Sperm DNA Fragmentation: Unraveling Its Imperative Impact on Male Infertility Based on Recent Evidence
Abstract
1. Introduction
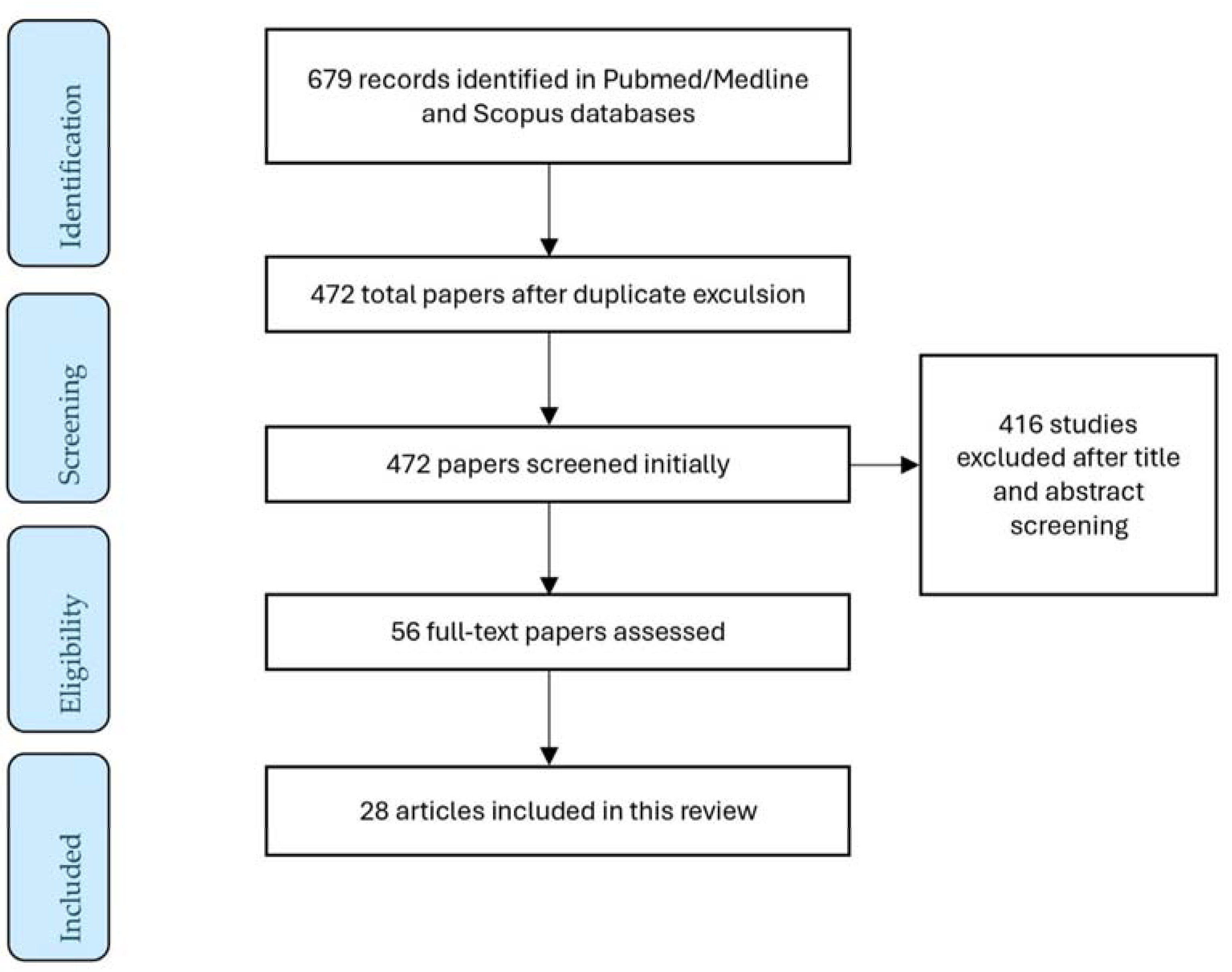
2. Literature Research
3. Impact of DFI on Seminal Parameters
4. Impact of DNA Fragmentation on Reproductive Outcomes
5. Discussion
6. Conclusions
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Group, E.C.W. A prognosis-based approach to infertility: Understanding the role of time. Hum. Reprod. 2017, 32, 1556–1559. [Google Scholar] [CrossRef]
- Potiris, A.; Perros, P.; Drakaki, E.; Mavrogianni, D.; Machairiotis, N.; Sfakianakis, A.; Karampitsakos, T.; Vrachnis, D.; Antonakopoulos, N.; Panagopoulos, P.; et al. Investigating the Association of Assisted Reproduction Techniques and Adverse Perinatal Outcomes. J. Clin. Med. 2024, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10. [Google Scholar] [CrossRef]
- Babakhanzadeh, E.; Nazari, M.; Ghasemifar, S.; Khodadadian, A. Some of the Factors Involved in Male Infertility: A Prospective Review. Int. J. Gen. Med. 2020, 13, 29–41. [Google Scholar] [CrossRef]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- Sunder, M.; Leslie, S.W. Semen Analysis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Vogiatzi, P.; Zini, A.; Arafa, M.; Agarwal, A. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef]
- Esteves, S.C.; Sanchez-Martin, F.; Sanchez-Martin, P.; Schneider, D.T.; Gosalvez, J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil. Steril. 2015, 104, 1398–1405. [Google Scholar] [CrossRef]
- Panagopoulos, P.; Mavrogianni, D.; Christodoulaki, C.; Drakaki, E.; Chrelias, G.; Panagiotopoulos, D.; Potiris, A.; Drakakis, P.; Stavros, S. Effects of endocrine disrupting compounds on female fertility. Best. Pract. Res. Clin. Obs. Gynaecol. 2023, 88, 102347. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J. Mens. Health 2020, 38, 412–471. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.; Assumpcao, M. Sperm DNA fragmentation: Causes and identification. Zygote 2020, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Seli, E.; Gardner, D.K.; Schoolcraft, W.B.; Moffatt, O.; Sakkas, D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil. Steril. 2004, 82, 378–383. [Google Scholar] [CrossRef]
- Borini, A.; Tarozzi, N.; Bizzaro, D.; Bonu, M.A.; Fava, L.; Flamigni, C.; Coticchio, G. Sperm DNA fragmentation: Paternal effect on early post-implantation embryo development in ART. Hum. Reprod. 2006, 21, 2876–2881. [Google Scholar] [CrossRef]
- Guideline Group on Unexplained, I.; Romualdi, D.; Ata, B.; Bhattacharya, S.; Bosch, E.; Costello, M.; Gersak, K.; Homburg, R.; Mincheva, M.; Norman, R.J.; et al. Evidence-based guideline: Unexplained infertility. Hum. Reprod. 2023, 38, 1881–1890. [Google Scholar] [CrossRef]
- Xie, D.; Lu, C.; Zhu, Y.; Zhu, S.; Yang, E.J.; Jin, X. Analysis on the association between sperm DNA fragmentation index and conventional semen parameters, blood microelements and seminal plasma ROS in male patients with infertility. Exp. Ther. Med. 2018, 15, 5173–5176. [Google Scholar] [CrossRef]
- Boushaba, S.; Belaaloui, G. Sperm DNA fragmentation and standard semen parameters in algerian infertile male partners. World J. Mens. Health 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Akhavizadegan, H.; Yamini, N.; Musavi, A.M.; Moradi, M.; Khatami, F. Sperm DNA Fragmentation Index in Abortion or in Vitro Fertilization Failure in Presence of Normal Semen Analysis. Prague Med. Rep. 2023, 124, 166–171. [Google Scholar] [CrossRef]
- Wang, Q.X.; Wang, X.; Yu, M.Y.; Sun, H.; Wang, D.; Zhong, S.P.; Guo, F. Random sperm DNA fragmentation index is not associated with clinical outcomes in day-3 frozen embryo transfer. Asian J. Androl. 2022, 24, 109–115. [Google Scholar] [CrossRef]
- Antonouli, S.; Papatheodorou, A.; Panagiotidis, Y.; Petousis, S.; Prapas, N.; Nottola, S.A.; Palmerini, M.G.; Macchiarelli, G.; Prapas, Y. The impact of sperm DNA fragmentation on ICSI outcome in cases of donated oocytes. Arch. Gynecol. Obs. 2019, 300, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Green, K.A.; Patounakis, G.; Dougherty, M.P.; Werner, M.D.; Scott, R.T., Jr.; Franasiak, J.M. Sperm DNA fragmentation on the day of fertilization is not associated with embryologic or clinical outcomes after IVF/ICSI. J. Assist. Reprod. Genet. 2020, 37, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, J.; Liang, Z.; Wu, J.; Li, L.; Chen, C.; Jin, F.; Tian, Y. Sperm DNA fragmentation and male fertility: A retrospective study of 5114 men attending a reproductive center. J. Assist. Reprod. Genet. 2021, 38, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.C.; Jing, J.; Chen, L.; Ge, Y.F.; Feng, R.X.; Liang, Y.J.; Yao, B. Analysis of human sperm DNA fragmentation index (DFI) related factors: A report of 1010 subfertile men in China. Reprod. Biol. Endocrinol. 2018, 16, 23. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, J.; Cheng, Z.; Wang, C.; Feng, Y. Mean number of DNA breakpoints: Illuminating sperm DNA integrity and in vitro fertilization outcomes. Fertil. Steril. 2024, 121, 264–270. [Google Scholar] [CrossRef]
- Le, M.T.; Nguyen, T.A.T.; Nguyen, H.T.T.; Nguyen, T.T.T.; Nguyen, V.T.; Le, D.D.; Nguyen, V.Q.H.; Cao, N.T. Does sperm DNA fragmentation correlate with semen parameters? Reprod. Med. Biol. 2019, 18, 390–396. [Google Scholar] [CrossRef]
- Vinnakota, C.; Cree, L.; Peek, J.; Morbeck, D.E. Incidence of high sperm DNA fragmentation in a targeted population of subfertile men. Syst. Biol. Reprod. Med. 2019, 65, 451–457. [Google Scholar] [CrossRef]
- Ferrigno, A.; Ruvolo, G.; Capra, G.; Serra, N.; Bosco, L. Correlation between the DNA fragmentation index (DFI) and sperm morphology of infertile patients. J. Assist. Reprod. Genet. 2021, 38, 979–986. [Google Scholar] [CrossRef]
- Jakubik-Uljasz, J.; Gill, K.; Rosiak-Gill, A.; Piasecka, M. Relationship between sperm morphology and sperm DNA dispersion. Transl. Androl. Urol. 2020, 9, 405–415. [Google Scholar] [CrossRef]
- Hosseinifar, H.; Yazdanikhah, S.; Modarresi, T.; Totonchi, M.; Sadighi Gilani, M.A.; Sabbaghian, M. Correlation between sperm DNA fragmentation index and CMA3 positive spermatozoa in globozoospermic patients. Andrology 2015, 3, 526–531. [Google Scholar] [CrossRef]
- Wang, Q.; Gu, X.; Chen, Y.; Yu, M.; Peng, L.; Zhong, S.; Wang, X.; Lv, J. The effect of sperm DNA fragmentation on in vitro fertilization outcomes of unexplained infertility. Clinics 2023, 78, 100261. [Google Scholar] [CrossRef] [PubMed]
- Asgari, F.; Gavahi, A.; Karimi, M.; Vatannejad, A.; Amjadi, F.; Aflatoonian, R.; Zandieh, Z. Risk of embryo aneuploidy is affected by the increase in sperm DNA damage in recurrent implantation failure patients under ICSI-CGH array cycles. Hum. Fertil. 2022, 25, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.C.; Zhang, Y.; Li, H.T.; Liu, X.M.; Yi, D.X.; Tian, L.; Liu, Y.X. Sperm DNA fragmentation index, as measured by sperm chromatin dispersion, might not predict assisted reproductive outcome. Taiwan. J. Obs. Gynecol. 2018, 57, 493–498. [Google Scholar] [CrossRef]
- Tello-Mora, P.; Hernandez-Cadena, L.; Pedraza, J.; Lopez-Bayghen, E.; Quintanilla-Vega, B. Acrosome reaction and chromatin integrity as additional parameters of semen analysis to predict fertilization and blastocyst rates. Reprod. Biol. Endocrinol. 2018, 16, 102. [Google Scholar] [CrossRef]
- Wdowiak, A.; Bakalczuk, S.; Bakalczuk, G. The effect of sperm DNA fragmentation on the dynamics of the embryonic development in intracytoplasmatic sperm injection. Reprod. Biol. 2015, 15, 94–100. [Google Scholar] [CrossRef]
- Dar, S.; Grover, S.A.; Moskovtsev, S.I.; Swanson, S.; Baratz, A.; Librach, C.L. In vitro fertilization-intracytoplasmic sperm injection outcome in patients with a markedly high DNA fragmentation index (>50%). Fertil. Steril. 2013, 100, 75–80. [Google Scholar] [CrossRef]
- AmirJannati, N.; Mohazzab, A.; Fathalian, M.; Akhavizadegan, H. Comparison of Embryological Results of Microinjection in Two Groups of Men with and without Requesting Sperm DNA Fragmentation Index Measurement. Biomed. Res. Int. 2024, 2024, 6769510. [Google Scholar] [CrossRef]
- Bibi, R.; Jahan, S.; Razak, S.; Hammadeh, M.E.; Almajwal, A.; Amor, H. Protamines and DNA integrity as a biomarkers of sperm quality and assisted conception outcome. Andrologia 2022, 54, e14418. [Google Scholar] [CrossRef]
- Rex, A.S.; Wu, C.; Aagaard, J.; Fedder, J. DNA Fragmentation in Human Spermatozoa and Pregnancy Rates after Intrauterine Insemination. Should the DFI Threshold Be Lowered? J. Clin. Med. 2021, 10, 1310. [Google Scholar] [CrossRef]
- Malic Voncina, S.; Stenqvist, A.; Bungum, M.; Schyman, T.; Giwercman, A. Sperm DNA fragmentation index and cumulative live birth rate in a cohort of 2,713 couples undergoing assisted reproduction treatment. Fertil. Steril. 2021, 116, 1483–1490. [Google Scholar] [CrossRef]
- Siddhartha, N.; Reddy, N.S.; Pandurangi, M.; Muthusamy, T.; Vembu, R.; Kasinathan, K. The Effect of Sperm DNA Fragmentation Index on the Outcome of Intrauterine Insemination and Intracytoplasmic Sperm Injection. J. Hum. Reprod. Sci. 2019, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Al Omrani, B.; Al Eisa, N.; Javed, M.; Al Ghedan, M.; Al Matrafi, H.; Al Sufyan, H. Associations of sperm DNA fragmentation with lifestyle factors and semen parameters of Saudi men and its impact on ICSI outcome. Reprod. Biol. Endocrinol. 2018, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, L.L.; Jiang, H.S.; Chen, H.; Chen, Y.; Dai, Y.T. Predictors of pregnancy outcome for infertile couples attending IVF and ICSI programmes. Andrologia 2016, 48, 874–881. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Ambar, R.F.; Agarwal, A.; Henkel, R. Etiologies of sperm DNA damage and its impact on male infertility. Andrologia 2021, 53, e13706. [Google Scholar] [CrossRef]
- Derbel, R.; Sellami, H.; Sakka, R.; Ben Slima, A.; Mkaddem, I.; Gdoura, R.; McElreavey, E.; Ammar-Keskes, L. Relationship between nuclear DNA fragmentation, mitochondrial DNA damage and standard sperm parameters in spermatozoa of infertile patients with leukocytospermia. J. Gynecol. Obs. Hum. Reprod. 2021, 50, 102101. [Google Scholar] [CrossRef]
- Ogawa, S.; Ota, K.; Nishizawa, K.; Shinagawa, M.; Katagiri, M.; Kikuchi, H.; Kobayashi, H.; Takahashi, T.; Yoshida, H. Micronutrient Antioxidants for Men (Menevit((R))) Improve Sperm Function by Reducing Oxidative Stress, Resulting in Improved Assisted Reproductive Technology Outcomes. Antioxidants 2024, 13, 635. [Google Scholar] [CrossRef]
- Linn, E.; Ghanem, L.; Bhakta, H.; Greer, C.; Avella, M. Genes Regulating Spermatogenesis and Sperm Function Associated With Rare Disorders. Front. Cell. Dev. Biol. 2021, 9, 634536. [Google Scholar] [CrossRef]
- Cheung, S.; Parrella, A.; Rosenwaks, Z.; Palermo, G.D. Genetic and epigenetic profiling of the infertile male. PLoS ONE 2019, 14, e0214275. [Google Scholar] [CrossRef]
- Stavros, S.; Mavrogianni, D.; Papamentzelopoulou, M.; Basamakis, E.; Khudeir, H.; Psarris, A.; Drakakis, P. Association of Tumor Necrosis Factor-alpha -308G>A, -238G>A and -376G>A polymorphisms with recurrent pregnancy loss risk in the Greek population. Fertil. Res. Pract. 2021, 7, 9. [Google Scholar] [CrossRef]
- Jing, R.; Zhang, H.; Kong, Y.; Li, K.; Dong, X.; Yan, J.; Han, J.; Feng, L. Different functions of biogenesis of lysosomal organelles complex 3 subunit 1 (Hps1) and adaptor-related protein complex 3, beta 1 subunit (Ap3b1) genes on spermatogenesis and male fertility. Reprod. Fertil. Dev. 2019, 31, 972–982. [Google Scholar] [CrossRef]
- Riel, J.M.; Yamauchi, Y.; Ruthig, V.A.; Malinta, Q.U.; Blanco, M.; Moretti, C.; Cocquet, J.; Ward, M.A. Rescue of Sly Expression Is Not Sufficient to Rescue Spermiogenic Phenotype of Mice with Deletions of Y Chromosome Long Arm. Genes 2019, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Guran, T.; Yesil, G.; Turan, S.; Atay, Z.; Bozkurtlar, E.; Aghayev, A.; Gul, S.; Tinay, I.; Aru, B.; Arslan, S.; et al. PPP2R3C gene variants cause syndromic 46,XY gonadal dysgenesis and impaired spermatogenesis in humans. Eur. J. Endocrinol. 2019, 180, 291–309. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Nie, H.; Meng, L.; Yuan, S.; He, W.; Luo, A.; Li, H.; Li, W.; Du, J.; Lu, G.; et al. Identification of DNAH6 mutations in infertile men with multiple morphological abnormalities of the sperm flagella. Sci. Rep. 2019, 9, 15864. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sha, Y.W.; Wang, W.T.; Cui, Y.Q.; Chen, J.; Yan, W.; Hou, X.T.; Mei, L.B.; Yu, C.C.; Wang, J. Novel IFT140 variants cause spermatogenic dysfunction in humans. Mol. Genet. Genom. Med. 2019, 7, e920. [Google Scholar] [CrossRef]
- Yang, P.; Chen, D.; Wang, Y.X.; Zhang, L.; Huang, L.L.; Lu, W.Q.; Zeng, Q. Mediation of association between polycyclic aromatic hydrocarbon exposure and semen quality by spermatogenesis-related microRNAs: A pilot study in an infertility clinic. J. Hazard. Mater. 2020, 384, 121431. [Google Scholar] [CrossRef]
- Zamore, P.D.; Haley, B. Ribo-gnome: The big world of small RNAs. Science 2005, 309, 1519–1524. [Google Scholar] [CrossRef]
- Godia, M.; Estill, M.; Castello, A.; Balasch, S.; Rodriguez-Gil, J.E.; Krawetz, S.A.; Sanchez, A.; Clop, A. A RNA-Seq Analysis to Describe the Boar Sperm Transcriptome and Its Seasonal Changes. Front. Genet. 2019, 10, 299. [Google Scholar] [CrossRef]
- Lian, J.; Tian, H.; Liu, L.; Zhang, X.S.; Li, W.Q.; Deng, Y.M.; Yao, G.D.; Yin, M.M.; Sun, F. Downregulation of microRNA-383 is associated with male infertility and promotes testicular embryonal carcinoma cell proliferation by targeting IRF1. Cell. Death Dis. 2010, 1, e94. [Google Scholar] [CrossRef]
- Rahbar, S.; Novin, M.G.; Alizadeh, E.; Shahnazi, V.; Pashaei-Asl, F.; AsrBadr, Y.A.; Farzadi, L.; Ebrahimie, E.; Pashaiasl, M. New insights into the expression profile of MicroRNA-34c and P53 in infertile men spermatozoa and testicular tissue. Cell. Mol. Biol. 2017, 63, 77–83. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Iommiello, V.M.; Albani, E.; Di Rosa, A.; Marras, A.; Menduni, F.; Morreale, G.; Levi, S.L.; Pisano, B.; Levi-Setti, P.E. Ejaculate oxidative stress is related with sperm DNA fragmentation and round cells. Int. J. Endocrinol. 2015, 2015, 321901. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.J.; Nixon, B.; Roman, S.D.; Aitken, R.J. Improved methods of DNA extraction from human spermatozoa that mitigate experimentally-induced oxidative DNA damage. PLoS ONE 2018, 13, e0195003. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.J.; Nixon, B.; Roman, S.D.; Scott, R.J.; Drevet, J.R.; Aitken, R.J. Paternal impacts on development: Identification of genomic regions vulnerable to oxidative DNA damage in human spermatozoa. Hum. Reprod. 2019, 34, 1876–1890. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Darand, M.; Salimi, Z.; Ghorbani, M.; Sadeghi, N.; Babaie, S.; Hosseinzadeh, M. Obesity is associated with quality of sperm parameters in men with infertility: A cross-sectional study. Reprod. Health 2023, 20, 134. [Google Scholar] [CrossRef]
- Wang, E.Y.; Huang, Y.; Du, Q.Y.; Yao, G.D.; Sun, Y.P. Body mass index effects sperm quality: A retrospective study in Northern China. Asian J. Androl. 2017, 19, 234–237. [Google Scholar] [CrossRef]
- Moustakli, E.; Zikopoulos, A.; Skentou, C.; Bouba, I.; Tsirka, G.; Stavros, S.; Vrachnis, D.; Vrachnis, N.; Potiris, A.; Georgiou, I.; et al. Sperm Mitochondrial Content and Mitochondrial DNA to Nuclear DNA Ratio Are Associated with Body Mass Index and Progressive Motility. Biomedicines 2023, 11, 3014. [Google Scholar] [CrossRef]
- Potiris, A.; Voitse, A.; Mavrogianni, D.; Machairiotis, N.; Drakaki, E.; Papamentzelopoulou, M.; Karampitsakos, T.; Zikopoulos, A.; Evgeni, E.; Drakakis, P.; et al. Association of GSTM1 Polymorphism and Redox Potential with Idiopathic Male Infertility. J. Clin. Med. 2023, 12, 6775. [Google Scholar] [CrossRef]
- Sharma, R.; Iovine, C.; Agarwal, A.; Henkel, R. TUNEL assay-Standardized method for testing sperm DNA fragmentation. Andrologia 2021, 53, e13738. [Google Scholar] [CrossRef]
- Farkouh, A.; Salvio, G.; Kuroda, S.; Saleh, R.; Vogiatzi, P.; Agarwal, A. Sperm DNA integrity and male infertility: A narrative review and guide for the reproductive physicians. Transl. Androl. Urol. 2022, 11, 1023–1044. [Google Scholar] [CrossRef]
- Agarwal, A.; Farkouh, A.; Saleh, R.; Hamoda, T.A.A.; Salvio, G.; Boitrelle, F.; Harraz, A.M.; Ghayda, R.A.; Kavoussi, P.; Gul, M.; et al. Technical Aspects and Clinical Limitations of Sperm DNA Fragmentation Testing in Male Infertility: A Global Survey, Current Guidelines, and Expert Recommendations. World J. Mens. Health 2024, 42, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Gutierrez, E.I.; Lopez-Fernandez, C.; Fernandez, J.L.; Davila-Rodriguez, M.I.; Johnston, S.D.; Gosalvez, J. Interpreting sperm DNA damage in a diverse range of mammalian sperm by means of the two-tailed comet assay. Front. Genet. 2014, 5, 404. [Google Scholar] [CrossRef][Green Version]
- Cho, C.L.; Agarwal, A.; Majzoub, A.; Esteves, S.C. Clinical utility of sperm DNA fragmentation testing: Concise practice recommendations. Transl. Androl. Urol. 2017, 6, S366–S373. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.L.; Muriel, L.; Rivero, M.T.; Goyanes, V.; Vazquez, R.; Alvarez, J.G. The sperm chromatin dispersion test: A simple method for the determination of sperm DNA fragmentation. J. Androl. 2003, 24, 59–66. [Google Scholar] [CrossRef]
| Study | Study Type | Sample | Outcome |
|---|---|---|---|
| Boushaba and Belaaoui (2015) [19] | Cohort study | 26 infertile men | No significant correlation of DFI with sperm morphology and volume was found. |
| Xie et al. (2018) [18] | Case–control study | 80 infertile men and 20 fertile men | The DFI showed no correlation between conventional semen parameters |
| Hosseinifar et al. (2015) [31] | Cohort study | 20 men | The mean DFI was significantly higher in patients with teratozoospermia, compared to the control group (p < 0.001). |
| Lu et al. (2018) [25] | Cohort study | 1010 infertile men |
|
| Vinnakota et al. (2019) [28] | Cohort study | 1082 infertile men and 234 sperm donors | Men with a high DFI were older and had lower sperm motility than those with a normal DFI. |
| Le et al. (2019) [27] | Case–control study | 318 infertile men |
|
| Jakubik-Uljasz et al.(2020) [30] | Cross-sectional study | 523 men with teratozoospermia | A higher proportion of individuals with teratozoospermia had high SDF levels (>30%) and a higher odds ratio for high SDF levels compared to those without. |
| Zhang et al. (2021) [24] | Cohort study | 2760 infertile men and 2354 men with women with unexplained miscarriage |
|
| Green et al. (2020) [23] | Cohort study | 234 couples | Men with a DFI >15% had significantly lower total motile sperm and sperm concentration compared to those with a DFI ≤ 15%. |
| Ferrigno et al. (2021) [29] | Cohort study | 125 infertile men | Spermatozoa with abnormal morphology were more likely to have DNA damage (p < 0.001). |
| Antonouli et al. (2019) [22] | Clinical trial | 150 couples |
|
| Wang et al. (2022) [21] | Clinical trial | 381 couples | The DFI showed a negative correlation with sperm motility (r = −0.640, p < 0.01), sperm concentration (r = −0.289, p < 0.01), and the fertilization rate of IVF cycles. |
| Zhou et al. (2023) [26] | Cross-sectional study | 93 couples | Logistic regression analysis indicated that the DFI had a negative correlation with asthenospermia (r = −0.37, p < 0.01). |
| Akhavizadegan et al. (2023) [20] | Cohort study | 172 couples | Patients with abnormal semen analysis had significantly higher DFI levels compared to patients with normal or slightly abnormal semen analysis. |
| Study | Study Type | Sample | Outcome |
|---|---|---|---|
| Wang et al. (2022) [21] | Clinical trial | 381 couples |
|
| Wdowiak et al. (2015) [36] | Clinical trial | 165 couples who underwent ICSI | Lower DFI levels were associated with faster embryo development at the blastocyst stage. |
| Esteves et al. (2015) [10] | Cohort study | 147 couples who underwent IVF-ICSI gave 77 testicular sperm samples (with lower DFI) and 87 ejaculated samples (with high DFI) | The clinical pregnancy rate was 51.9% using testicular samples versus 40.2% using ejaculated samples (p = 0.131); similarly, the miscarriage rate was 10.0% versus 34.3% (p = 0.012) and the live birth rate was 46.7% versus 26.4% (p = 0.007). |
| Zhang et al. (2016) [44] | Cross-sectional study | 1316 couples in IVF and 266 couples in ICSI | The corresponding odds ratio (OR) of pregnant versus not pregnant was 10%.The DFI increase was 0.849 (95% CI, 0.738–0.976, p = 0.022) and 0.707 (95% CI, 0.559–0.893, p = 0.004) in the IVF andICSI programs, respectively. |
| Tello-Mora et al. (2018) [35] | Cross-sectional study | 69 couples | The DFI was inversely correlated with viable blastocyst rates. |
| Omrani et al. (2018) [43] | Cross-sectional study | 94 infertile men |
|
| Sun et al. (2018) [34] | Cohort study | 390 couples | No significant differences in fertilization rate, euploid embryos, or pregnancy rate between the high- (≥30%) and low (<30%)-DFI groups after IVF or ICSI were found. |
| Siddhartha et al. (2019) [42] | Cohort study | 105 infertile men | The pregnancy rate using ICSI was significantly lower in the positive-DFI group, compared to the group with negative DFI values (16.7% vs. 47.4%, p = 0.046). |
| Voncina et al. (2021) [41] | Cohort study | 2713 infertile couples |
|
| Agsari et al. (2021) [33] | Clinical trial | 42 couples | The higher DFI group showed a significant (p = 0.04) increase in the number of aneuploid embryos compared to the low-DFI group. |
| Wang et al. (2023) [32] | Case–control study | 176 men | A negative correlation between the DFI and number of good quality embryos (rs = −0.347, p < 0.001) and live birth rate (rs = −0.185, p = 0.028) was found. |
| Bibi et al. (2022) [39] | Cohort study | 700 couples | Sperm chromatin structural anomalies are significantly associated with a decreased fertilization rate (p = 0.009) and live birth rate (p = 0.006). |
| AmirJannati et al. (2024) [38] | Case–control study | 870 couples | No significant differences in the fertilization rate, number, and quality of embryos were found. |
| Dar et al. (2013) [37] | Cohort study | 150 men | No significant differences in fertilization and clinical pregnancy rates were observed. |
| Rex et al. (2021) [40] | Cohort study | 357 couples |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavros, S.; Potiris, A.; Molopodi, E.; Mavrogianni, D.; Zikopoulos, A.; Louis, K.; Karampitsakos, T.; Nazou, E.; Sioutis, D.; Christodoulaki, C.; et al. Sperm DNA Fragmentation: Unraveling Its Imperative Impact on Male Infertility Based on Recent Evidence. Int. J. Mol. Sci. 2024, 25, 10167. https://doi.org/10.3390/ijms251810167
Stavros S, Potiris A, Molopodi E, Mavrogianni D, Zikopoulos A, Louis K, Karampitsakos T, Nazou E, Sioutis D, Christodoulaki C, et al. Sperm DNA Fragmentation: Unraveling Its Imperative Impact on Male Infertility Based on Recent Evidence. International Journal of Molecular Sciences. 2024; 25(18):10167. https://doi.org/10.3390/ijms251810167
Chicago/Turabian StyleStavros, Sofoklis, Anastasios Potiris, Ermioni Molopodi, Despoina Mavrogianni, Athanasios Zikopoulos, Konstantinos Louis, Theodoros Karampitsakos, Eleni Nazou, Dimdos Sioutis, Chrysi Christodoulaki, and et al. 2024. "Sperm DNA Fragmentation: Unraveling Its Imperative Impact on Male Infertility Based on Recent Evidence" International Journal of Molecular Sciences 25, no. 18: 10167. https://doi.org/10.3390/ijms251810167
APA StyleStavros, S., Potiris, A., Molopodi, E., Mavrogianni, D., Zikopoulos, A., Louis, K., Karampitsakos, T., Nazou, E., Sioutis, D., Christodoulaki, C., Skentou, C., Gerede, A., Zachariou, A., Christopoulos, P., Panagopoulos, P., Domali, E., & Drakakis, P. (2024). Sperm DNA Fragmentation: Unraveling Its Imperative Impact on Male Infertility Based on Recent Evidence. International Journal of Molecular Sciences, 25(18), 10167. https://doi.org/10.3390/ijms251810167









