Endocrine-Disrupting Chemicals and the Development of Diabetes Mellitus Type 1: A 5-Year Systematic Review
Abstract
:1. Introduction
1.1. Endocrine Disrupting Chemicals
1.2. Diabetes Mellitus
1.2.1. Diabetes Mellitus Type 1
1.2.2. Diabetes Mellitus Type 1 and EDCs (Figure 1)
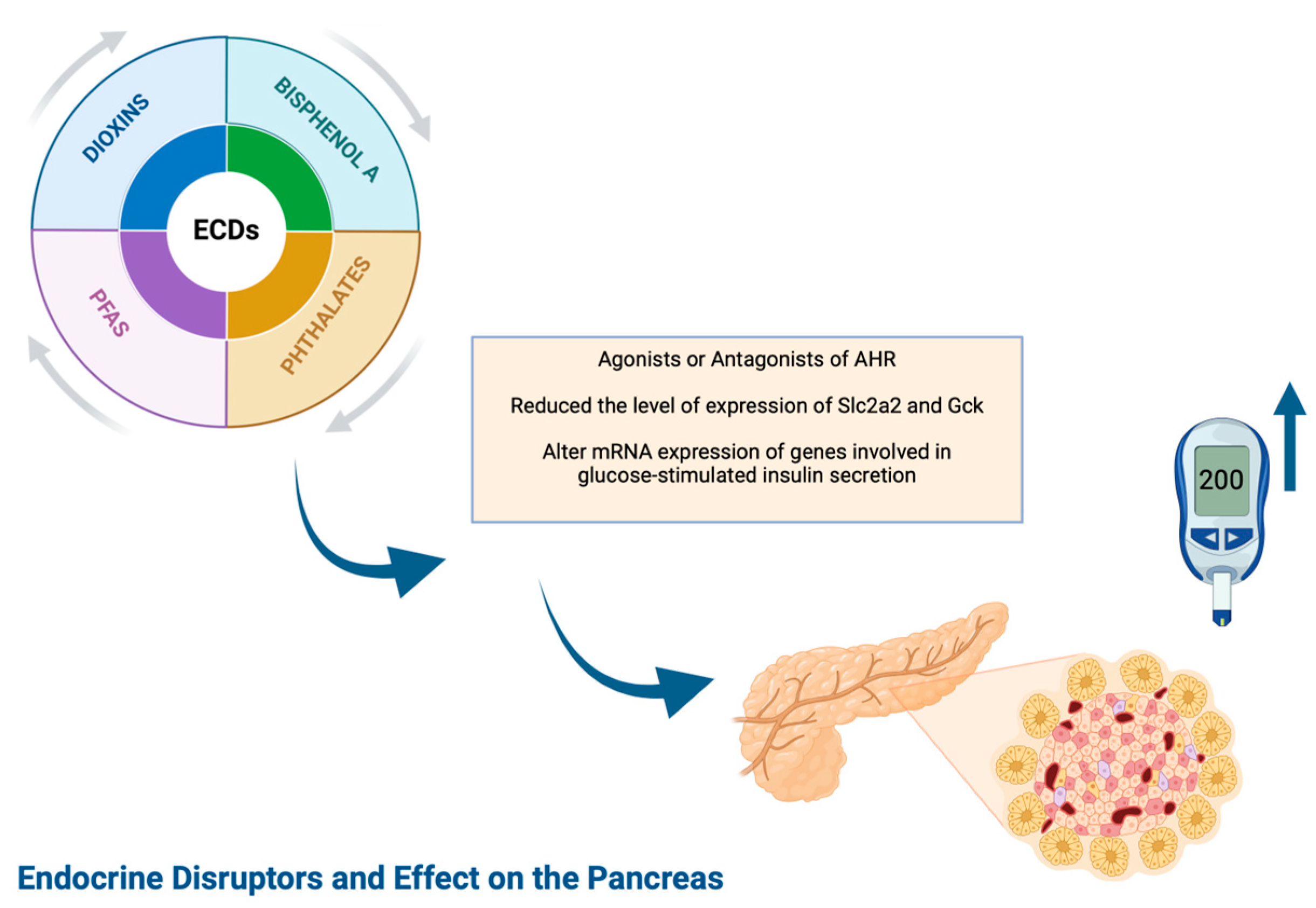
1.2.3. Epidemiology
1.2.4. Stages of Diabetes Mellitus Type 1
1.2.5. Diagnostic Criteria of T1DM (Figure 2)
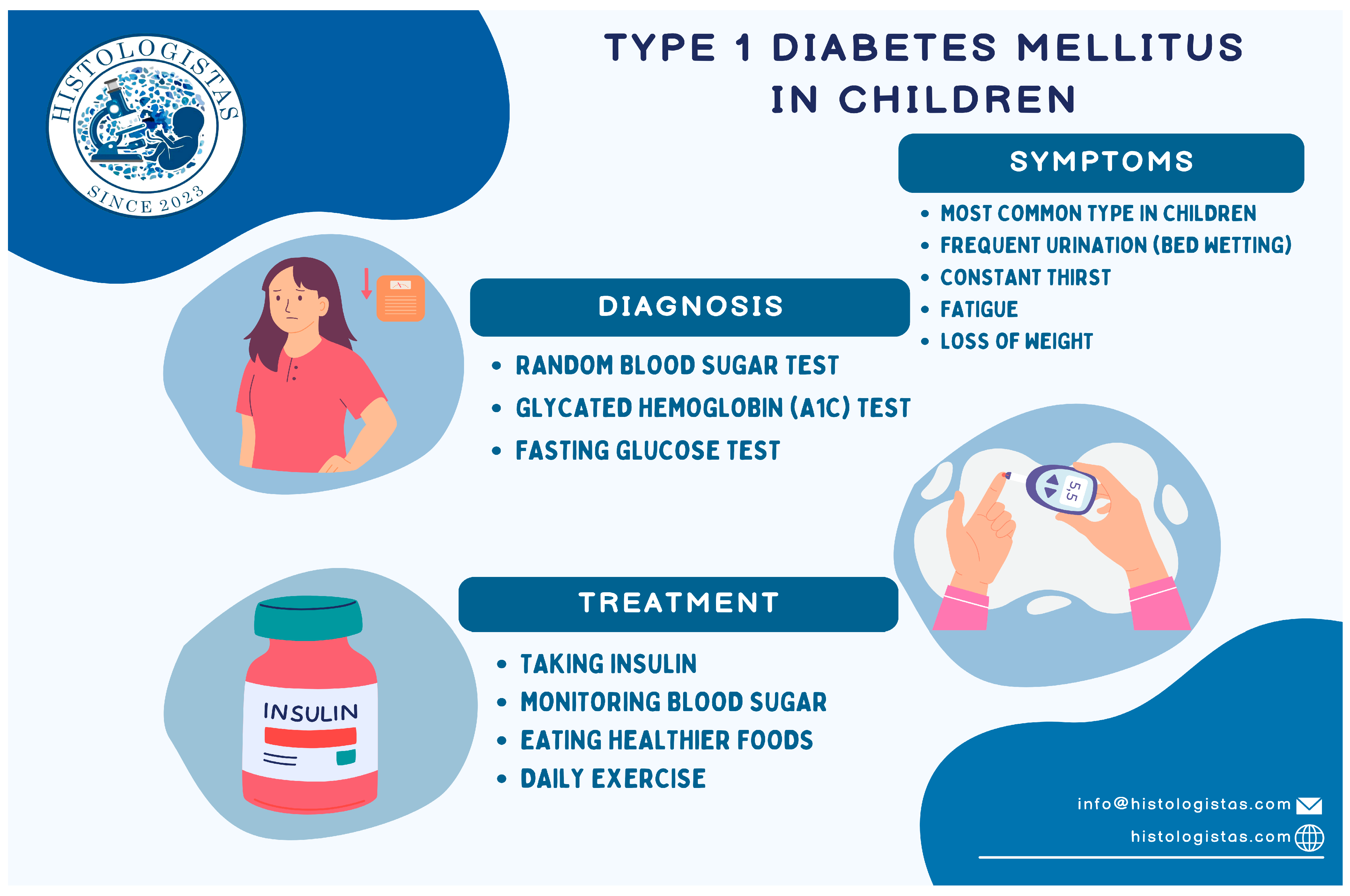
1.2.6. Basic Treatment Options for T1DM (Figure 2)
2. Methods
2.1. Purpose of Study
2.2. PICO Table–PRISMA Flow Diagram–Table–Quality Assessment
3. Results
3.1. PICO Table
3.2. PRISMA Flow Diagram
3.3. Quality Assessment
3.3.1. Newcastle–Ottawa Scale (NOS)
3.3.2. ARRIVE Quality Assessment Scale
| Authors | Study Type | Type of ECDS | ECDS Correlation with T1DM | Number of Population | Method of Assessment | Risk Factors | Sex | Age |
|---|---|---|---|---|---|---|---|---|
| Salo HM, 2019 [10] | Cohort | 1. Persistent organic pollutants (POPs) 2. dioxins 3. polychlorinated biphenyls (PCBs) 4. Pesticides 5. Brominated flame-retardants | Could not observe any definite associations between increased exposure to chemical pollutants at birth, at 12 or at 48 months of age, and risk of β-cell autoimmunity. The current work indicates that prenatal or early childhood exposure to POPs, including PFASs, is not an apparent risk factor for later β-cell autoimmunity. In 48-month-old children, PFDA was above the LOQ in 34% of the autoantibody-negative children, in 63% of the autoantibody-positive children, and in 88% of the children diagnosed with type 1 diabetes. PFDA has been demonstrated to interfere with the function of thyroid hormones in in vitro studies, and endocrine disruption is an interesting mode of action of PFDA in biological systems. | 136 | 1. FINDIA pilot study 2. DIABIMMUNE study | Autoantibody positive cases, HLA risk genotype, Breastfeeding, Formula feeding | XX, XY | from newborns to 6 years old |
| Bresson SE, 2019, [12] | Cohort | 1. Polychlorinated biphenyls (PCBs) 2. Organochlorine pesticides 3. POPs | There was a significant association between T1D/IS and several POPs including p, p’-DDE, trans-nonachlor and PCB-153. PCB-153 and p, p’-DDE reduce insulin production and secretion in β-cells. Interestingly, PCB-153 but not p, p’-DDE, reduced the expression level of Slc2a2 and Gck. PCB-153 and p, p’-DDE alter mRNA expression of genes involved in glucose-stimulated insulin secretion. | 442 | SEARCH Case-Control (SEARCH-CC) | BMI, Insulin Sensitivity, Insulin Resistance | XX, XY | 10 to 22 years |
| Dufour, P, 2023 [13] | Cohort | 7 phthalate metabolites, 4 parabens, 7 bisphenols, benzophenone 3 and triclosan were measured in urine, while 15 organochlorine pesticides, 4 polychlorinated biphenyls (PCBs) and 7 perfluoroalkyl substances 1. Phthalate metabolites 2. PCBs 3. Bisphenols 4. PCB 5. Miscellaneous, OCP, PFAS | This work investigated the link between the exposure to some environmental pollutants, from persistent organic pollutants to some non-persistent plasticizers and antimicrobials, and thyroid disorders in type 1 diabetes children. Associations between the levels (in serum or urine) of some PFASs, PCBs, phthalates and bisphenols and thyroid hormone were highlighted, suggesting an impact of these pollutants on the thyroid function in this population suspected to be particularly vulnerable toward endocrine disruption. | 54 | Urine and Blood Analysis | - | XX, XY | 3 to 18 years |
| Lee, I, 2021 [11] | Cohort | Phthalates and bisphenol A (BPA), parabens | BPA in urine was associated with higher rates of DM | 3787 | 1. Korean National Environmental Health Survey (KoNEHS) 2. Concentrations of phthalate metabolites, BPA, and parabens were measured in the spot urine samples using liquid–liquid extraction and ultraperformance liquid chromatography mass spectrometry separation, followed by electrospray ionization and tandem mass spectrometry 3. Urinary dilution, in addition to two traditional methods (i.e., Cr adjustment and SG adjustment), a CAS was used | - | XX, XY | 19 and older |
| Authors | Type of Study | Type of ECDS | ECDS Correlation with T1DM | Number of Population | Method of Assessment | Sex | Age |
|---|---|---|---|---|---|---|---|
| Xu J, 2019 [14] | Basic Research Study | Bisphenol A (BPA) | Not statistically significant, but a shift towards accelerated T1D development was observed | 30 | BW and non-fasting BGLs were measured every 1–2 weeks. Accu-Chek Diabetes monitoring kit (Roche Diagnostics, Indianapolis, IN, USA) or Contour Blood Glucose Meter (Ascensia Diabetes Care, Parsippany, NJ, USA) were used to measure BGLs from a small sample of venous blood (tail nick). | XX | 8 to 12 weeks old |
| McDonough CM, 2022 [15] | Basic Research Study | Bisphenol S (BPS) | Significant adverse effect | 12 | Body weight, blood glucose measurement, diabetic incidence, GTT and ITT, behaviour test, Y-maze test, Flow Cytometric Analysis | XX, XY | 10 weeks |
| Sinioja T, 2022 [16] | Basic Research Study | Persistent organic pollutants (POPs), organochlorides, organobromides, and per- and polyfluoroalkyl substances (PFAS) | Risk of T1D | 29 | Folch procedure for Lipidomic Analysis | XX, XY | 8 to 10 weeks old |
| Xu J, 2019 [17] | Basic Research Study | Bisphenol A (BPA) | Sex plays an important role in BPA altering T1D risk. BPA accelerated T1D development in adult NOD females but delayed T1D development in male mice. | - | Tolerance tests and insulin measurement, antibody measurement, cytokine/chemokine measurement, GMB, bioinformatics and metabolomics and bioinformatics analysis | XX, XY | 8 to 12 weeks old |
| Xu J, 2019 [18] | Basic Research Study | Bisphenol S (BPS) | PS exposure had dose-related protective effects on T1D in females. This suggests that BPS uses different mechanisms from BPA to alter glucose homeostasis and T1D | - | Tolerance tests and insulin measurement, antibody measurement, cytokine/chemokine measurement, GMB, bioinformatics and metabolomics and bioinformatics analysis | 8 to 15 weeks old |
| Authors | Study Type | Type of ECDS | ECDS Correlation with T1DM | Doi |
|---|---|---|---|---|
| Melissa and Cairro, 2023 [19] | Review | Phthalates | There is a possible link between the exposure to phthalates and the development of DM. Phthalates may lead to insulin resistance and consequent diabetes mellitus through oxidative stress, the activation of different hormone receptors (PPAR and ER), and impaired inflammatory factors. | 10.3390/metabo13060746 |
| Prediere et al., 2020 [20] | Review | Chemical substances (bisphenol A; pesticides; phthalates; polychlorinated biphenyls; polyfluorinated substances) | They could affect the development and the function of the immune system or of the β-cells function, promoting autoimmunity and increasing the susceptibility to autoimmune attack. However, the studies are few and demonstrated contradictory results, according to gender and age. | 10.3390/ijms21082937 |
| HInault et al., 2023 [21] | Review | Organochlorine (OCs) pesticides: dichloro-diphenyl-trichloroethane (DDT) and its metabolites (chlordane) and industrial chemicals: polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDE), dioxins (TCDD: 2,3,7,8-tétrachlorodibenzo-p-dioxine), and per- and polyfluoroalkyl substances (PFAs), bisphenol A (BPA) | It remains difficult to form a comprehensive view on the causal relationship between EDCs and diabetes (both T1DM and T2DM), and further experiments are required. | 10.3390/ijms24054537 |
| Khali et al., 2023 [22] | Review | Ambient air pollution, persistent organic pollutants (POPs), metals (bisphenol A [BPA]), phthalates, polybrominated diphenyl ethers (PBDEs) | The research has shown inconsistent results regarding direct pathogenesis, since these diseases are multifactorial. Many studies attempted to determine the impact of isolated atmospheric compounds but did not taking into consideration their compounding effects, and others used smaller sample sizes. | 10.3390/ijms24108870 |
| Heo and Kim, 2021 [23] | Review | Ambient air pollution (PM, NO2, and NOx) | Altered immune response, oxidative stress, neuroinflammation, inadequate placental development, and epigenetic modulation are some of the underlying factors that have been identified. However, it is difficult to demonstrate causality. | 10.6065/apem.2142132.066 |
| Jiang et al., 2023 [24] | Review | bisphenol A (BPA) | BPA exposure is associated with target organ damage in DM and may exacerbate the progression of some chronic complications of DM. | 10.1016/j.heliyon. 2023.e16340 |
| Ibarra et al., 2020 [25] | Review | bisphenol A (BPA) & phthalates | There is association with a wide range of reproductive, metabolic and neurological diseases, as well as hormone-related cancers. | 10.1016/j.envpol.2020.116380 |
| Selection | Comparability | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|
| Source | Representativeness of the Sample | Sample Size | Non–Respondents | Ascertainment of the Exposure | The Subjects in Different Outcome Groups Are Comparable | Assessment of the Outcome | Statistical Test | Quality |
| Salo HM, 2019 [10] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Bresson SE, 2019, [11] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 6 |
| Lee, I, 2021 [12] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Dufour, P, 2023 [13] | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 5 |
| Source | Study Design | Sample Size | Inclusion and Exclusion Criteria | Randomisation | Blinding | Outcome Measures | Statistical Methods | Experimental Animals | Experimental Procedures | Results | Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Xu J, 2019 [14] | + | + | + | + | ? | + | + | + | + | + | 9 |
| McDonough CM, 2022 [15] | + | + | ? | ? | ? | + | + | + | + | + | 7 |
| Sinioja T, 2022 [16] | + | + | - | + | ? | ? | + | + | + | + | 7 |
| Xu J, 2019 [17] | + | - | - | + | ? | + | + | + | + | + | 7 |
| Xu J, 2019 [18] | + | - | - | + | ? | + | + | + | + | + | 7 |
4. Discussion
4.1. Correlation between ECDs and T1DM Based on Human Cohort Studies
4.2. Correlation between ECDs and T1DM Based on Basic Research Animal Studies
4.3. Correlation between ECDs and T1DM Based on the Literature and Systematic Reviews
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Endocrine Society. Endocrine-Disrupting Chemicals (EDCs) [Internet]; Endocrine Society: Washington, DC, USA, 2023; Available online: https://www.endocrine.org/patient-engagement/endocrine-library/edcs (accessed on 11 August 2024).
- National Institute of Environmental Health Sciences. Endocrine Disruptors [Internet]; NIEHS: Durham, NC, USA, 2023. Available online: https://www.niehs.nih.gov/health/topics/agents/endocrine (accessed on 11 August 2024).
- Wiley Online Library [Internet]; Wiley: Hoboken, NJ, USA, 2024; Available online: https://onlinelibrary.wiley.com/ (accessed on 12 June 2024).
- Lucier, J. Type 1 Diabetes [Internet]. StatPearls. 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507713/#:~:text=Type%201%20diabetes%20mellitus%20(T1D,an%20automated%20insulin%20delivery%20system (accessed on 12 June 2024).
- Diabetes Mellitus in Children and Adolescents—Pediatrics [Internet]. MSD Manual Professional Edition. Available online: https://www.msdmanuals.com/professional/pediatrics/endocrine-disorders-in-children/diabetes-mellitus-in-children-and-adolescents# (accessed on 12 June 2024).
- BridgetChapple. Ketones and Diabetes [Internet]. Diabetes UK. Available online: https://www.diabetes.org.uk/guide-to-diabetes/managing-your-diabetes/ketones-and-diabetes#:~:text=Ketones%20are%20a%20type%20of,without%20it%20being%20a%20problem (accessed on 12 June 2024).
- Diabetic Ketoacidosis (DKA)—Endocrine and Metabolic Disorders [Internet]. MSD Manual Professional Edition. Available online: https://www.msdmanuals.com/professional/endocrine-and-metabolic-disorders/diabetes-mellitus-and-disorders-of-carbohydrate-metabolism/diabetic-ketoacidosis-dka (accessed on 12 June 2024).
- Kim, K. The Role of Endocrine Disruption Chemical-Regulated Aryl Hydrocarbon Receptor Activity in the Pathogenesis of Pancreatic Diseases and Cancer. Int. J. Mol. Sci. 2024, 25, 3818. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pandol, S.J. Pancreatic Embryology and Development [Internet]. The Exocrine Pancreas. 1970. Available online: https://www.ncbi.nlm.nih.gov/books/NBK54135/ (accessed on 12 June 2024).
- Salo, H.M.; Koponen, J.; Kiviranta, H.; Rantakokko, P.; Honkanen, J.; Härkönen, T.; Ilonen, J.; Virtanen, S.M.; Tillmann, V.; Knip, M.; et al. No evidence of the role of early chemical exposure in the development of β-cell autoimmunity. Environ. Sci. Pollut. Res. Int. 2019, 26, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Park, Y.J.; Kim, M.J.; Kim, S.; Choi, S.; Park, J.; Cho, Y.H.; Hong, S.; Yoo, J.; Park, H.; et al. Associations of urinary concentrations of phthalate metabolites, bisphenol A, and parabens with obesity and diabetes mellitus in a Korean adult population: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ. Int. 2021, 146, 106227. [Google Scholar] [CrossRef] [PubMed]
- Bresson, S.E.; Isom, S.; Jensen, E.T.; Huber, S.; Oulhote, Y.; Rigdon, J.; Lovato, J.; Liese, A.D.; Pihoker, C.; Dabelea, D.; et al. Associations between persistent organic pollutants and type 1 diabetes in youth. Environ. Int. 2022, 163, 107175. [Google Scholar] [CrossRef] [PubMed]
- Dufour, P.; Pirard, C.; Lebrethon, M.C.; Charlier, C. Associations between endocrine disruptor contamination and thyroid hormone homeostasis in Belgian type 1 diabetic children. Int. Arch. Occup. Environ. Health 2023, 96, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, G.; Nagy, T.; Guo, T.L. Bisphenol A alteration of type 1 diabetes in non-obese diabetic (NOD) female mice is dependent on window of exposure. Arch. Toxicol. 2019, 93, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- McDonough, C.M.; Xu, J.; Guo, T.L. Behavioral changes and hyperglycemia in NODEF mice following bisphenol S exposure are affected by diets. Neurotoxicology 2021, 85, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Sinioja, T.; Bodin, J.; Duberg, D.; Dirven, H.; Berntsen, H.F.; Zimmer, K.; Nygaard, U.C.; Orešič, M.; Hyötyläinen, T. Exposure to persistent organic pollutants alters the serum metabolome in non-obese diabetic mice. Metabolomics 2022, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, G.; Guo, T.L. Sex-dependent effects of bisphenol A on type 1 diabetes development in non-obese diabetic (NOD) mice. Arch. Toxicol. 2019, 93, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, G.; Guo, T.L. Bisphenol S modulates type 1 diabetes development in non-obese diabetic (NOD) mice with diet- and sex-related effects. Toxics 2019, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Cairrao, E. The relationship between phthalates and diabetes: A review. Metabolites 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Predieri, B.; Bruzzi, P.; Bigi, E.; Ciancia, S.; Madeo, S.F.; Lucaccioni, L.; Iughetti, L. Endocrine disrupting chemicals and type 1 diabetes. Int. J. Mol. Sci. 2020, 21, 2937. [Google Scholar] [CrossRef] [PubMed]
- Hinault, C.; Caroli-Bosc, P.; Bost, F.; Chevalier, N. Critical overview on endocrine disruptors in diabetes mellitus. Int. J. Mol. Sci. 2023, 24, 4537. [Google Scholar] [CrossRef] [PubMed]
- Khalil, W.J.; Akeblersane, M.; Khan, A.S.; Moin, A.S.M.; Butler, A.E. Environmental pollution and the risk of developing metabolic disorders: Obesity and diabetes. Int. J. Mol. Sci. 2023, 24, 8870. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.J.; Kim, H.S. Ambient air pollution and endocrinologic disorders in childhood. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ding, K.; Huang, W.; Xu, F.; Lei, M.; Yue, R. Potential effects of bisphenol A on diabetes mellitus and its chronic complications: A narrative review. Heliyon 2023, 9, e16340. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ibarra, A.; Martínez-Razo, L.D.; MacDonald-Ramos, K.; Morales-Pacheco, M.; Vázquez-Martínez, E.R.; López-López, M.; Dorantes, M.R.; Cerbón, M. Multisystemic alterations in humans induced by bisphenol A and phthalates: Experimental, epidemiological and clinical studies reveal the need to change health policies. Environ Pollut. 2021, 271, 116380. [Google Scholar] [CrossRef] [PubMed]

| PICO | |
|---|---|
| P | Children and Adolescents and Young Adults (10–22 years old) |
| I | Endocrine-Disrupting Chemicals |
| C | Diabetes Mellitus Type 1 (T1DM) |
| O | Endocrine Disrupting Chemicals as Risk Factors of Diabetes Mellitus Type 1 (T1DM) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keskesiadou, G.-N.; Tsokkou, S.; Konstantinidis, I.; Georgaki, M.-N.; Sioga, A.; Papamitsou, T.; Karachrysafi, S. Endocrine-Disrupting Chemicals and the Development of Diabetes Mellitus Type 1: A 5-Year Systematic Review. Int. J. Mol. Sci. 2024, 25, 10111. https://doi.org/10.3390/ijms251810111
Keskesiadou G-N, Tsokkou S, Konstantinidis I, Georgaki M-N, Sioga A, Papamitsou T, Karachrysafi S. Endocrine-Disrupting Chemicals and the Development of Diabetes Mellitus Type 1: A 5-Year Systematic Review. International Journal of Molecular Sciences. 2024; 25(18):10111. https://doi.org/10.3390/ijms251810111
Chicago/Turabian StyleKeskesiadou, Georgia-Nektaria, Sophia Tsokkou, Ioannis Konstantinidis, Maria-Nefeli Georgaki, Antonia Sioga, Theodora Papamitsou, and Sofia Karachrysafi. 2024. "Endocrine-Disrupting Chemicals and the Development of Diabetes Mellitus Type 1: A 5-Year Systematic Review" International Journal of Molecular Sciences 25, no. 18: 10111. https://doi.org/10.3390/ijms251810111







