Components in SLPE Alleviate AD Model Nematodes by Up-Regulating Gene gst-5
Abstract
1. Introduction
2. Results
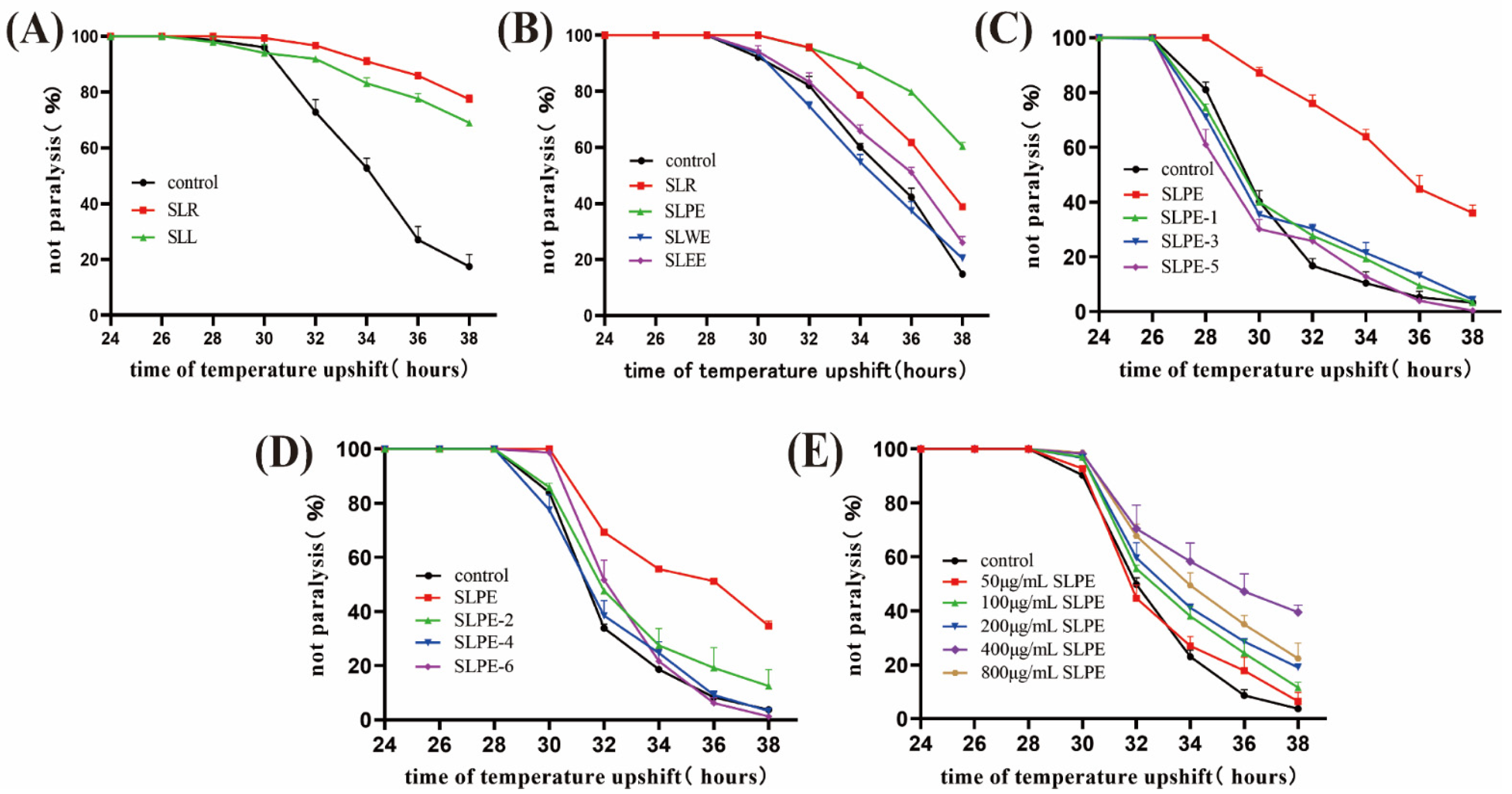
2.1. SLPE Delays CL4176 Paralysis Symptoms
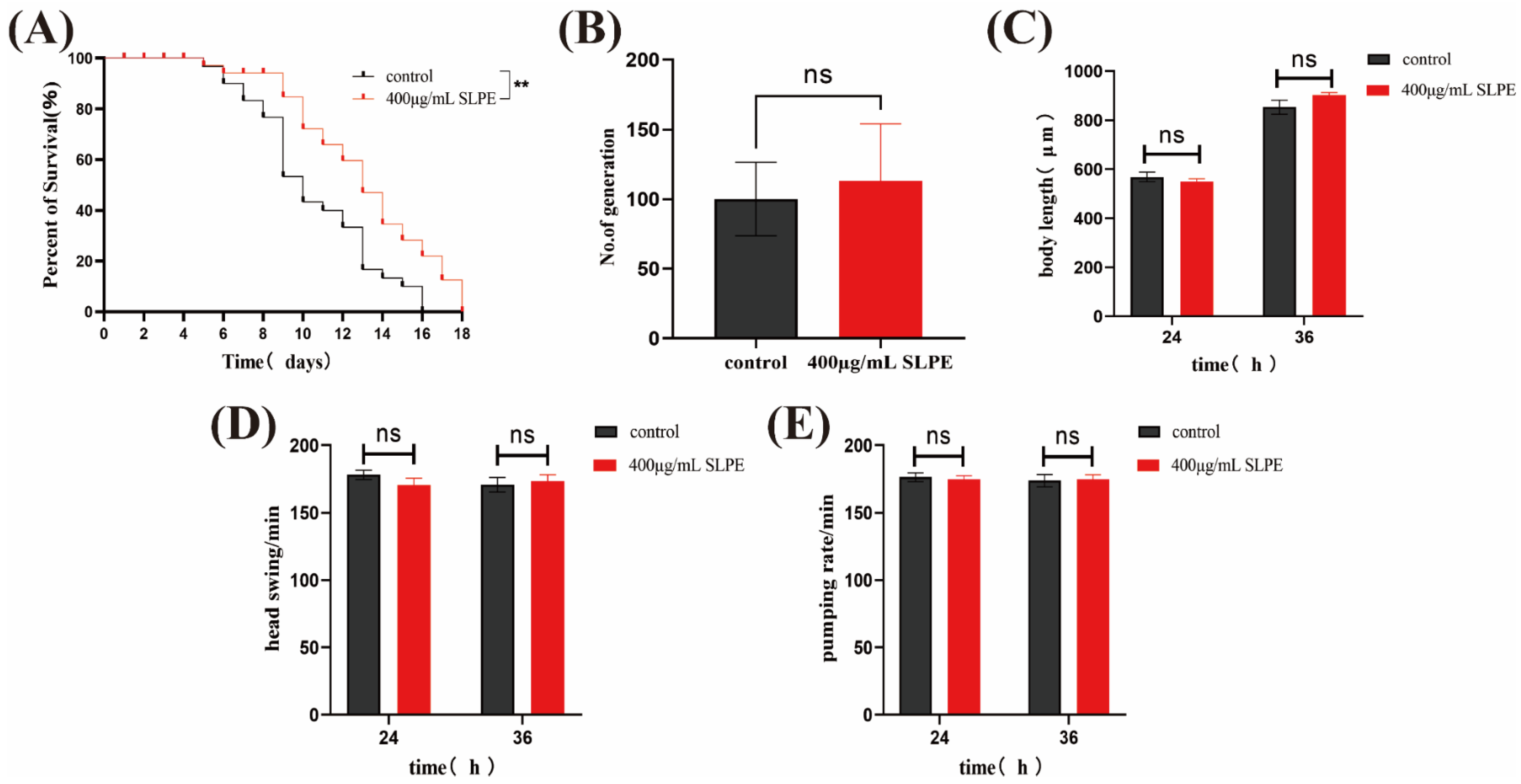
2.2. SLPE Extends the Lifespan of C. elegans without Affecting Other Basic Biological Characteristics
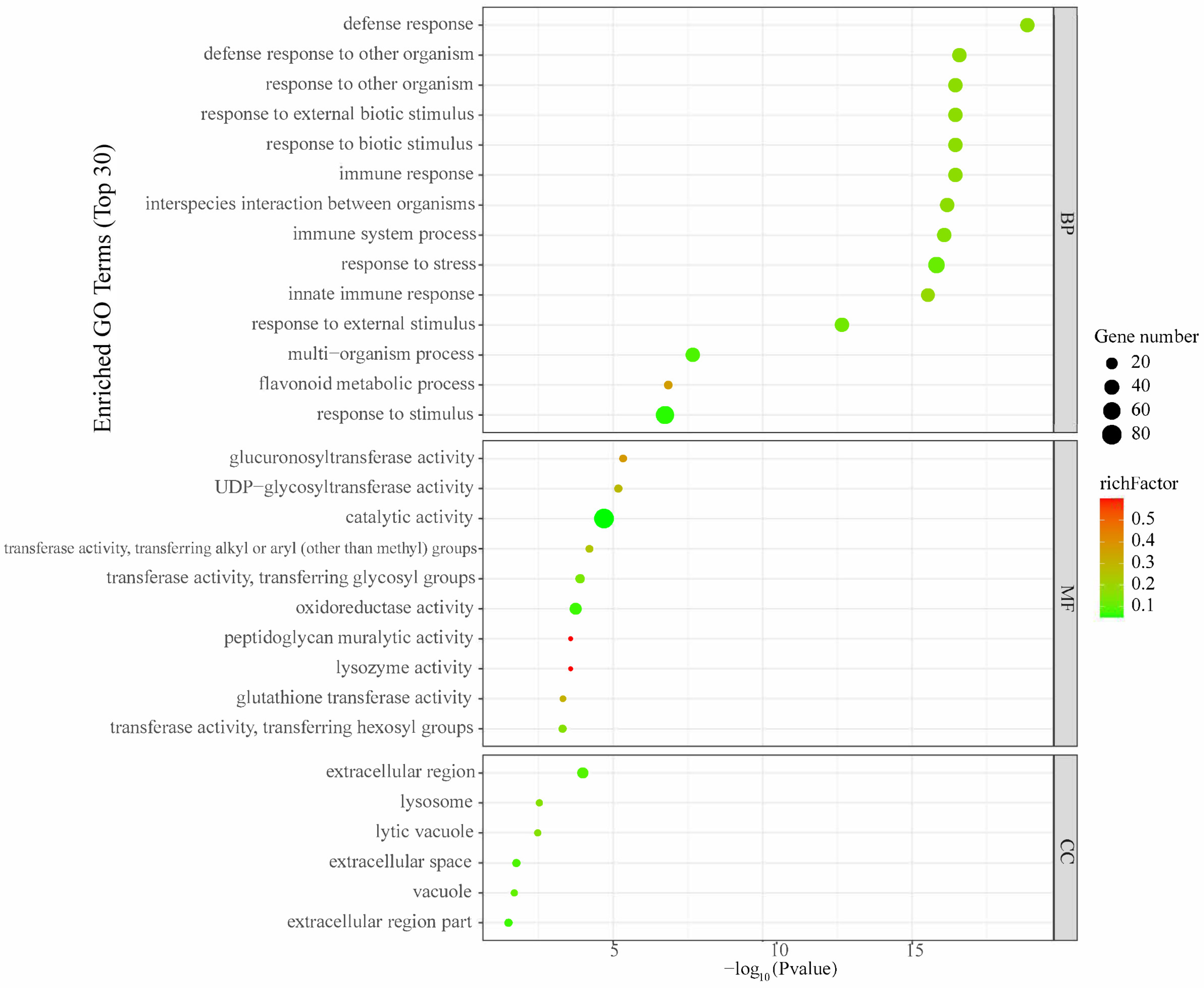
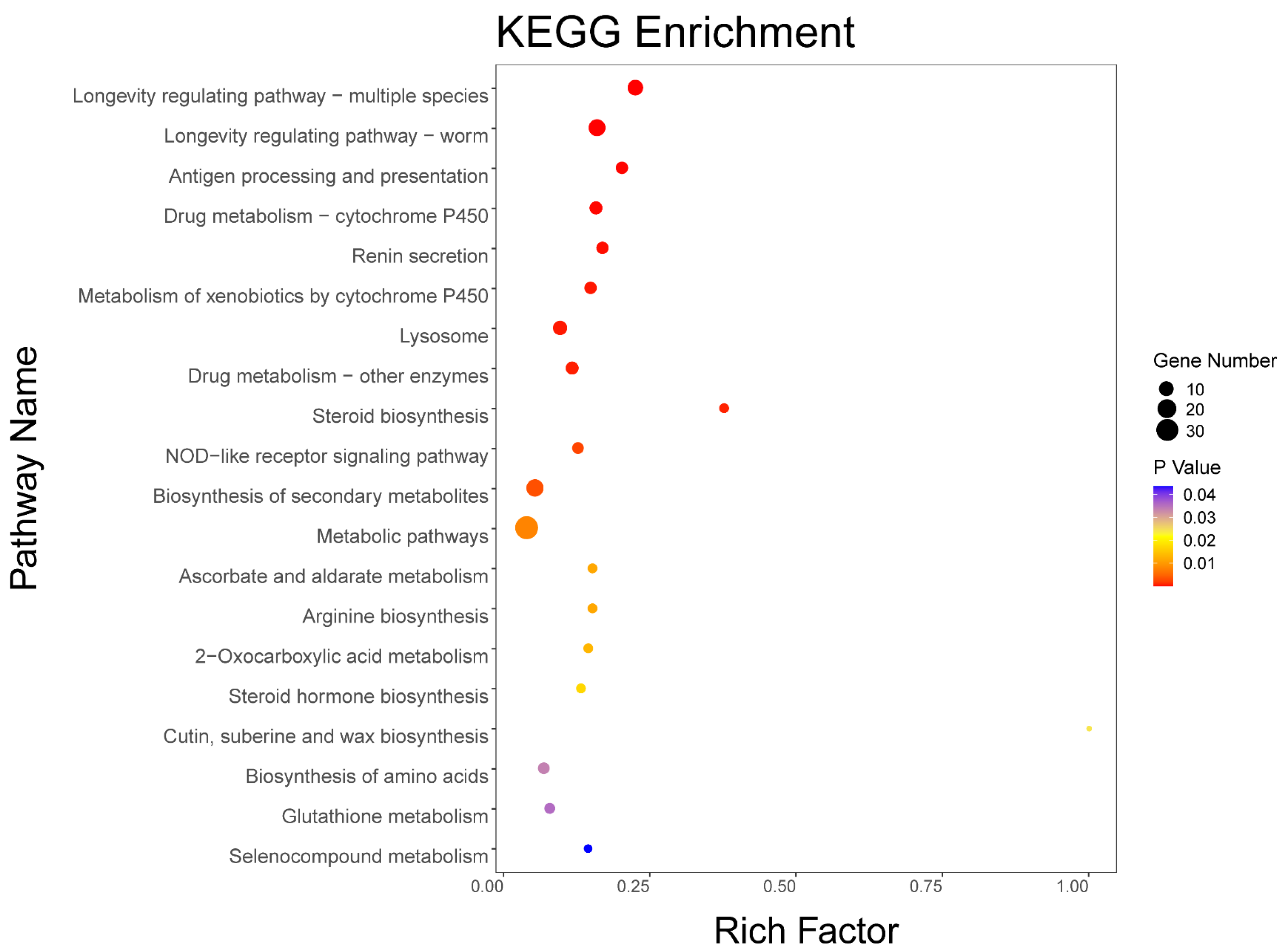
2.3. Effect of SLPE on the Expression of Different Genes and Results of GO and KEGG Enrichment Analysis
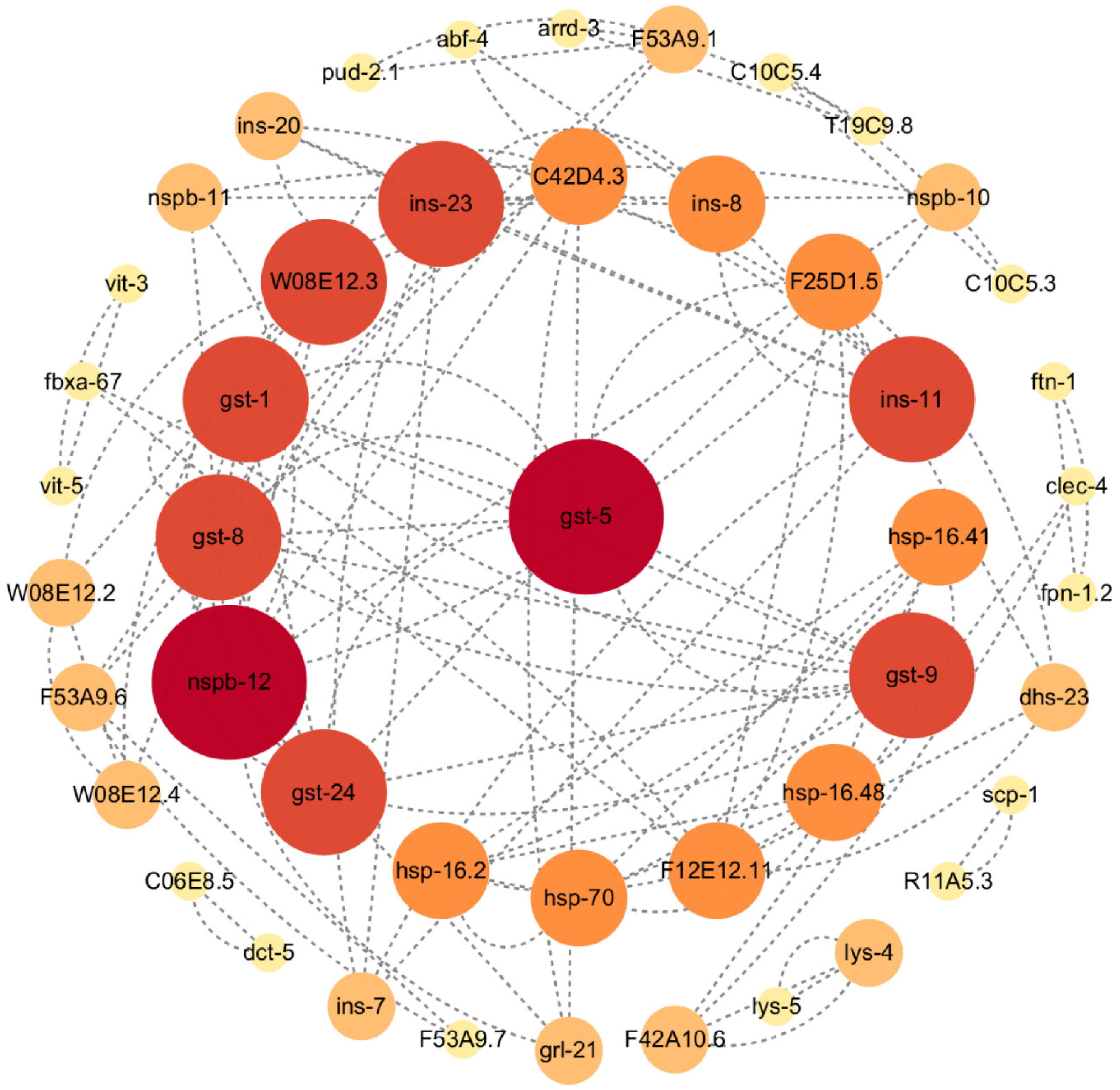
2.4. PPI Network Analyses of DEGs
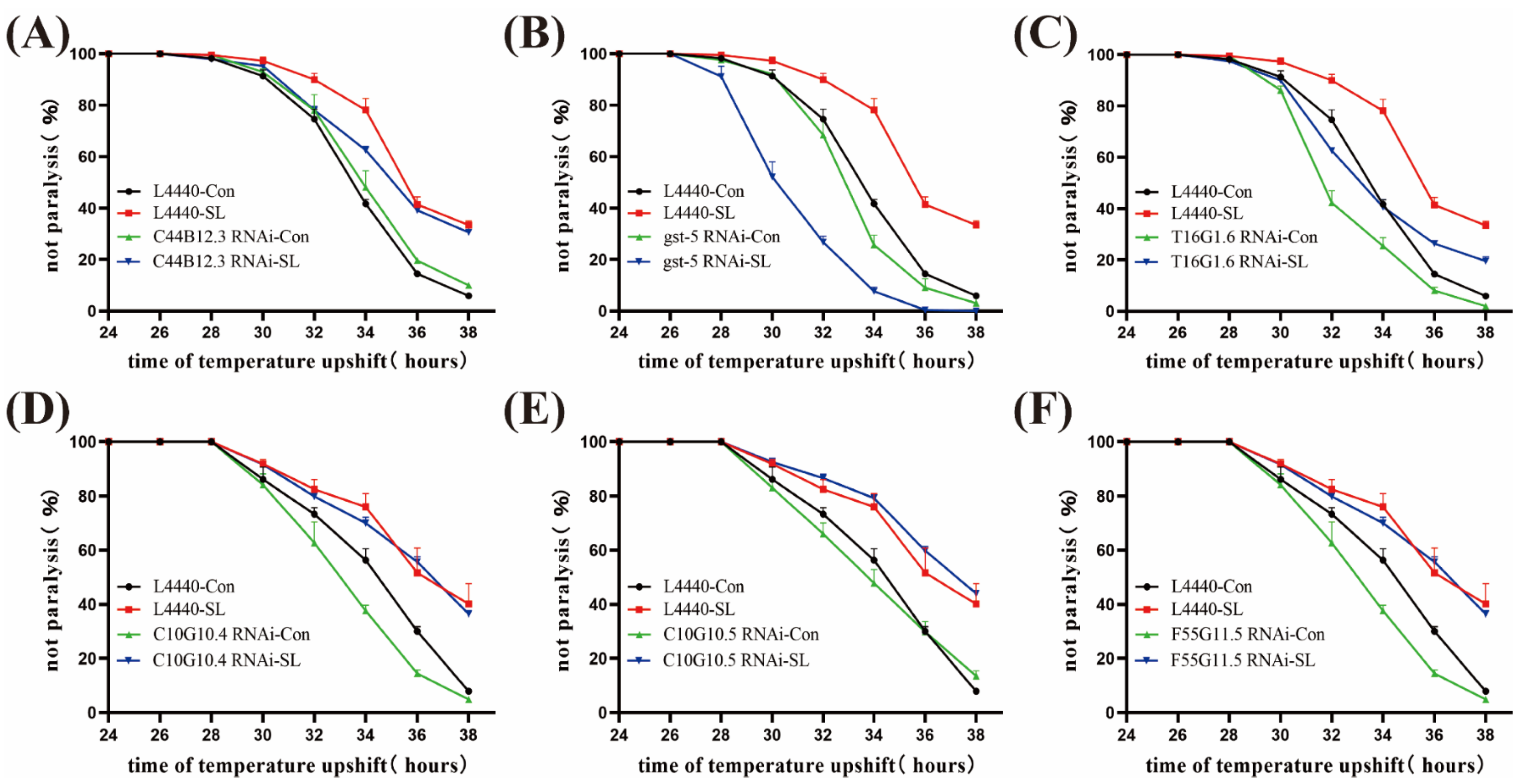
2.5. SLPE Exerts Anti-AD Effects through Genes Gst-5
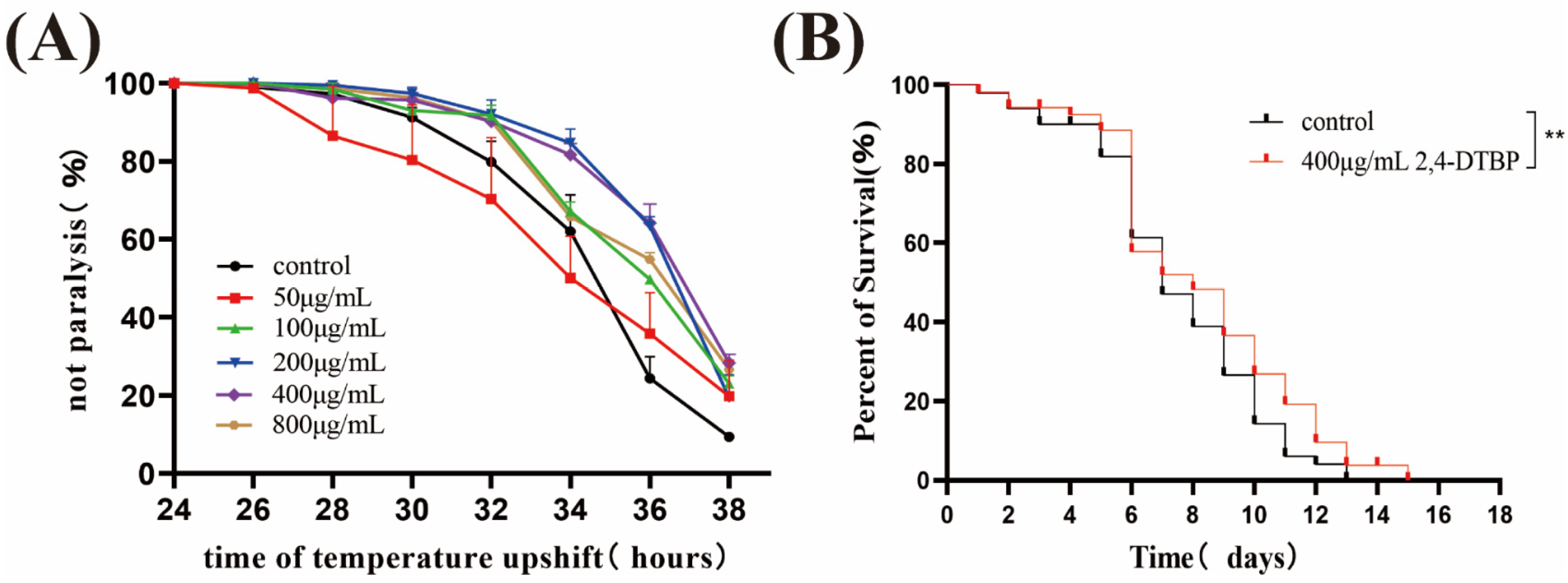
2.6. Compound 2,4-DTBP Delays CL4176 Paralysis and Prolongs the Lifespan of C. elegans N2 in the Heat Shock State
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Nematode Strains
4.2. Bioactivity Assay for Alzheimer’s Disease-like Phenotype of Delaying Paralysis in C. elegans
4.3. Phenotypic Experiments
4.4. PPI Network Construction
4.5. Transcriptome Sequencing and RNAi
4.6. GC-MS
4.7. Heat Shock Assays
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. Dementia. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Chen, C.H.; Chou, M.C.; Li, C.H.; Liu, C.K.; Chen, S.H. Concentration of donepezil to the cognitive response in Alzheimer disease. J. Clin. Psychopharmacol. 2013, 33, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Kondak, C.; Leith, M.; Baddeley, T.C.; Santos, R.X.; Harrington, C.R.; Wischik, C.M.; Riedel, G.; Klein, J. Mitochondrial Effects of Hydromethylthionine, Rivastigmine and Memantine in Tau-Transgenic Mice. Int. J. Mol. Sci. 2023, 24, 10810. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, S.; Van Baelen, B.; Schauble, B. Long-term effects of galantamine on cognitive function in Alzheimer’s disease: A large-scale international retrospective study. J. Alzheimers Dis. 2011, 27, 521–530. [Google Scholar] [CrossRef]
- Chen, H.S.; Lipton, S.A. The chemical biology of clinically tolerated NMDA receptor antagonists. J. Neurochem. 2006, 97, 1611–1626. [Google Scholar] [CrossRef]
- Magyar, K. The pharmacology of selegiline. Int. Rev. Neurobiol. 2011, 100, 65–84. [Google Scholar] [CrossRef]
- Rani, N.; Alam, M.M.; Jamal, A.; Ghaffar, O.B.; Parvez, S. Caenorhabditis elegans: A transgenic model for studying age-associated neurodegenerative diseases. Ageing Res. Rev. 2023, 91, 102036. [Google Scholar] [CrossRef]
- Zheng, J.; Greenway, F.L. Caenorhabditis elegans as a model for obesity research. Int. J. Obes. 2012, 36, 186–194. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans; WormBook: New York, NY, USA, 2006; pp. 1–11. [Google Scholar] [CrossRef]
- Xin, L.; Yamujala, R.; Wang, Y.; Wang, H.; Wu, W.H.; Lawton, M.A.; Long, C.; Di, R. Acetylcholineestarase-inhibiting alkaloids from Lycoris radiata delay paralysis of amyloid beta-expressing transgenic C. elegans CL4176. PLoS ONE 2013, 8, e63874. [Google Scholar] [CrossRef] [PubMed]
- Link, P.; Wetterauer, B.; Fu, Y.; Wink, M. Extracts of Glycyrrhiza uralensis and isoliquiritigenin counteract amyloid-beta toxicity in Caenorhabditis elegans. Planta Medica 2015, 81, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Dong, Y.Q.; Ye, B.P. Cranberry extract supplementation exerts preventive effects through alleviating Abeta toxicity in Caenorhabditis elegans model of Alzheimer’s disease. Chin. J. Nat. Med. 2016, 14, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Su, P.J.; Zhang, Z.P.; Cui, W.B.; Liu, X.; Wang, R.Y.; Zhi, D.J.; Qi, F.M.; Chen, X.H.; Li, Y.Q.; Djimabi, K.; et al. Polyoxygenated sesquiterpenoids from Salvia castanea and their potential anti-Alzheime’s disease bioactivities. Fitoterapia 2021, 151, 104867. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C.; Grayer, R.J.; Irwin, J.L.; Takeda, K. Flavonoid cellobiosides from Salvia uliginosa. Phytochemistry 1998, 48, 389–393. [Google Scholar] [CrossRef]
- Koch, K.; Weldle, N.; Baier, S.; Buchter, C.; Watjen, W. Hibiscus sabdariffa L. extract prolongs lifespan and protects against amyloid-beta toxicity in Caenorhabditis elegans: Involvement of the FoxO and Nrf2 orthologues DAF-16 and SKN-1. Eur. J. Nutr. 2020, 59, 137–150. [Google Scholar] [CrossRef]
- Kanaan, N.M.; Morfini, G.; Pigino, G.; LaPointe, N.E.; Andreadis, A.; Song, Y.; Leitman, E.; Binder, L.I.; Brady, S.T. Phosphorylation in the amino terminus of tau prevents inhibition of anterograde axonal transport. Neurobiol. Aging 2012, 33, 826.e15–826.e30. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Navarro-Hortal, M.D.; Varela-Lopez, A.; Osorio, R.; Quiles, J.L. Novel Polymeric Nanocarriers Reduced Zinc and Doxycycline Toxicity in the Nematode Caenorhabditis elegans. Antioxidants 2019, 8, 550. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Wang, H.; Hu, T. Identification of key genes and pathways in abdominal aortic aneurysm by integrated bioinformatics analysis. J. Int. Med. Res. 2020, 48, 300060519894437. [Google Scholar] [CrossRef]
- Keowkase, R.; Aboukhatwa, M.; Luo, Y. Fluoxetine protects against amyloid-beta toxicity, in part via daf-16 mediated cell signaling pathway, in Caenorhabditis elegans. Neuropharmacology 2010, 59, 358–365. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.D.; Wang, Y.D. DAF-16/FOXO Transcription Factor in Aging and Longevity. Front. Pharmacol. 2017, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Zečić, A.; Braeckman, B.P. DAF-16/FoxO in Caenorhabditis elegans and Its Role in Metabolic Remodeling. Cells 2020, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M.A.; Green, J.W.; Au, C.; Benedetto, A.; Thompson, M.A.; Clark, E.; Gilliat, A.F.; Young, A.; Schmeisser, K.; Gems, D. The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms. Aging Cell 2017, 16, 1191–1194. [Google Scholar] [CrossRef]
- Fang, E.F.; Waltz, T.B.; Kassahun, H.; Lu, Q.; Kerr, J.S.; Morevati, M.; Fivenson, E.M.; Wollman, B.N.; Marosi, K.; Wilson, M.A.; et al. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 2017, 7, 46208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural sources and bioactivities of 2, 4-di-tert-butylphenol and its analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Kim, J.K.; Kim, H.K.; Harris, K.; Kim, C.J.; Park, G.G.; Park, C.S.; Shin, D.H. 2,4-Di-tert-butylphenol from sweet potato protects against oxidative stress in PC12 cells and in mice. J. Med. Food 2013, 16, 977–983. [Google Scholar] [CrossRef]
- Ma, H.; Ma, Y.; Zhang, Z.; Zhao, Z.; Lin, R.; Zhu, J.; Guo, Y.; Xu, L. L-arginine enhances resistance against oxidative stress and heat stress in Caenorhabditis elegans. Int. J. Environ. Res. Public Health 2016, 13, 969. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Leung, M.C.K.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis elegans: An emerging model in biomedical and environmental toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef]
- Lindblom, T.H.; Dodd, A.K. Xenobiotic detoxification in the nematode Caenorhabditis elegans. J. Exp. Zool. A Comp. Exp. Biol. 2006, 305, 720–730. [Google Scholar] [CrossRef]
- McElwee, J.J.; Schuster, E.; Blanc, E.; Thomas, J.H.; Gems, D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J. Biol. Chem. 2004, 279, 44533–44543. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, L.; Yu, Z.; Zhang, T.; Ma, S.; Liu, J. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res. Int. 2016, 90, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Antioxidant treatment in Alzheimer’s disease: Current state. J. Mol. Neurosci. 2003, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Ayyadevara, S.; Engle, M.R.; Singh, S.P.; Dandapat, A.; Lichti, C.F.; Beneš, H.; Reis, R.J.S.; Liebau, E.; Zimniak, P. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell 2005, 4, 257–271. [Google Scholar] [CrossRef]
- Perluigi, M.; Coccia, R.; Butterfield, D.A. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: A toxic combination illuminated by redox proteomics studies. Antioxid. Redox Signal. 2012, 17, 1590–1609. [Google Scholar] [CrossRef]
- Sayre, L.M.; Zelasko, D.A.; Harris, P.L.; Perry, G.; Salomon, R.G.; Smith, M.A. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J. Neurochem. 1997, 68, 2092–2097. [Google Scholar] [CrossRef]
- Ayyadevara, S.; Dandapat, A.; Singh, S.P.; Siegel, E.R.; Shmooklerreis, R.; Zimniak, L.; Zimniak, P. Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal. Mech. Ageing Dev. 2007, 128, 196–205. [Google Scholar] [CrossRef]
- Golden, N.L.; Plagens, R.N.; Guisbert, K.S.K.; Guisbert, E.J.J. Standardized methods for measuring induction of the heat shock response in Caenorhabditis elegans. J. Vis. Exp. 2020, 161, e61030. [Google Scholar]
- Guisbert, E.; Morimoto, R.I. The regulation and function of the heat shock response. In Protein Quality Control in Neurodegenerative Diseases; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–18. [Google Scholar]
- Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C. Caenorhabditis elegans as a model organism to evaluate the antioxidant effects of phytochemicals. Molecules 2020, 25, 3194. [Google Scholar] [CrossRef] [PubMed]
- Link, C.D.; Taft, A.; Kapulkin, V.; Duke, K.; Kim, S.; Fei, Q.; Wood, D.E.; Sahagan, B.G. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiol. Aging 2003, 24, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Saar, V.; Leung, K.L.; Chen, L.; Wong, G. Human amyloid beta peptide and tau co-expression impairs behavior and causes specific gene expression changes in Caenorhabditis elegans. Neurobiol. Dis. 2018, 109, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.-B.; Zhang, Z.-P.; Bai, X.; Wang, S.-S.; Chen, X.-H.; Liu, X.; Su, P.-J.; Zhi, D.-J.; Fei, D.-Q.; Zhang, Z.-X.; et al. Cryptotanshinone Alleviates Oxidative Stress and Reduces the Level of Abnormally Aggregated Protein in Caenorhabditis elegans AD Models. Int. J. Mol. Sci. 2022, 23, 10030. [Google Scholar] [CrossRef]
- AlOkda, A.; Van Raamsdonk, J.M. Effect of DMSO on lifespan and physiology in C. elegans: Implications for use of DMSO as a solvent for compound delivery. Micropubl. Biol. 2022, 2022. [Google Scholar] [CrossRef]
- Li, R.; Tao, M.; Xu, T.; Huang, Y.; Zogona, D.; Pan, S.; Wu, T.; Xu, X. Artemisia selengensis Turcz. leaf extract promotes longevity and stress resistance in Caenorhabditis elegans. J. Sci. Food Agric. 2022, 102, 4532–4541. [Google Scholar] [CrossRef]

| Serial Number | Compounds | Matching Factor | Molecular Formula | CAS Number | Relative Content (%) |
|---|---|---|---|---|---|
| 1 | 2,4-Di-tert-butylphenol | 95.28641529 | C14H22O | 96-76-4 | 20.32 |
| 2 | p-Xylene | 92.08370814 | C8H10 | 106-42-3 | 3.34 |
| 3 | Mesitylene | 91.02907647 | C9H12 | 108-67-8 | 1.25 |
| 4 | Cyclohexasiloxane, dodecamethyl- | 90.74789501 | C12H36O6Si6 | 540-97-6 | 1.29 |
| 5 | Dodecane, 4,6-dimethyl- | 90.6694043 | C14H30 | 61141-72-8 | 3.19 |
| 6 | Undecane, 5,7-dimethyl- | 88.78228596 | C13H28 | 17312-83-3 | 0.97 |
| 7 | Nonane, 2-methyl- | 88.39238821 | C10H22 | 871-83-0 | 0.57 |
| 8 | Dibenzylamine | 88.06018078 | C14H15N | 103-49-1 | 11.45 |
| 9 | Decane, 2,3,7-trimethyl- | 87.51639754 | C13H28 | 62238-13-5 | 1.17 |
| 10 | 2,6-Dimethyldecane | 87.15292199 | C12H26 | 13150-81-7 | 1.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Wang, Z.; Liao, S.; Liao, Y.; Hu, S.; Qin, J.; Zhang, D.; Yan, X. Components in SLPE Alleviate AD Model Nematodes by Up-Regulating Gene gst-5. Int. J. Mol. Sci. 2024, 25, 10188. https://doi.org/10.3390/ijms251810188
Zhao P, Wang Z, Liao S, Liao Y, Hu S, Qin J, Zhang D, Yan X. Components in SLPE Alleviate AD Model Nematodes by Up-Regulating Gene gst-5. International Journal of Molecular Sciences. 2024; 25(18):10188. https://doi.org/10.3390/ijms251810188
Chicago/Turabian StyleZhao, Peng, Zifu Wang, Shimei Liao, Yangxin Liao, Shijun Hu, Jianchun Qin, Donghua Zhang, and Xiaohui Yan. 2024. "Components in SLPE Alleviate AD Model Nematodes by Up-Regulating Gene gst-5" International Journal of Molecular Sciences 25, no. 18: 10188. https://doi.org/10.3390/ijms251810188
APA StyleZhao, P., Wang, Z., Liao, S., Liao, Y., Hu, S., Qin, J., Zhang, D., & Yan, X. (2024). Components in SLPE Alleviate AD Model Nematodes by Up-Regulating Gene gst-5. International Journal of Molecular Sciences, 25(18), 10188. https://doi.org/10.3390/ijms251810188







