Dysbiosis in Human Urinary Microbiota May Differentiate Patients with a Bladder Cancer
Abstract
1. Introduction
2. Results
2.1. Sequencing Analysis of Human Urine Microbiota
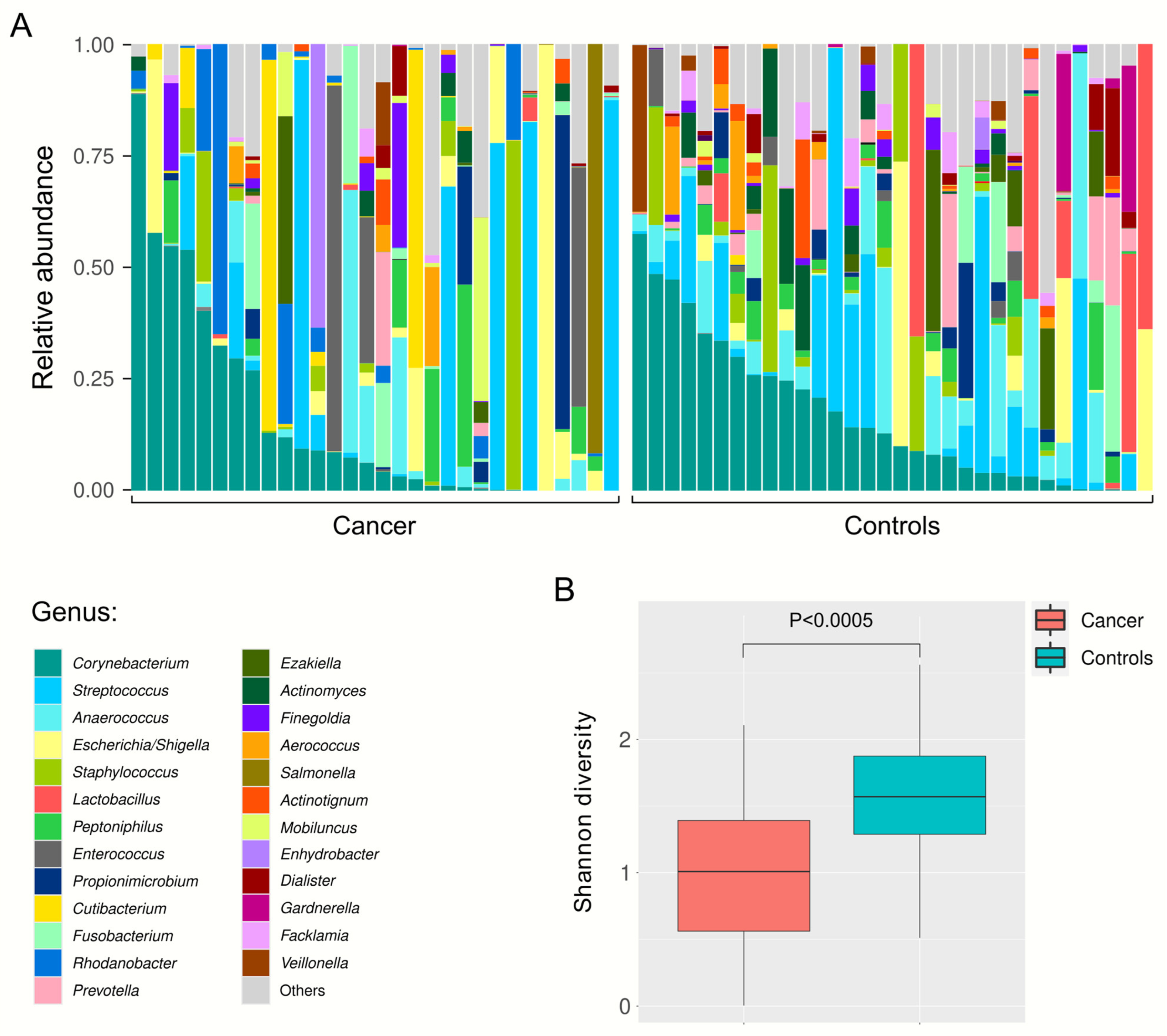
2.2. Relative Abundance of Urine Bacteria at Different Taxa Levels
2.3. Differential Urinary Microbiota Abundance Profiles between Bladder Cancer Patients and Healthy Volunteers
3. Discussion
4. Methods
4.1. Urine Sample Collection
4.2. DNA Extraction and Quantification
4.3. Next-Generation Sequencing (NGS) Experiments
4.4. Bioinformatics Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Silverman, D.T.; Hollenbeck, A.R.; Schatzkin, A.; Abnet, C.C. Association between smoking and risk of bladder cancer among men and women. JAMA 2011, 306, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M. Trends in Bladder Cancer Incidence and Mortality: Success or Disappointment? Eur. Urol. 2017, 71, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Bajic, P.; Wolfe, A.J.; Gupta, G.N. The Urinary Microbiome: Implications in Bladder Cancer Pathogenesis and Therapeutics. Urology 2019, 126, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Plottel, C.S.; Blaser, M.J. Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Zheng, X.; Ren, L.; Yang, Y.; Li, W.; Fu, W.; Wang, J.; Du, G. Tumorigenic bacteria in colorectal cancer: Mechanisms and treatments. Cancer Biol. Med. 2021, 19, 147–162. [Google Scholar] [CrossRef]
- Kashyap, D.; Rele, S.; Bagde, P.H.; Saini, V.; Chatterjee, D.; Jain, A.K.; Pandey, R.K.; Jha, H.C. Comprehensive insight into altered host cell-signaling cascades upon Helicobacter pylori and Epstein-Barr virus infections in cancer. Arch. Microbiol. 2023, 205, 262. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, F. The role of the gut microbiota in tumor, immunity, and immunotherapy. Front. Immunol. 2024, 15, 1410928. [Google Scholar] [CrossRef]
- Grine, G.; Lotte, R.; Chirio, D.; Chevalier, A.; Raoult, D.; Drancourt, M.; Ruimy, R. Co-culture of Methanobrevibacter smithii with enterobacteria during urinary infection. EBioMedicine 2019, 43, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Komesu, Y.M.; Richter, H.E.; Carper, B.; Dinwiddie, D.L.; Lukacz, E.S.; Siddiqui, N.Y.; Sung, V.W.; Zyczynski, H.M.; Ridgeway, B.; Rogers, R.G.; et al. The urinary microbiome in women with mixed urinary incontinence compared to similarly aged controls. Int. Urogynecol. J. 2018, 29, 1785–1795. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.M.; Hilt, E.E.; Rosenfeld, A.B.; Zilliox, M.J.; Thomas-White, K.; Fok, C.; Kliethermes, S.; Schreckenberger, P.C.; Brubaker, L.; Gai, X.; et al. The female urinary microbiome: A comparison of women with and without urgency urinary incontinence. mBio 2014, 5, e01283-e14. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.F.; Muthusamy, A.; Al-Ghalith, G.A.; Knights, D.; Guo, B.; Wu, B.; Remmel, R.P.; Schladt, D.P.; Alegre, M.L.; Oetting, W.S.; et al. Urinary microbiome associated with chronic allograft dysfunction in kidney transplant recipients. Clin. Transpl. 2018, 32, e13436. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Tian, Y.; Song, C.; Li, J.; Liu, T.; Chen, Z.; Chen, C.; Huang, Y.; Zhang, Y. Urinary microbiota—A potential biomarker and therapeutic target for bladder cancer. J. Med. Microbiol. 2019, 68, 1471–1478. [Google Scholar] [CrossRef]
- Bucevic Popovic, V.; Situm, M.; Chow, C.T.; Chan, L.S.; Roje, B.; Terzic, J. The urinary microbiome associated with bladder cancer. Sci. Rep. 2018, 8, 12157. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, G.; Zhao, J.; Chen, J.; Chen, Y.; Huang, W.; Zhong, J.; Zeng, J. Profiling the Urinary Microbiota in Male Patients With Bladder Cancer in China. Front. Cell Infect. Microbiol. 2018, 8, 167. [Google Scholar] [CrossRef]
- Aso, Y.; Akaza, H.; Kotake, T.; Tsukamoto, T.; Imai, K.; Naito, S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur. Urol. 1995, 27, 104–109. [Google Scholar] [CrossRef]
- Lamm, D.L. BCG immunotherapy for transitional-cell carcinoma in situ of the bladder. Oncology 1995, 9, 947–952, 955; discussion 955–965. [Google Scholar]
- Boursi, B.; Mamtani, R.; Haynes, K.; Yang, Y.X. Recurrent antibiotic exposure may promote cancer formation--Another step in understanding the role of the human microbiota? Eur. J. Cancer. 2015, 51, 2655–2664. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Viaud, S.; Vétizou, M.; Daillère, R.; Merad, M.; Kroemer, G. Cancer and the gut microbiota: An unexpected link. Sci. Transl. Med. 2015, 7, 271ps1. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.A.; Elsayed, A.S.; Durrani, M.; Jing, Z.; Iqbal, U.; Gomez, E.C.; Singh, P.K.; Liu, S.; Smith, G.; Tang, L.; et al. Investigating the association between the urinary microbiome and bladder cancer: An exploratory study. Urol. Oncol. 2021, 39, 370.e9–370.e19. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, L.; Lee, P.; Huang, W.C.; Nossa, C.; Ma, Y.; Deng, F.M.; Zhou, M.; Melamed, J.; Pei, Z. Mini-review: Perspective of the microbiome in the pathogenesis of urothelial carcinoma. Am. J. Clin. Exp. Urol. 2014, 2, 57–61. [Google Scholar]
- Vendrell, J.A.; Henry, S.; Cabello-Aguilar, S.; Heckendorn, E.; Godreuil, S.; Solassol, J. Determination of the Optimal Bacterial DNA Extraction Method to Explore the Urinary Microbiota. Int. J. Mol. Sci. 2022, 23, 1336. [Google Scholar] [CrossRef]
- Gottschick, C.; Deng, Z.L.; Vital, M.; Masur, C.; Abels, C.; Pieper, D.H.; Wagner-Döbler, I. The urinary microbiota of men and women and its changes in women during bacterial vaginosis and antibiotic treatment. Microbiome 2017, 5, 99. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, G.; Chen, C.; Li, K.; Wen, Y.; Zhao, J.; Wu, P. Alterations in Urobiome in Patients with Bladder Cancer and Implications for Clinical Outcome: A Single-Institution Study. Front. Cell Infect. Microbiol. 2020, 10, 555508. [Google Scholar] [CrossRef] [PubMed]
- Chipollini, J.; Wright, J.R.; Nwanosike, H.; Kepler, C.Y.; Batai, K.; Lee, B.R.; Spiess, P.E.; Stewart, D.B.; Lamendella, R. Characterization of urinary microbiome in patients with bladder cancer: Results from a single-institution, feasibility study. Urol. Oncol. 2020, 38, 615–621. [Google Scholar] [CrossRef]
- Liu, F.; Liu, A.; Lu, X.; Zhang, Z.; Xue, Y.; Xu, J.; Zeng, S.; Xiong, Q.; Tan, H.; He, X.; et al. Dysbiosis signatures of the microbial profile in tissue from bladder cancer. Cancer Med. 2019, 8, 6904–6914. [Google Scholar] [CrossRef]
- Aso, Y.; Akazan, H. Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. BLP Study Group. Urol. Int. 1992, 49, 125–129. [Google Scholar] [CrossRef]
- Naito, S.; Koga, H.; Yamaguchi, A.; Fujimoto, N.; Hasui, Y.; Kuramoto, H.; Iguchi, A.; Kinukawa, N. Prevention of recurrence with epirubicin and lactobacillus casei after transurethral resection of bladder cancer. J. Urol. 2008, 179, 485–490. [Google Scholar] [CrossRef]
- Miyake, M.; Oda, Y.; Owari, T.; Iida, K.; Ohnishi, S.; Fujii, T.; Nishimura, N.; Miyamoto, T.; Shimizu, T.; Ohnishi, K.; et al. Probiotics enhances anti-tumor immune response induced by gemcitabine plus cisplatin chemotherapy for urothelial cancer. Cancer Sci. 2023, 114, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Senthakumaran, T.; Moen, A.E.F.; Tannæs, T.M.; Endres, A.; Brackmann, S.A.; Rounge, T.B.; Bemanian, V.; Tunsjø, H.S. Microbial dynamics with CRC progression: A study of the mucosal microbiota at multiple sites in cancers, adenomatous polyps, and healthy controls. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 305–322. [Google Scholar] [CrossRef]
- Wu, Z.F.; Zou, K.; Wu, G.N.; Jin, Z.J.; Xiang, C.J.; Xu, S.; Wang, Y.H.; Wu, X.Y.; Chen, C.; Xu, Z.; et al. A Comparison of Tumor-Associated and Non-Tumor-Associated Gastric Microbiota in Gastric Cancer Patients. Dig. Dis. Sci. 2021, 66, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.X.; Xia, Q.D.; Zhong, X.Y.; Liu, Z.; Wang, S.G. The bladder microbiome of NMIBC and MIBC patients revealed by 2bRAD-M. Front. Cell Infect. Microbiol. 2023, 13, 1182322. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Ai, B.; Wang, Z.; Shen, B.; Xue, G.; Yu, D. Uncovering Microbial Composition of the Tissue Microenvironment in Bladder Cancer using RNA Sequencing Data. J. Cancer. 2024, 15, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, V.; Inoue, L.T.; Asprino, P.F.; Bettoni, F.; Mariotti, A.C.H.; Bastos, D.A.; Jardim, D.L.F.; Arap, M.A.; Camargo, A.A. Choice of 16S Ribosomal RNA Primers Impacts Male Urinary Microbiota Profiling. Front. Cell Infect. Microbiol. 2022, 12, 862338. [Google Scholar] [CrossRef]
- Jingushi, K.; Kawashima, A.; Tanikawa, S.; Saito, T.; Yamamoto, A.; Uemura, T.; Sassi, N.; Ishizuya, Y.; Yamamoto, Y.; Kato, T.; et al. Cutibacterium acnes-derived extracellular vesicles promote tumor growth in renal cell carcinoma. Cancer Sci. 2024, 115, 2578–2587. [Google Scholar] [CrossRef]
- Jia, X.; Lu, S.; Zeng, Z.; Liu, Q.; Dong, Z.; Chen, Y.; Zhu, Z.; Hong, Z.; Zhang, T.; Du, G.; et al. Characterization of Gut Microbiota, Bile Acid Metabolism, and Cytokines in Intrahepatic Cholangiocarcinoma. Hepatology 2020, 71, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Png, C.W.; Lee, W.J.J.; Chua, S.J.; Zhu, F.; Yeoh, K.G.; Zhang, Y. Mucosal microbiome associates with progression to gastric cancer. Theranostics 2022, 12, 48–58. [Google Scholar] [CrossRef]
- Jiao, J.; Zheng, Y.; Zhang, Q.; Xia, D.; Zhang, L.; Ma, N. Saliva microbiome changes in thyroid cancer and thyroid nodules patients. Front. Cell Infect. Microbiol. 2022, 12, 989188. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. CUTADAPT removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]


| Clinical and Demographic Parameters | Cancer Patients (n = 30) | Healthy Volunteers (n = 32) | p | |
|---|---|---|---|---|
| Age at sampling (y: mean ± SD) | 72.6 ± 10.0 | 70.6 ± 13.8 | NS (0.37) a | |
| Sex | Male | 24 | 21 | NS (0.21) b |
| Female | 6 | 11 | ||
| Smoking history | ||||
| Have smoked | 4 | NA | ||
| Smoker | 11 | |||
| Non-smoker | 13 | |||
| History of BCG therapy | ||||
| Yes | 18 | NA | NA | |
| No | 10 | |||
| Unknown | 2 | |||
| Age at cancer diagnosis (y: mean ± SD) | 70.4 ± 10.8 | NA | NA | |
| Invaded muscle at bladder cancer diagnosis | ||||
| NMIBC | 27 | NA | ||
| MIBC | 2 | |||
| Unknown | 1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vendrell, J.A.; Cabello-Aguilar, S.; Senal, R.; Heckendorn, E.; Henry, S.; Godreuil, S.; Solassol, J. Dysbiosis in Human Urinary Microbiota May Differentiate Patients with a Bladder Cancer. Int. J. Mol. Sci. 2024, 25, 10159. https://doi.org/10.3390/ijms251810159
Vendrell JA, Cabello-Aguilar S, Senal R, Heckendorn E, Henry S, Godreuil S, Solassol J. Dysbiosis in Human Urinary Microbiota May Differentiate Patients with a Bladder Cancer. International Journal of Molecular Sciences. 2024; 25(18):10159. https://doi.org/10.3390/ijms251810159
Chicago/Turabian StyleVendrell, Julie A., Simon Cabello-Aguilar, Romain Senal, Elise Heckendorn, Steven Henry, Sylvain Godreuil, and Jérôme Solassol. 2024. "Dysbiosis in Human Urinary Microbiota May Differentiate Patients with a Bladder Cancer" International Journal of Molecular Sciences 25, no. 18: 10159. https://doi.org/10.3390/ijms251810159
APA StyleVendrell, J. A., Cabello-Aguilar, S., Senal, R., Heckendorn, E., Henry, S., Godreuil, S., & Solassol, J. (2024). Dysbiosis in Human Urinary Microbiota May Differentiate Patients with a Bladder Cancer. International Journal of Molecular Sciences, 25(18), 10159. https://doi.org/10.3390/ijms251810159







