C.; Wieler, L.H. High dietary zinc feeding promotes persistence of multi-resistant E. coli in the swine gut. PLoS ONE 2018, 13, e0191660. [Google Scholar] [CrossRef]
- Ekhlas, D.; Sanjuán, J.M.O.; Manzanilla, E.G.; Leonard, F.C.; Argüello, H.; Burgess, C.M. Comparison of antimicrobial resistant Escherichia coli isolated from Irish commercial pig farms with and without zinc oxide and antimicrobial usage. Gut Pathog. 2023, 15, 8. [Google Scholar] [CrossRef]
- Slifierz, M.J.; Friendship, R.; Weese, J.S. Zinc oxide therapy increases prevalence and persistence of methicillin-resistant Staphylococcus aureus in pigs, a randomized controlled trial. Zoonoses Public Health 2015, 62, 301–308. [Google Scholar] [CrossRef]
- Slifierz, M.J.; Park, J.; Friendship, R.M.; Weese, J.S. Zinc-resistance gene CzrC identified in methicillin-resistant Staphylococcus hyicus isolated from pigs with exudative epidermitis. Can. Vet. J. 2014, 55, 489–490. [Google Scholar]
- Augspurger, N.R.; Spencer, J.D.; Webel, D.M.; Baker, D.H. Pharmacological zinc levels reduce the phosphorus-releasing efficacy of phytase in young pigs and chickens. J. Anim. Sci. 2004, 82, 1732–1739. [Google Scholar] [CrossRef]
- Martínez, M.M.; Link, J.E.; Hill, G.M. Dietary pharmacological or excess zinc and phytase effects on tissue mineral concentrations, metallothionein, and apparent mineral retention in the newly weaned pig. Biol. Trace Elem. Res. 2005, 105, 97–115. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, J.W.; Ma, W.Q.; Feng, J.; Feng, J. Dietary zinc glycine chelate on growth performance, tissue mineral concentrations, and serum enzyme activity in weanling piglets. Biol. Trace Elem. Res. 2010, 133, 325–334. [Google Scholar] [CrossRef]
- Jang, K.B.; Moita, V.H.C.; Martinez, N.; Sokale, A.; Kim, S.W. Efficacy of zinc glycinate reducing zinc oxide on intestinal health and growth of nursery pigs challenged with F18+ Escherichia coli. J. Anim. Sci. 2023, 101, skad035. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, T.; Wu, H.; Zhang, W.; Xue, J.; He, D.; Zhang, L. Effects of nano zinc oxide as an alternative to pharmacological dose of zinc oxide on growth performance, diarrhea, immune responses, and intestinal microflora profile in weaned piglets. Anim. Feed Sci. Technol. 2019, 258, 114312. [Google Scholar] [CrossRef]
- Dong, X.; Xu, Q.; Wang, C.; Zou, X.; Lu, J. Supplemental-coated zinc oxide relieves diarrhoea by decreasing intestinal permeability in weanling pigs. J. Appl. Anim. Res. 2019, 47, 362–368. [Google Scholar] [CrossRef]
- Byun, Y.J.; Lee, C.Y.; Kim, M.H.; Jung, D.Y.; Han, J.H.; Jang, I.; Song, Y.M.; Park, B.C. Effects of dietary supplementation of a lipid-coated zinc oxide product on the fecal consistency, growth, and morphology of the intestinal mucosa of weanling pigs. J. Anim. Sci. Technol. 2018, 59, 29. [Google Scholar] [CrossRef]
- Ouyang, Z.; Ren, P.; Zheng, D.; Huang, L.; Wei, T.; Yang, C.; Kong, X.; Yin, Y.; He, Q. Hydrothermal synthesis of a new porous zinc oxide and its antimicrobial evaluation in weanling piglets. Livest. Sci. 2021, 248, 104499. [Google Scholar] [CrossRef]
- Long, L.; Chen, J.; Zhang, Y.; Liang, X.; Ni, H.; Zhang, B.; Yin, Y. Comparison of porous and nano zinc oxide for replacing high-dose dietary regular zinc oxide in weaning piglets. PLoS ONE 2017, 12, e0182550. [Google Scholar]
- Chaudhry, Q.; Castle, L. Food applications of nanotechnologies: An overview of opportunities and challenges for developing countries. Trends Food. Sci. Technol. 2011, 22, 593–603. [Google Scholar] [CrossRef]
- McClements, D.J.; Xiao, H. Is nano safe in foods? establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci. Food 2017, 1, 6. [Google Scholar] [CrossRef]
- Moreno-Olivas, F.; Tako, E.; Mahler, G.J. ZnO nanoparticles affect nutrient transport in an in vitro model of the small intestine. Food Chem. Toxicol. 2019, 124, 112–127. [Google Scholar] [CrossRef]
- Wei, X.; Tsai, T.; Howe, S.; Zhao, J. Weaning Induced Gut Dysfunction and Nutritional Interventions in Nursery Pigs: A Partial Review. Animals 2021, 11, 1279. [Google Scholar] [CrossRef]
- Verma, S.K.; Jha, E.; Panda, P.K.; Das, J.K.; Thirumurugan, A.; Suar, M.; Parashar, S. Molecular aspects of core-shell intrinsic defect induced enhanced antibacterial activity of ZnO nanocrystals. Nanomedicine 2018, 13, 43–68. [Google Scholar] [CrossRef]
- Xie, J.; Li, H.; Zhang, T.; Song, B.; Wang, X.; Gu, Z. Recent Advances in ZnO Nanomaterial-Mediated Biological Applications and Action Mechanisms. Nanomaterials 2023, 13, 1500. [Google Scholar] [CrossRef]
- Kim, T.; Kim, M.; Lee, J.; Moturi, J.; Ha, S.; Tajudeen, H.; Mun, J.; Hosseindoust, A.; Chae, B. Supplementation of nano-zinc in lower doses as an alternative to pharmacological doses of ZnO in weanling pigs. J. Anim. Sci. Technol. 2022, 64, 70–83. [Google Scholar] [CrossRef]
- Liang, S.; Sun, K.; Wang, Y.; Dong, S.; Wang, C.; Liu, L.; Wu, Y. Role of Cyt-C/caspases-9,3, Bax/Bcl-2 and the FAS death receptor pathway in apoptosis induced by zinc oxide nanoparticles in human aortic endothelial cells and the protective effect by alpha-lipoic acid. Chem. Biol. Interact. 2016, 258, 40–51. [Google Scholar] [CrossRef]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain, Part 1, human and animal health. EFSA J. 2018, 16, e05327. [Google Scholar]
- Zhang, G.; Zhao, J.; Lin, G.; Guo, Y.; Li, D.; Wu, Y. Effects of protein-chelated zinc combined with mannan-rich fraction to replace high-dose zinc oxide on growth performance, nutrient digestibility, and intestinal health in weaned piglets. Animals 2022, 12, 3407. [Google Scholar] [CrossRef]
- Buff, C.E.; Bollinger, D.W.; Ellersieck, M.R.; Brommelsiek, W.A.; Veum, T.L. Comparison of growth performance and zinc absorption, retention, and excretion in weanling pigs fed diets supplemented with zinc-polysaccharide or zinc oxide. J. Anim. Sci. 2005, 83, 2380–2386. [Google Scholar] [CrossRef]
- Hollis, G.R.; Carter, S.D.; Cline, T.R.; Crenshaw, T.D.; Cromwell, G.L.; Hill, G.M.; Kim, S.W.; Lewis, A.J.; Mahan, D.C.; Miller, P.S.; et al. Effects of replacing pharmacological levels of dietary zinc oxide with lower dietary levels of various organic zinc sources for weanling pigs. J. Anim. Sci. 2005, 83, 2123–2129. [Google Scholar] [CrossRef]
- Song, Y.M.; Kim, M.H.; Kim, H.N.; Jang, I.; Han, J.H.; Fontamillas, G.A.; Lee, C.Y.; Park, B.C. Effects of dietary supplementation of lipid-coated zinc oxide on intestinal mucosal morphology and expression of the genes associated with growth and immune function in weanling pigs. Asian Australas. J. Anim. Sci. 2018, 31, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Kwon, C.H.; Ha, D.M.; Jung, D.Y.; Kang, S.Y.; Park, M.J.; Han, J.H.; Park, B.C.; Lee, C.Y. Effects of a lipid-encapsulated zinc oxide supplement on growth performance and intestinal morphology and digestive enzyme activities in weanling pigs. J. Anim. Sci. Technol. 2014, 56, 29. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, A.; Gedanken, A. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Long, L.; Zhao, X.; Chen, J.; Wang, Z.; Tang, Y.; Huang, J.; Yin, Y. Piglet growth performance improved by dietary supplementation of porous or nano particles of zinc oxide may be related to the gut microbiota. Anim. Nutr. 2023, 15, 159–172. [Google Scholar] [CrossRef]
- Vahjen, W.; Zentek, J.; Durosoy, S. Inhibitory action of two zinc oxide sources on the ex vivo growth of porcine small intestine bacteria. J. Anim. Sci. 2012, 90, 334–336. [Google Scholar] [CrossRef]
- Morales, J.; Cordero, G.; Piñeiro, C.; Durosoy, S. Zinc oxide at low supplementation level improves productive performance and health status of piglets. J. Anim. Sci. 2012, 90, 436–438. [Google Scholar] [CrossRef][Green Version]
- Cho, J.H.; Liu, S.D.; Yun, W.; Kim, K.S.; Kim, I.H. Effect of supplemented microencapsulated zinc oxide and organic acids and pure botanicals on growth performance, nutrient digestibility, blood profiles, feces microflora, and zinc level of feces in weanling pigs. Can. J. Anim. Sci. 2019, 99, 63–67. [Google Scholar] [CrossRef]

| Species | Forms of Zinc | Doses | Major Health Benefits | Reference |
|---|---|---|---|---|
| Rat | ZnO-NPs | 4 mg/kg BW | reproductive toxicity | Lokman et al. [54] |
| Mice | ZnO-NPs | 20 μg/kg | IL-6↓, IL-8↓, TNF-α↓, inflammatory cells penetration↓ | Chen et al. [55] |
| Mice | ZnO-NPs | 20 μg/kg | IL-6↓, IL-8↓, TNF-α↓, inflammatory cells penetration↓ | Yu et al. [56] |
| Rabbit | ZnO-NPs | 20–80 mg/kg diet | growth performance↑, meat physicochemical properties↑, blood biochemistry parameters↑ | Abdel-Wareth et al. [57] |
| Rabbit | ZnO-NPs | 50 mg/kg diet | Digestibility of crude protein and crude fat↑, cecal Lactobacilli spp. ↑, serum testosterone levels↑, serum ALT↓, serum AST ↓ | Abdel-Wareth et al. [58] |
| Rabbit | Zn-CNPs | 50, 75, and 100 ppm | immune functions↑ | Hassan et al. [59] |
| Goat | NZn | 25, 50 mg/kg Zn | immune functions↑, serum hormone profiles↑ | Swain et al. [60] |
| Calves | ZnO-NPs | 50 mg Zn/kg dry matter | incidence of diarrhea↓, pneumonia↓, feed intake↑, digestibility↑, blood Zn concentration↑ | Abdollahi et al. [61] |
| Duck | Zn-Met | 0, 30, 60, and 120 mg Zn/kg diet | growth performance↑, immune function↑, intestinal health↑ | Chang et al. [62] |
| Broiler Chickens | ZnO-NPs | 20, 40, and 60 mg/kg diet | growth performance↑, nutrient digestibility↑, carcass criteria↑, liver and kidney functions↑ | Abdel-Wareth et al. [63] |
| Broiler Chickens | ZnO-NPs | 40 or 60 mg/kg diet | productive performance↑, physiological status↑ | Hatab et al. [64] |
| Broiler Chickens | Zn-POS | 0, 200, 400, 600, and 800 mg/kg diet | productive performance↑, Zn enrichment in the metabolic organs↑ | Wang et al. [65] |
| Broiler Chickens | Gly-Zn | 60 mg Zn/kg diet | growth performance↑, serum indexes↑, intestinal morphology↑ | Zhu et al. [66] |
| Broiler Chickens | ZnO-NPs | 90 mg/kg diet | body weight↑, antibacterial activity↑ | Radi et al. [67] |
| Laying Hens | Zn-Met | 40 and 80 mg Zn/kg diet | Zn contents in liver, duodenum, and jejunum↑, intestinal morphology↑, metallothionein mRNA expression↑ | Li et al. [68] |
| Laying Hens | ZnCP | 40.25 and 80.50 mg Zn/kg diet | blood iron (Fe) content↑, jejunal MT-4 mRNA abundance↑, liver Zn content↑, pancreas Zn content↑ | Li et al. [69] |
| Laying Hens | ZnO | 25 or 75 mg diet | feed intake↑, antioxidant ability↑, serum zinc status↑ | Abd El-Hack et al. [70] |
| Weaned Piglets | ZnO | 3000 mg/kg | diarrhea rate↓, growth performance↑, intestinal barrier function↑, immune function↑ | Zhu et al. [33] |
| Weaned Piglets | ZnSO4, Gly-Zn, zinc lactate | 100 mg/kg diet | growth performance↑, intestinal barrier function↑ | Diao et al. [71] |
| Weaned Piglets | TBZC | 1000 mg Zn/kg diet | zinc excretion↓, growth performance↑ | Zhang et al. [72] |
| Weaned Piglets | HME-Zn | 80 mg/kg diet | growth performance↑, antioxidant capacity↑, pancreatic enzyme activity↑, intestinal morphology↑, nutrient digestibility↑ | Lee et al. [73] |
| Weaned Piglets | HME-ZnO | 500, 1000, or 2500 ppm | digestibility of protein↑, intestinal Coliform and Clostridium↑, intestinal morphology↑ | Oh et al. [74] |
| Novel Zinc Oxide Forms | Dose | Does of ZnO | Substitution Effect | Reference |
|---|---|---|---|---|
| ZnO-NPs | 400, 800 mg/kg | 3000 mg/kg | ADG↑, diarrhea rate↓, intestinal morphology↑, occludin↑, ZO-1↑ | Wang et al. [53] |
| ZnO-NPs | 200 mg/kg | 2500 mg/kg | diarrhea rate↓, dry matter and gross energy digestibility↑, IL-6↓, IL-8↓, Succinivibrio↑ | Oh et al. [137] |
| ZnO-NPs | 300, 400, 500, 600 mg/kg | 2000 mg/kg | ADG↑, diarrhea rate↓, serum ALP, IgG, SOD↑, lactic acid bacteria and total anaerobic bacteria↑, E. coli↓ | Sun et al. [175] |
| ZnO-NPs | 150, 300, or 450 mg/kg | 3000 mg/kg | ADG↑, ADFI↑, intestinal morphology↑, diarrhea rate↓, Escherichia colii↓ | Pei et al. [115] |
| ZnO-NPs | 600 mg Zn/kg | 2000 mg/kg | diarrhea rate↓, antioxidant enzymes↑, tight junction proteins↑, IL-1β↓, IFN-γ↓, NF-κB↓, TNF-α↓, Streptococcus↑, Lactobacillus↑, Lactobacillus↓, Oscillospira↓, Prevotella↓ | Xia et al. [131] |
| zinc glycine chelate | 50, and 100 mg/kg | 3000 mg/kg | ADG↑, ALP↑, Cu/Zn SOD↑ | Wang et al. [173] |
| ZnGly | 400, 800, and 1200 mg/kg | 2500 mg/kg | fecal score↓, TNF-α↓, Actinobacteria↑, ZIP4↑, ZnT5↑, Enterobacteriaceae↓ | Jang et al. [174] |
| CS-Zn | 50, 100 mg/kg | 3000 mg/kg | serum DAO activities↓, D-lactate levels↓, endotoxin contents↓, intestinal morphology↑, sIgA↑, apoptotic cells↓ | Han et al. [140] |
| CS-Zn | 100 mg/kg | 1600 mg/kg | intestinal morphology↑, Lactobacillus↑, Streptococcus, Escherichia shigella↓, Actinobacillus↓, Clostridium sensu stricto 6↓, propionate↑, butyrate↑, lactate↑, IL-1β↓, TNF-α↓, MPO↓, INF-γ↓ | Hou et al. [144] |
| coated ZnO | 250, 380, 570, 760 and 1140 mg/kg | 2250 mg/kg | diarrhea rate↓, intestinal morphology↑, IGF1↑, ZO-1↑, occludin↑, IL-10↑, sIgA↑, microbiota richness↑ | Shen et al. [141] |
| coated ZnO | 100 mg/kg | 2500 mg/kg | ADG↑, goblet cell density ↑, intestinal morphology↑, fecal consistency score↓ | Kwon et al. [116] |
| coated ZnO | 562.5 mg/kg | 2250 mg/kg | diarrhea rate↓, plasma D-lactate level↓, intestinal morphology↑, occludin↑, ZO-1↑, T-AOC↑, MDA↓ | Dong et al. [176] |
| coated ZnO | 100, 200 mg/kg | 2500 mg/kg | ADG↓, gain:feed ratio↓, diarrhea rate↓ | Byun et al. [177] |
| coated ZnO | 500 mg Zn/kg | 2000 mg/kg | growth performance↑, diarrhea rate↓, barrier function↑, SCFAs↑ | Sun et al. [109] |
| coated ZnO | 100 mg/kg | 2400 mg/kg | ADG↑, intestinal morphology↑, goblet cell density↑, fecal consistency score↓, | Kim et al. [117] |
| porous ZnO | 200 mg/kg, 500 mg/kg | 2000 mg/kg | ADG↑, ADFI↑, diarrhea rate↓, Lactobacillus spp.↑, Escherichia colii, Clostridium coccoides, and Clostridium↓, intestinal morphology↑ | Peng et al. [40] |
| porous ZnO | 500, 1000 mg/kg | 3000 mg/kg | FCR↑, diarrhea rate↓ | Ouyang et al. [178] |
| porous ZnO | 500 mg /kg | 3000 mg/kg | ADG↑, ADFI↑, intestinal morphology↑, diarrhea rate↓ | Long et al. [179] |
| porous ZnO | 750 mg/kg, 1500 mg/kg | 3000 mg/kg | ADG↑, FCR↑, diarrhea rate↓, serum ALP↑, IgG↑, IGF-1↑, TGF-β↓, zonula occludens-1↑, occludin↑, IL-8↓, AQP3↓ | Peng et al. [38] |
| DS-ZnO | 500 mg Zn/kg | 2250 mg/kg | ADG↑, ADFI↑, post-weaning scour scores↓, intestinal morphology↑, occludin↑, claudin-1↑, ZO-1↑, TNF-α↓, IL-6↓, IFN-γ↓ | Hu et al. [112] |
| mZnO | 150 or 400 mg/kg | 3000 mg/kg | ADG↑, intestinal morphology↑, TNF-α↓, IFN-γ↓, occludin↑, ZO-1↑ | Grilli et al. [113] |
| SR-ZnO | 500 mg/kg | 1500 mg/kg | diarrhea rate↓, serum ALP↑, zinc bioavailability↑, Campylobacters↓, Clostridium↑ | Wang et al. [31] |
| ZnO-MMT | 250, 500, and 750 mg/kg | 2000 mg/kg | ADG↑, ADFI↑, fecal scores↓, intestinal permeability↓, digestive enzyme activities↑ | Hu et al. [120] |
| alternative ZnO | 300 mg/kg | 3000 mg/kg | diarrhea rate↓, digestive enzyme activities↑, zinc transporter proteins↑, faecal zinc emissions↓, TJ proteins↑, mucins↑, antimicrobial peptides↑, Lactobacillus↑ | Su et al. [32] |
| PNZ | 700, 1000, or 1300 mg/kg | 3000 mg/kg | intestinal morphology↑, goblet cells↑, TNF-α↓, IL-1β↓, sIgA↑, IL-4↑, MCU2↑, ZO-1↑, TLR4↓, MyD88↓ | Yu et al. [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Xiong, K.; Zeng, Y.; Fang, R. The Mechanism of Zinc Oxide in Alleviating Diarrhea in Piglets after Weaning: A Review from the Perspective of Intestinal Barrier Function. Int. J. Mol. Sci. 2024, 25, 10040. https://doi.org/10.3390/ijms251810040
Tang X, Xiong K, Zeng Y, Fang R. The Mechanism of Zinc Oxide in Alleviating Diarrhea in Piglets after Weaning: A Review from the Perspective of Intestinal Barrier Function. International Journal of Molecular Sciences. 2024; 25(18):10040. https://doi.org/10.3390/ijms251810040
Chicago/Turabian StyleTang, Xiaopeng, Kangning Xiong, Yan Zeng, and Rejun Fang. 2024. "The Mechanism of Zinc Oxide in Alleviating Diarrhea in Piglets after Weaning: A Review from the Perspective of Intestinal Barrier Function" International Journal of Molecular Sciences 25, no. 18: 10040. https://doi.org/10.3390/ijms251810040
APA StyleTang, X., Xiong, K., Zeng, Y., & Fang, R. (2024). The Mechanism of Zinc Oxide in Alleviating Diarrhea in Piglets after Weaning: A Review from the Perspective of Intestinal Barrier Function. International Journal of Molecular Sciences, 25(18), 10040. https://doi.org/10.3390/ijms251810040






