BMP4 and Temozolomide Synergize in the Majority of Patient-Derived Glioblastoma Cultures
Abstract
1. Introduction
2. Results
2.1. Simultaneous Administration of TMZ and BMP4 to Patient-Derived GBM Cell Cultures Is More Effective in Decreasing Cell Viability than Sequential Treatment
2.2. BMP4 Synergizes with TMZ in the Majority of Patient-Derived GBM Cultures
2.3. Decreased Cell Viability after Synergistic TMZ + BMP4 Results from Increased Apoptosis
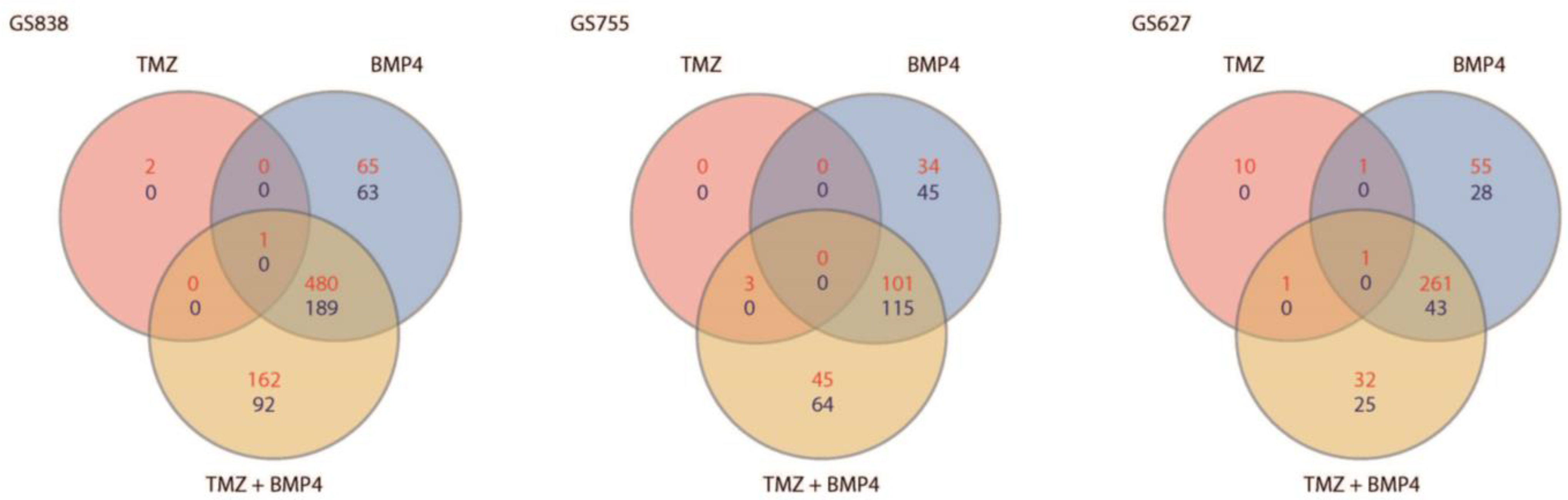
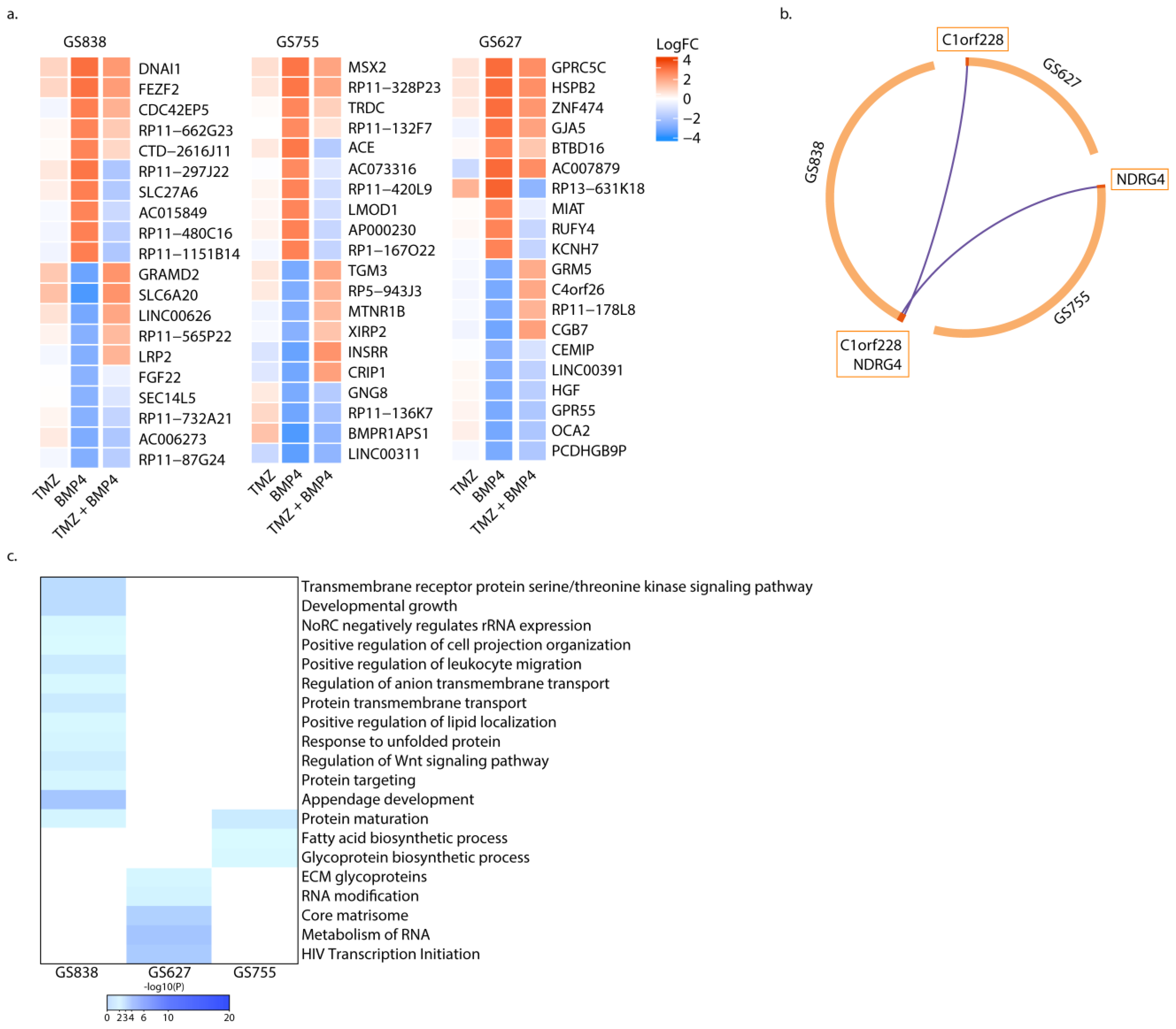
2.4. Gene Expression Profiles Associated with BMP4 Monotherapy, and Not with Combination Therapy, Are Unique per Culture
2.5. Synergy of TMZ + BMP4 Is Associated with Upregulated BHLHE40, and Decreased MAPK Signaling and Purine Metabolism
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Assay
4.3. Synergy Analysis
4.4. EdU Incorporation Assay
4.5. Annexin-V Assay
4.6. cDNA Library Preparation and Sequencing
4.7. RNA-Seq Bioinformatics Analysis
4.8. Quantitative PCR
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Carlo, D.T.; Cagnazzo, F.; Benedetto, N.; Morganti, R.; Perrini, P. Multiple high-grade gliomas: Epidemiology, management, and outcome. A systematic review and meta-analysis. Neurosurg. Rev. 2019, 42, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology 2015, 17 (Suppl. S4), iv1–iv62. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Yu, T.S.; McKay, R.M.; Burns, D.K.; Kernie, S.G.; Parada, L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012, 488, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Verploegh, I.S.C.; Conidi, A.; Brouwer, R.W.W.; Balcioglu, H.E.; Karras, P.; Makhzami, S.; Korporaal, A.; Marine, J.C.; Lamfers, M.; van IJcken, W.F.; et al. Comparative single-cell RNA-sequencing profiling of BMP4-treated primary glioma cultures reveals therapeutic markers. Neuro-Oncology 2022, 24, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.E.; Mehler, M.F.; Mabie, P.C.; Zang, Z.; Santschi, L.; Kessler, J.A. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 1996, 17, 595–606. [Google Scholar] [CrossRef]
- Bago, J.R.; Alfonso-Pecchio, A.; Okolie, O.; Dumitru, R.; Rinkenbaugh, A.; Baldwin, A.S.; Miller, C.R.; Magness, S.T.; Hingtgen, S.D. Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma. Nat. Commun. 2016, 7, 10593. [Google Scholar] [CrossRef]
- Piccirillo, S.G.; Reynolds, B.A.; Zanetti, N.; Lamorte, G.; Binda, E.; Broggi, G.; Brem, H.; Olivi, A.; Dimeco, F.; Vescovi, A.L. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature 2006, 444, 761–765. [Google Scholar] [CrossRef]
- Caren, H.; Stricker, S.H.; Bulstrode, H.; Gagrica, S.; Johnstone, E.; Bartlett, T.E.; Feber, A.; Wilson, G.; Teschendorff, A.E.; Bertone, P.; et al. Glioblastoma Stem Cells Respond to Differentiation Cues but Fail to Undergo Commitment and Terminal Cell-Cycle Arrest. Stem Cell Rep. 2015, 5, 829–842. [Google Scholar] [CrossRef]
- Sachdeva, R.; Wu, M.; Johnson, K.; Kim, H.; Celebre, A.; Shahzad, U.; Graham, M.S.; Kessler, J.A.; Chuang, J.H.; Karamchandani, J.; et al. BMP signaling mediates glioma stem cell quiescence and confers treatment resistance in glioblastoma. Sci. Rep. 2019, 9, 14569. [Google Scholar] [CrossRef]
- Bos, E.M.; Binda, E.; Verploegh, I.S.C.; Wembacher, E.; Hoefnagel, D.; Balvers, R.K.; Korporaal, A.L.; Conidi, A.; Warnert, E.A.H.; Trivieri, N.; et al. Local delivery of hrBMP4 as an anticancer therapy in patients with recurrent glioblastoma: A first-in-human phase 1 dose escalation trial. Mol. Cancer 2023, 22, 129. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ntafoulis, I.; Kleijn, A.; Ju, J.; Jimenez-Cowell, K.; Fabro, F.; Klein, M.; Chi Yen, R.T.; Balvers, R.K.; Li, Y.; Stubbs, A.P.; et al. Ex vivo drug sensitivity screening predicts response to temozolomide in glioblastoma patients and identifies candidate biomarkers. Br. J. Cancer 2023, 129, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Schilling, S.H.; Hjelmeland, A.B.; Radiloff, D.R.; Liu, I.M.; Wakeman, T.P.; Fielhauer, J.R.; Foster, E.H.; Lathia, J.D.; Rich, J.N.; Wang, X.F.; et al. NDRG4 is required for cell cycle progression and survival in glioblastoma cells. J. Biol. Chem. 2009, 284, 25160–25169. [Google Scholar] [CrossRef]
- Ionescu, A.M.; Drissi, H.; Schwarz, E.M.; Kato, M.; Puzas, J.E.; McCance, D.J.; Rosier, R.N.; Zuscik, M.J.; O’Keefe, R.J. CREB Cooperates with BMP-stimulated Smad signaling to enhance transcription of the Smad6 promoter. J. Cell. Physiol. 2004, 198, 428–440. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Liu, J.; Cao, X.; Wang, X.; Wang, D.; Seo, H.; Gao, B. The membrane topological analysis of 3β-hydroxysteroid-Δ24 reductase (DHCR24) on endoplasmic reticulum. J. Mol. Endocrinol. 2012, 48, 1–9. [Google Scholar] [CrossRef]
- Talasila, K.M.; Røsland, G.V.; Hagland, H.R.; Eskilsson, E.; Flønes, I.H.; Fritah, S.; Azuaje, F.; Atai, N.; Harter, P.N.; Mittelbronn, M.; et al. The angiogenic switch leads to a metabolic shift in human glioblastoma. Neuro-Oncology 2017, 19, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Zhang, C.; Yan, W.; Liu, Y.; Li, M.; Zhang, W.; Jiang, T. BMP4, a strong better prognosis predictor, has a subtype preference and cell development association in gliomas. J. Transl. Med. 2013, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Q.; Tian, D.; Wu, L.; Dong, H.; Wang, J.; Ji, B.; Zhu, X.; Cai, Q.; Wang, L.; et al. BMP4 reverses multidrug resistance through modulation of BCL-2 and GDNF in glioblastoma. Brain Res. 2013, 1507, 115–124. [Google Scholar] [CrossRef]
- Russo, K.; Wharton, K.A. BMP/TGF-β signaling as a modulator of neurodegeneration in ALS. Dev. Dyn. 2022, 251, 10–25. [Google Scholar] [CrossRef]
- Fiori, J.L.; Billings, P.C.; de la Peña, L.S.; Kaplan, F.S.; Shore, E.M. Dysregulation of the BMP-p38 MAPK signaling pathway in cells from patients with fibrodysplasia ossificans progressiva (FOP). J. Bone Min. Res 2006, 21, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-T.; Lee, I.-N.; Chen, C.-H.; Lu, F.-J.; Chung, C.-Y.; Lee, M.-H.; Cheng, Y.-C.; Chen, K.-T.; Peng, J.-Y.; Chen, C.-H. Gallic Acid Enhances the Anti-Cancer Effect of Temozolomide in Human Glioma Cell Line via Inhibition of Akt and p38-MAPK Pathway. Processes 2022, 10, 448. [Google Scholar] [CrossRef]
- Shireman, J.M.; Atashi, F.; Lee, G.; Ali, E.S.; Saathoff, M.R.; Park, C.H.; Savchuk, S.; Baisiwala, S.; Miska, J.; Lesniak, M.S.; et al. De novo purine biosynthesis is a major driver of chemoresistance in glioblastoma. Brain J. Neurol. 2021, 144, 1230–1246. [Google Scholar] [CrossRef]
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1999, 1473, 4–8. [Google Scholar] [CrossRef]
- Cuello, H.A.; Ferreira, G.M.; Gulino, C.A.; Toledo, A.G.; Segatori, V.I.; Gabri, M.R. Terminally sialylated and fucosylated complex N-glycans are involved in the malignant behavior of high-grade glioma. Oncotarget 2020, 11, 4822–4835. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hernandez, M.; Shen, S.; Sabo, J.K.; Kelkar, D.; Wang, J.; O’Leary, R.; Phillips, G.R.; Cate, H.S.; Casaccia, P. Differential modulation of the oligodendrocyte transcriptome by sonic hedgehog and bone morphogenetic protein 4 via opposing effects on histone acetylation. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 6651–6664. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, D.; Sun, Q.; Nie, H.; Zhang, Y.; Wang, X.; Huang, Y.; Sun, Y. Single-Cell and Spatial Transcriptome Profiling Identifies the Transcription Factor BHLHE40 as a Driver of EMT in Metastatic Colorectal Cancer. Cancer Res. 2024, 84, 2202–2217. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, J.; Fan, Y.; Qi, Y.; Wang, S.; Zhao, S.; Guo, X.; Xue, H.; Deng, L.; Zhao, R.; et al. PDIA3P1 promotes Temozolomide resistance in glioblastoma by inhibiting C/EBPβ degradation to facilitate proneural-to-mesenchymal transition. J. Exp. Clin. Cancer Res. 2022, 41, 223. [Google Scholar] [CrossRef]
- Balvers, R.K.; Kleijn, A.; Kloezeman, J.J.; French, P.J.; Kremer, A.; van den Bent, M.J.; Dirven, C.M.; Leenstra, S.; Lamfers, M.L. Serum-free culture success of glial tumors is related to specific molecular profiles and expression of extracellular matrix-associated gene modules. Neuro-Oncology 2013, 15, 1684–1695. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 2.0: Visual analytics of multi-drug combination synergies. Nucleic Acids Res. 2020, 48, W488–W493. [Google Scholar] [CrossRef]
- Berenbaum, M.C. What is synergy? Pharmacol. Rev. 1989, 41, 93–141. [Google Scholar] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Mackey, K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques 1995, 19, 942–945. [Google Scholar] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]




| Primer | Sequence |
|---|---|
| GAPDH for | 5′-AATCCCATCACCATCTTCCA-3′ |
| GAPDH rev | 5′-CATCATGCAGCACCTCAGGT-3′ |
| RPS6KA4 for | 5′-GATCACAGAAGCCAACCTG-3′ |
| RPS6KA4 rev | 5′-GAAGTTCTCCACGCTCAC-3′ |
| DHCR24 for | 5′-CCGTGGTTCTTTAAGCATGT-3′ |
| DHCR24 rev | 5′-AAAGGGGATAATGTCCTGGAG-3′ |
| FIG4 for | 5′-TCTGTATGAGACTAGAGCTAGATA-3′ |
| FIG4 rev | 5′-CTGCATTATTGCTCCCAACT-3′ |
| CDH10 for | 5′-CATTCGAGTGTGTGCTTGT-3′ |
| CDH10 rev | 5′-TGCAAACAGTACTACTATAACCAG-3′ |
| BHLHE40 for | 5′-GGAGACCTACCAGGGATGTA-3′ |
| BHLHE40 rev | 5′-TATTCCCCGTCTTGACTTGT-3′ |
| LPAR2 for | 5′-ACACCCGCATTTTCTTCTAC-3′ |
| LPAR2 rev | 5′-GAACGCCCCCAGGATG-3′ |
| NT5DC1 for | 5′-GCCATCTCTGGATAAACCTG-3′ |
| NT5DC1 rev | 5′-ACCAAAATAAACAACCTTGGGT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verploegh, I.S.C.; Conidi, A.; El Hassnaoui, H.; Verhoeven, F.A.M.; Korporaal, A.L.; Ntafoulis, I.; van den Hout, M.C.G.N.; Brouwer, R.W.W.; Lamfers, M.L.M.; van IJcken, W.F.J.; et al. BMP4 and Temozolomide Synergize in the Majority of Patient-Derived Glioblastoma Cultures. Int. J. Mol. Sci. 2024, 25, 10176. https://doi.org/10.3390/ijms251810176
Verploegh ISC, Conidi A, El Hassnaoui H, Verhoeven FAM, Korporaal AL, Ntafoulis I, van den Hout MCGN, Brouwer RWW, Lamfers MLM, van IJcken WFJ, et al. BMP4 and Temozolomide Synergize in the Majority of Patient-Derived Glioblastoma Cultures. International Journal of Molecular Sciences. 2024; 25(18):10176. https://doi.org/10.3390/ijms251810176
Chicago/Turabian StyleVerploegh, Iris S. C., Andrea Conidi, Hoesna El Hassnaoui, Floor A. M. Verhoeven, Anne L. Korporaal, Ioannis Ntafoulis, Mirjam C. G. N. van den Hout, Rutger W. W. Brouwer, Martine L. M. Lamfers, Wilfred F. J. van IJcken, and et al. 2024. "BMP4 and Temozolomide Synergize in the Majority of Patient-Derived Glioblastoma Cultures" International Journal of Molecular Sciences 25, no. 18: 10176. https://doi.org/10.3390/ijms251810176
APA StyleVerploegh, I. S. C., Conidi, A., El Hassnaoui, H., Verhoeven, F. A. M., Korporaal, A. L., Ntafoulis, I., van den Hout, M. C. G. N., Brouwer, R. W. W., Lamfers, M. L. M., van IJcken, W. F. J., Huylebroeck, D., & Leenstra, S. (2024). BMP4 and Temozolomide Synergize in the Majority of Patient-Derived Glioblastoma Cultures. International Journal of Molecular Sciences, 25(18), 10176. https://doi.org/10.3390/ijms251810176









