Colchicine, Caffeine, Gramine, and Their Derivatives as Potential Herbicides, Fungicides, and Insecticides
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Compound Preparation
3.2. In Silico Screening/Calculations
3.3. Fungicidal Tests
3.3.1. Activity against Phytophthora Infestans in the Microtiter Plate Assay
3.3.2. Microtiter Plate Activity against Zymoseptoria Tritici
3.3.3. Microtiter Plate Activity against Botrytis Cinerea
3.3.4. Activity against Fusarium Culmorum in the Microtiter Plate Test
3.4. Insecticidal Tests
3.4.1. Test to Evaluate the Control of Vetch Aphid (Megoura viciae) by Contact or Systemic Means
3.4.2. Test to Evaluate the Control of Caenorhabditis elegans by Contact or Systemic Means
3.4.3. Test to Evaluate the Control of Green Peach Aphid (Myzus persicae) by Systemic Means
3.4.4. Boll Weevil (Anthonomus grandis) Control Test
3.4.5. Test to Evaluate the Control of Mediterranean Fruit Fly (Ceratitis capitata)
3.4.6. Test to Evaluate the Control of Tobacco Budworm (Heliothis virescens)
3.4.7. Test to Evaluate the Control of Tobacco Budworm Yellow Fever Mosquito (Aedes aegypti) Control Assay
3.4.8. Test to Evaluate Control of Greenhouse Whitefly (Trialeurodes vaporariorum)
3.5. Herbicidal Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petit, S.; Boursault, A.; LeGuilloux, M. Weeds in Agricultural Landscapes. A Review. Agron. Sustain. Dev. 2011, 31, 309–317. [Google Scholar] [CrossRef]
- Lechenet, M.; Bretagnolle, V.; Bockstaller, C.; Boissinot, F.; Petit, M.-S.; Petit, S.; Munier-Jolain, N.M. Reconciling Pesticide Reduction with Economic and Environmental Sustainability in Arable Farming. PLoS ONE 2014, 9, e97922. [Google Scholar] [CrossRef] [PubMed]
- Lechenet, M.; Dessaint, F.; Py, G.; Makowski, D.; Munier-Jolain, N. Reducing pesticide use while preserving crop productivity and profitability on arable farms. Nat. Plants Lett. 2017, 3, 17008. [Google Scholar] [CrossRef] [PubMed]
- Mitich, L.W. Annual bluegrass (Poa annua L.). Weed Technol. 2017, 12, 414–416. [Google Scholar] [CrossRef]
- Warwick, S.I. The biology of Canadian weeds.: 37 Poa annua L. Can. J. Plant Sci. 1979, 59, 1053–1066. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Herbicide Resistant Weeds. 2019. Available online: http://www.weedscience.com (accessed on 12 December 2023).
- Mao, Q.; Huff, D.R. The evolutionary origin of Poa annua L. Crop Sci. 2012, 52, 1910–1922. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Mesarich, C.H.; Harrington, K.C. Molecular characteristics of the first case of haloxyfop-resistant Poa annua. Sci. Rep. 2020, 10, 4231. [Google Scholar] [CrossRef]
- Aksoy, A.; Dixon, J.M.; Hale, W.H. Biological flora of the British Isles. Capsella bursa-pastoris (L.) Medikus (Thlaspi bursapastoris L., Bursa bursa-pastoris (L.) Shull, Bursa pastoris (L.) Weber). J. Ecol. 1998, 86, 171–186. [Google Scholar] [CrossRef]
- Stukenbrock, E.H.; Jørgensen, F.G.; Zala, M.; Hansen, T.T.; McDonald, B.A.; Schierup, M.H. Whole-Genome and Chromosome Evolution Associated with Host Adaptation and Speciation of the Wheat Pathogen Mycosphaerella graminicola. PLoS Genet. 2010, 6, 12. [Google Scholar] [CrossRef]
- Thomas, M.C.; Heppner, J.B.; Woodruff, R.E.; Weems, H.V.; Steck, G.J.; Fasulo, T.R. Mediterranean Fruit Fly, Ceratitis capitata (Wiedemann) (Insecta: Diptera: Tephritidae) DPI Entomology Circulars; Florida Department of Agriculture & Consumer Services: Tallahassee, FL, USA, 2001; pp. 1–17. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Herbaceous Plants and Shrubs; Wiley & Sons: Chichester, UK, 2006; Volumes 1 and 2. [Google Scholar]
- Sifri, C.D.; Begun, J.; Ausubel, F.M.; Calderwood, S.B. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 2003, 71, 2208–2217. [Google Scholar] [CrossRef]
- Blanco, C.A. Heliothis virescens and Bt cotton in the United States. GM Crops Food Biotechnol. Agric. Food Chain 2012, 3, 201–212. [Google Scholar] [CrossRef] [PubMed]
- McLeod, P.J.; Steinkraus, D.C.; Correll, J.C.; Morelock, T.E. Prevalence of Erynia neoaphidis (Entomophthorales: Entomophthoraceae) infections of green peach aphid (Homoptera: Aphididae) on spinach in the Arkansas River Valley. Environ. Entomol. 1998, 27, 796–800. [Google Scholar] [CrossRef]
- Balliu, A.; Cota, E. Biological control of main greenhouse pests in Albania. Acta Hortic. 2007, 729, 489–492. [Google Scholar] [CrossRef]
- Eisen, L.; Moore, C.G. Aedes (Stegomyia) aegypti in the Continental United States: A Vector at the Cool Margin of Its Geographic Range. J. Med. Entomol. 2013, 50, 467–478. [Google Scholar] [CrossRef]
- Enforcer Products Inc.; PO Box 1060 Cartersville, GA 30120. Office of Pesticide Programs Environmental Protection Agency: Washington, DC, USA, 2016.
- Osborne, L.S.; Henley, R.W. Evaluation of Safer Agro-Chems Insecticidal Soap for the Control of Mites in the Interior Environment. Foliage Dig. 1982, 5, 10–11. [Google Scholar]
- Sporleder, M.; Lacey, L.A. Chapter 16. Biopesticides. In Insect Pests of Potato; Elsevier: Amsterdam, The Netherlands, 2013; pp. 463–497. [Google Scholar]
- Vaughan, M.A.; Vaughn, K.C. Mitotic disrupters from higher plants and their potential uses as herbicides. Weed Technol. 1988, 2, 533–539. [Google Scholar] [CrossRef]
- Cai, Q.N.; Han, Y.; Cao, Y.Z.; Hu, Y.; Zhao, X.; Bi, J.L. Detoxification of gramine by the cereal aphid sitobion avenae. J. Chem. Ecol. 2009, 35, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Hu, H.Y.; Xie, X.; Sakoda, A.; Sagehashi, M.; Li, F.M. Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2009, 91, 262–269. [Google Scholar] [CrossRef]
- Hong, Y.; Hu, H.Y.; Sakoda, A.; Sagehashi, M. Effects of allelochemical gramine on photosynthetic pigments of cyanobacterium Microcystis aeruginosa. World Acad. Sci. Eng. Technol. 2010, 47, 831–835. [Google Scholar]
- Feng, K.; Li, X.; Yu, L. Synthesis, antibacterial activity, and application in the antifouling marine coatings of novel acylamino compounds containing gramine groups. Prog. Org. Coat. 2018, 118, 141–147. [Google Scholar] [CrossRef]
- Iwata, S.; Saito, S.Y.; Kon-ya, K.; Shizuri, Y.; Ohizumi, Y. Novel marine-derived halogen-containing gramine analogues induce vasorelaxation in isolated rat aorta. Eur. J. Pharmacol. 2001, 432, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Wang, T.; Hui, H.; Wei, X.; Cui, W.; Zhou, C.; Li, H.; Wang, Z.; Guo, J.; Ma, D.; et al. Natural Products for Drug Discovery: Discovery of Gramines as Novel Agents against a Plant Virus. J. Agric. Food Chem. 2019, 67, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Petroski, R.J.; Stanley, D.W. Natural Compounds for Pest and Weed Control. J. Agric. Food Chem. 2009, 57, 8171–8179. [Google Scholar] [CrossRef]
- Miranda, F.; Fernandes, K.M.; Bernardes, R.C.; Martins, G.F. Biological, histological and immunohistochemical studies on the toxicity of spent coffee grounds and caffeine on the larvae of Aedes aegypti (Diptera: Culicidae). Environ. Pollut. 2021, 271, 116307–116310. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, B.; Guo, Y.; Zhou, Y.; Pan, L.; Xiong, L.; Yu, S.; Li, Z. Synthesis and Biological Activities of Novel Methyl Xanthine Derivatives. Chem. Res. Chin. Univ. 2014, 30, 98–102. [Google Scholar] [CrossRef]
- Kurek, J.; Boczoń, W.; Przybylski, P.; Brzeziński, B. Complexes with Lithium, Sodium and Potassium Salts. J. Mol. Struct. 2007, 846, 13–27. [Google Scholar] [CrossRef]
- Kurek, J.; Kwaśniewska-Sip, P.; Myszkowski, K.; Cofta, G.; Barczyński, P.; Murias, M.; Kurczab, R.; Śliwa, P.; Przybylski, P. Antifungal, anticancer and docking studies of colchiceine complexes with monovalent metal cation salts. Chem. Biol. Drug Des. 2019, 94, 1930–1946. [Google Scholar] [CrossRef]
- Kurek, J.; Kwaśniewska-Sip, P.; Myszkowski, K.; Cofta, G.; Barczyński, P.; Pietruś, W.; Murias, M. Alkaloid from Colchicum species in complexes with lithium, sodium, potassium and magnesium cations spectroscopic characterization, semiempirical and theoretical calculation, fungicidal and cytotoxic activity. J. Mol. Struct. 2020, 1204, 127520. [Google Scholar] [CrossRef]
- Kurek, J.; Bartkowiak, G.; Jankowski, W.; Kwaśniewska-Sip, P.; Schroeder, G.; Hoffmann, M.; Cofta, G.; Barczyński, P. Human Body Fluid Ions In colchicine complexes ESI MS, MADLI MS, Spectroscopic, DFT Studies and Fungicidal Activity of colchicine complexes With Sodium, Potassium, Magnesium and calcium carbonates and Sulphates. IOSR J. Pharm. 2016, 6, 40–54. [Google Scholar] [CrossRef]
- Kurek, J.; Barczyński, P. Colchiceine complexes with lithium, sodium and potassium salts Spectroscopic studies. Croat. Chem. Acta 2016, 89, 297–308. [Google Scholar] [CrossRef]
- Kurek, J.; Myszkowski, K.; Boczoń, W.; Borowiak, T.; Wolska, I.; Murias, M. Synthesis of sulfur containing colchicine derivatives and their biological evaluation as cytotoxic agents. Lett. Drug Disc. Des. 2014, 11, 279–286. [Google Scholar] [CrossRef]
- Kurek, J.; Kwaśniewska-Sip, P.; Myszkowski, K.; Jasiewicz, B.; Murias, M.; Cofta, G.; Barczynski, P.; Kurczab, R. 7-Deacetyl-10-alkilthiocolchicine derivatives—New compounds with potent anticancer and fungicidal activity. Med. Chem. Comm. 2018, 9, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Kurek, J.; Kwaśniewska-Sip, P.; Myszkowski, K.; Cofta, G. 10-Methylthiocolchicine complexes with lithium, sodium, potassium, rubidium and cesium metal cations salts-cytotoxic, semiempirical and molecular modeling studies. Polyhedron 2020, 190, 114791–114801. [Google Scholar] [CrossRef]
- Kwaśniewska-Sip, P.; Cofta, G.; Kurek, J.; Barczyński, P. Fungicidal activity of alkaloid from Colchicum species and its complexes. Ann. WULS-SGGW For. Wood Technol. 2018, 104, 252–254. [Google Scholar]
- Al-Ani, E.H.; Al-Khazraji, H.I.; Al-Shaibani, M.B. The Use of Alkaloids as Botanical Insecticides. ACE Res. J. Microbiol. Biotechnol. 2022, 33, 33–41. [Google Scholar]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Muzquiz, M.; de la Cuadra, C.; Cuadrado, C.; Burbano, C.; Calvo, R. Herbicide-like effect of Lupinus alkaloids. Ind. Crops Prod. 1994, 2, 273–280. [Google Scholar] [CrossRef]
- El Saved, K.A. Natural products as antiviral agent. Stud. Nat. Prod. Chem. 2000, 24, 473–572. [Google Scholar]
- Zhang, J.; Jia, Q.; Li, N.; Gu, L.; Dan, W.; Dai, J. Recent Developments of Gramine: Chemistry and Biological Activity. Molecules 2023, 28, 5695. [Google Scholar] [CrossRef]
- AlEraky, D.M.; Abuohashish, H.M.; Gad, M.M.; Alshuyukh, M.H.; Bugshan, A.S.; Almulhim, K.S.; Mahmoud, M.M. The Antifungal and Antibiofilm Activities of Caffeine against Candida albicans on Polymethyl Methacrylate Denture Base Material. Biomedicines 2022, 10, 2078. [Google Scholar] [CrossRef]
- Meredith, S.E.; Juliano, L.M.; Hughes, J.R.; Griffiths, R.R. Caffeine Use Disorder: A Comprehensive Review and Research Agenda. J. Caffeine Res. 2013, 3, 114–130. [Google Scholar] [CrossRef]
- Kozanecka-Okupnik, W.; Sierakowska, A.; Berdzik, N.; Kowalczyk, I.; Mrówczyńska, L.; Jasiewicz, B. New triazole-bearing gramine derivatives–synthesis, structural analysis and protective effect against oxidative haemolysis. Nat. Prod. Res. 2022, 36, 3413–3419. [Google Scholar] [CrossRef] [PubMed]
- Jasiewicz, B.; Sierakowska, A.; Jankowski, W.; Hoffmann, M.; Piorońska, W.; Górnicka, A.; Bielawska, A.; Bielawski, K.; Mrówczyńska, L. Antioxidant and cytotoxic activity of new di- and polyamine caffeine analogues. Free. Radic. Res. 2018, 52, 724–736. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Kozanecka-Okupnik, W.; Przygodzki, M.; Warżajtis, B.; Rychlewska, U.; Pospieszny, T.; Mrówczyńska, L. Synthesis, antioxidant and cytoprotective activity evaluation od C-3 substituted indole derivatives. Sci. Rep. 2021, 10, 15425. [Google Scholar] [CrossRef]
- Jasiewicz, B.; Sierakowska, A.; Wandyszewska, N.; Warżajtis, B.; Rychlewska, U.; Wawrzyniak, R.; Mrówczyńska, L. Antioxidant properties of thio-caffeine derivatives: Identification of the newly synthesized 8-[(pyrrolidin-1-ylcarbonothioyl)sulfanyl]caffeine as antioxidant and highly potent cytoprotective agent. Bioorg. Med. Chem. Lett. 2016, 26, 3994–3998. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Zimmer, A.; Labs, S.; Buchholz, S.; Hinz, S. Life & Brain GMBH. 8-Triazolylxanthine derivatives, processes for their production and their use as adenosine receptor antagonists. WO2012/76974, A1, 14 June 2012. [Google Scholar]
- Kaplánek, R.; Jakubek, M.; Rak, J.; Kejik, Z.; Havlik, M.; Dolenskŷ, B.; Frydrych, I.; Hajdŭch, M.; Kolář, M.; Bogdanová, K.; et al. Caffeine–hydrazones as anticancer agents with pronounced selectivity toward T-lymphoblastic leukemia cells. Bioorg. Chem. 2015, 60, 19–29. [Google Scholar] [CrossRef]
- Sierakowska, A.; Jasiewicz, B.; Piosik, Ł.; Mrówczyńska, L. New C8-substituted caffeine derivatives as promising antioxidants and cytoprotective agents in human erythrocytes. Sci. Rep. 2023, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. In Silico Prediction of Aqueous Solubility Using Simple QSPR Models: The Importance of Phenol and Phenol-like Moieties. J. Chem. Inf. Model. 2012, 52, 2950–2957. [Google Scholar] [CrossRef]
- SILICOS-IT Fragmental Method Calculated by FILTER-IT Program, Version 1.0.2., Courtesy of SILICOS-IT. Available online: http://www.silicos-it.com (accessed on 20 June 2024).
- MolInspiration Cheminformatics. Available online: https://www.molinspiration.com/ (accessed on 20 June 2024).
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef]
- Log P (XLOGP3) Atomistic and knowledge-based method calculated by XLOGP program, version 3.2.2., courtesy of CCBG, Shanghai Institute of Organic Chemistry.
- Wildman, S.A.; Crippen, G.M. Prediction of Physicochemical Parameters by Atomic Contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Morigichi, I.; Hirono, S.; Liu, Q.; Nakagome, I.; Matsushita, Y. Simple method of calculating octanol/water partition coefficient. Chem. Pharm. Bull. 1992, 40, 127–130. [Google Scholar] [CrossRef]
- Lipinski, P.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Log P (MLOGP) Hybrid Fragmental/Topological Method Calculated by FILTER-IT Program, Version 1.0.2, Courtesy of SILICOS-IT. Available online: http://www.silicos-it.com (accessed on 20 June 2024).
- Log P (SILICOS-IT). Available online: http://www.swissadme.ch/ (accessed on 20 June 2024).
- Consensus Log P average of All Five Predictions 2–6. Available online: http://www.swissadme.ch/ (accessed on 20 June 2024).
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
 | No. | R | R1 | Ref. |
| 1 | -OCH3 colchicine | -COCH3 | - | |
| 2 | -OH colchiceine | -COCH3 | [35] | |
| 3 | -SCH3 | -COCH3 | [36] | |
| 4 | -SCH2CH3 | -COCH3 | [36] | |
| 5 | -SCH2CH2CH3 | -COCH3 | [36] | |
| 6 | -SCH(CH3)2 | -COCH3 | [36] | |
| 7 | -SCH2CH2CH2CH3 | -COCH3 | [36] | |
| 8 | -SCH3 | -H | [37] | |
| 9 | -SCH2CH3 | -H | [37] | |
| 10 | -SCH2CH2CH3 | -H | [37] | |
| 11 | -SCH(CH3)2 | -H | [37] | |
| 12 | -SCH2CH2CH2CH3 | -H | [37] |
| Compounds | No. | R1 | R2 | Ref. |
|---|---|---|---|---|
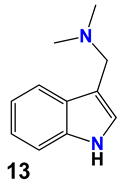  | 13 | -N(CH3)2 gramine | -H | |
| 14 | -OCOCH3 | -COCH3 | [47] | |
| 15 | -OC CH | -H | [47] | |
| 16 | -OCH2CH3 | -(CH2)3Br | [47] | |
| 17 | -OCH2CH3 | -(CH2)3N3 | [47] | |
| 18 | -OCH2CH3 | -(CH2)5Br | [47] | |
| 19 | -OCH2CH3 | -(CH2)5N3 | [47] | |
| 20 | 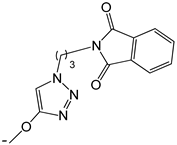 | -H | [47] | |
| 21 | 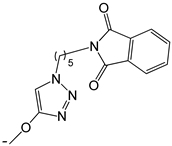 | -H | [47] | |
| 22 | 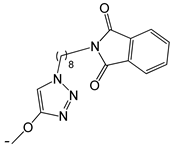 | -H | [47] | |
| 23 | 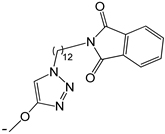 | -H | [47] | |
| 24 | -OC2H5 | 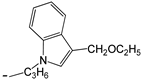 | [47] | |
| 25 | -OC2H5 | 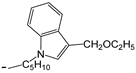 | [47] | |
| 26 | -OH | -COCH3 | [48] | |
| 27 | -OC2H5 | -H | [49] | |
| 28 | -OCH2CH2CH3 | -H | [49] | |
| 29 | -OCH2CH2CH2CH3 | -H | [49] | |
| 30 | -OCH2CH2CH2CH2CH3 | -H | [49] | |
| 31 | -OCH(CH3)2 | -H | [49] | |
| 32 | -OCH2CH2CH(CH3)2 | -H | [49] | |
| 33 |  | -H | [49] | |
| 34 | 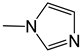 | -H | [49] | |
| 35 | 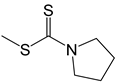 | -H | [49] |
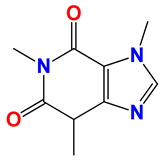 Caffeine 36 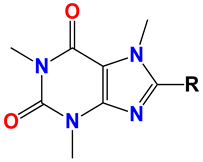 | No. | R1 | Ref. |
| 36 | -H | - | |
| 37 | -Br | [50] | |
| 38 | -N3 | [51] | |
| 39 | -SCH3 | [50] | |
| 40 | -SC2H5 | [50] | |
| 41 | -SCH2CH2CH3 | [50] | |
| 42 | -SCH(CH3)2 | [50] | |
| 43 | -SC(CH3)3 | [50] | |
| 44 | 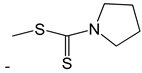 | [50] | |
| 45 | -NHNH2 | [52] | |
| 46 | -NH(CH2)2NH2 | [48] | |
| 47 | 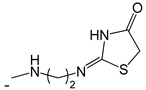 | [53] | |
| 48 | 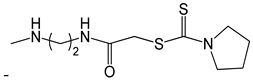 | [53] |
| Comp. No. | Fungi Stains | |||
|---|---|---|---|---|
| Botrytis cinerea | Fusarium culmorum | Phytophthora infestans | Zymoseptoria tritici | |
| 13 | 3 | 3 | 3 | 3 |
| 14 | 3 | 3 | 2 | 2 |
| 15 | 3 | 3 | 3 | 3 |
| 17 | 3 | 3 | 3 | 3 |
| 26 | 3 | 3 | 3 | 3 |
| 27 | 2 | 3 | 3 | 3 |
| 28 | 3 | 3 | 3 | 3 |
| 29 | 1 | 2 | 3 | 1 |
| 30 | 3 | 3 | 3 | 3 |
| 31 | 2 | 3 | 3 | 2 |
| 32 | 3 | 3 | 3 | 3 |
| 33 | 3 | 3 | 3 | 3 |
| 34 | 3 | 3 | 3 | 3 |
| 35 | 1 | 1 | 2 | 1 |
| Comp. | Water Solubility | ||
|---|---|---|---|
| LogS (ESOL) [54] /Class | Log S (Ali) [55] /Class | Log S (SILICOS-IT) [56] /Class | |
| 13 | −2.42/soluble | −1.80/very soluble | −3.79/soluble |
| 14 | −2.39/soluble | −2.19/soluble | −3.28/soluble |
| 15 | −2.69/soluble | −2.39/soluble | −3.51/soluble |
| 17 | −3.35/soluble | −4.22/moderate soluble | −4.76/moderate soluble |
| 26 | −1.99/very soluble | −1.48/very soluble | −2.63/soluble |
| 27 | −2.48/soluble | −2.11/soluble | −4.22/moderate soluble |
| 28 | −2.79/soluble | −2.66/soluble | −4.63/moderate soluble |
| 29 | −3.01/soluble | −3.03/soluble | −5.04 moderate soluble |
| 30 | −3.34/soluble | −3.59/soluble | −5.44/moderate soluble |
| 31 | −2.80/soluble | −2.57/soluble | −4.25 moderate soluble |
| 32 | −3.35/soluble | −3.49/soluble | −5.07/moderate soluble |
| 33 | −3.54/soluble | −3.28/soluble | −5.87/moderate soluble |
| 34 | −2.65/soluble | −1.95/very soluble | −4.22/moderate soluble |
| 35 | −3.73/soluble | −4.56/moderate soluble | −4.39/moderate soluble |
| Comp. | Lipophilicity | |||||||
|---|---|---|---|---|---|---|---|---|
| LogP [57] | LogP (iLOGP) [58] | LogP (XLOGP3) [59] | LogP (WLOGP) [60,61,62] | LogP (MLOGP) [63] | LogP (SILICOS-IT) [64] | Consensus LogP [65] | Log P [66] | |
| 13 | 1.89 | 1.88 | 1.78 | 2.08 | 1.55 | 2.41 | 1.94 | 2.23 |
| 14 | 2.45 | 2.43 | 1.57 | 2.21 | 1.84 | 2.06 | 2.02 | 2.36 |
| 15 | 1.82 | 2.03 | 2.23 | 2.20 | 1.47 | 3.00 | 2.19 | 2.28 |
| 17 | 3.46 | 3.02 | 3.21 | 3.73 | 1.27 | 1.98 | 2.64 | 3.33 |
| 26 | 1.75 | 1.77 | 1.00 | 1.64 | 1.40 | 1.64 | 1.49 | 1.79 |
| 27 | 2.42 | 2.01 | 1.96 | 2.55 | 1.55 | 3.18 | 2.25 | 2.7 |
| 28 | 2.92 | 2.26 | 2.49 | 2.94 | 1.84 | 3.53 | 2.61 | 3.09 |
| 29 | 3.48 | 2.58 | 2.85 | 3.33 | 2.11 | 3.89 | 2.95 | 3.48 |
| 30 | 3.98 | 2.85 | 3.39 | 3.72 | 2.37 | 4.26 | 3.32 | 3.87 |
| 31 | 2.78 | 2.29 | 2.40 | 2.94 | 1.84 | 3.35 | 2.56 | 3.09 |
| 32 | 3.69 | 2.67 | 3.29 | 3.58 | 2.37 | 4.09 | 3.20 | 3.73 |
| 33 | 3.48 | 2.05 | 2.52 | 2.43 | 2.75 | 3.41 | 2.63 | 2.90 |
| 34 | 1.95 | 1.48 | 1.63 | 2.41 | 1.16 | 2.42 | 1.82 | 2.41 |
| 35 | 3.42 | 2.61 | 3.28 | 3.25 | 2.29 | 4.63 | 3.21 | 3.72 |
| Compounds Structures | Dotted Models | Space-Filling CPK Models |
|---|---|---|
Gramine 13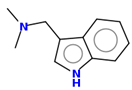 |   | |
14 |   | |
15 |   | |
17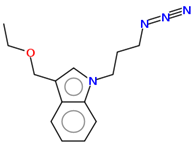 |   | |
26 |   | |
27 |   | |
28 |   | |
29 |   | |
30 |   | |
31 |   | |
32 |   | |
33 |   | |
34 |   | |
35 |   | |
| Comp. | Molecular Weight | Number of Hydrogen Bond Acceptors | Number of Hydrogen Bond Donors | Number of Atoms | Number of Bonds | Number of Rotable Bonds | Molecular Refractivity | Topological Polar Surface Area | Predicted LD50 mg/kg | Precicted Toxicity Active 1–6 Inactive |
|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 174.24 | 15 | 1 | 27 | 28 | 2 | 56.77 | 19.03 | 380 | 4 |
| 14 | 231.25 | 16 | 0 | 30 | 31 | 4 | 64.29 | 48.3 | 500 | 4 |
| 15 | 171.2 | 10 | 1 | 22 | 23 | 2 | 52.13 | 25.02 | 1420 | 4 |
| 17 | 258.32 | 20 | 0 | 37 | 38 | 7 | 73.3 | 63.91 | 500 | 4 |
| 26 | 189.21 | 13 | 1 | 25 | 26 | 2 | 54.56 | 42.23 | 500 | 4 |
| 27 | 175.23 | 14 | 1 | 26 | 27 | 3 | 53.93 | 25.02 | 1425 | 4 |
| 28 | 189.25 | 16 | 1 | 29 | 30 | 4 | 58.77 | 25.0 | 5000 | 5 |
| 29 | 203.28 | 18 | 1 | 32 | 33 | 5 | 63.58 | 25.02 | 1000 | 4 |
| 30 | 217.31 | 20 | 1 | 35 | 36 | 6 | 68.38 | 25.02 | 1000 | 4 |
| 31 | 189.25 | 16 | 1 | 29 | 30 | 3 | 58.77 | 25.02 | 1425 | 4 |
| 32 | 217.31 | 20 | 1 | 35 | 36 | 5 | 68.38 | 25.02 | 1000 | 4 |
| 33 | 276.29 | 15 | 1 | 33 | 36 | 2 | 83.06 | 53.17 | 2000 | 4 |
| 34 | 197.24 | 12 | 1 | 26 | 28 | 2 | 59.83 | 33.61 | 1000 | 4 |
| 35 | 276.42 | 19 | 1 | 34 | 36 | 4 | 87.38 | 76.42 | 5600 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurek, J.; Sierakowska, A.; Berdzik, N.; Jasiewicz, B. Colchicine, Caffeine, Gramine, and Their Derivatives as Potential Herbicides, Fungicides, and Insecticides. Int. J. Mol. Sci. 2024, 25, 10081. https://doi.org/10.3390/ijms251810081
Kurek J, Sierakowska A, Berdzik N, Jasiewicz B. Colchicine, Caffeine, Gramine, and Their Derivatives as Potential Herbicides, Fungicides, and Insecticides. International Journal of Molecular Sciences. 2024; 25(18):10081. https://doi.org/10.3390/ijms251810081
Chicago/Turabian StyleKurek, Joanna, Arleta Sierakowska, Natalia Berdzik, and Beata Jasiewicz. 2024. "Colchicine, Caffeine, Gramine, and Their Derivatives as Potential Herbicides, Fungicides, and Insecticides" International Journal of Molecular Sciences 25, no. 18: 10081. https://doi.org/10.3390/ijms251810081





