Transplanted Murine Tumours SPECT Imaging with 99mTc Delivered with an Artificial Recombinant Protein
Abstract
:1. Introduction
1.1. 99mTc Carriers for SPECT/CT Tumour Diagnosis
1.2. Known Types of Therapeutically Compatible 99mTc Binding Groups
1.3. Artificial Metal-Binding Proteins as a Novel Approach to 99mTc Delivery to Tumours
2. Results
2.1. Novel Recombinant Artificial Protein E1b
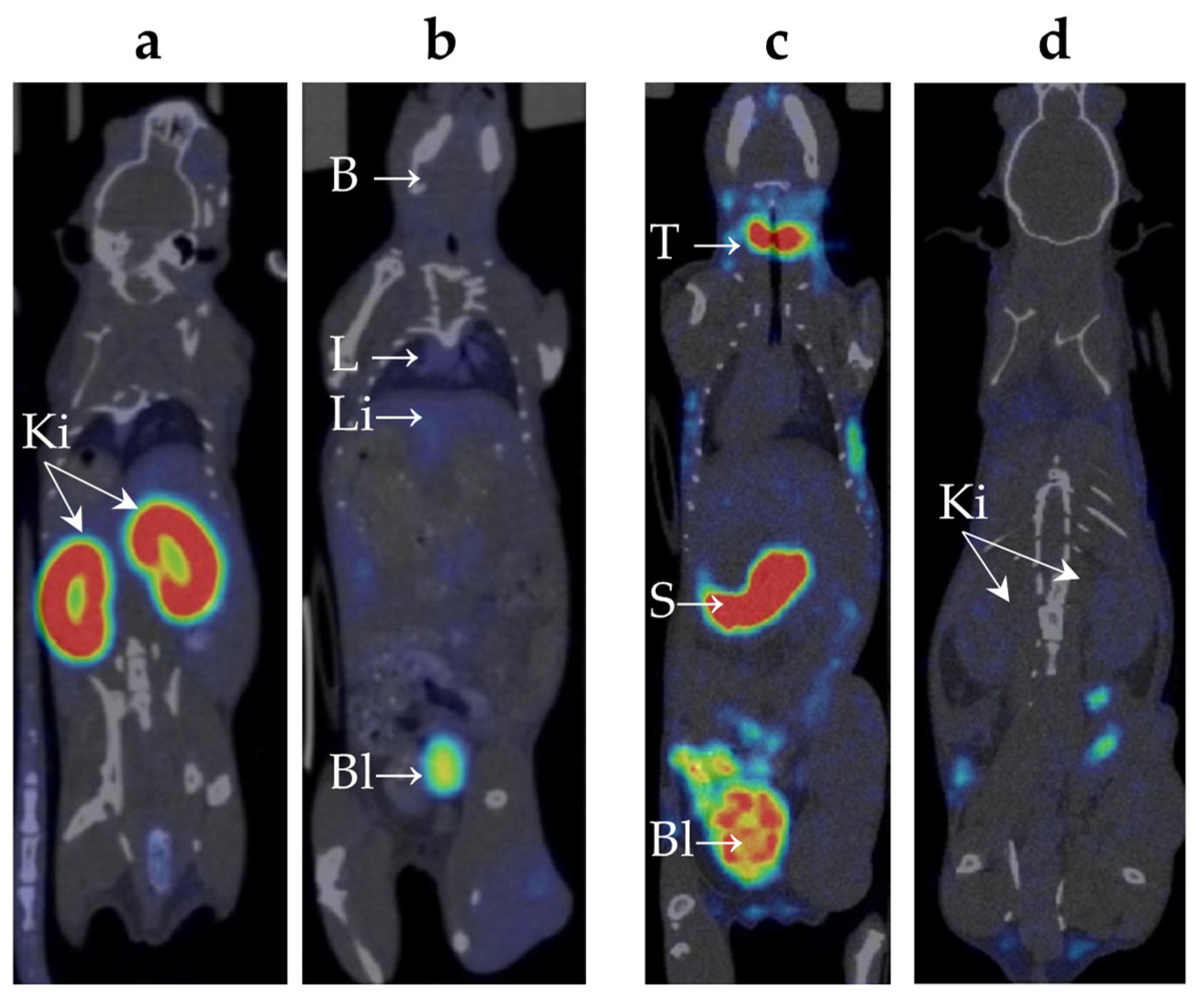
2.2. Biodistribution of 99mTc-E1b Protein in Mice
2.3. Biodistribution of 99mTc-Pertechnetate in Mice
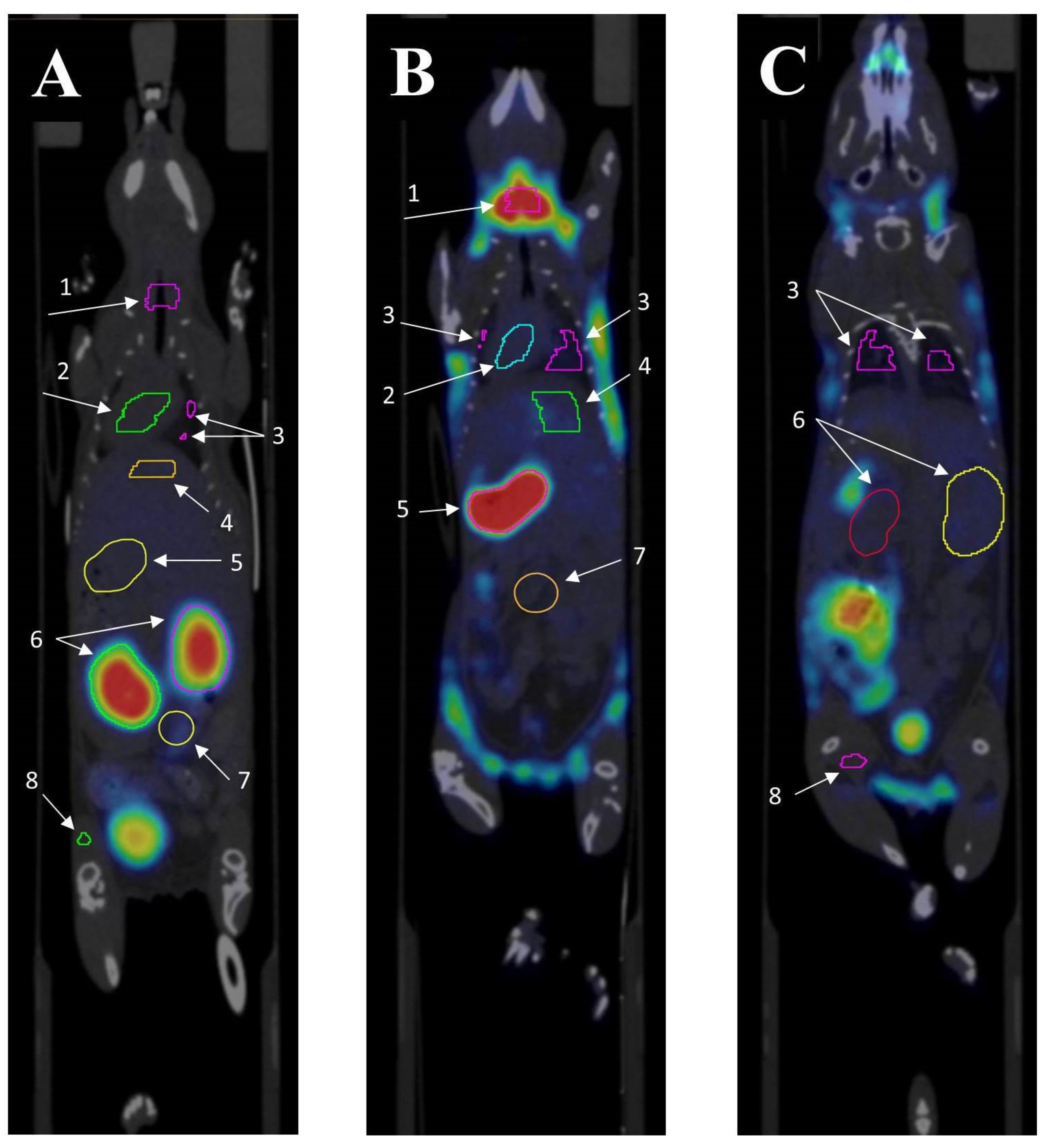
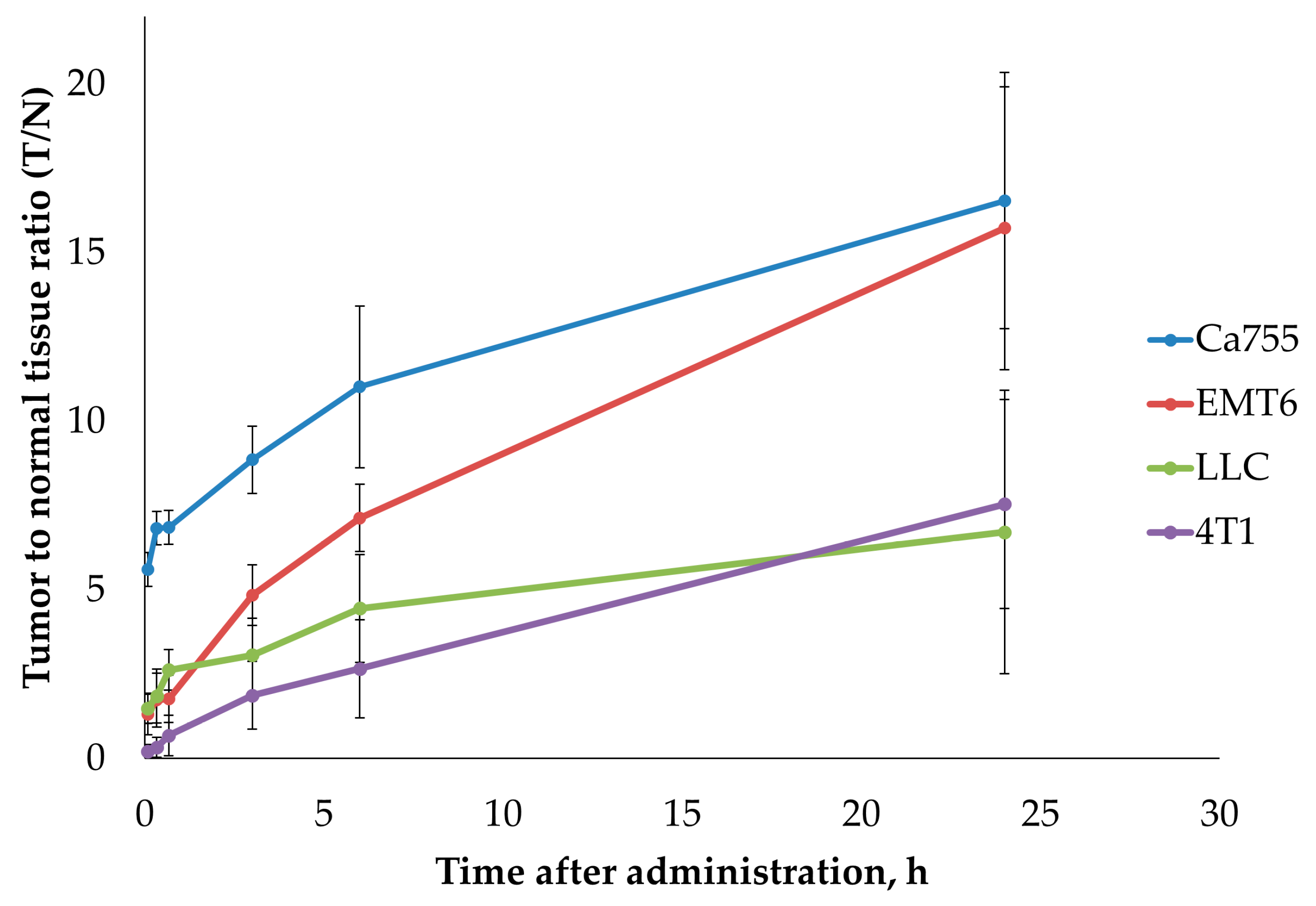
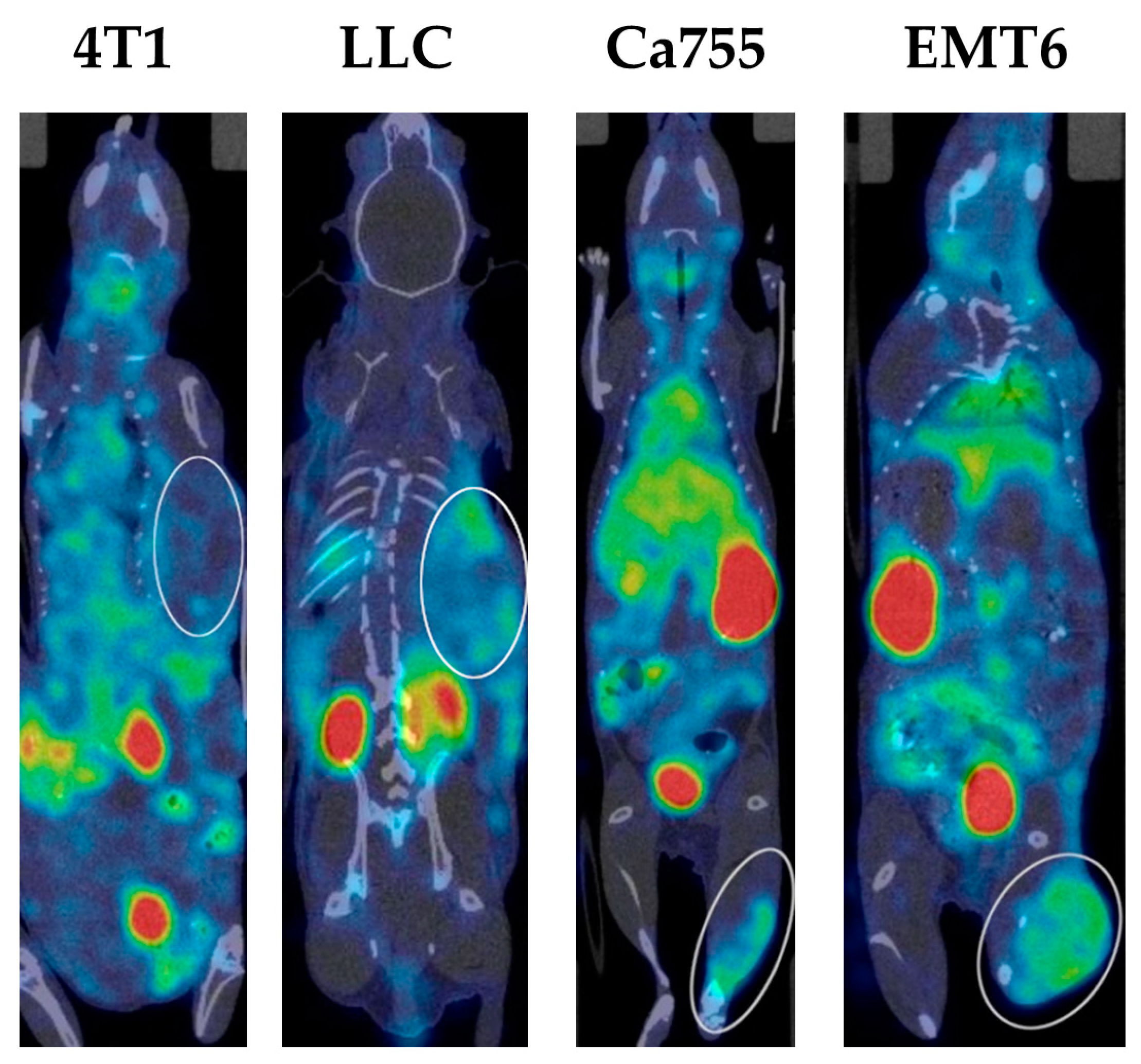
2.4. Tumour Uptake of 99mTc-E1b
3. Discussion
4. Materials and Methods
4.1. Chemicals and Disposables
4.2. Genetic Constructs
4.3. Software
4.4. Murine Tumour Cell Cultures
4.5. Production and Purification of E1b Protein
4.5.1. Genetic Construct pE1b
4.5.2. E1b Protein Production and Purification
4.6. Labelling E1b Protein with 99mTc and Purification of the Complex
4.7. Tumour Animal Model
4.8. SPECT/CT In Vivo Imaging
4.9. Processing of SPECT/CT Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 99mTc | Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 symbolized as 99mTc |
| ELP repeats | Elastin-like polypeptides |
| SPECT | Single-Photon Emission Computed Tomography |
| CT | X-ray Computed Tomography |
| G4EC | Oligopeptide with amino acid composition GGGGEC |
| HER-2 | Cancer Marker (Epidermal Growth Factor Receptor) |
| TNFα | Tumour Necrosis Factor A |
| SUV | Standardized uptake value |
| His-tags | Polyhistidine-tag is an amino acid motif in proteins that typically consists of at least six histidine (His) |
| T/N ratio | Tumour-to-normal tissue ratio |
| SCID | Severe combined immunodeficiency |
| RGD | Peptide—peptide containing the RGD (Arginylglycylaspartic acid) |
| F3 peptide | A 31 amino acid fragment of HMGN2 protein, specifically bound to the nucleolin oncomarker |
| GFP | green fluorescent protein |
| E. coli | Escherichia coli |
| IMAC | Immobilized Metal Chelate Affinity Chromatography |
| 99mTc-E1b | Tight complex of 99mTc radioactive isotopes and E1b protein |
| EDTA | ethylenediaminetetraacetic acid |
| SDS-PAGE | Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis |
| DTNB | Ellman’s reagent, 5,5′-dithiobis-(2-nitrobenzoic acid) |
| E2-13W4 | protein composed from multiple metal-binding centres and the universal tumour-specific ligands: RGD (Arg-Gly-Asp) and F3-peptide (nucleolin-binding peptide) |
| BCA | Bicinchoninic acid |
| ATCC | American Type Culture Collection |
| Blokhin NMRCO | Blokhin National Medical Research Centre of Oncology of the Ministry of Health of the Russian Federation |
| MW | Molecular weight |
Appendix A. The E1b Protein 3D-Structure Theoretical Model
Appendix B. The Procedure for Contouring Organs up on SPECT/CT
References
- Ranadive, G.N.; Rosenzweig, H.S.; Epperly, M.W.; Seskey, T.; Bloomer, W.D. A new method of technetium-99m labeling of monoclonal antibodies through sugar residues. A study with TAG-72 specific CC-49 antibody. Nucl. Med. Biol. 1993, 20, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, T.; Shi, L.; Zhang, X.; Guo, X.; Hu, B.; Yao, M.; Zhu, H.; Yang, Z.; Jia, B.; et al. HER2-targeted dual radiotracer approach with clinical potential for noninvasive imaging of trastuzumab-resistance caused by epitope masking. Theranostics 2022, 12, 5551–5563. [Google Scholar] [CrossRef]
- Shi, D.; Fu, W.; Tan, H.; Lin, Q.; Shi, H.; Cheng, D. Preclinical Evaluation of 99mTc-MAG3-5-Fab Targeting TREM2 in Lung Cancer Mouse Models: A Comparison with 99mTc-MAG3-5-F(ab’)2. Mol. Pharm. 2024, 21, 303–312. [Google Scholar] [CrossRef]
- Chapeau, D.; Koustoulidou, S.; Handula, M.; Beekman, S.; de Ridder, C.; Stuurman, D.; de Blois, E.; Buchatskaya, Y.; van der Schilden, K.; de Jong, M.; et al. [212Pb]Pb-eSOMA-01: A Promising Radioligand for Targeted Alpha Therapy of Neuroendocrine Tumors. Pharmaceuticals 2023, 16, 985. [Google Scholar] [CrossRef]
- Shi, J.; Liu, S. Clinical application of 99mTc-labeled peptides for tumor imaging: Current status and future directions. iRADIOLOGY 2024, 2, 17–34. [Google Scholar] [CrossRef]
- Biabani Ardakani, J.; Abedi, S.M.; Mardanshahi, A.; Shojaee, L.; Zaboli, E.; Khorramimoghaddam, A.; Nosrati, A.; Sabahno, H.; Banimostavafi, E.S.; Hosseinimehr, S.J. Molecular Imaging of HER2 Expression in Breast Cancer patients Using the [99mTc] Tc-Labeled Small Peptide. Clin. Breast Cancer 2023, 23, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Yao, J.; Zhao, Z.; Zhang, X.; Lu, J. Application of 99mTc-Labeled WL12 Peptides as a Tumor PD-L1-Targeted SPECT Imaging Agent: Kit Formulation, Preclinical Evaluation, and Study on the Influence of Coligands. Pharmaceuticals 2024, 17, 906. [Google Scholar] [CrossRef] [PubMed]
- Stafford, J.H.; Thorpe, P.E. Increased exposure of phosphatidylethanolamine on the surface of tumor vascular endothelium. Neoplasia 2011, 13, 299–308. [Google Scholar] [CrossRef]
- Delvaeye, T.; Wyffels, L.; Deleye, S.; Lemeire, K.; Gonçalves, A.; Decrock, E.; Staelens, S.; Leybaert, L.; Vandenabeele, P.; Krysko, D.V. Noninvasive Whole-Body Imaging of Phosphatidylethanolamine as a Cell Death Marker Using 99mTc-Duramycin during TNF-Induced SIRS. J. Nucl. Med. 2018, 59, 1140–1145. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Z.; Bugenhagen, S. 99mTc-labeled duramycin as a novel phosphatidylethanolamine-binding molecular probe. J. Nucl. Med. 2008, 49, 1345–1352. [Google Scholar] [CrossRef]
- Elvas, F.; Vangestel, C.; Rapic, S.; Verhaeghe, J.; Gray, B.; Pak, K.; Stroobants, S.; Staelens, S.; Wyffels, L. Characterization of [99mTc]Duramycin as a SPECT Imaging Agent for Early Assessment of Tumor Apoptosis. Mol. Imaging Biol. 2015, 17, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Veres, D.S.; Máthé, D.; Hegedűs, N.; Horváth, I.; Kiss, F.J.; Taba, G.; Tóth-Bodrogi, E.; Kovács, T.; Szigeti, K. Radiomic detection of microscopic tumorous lesions in small animal liver SPECT imaging. EJNMMI Res. 2019, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Kręcisz, P.; Czarnecka, K.; Królicki, L.; Mikiciuk-Olasik, E.; Szymański, P. Radiolabeled Peptides and Antibodies in Medicine. Bioconjug Chem. 2021, 32, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Uccelli, L.; Martini, P. A Picture of Modern Tc-99m Radiopharmaceuticals: Production, Chemistry, and Applications in Molecular Imaging. Appl. Sci. 2019, 9, 2526. [Google Scholar] [CrossRef]
- Lanteigne, D.; Hnatowich, D.J. The labeling of DTPA-coupled proteins with 99mTc. Int. J. Appl. Radiat. Isot. 1984, 35, 617–621. [Google Scholar] [CrossRef]
- Badar, A.; Williams, J.; de Rosales, R.T.; Tavaré, R.; Kampmeier, F.; Blower, P.J.; Mullen, G.E. Optimising the radiolabelling properties of technetium tricarbonyl and His-tagged proteins. EJNMMI Res. 2014, 4, 14. [Google Scholar] [CrossRef]
- Cappenberg, T.M.; De Schepper, S.; Vangestel, C.; De Lombaerde, S.; Wyffels, L.; Van den Wyngaert, T.; Mattis, J.; Gray, B.; Pak, K.; Stroobants, S.; et al. First-in-human study of a novel cell death tracer [99mTc]Tc-Duramycin: Safety, biodistribution and radiation dosimetry in healthy volunteers. EJNMMI Radiopharm. Chem. 2023, 8, 20. [Google Scholar] [CrossRef]
- Pozdniakova, N.V.; Ryabaya, O.V.; Semkina, A.S.; Skribitsky, V.A.; Shevelev, A.B. Using ELP Repeats as a Scaffold for De Novo Construction of Gadolinium-Binding Domains within Multifunctional Recombinant Proteins for Targeted Delivery of Gadolinium to Tumour Cells. Int. J. Mol. Sci. 2022, 23, 3297. [Google Scholar] [CrossRef]
- Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models. Cells 2019, 8, 889. [Google Scholar] [CrossRef]
- Franken, P.R.; Guglielmi, J.; Vanhove, C.; Koulibaly, M.; Defrise, M.; Darcourt, J.; Pourcher, T. Distribution and dynamics of 99mTc-pertechnetate uptake in the thyroid and other organs assessed by single-photon emission computed tomography in living mice. Thyroid 2010, 20, 519–526. [Google Scholar] [CrossRef]
- Tanwar, K.S.; Rana, N.; Mittal, B.R.; Bhattacharya, A. Early Quantification of Salivary Gland Function after Radioiodine Therapy. Indian. J. Nucl. Med. 2021, 36, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jiang, G.; Meng, Y.; Chen, J.; Liu, J. 99mTc-pertechnetate thyroid static scintigraphy unexpectedly revealed ectopic gastric mucosa of upper esophagus. Hell. J. Nucl. Med. 2023, 26, 157–159. [Google Scholar] [CrossRef]
- Niu, S.W.; Wu, C.H.; Chen, H.C.; Yang, C.J.; Chang, J.M.; Chang, E.E.; Chuang, H.H.; Chiu, Y.W.; Zhen, Y.Y.; Hung, C.C.; et al. Proteins Secreted by Lung Cancer Cells Induce the Onset of Proteinuria via Focal Adhesion Kinase Signaling in Mice. Lab. Investig. 2023, 103, 100156. [Google Scholar] [CrossRef]
- Picchio, M.; Beck, R.; Haubner, R.; Seidl, S.; Machulla, H.J.; Johnson, T.D.; Wester, H.J.; Reischl, G.; Schwaiger, M.; Piert, M. Intratumoral spatial distribution of hypoxia and angiogenesis assessed by 18F-FAZA and 125I-Gluco-RGD autoradiography. J. Nucl. Med. 2008, 49, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Moura, V.; Simões, S.; Moreira, J.N. Anticancer activity and antibody-dependent cell-mediated cytotoxicity of novel anti-nucleolin antibodies. Sci. Rep. 2018, 8, 7450. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Zhang, H.; Peng, Y.; Fu, Q.; Yue, Q.; Zhao, Y.; Guo, L.; Wu, Y. Dual-targeting liposomes with active recognition of GLUT5 and αvβ3 for triple-negative breast cancer. Eur. J. Med. Chem. 2019, 183, 111720. [Google Scholar] [CrossRef]
- Bertram, J.S.; Janik, P. Establishment of a cloned line of Lewis Lung Carcinoma cells adapted to cell culture. Cancer Lett. 1980, 11, 63–73. [Google Scholar] [CrossRef]
- Sharma, S.; Stolina, M.; Lin, Y.; Gardner, B.; Miller, P.W.; Kronenberg, M.; Dubinett, S.M. T cell-derived IL-10 promotes lung cancer growth by suppressing both T cell and APC function. J. Immunol. 1999, 163, 5020–5028. [Google Scholar] [CrossRef]
- Straus, M.J.; Sege, V.; Choi, S.C. The effect of surgery and pretreatment or posttreatment adjuvant chemotherapy on primary tumor growth in an animal model. J. Surg. Oncol. 1975, 7, 497–512. [Google Scholar] [CrossRef]
- Rockwell, S.C.; Kallman, R.F.; Fajardo, L.F. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. J. Natl. Cancer Inst. 1972, 49, 735–749. [Google Scholar]
- Straus, M.J.; Mantel, N.; Goldin, A. The effect of the sequence of administration of cytoxan and methotrexate on the life-span of L1210 leukemic mice. Cancer Res. 1972, 32, 200–207. [Google Scholar]
- Straus, M.J.; Goldin, A. Effects of priming dose schedules in cytoxan treatment of mouse leukemia L1210. Proc. Soc. Exp. Biol. Med. 1974, 145, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Pulaski, B.A.; Clements, V.K.; Pipeling, M.R.; Ostrand-Rosenberg, S. Immunotherapy with vaccines combining MHC class II/CD80+ tumor cells with interleukin-12 reduces established metastatic disease and stimulates immune effectors and monokine induced by interferon gamma. Cancer Immunol. Immunother. 2000, 49, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.L.; Bonawitz, N.D. Spectrophotometric determination of reaction rates and kinetic parameters of a BAHD acyltransferase using DTNB (5,5’-dithio-bis-[2-nitrobenzoic acid]). Plant Sci. 2018, 269, 148–152. [Google Scholar] [CrossRef]
- Directive 2010/63/EU of the European Parliament and of the Council of the European Union of September 22, 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:EN:PDF (accessed on 30 July 2024).
- Treshchalina, E.M.; Zhukova, O.S.; Gerasimova, G.K.; Andronova, N.V.; Garin, A.M. Methodological guidelines for the study of antitumour activity of pharmacological substances. In Guidelines for the Experimental (Preclinical) Study of New Pharmacological Substances; Khabriev, R.U., Ed.; Medicine: Moscow, Russia, 2005; pp. 637–651. (In Russian) [Google Scholar]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef]
- Wang, J.; Youkharibache, P.; Zhang, D.; Lanczycki, C.J.; Geer, R.C.; Madej, T.; Phan, L.; Ward, M.; Lu, S.; Marchler, G.H.; et al. iCn3D, a web-based 3D viewer for sharing 1D/2D/3D representations of biomolecular structures. Bioinformatics 2020, 36, 131–135. [Google Scholar] [CrossRef]
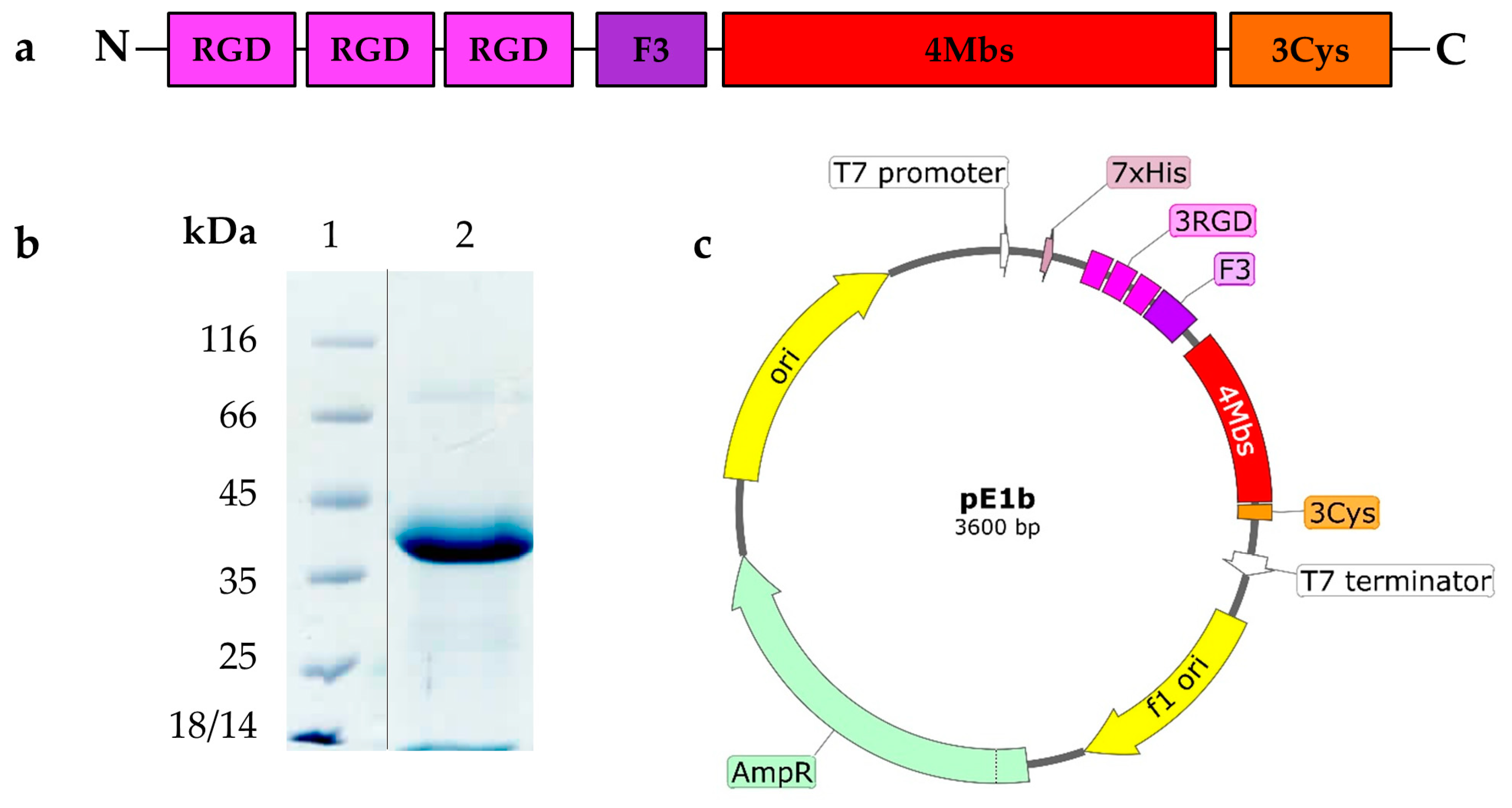

| Functional Elements | Number per Molecule | Function |
|---|---|---|
| His-taq | 1 | Protein purification by IMAC |
| RGD-motif | 3 | Binding integrin αVβ3 |
| F3-peptide | 1 | Nucleolin ligand binding |
| Metal binding site (Mbs) | 4 | Radionuclide binding in coordinated complex |
| Free cysteine | 3 | Radionuclide covalent coupling |
| ELP repeats (5 amino acids) | 13 | Immune-compatible molecular framework |
| Organ | Time Point | 99mTc-E1b Protein (SUV) | 99mTc-Pertechnetate (SUV) |
|---|---|---|---|
| Heart | 5 min | 3.5 ± 0.5 | 2.1 ± 0.3 |
| 20 min | 2.1 ± 0.3 | 1.2 ± 0.2 | |
| 40 min | 1.4 ± 0.4 | 0.8 ± 0.2 | |
| 2 h | 0.6 ± 0.3 | ||
| 3 h | 0.4 ± 0.2 | 0.7 ± 0.1 | |
| 6 h | 0.1 ± 0.1 | ||
| 24 h | 0.1 ± 0.1 | 0.05 ± 0.01 | |
| Lungs | 5 min | 1.8 ± 0.2 | 1.3 ± 0.3 |
| 20 min | 1.2 ± 0.4 | 0.9 ± 0.2 | |
| 40 min | 0.8 ± 0.3 | 0.6 ± 0.2 | |
| 2 h | 0.3 ± 0.2 | ||
| 3 h | 0.2 ± 0.2 | 0.4 ± 0.1 | |
| 6 h | 0.07 ± 0.02 | ||
| 24 h | 0.04 ± 0.02 | 0.04 ± 0.02 | |
| Liver | 5 min | 1.8 ± 0.2 | 1.6 ± 0.3 |
| 20 min | 1.3 ± 0.2 | 1.5 ± 0.4 | |
| 40 min | 1.0 ± 0.1 | 1.4 ± 0.2 | |
| 2 h | 0.7 ± 0.1 | ||
| 3 h | 0.6 ± 0.1 | 0.8 ± 0.2 | |
| 6 h | 0.5 ± 0.1 | ||
| 24 h | 0.3 ± 0.1 | 0.1 ± 0.1 | |
| Kidneys | 5 min | 14 ± 1 | 1.4 ± 0.3 |
| 20 min | 18 ± 2 | 0.9 ± 0.3 | |
| 40 min | 18 ± 1 | 0.7 ± 0.2 | |
| 2 h | 24 ± 6 | ||
| 3 h | 22 ± 5 | 0.6 ± 0.1 | |
| 6 h | 22 ± 7 | ||
| 24 h | 13 ± 1 | 0.1 ± 0.1 | |
| Stomach | 5 min | 0.5 ± 0.1 | 5 ± 1 |
| 20 min | 0.3 ± 0.1 | 7 ± 1 | |
| 40 min | 0.2 ± 0.07 | 12 ± 2 | |
| 2 h | 0.1 ± 0.05 | ||
| 3 h | 0.1 ± 0.05 | 24 ± 2 | |
| 6 h | 0.08 ± 0.01 | ||
| 24 h | 0.05 ± 0.01 | 1.4 ± 0.3 | |
| Neck (thyroid and salivary gland area) | 5 min | 0.2 ± 0.1 | 5 ± 2 |
| 20 min | 0.2 ± 0.1 | 9 ± 2 | |
| 40 min | 0.1 ± 0.1 | 12 ± 1 | |
| 2 h | 0.1 ± 0.1 | ||
| 3 h | 0.06 ± 0.04 | 23 ± 3 | |
| 6 h | 0.02 ± 0.01 | ||
| 24 h | 0.02 ± 0.01 | 0.9 ± 0.2 | |
| Muscle | 5 min | 0.3 ± 0.1 | 0.3 ± 0.1 |
| 20 min | 0.2 ± 0.1 | 0.2 ± 0.2 | |
| 40 min | 0.17 ± 0.07 | 0.2 ± 0.1 | |
| 2 h | 0.07 ± 0.05 | ||
| 3 h | 0.05 ± 0.05 | 0.1 ± 0.1 | |
| 6 h | 0.03 ± 0.01 | ||
| 24 h | 0.02 ± 0.01 | 0.01 ± 0.01 |
| T, min | Ca755 | EMT6 | LLC | 4T1 |
|---|---|---|---|---|
| 2 | 5.6 ± 0.5 | 1.3 ± 0.6 | 1.5 ± 0.5 | 0.2 ± 0.2 |
| 20 | 6.8 ± 0.5 | 1.7 ± 0.8 | 1.8 ± 0.8 | 0.3 ± 0.3 |
| 40 | 6.8 ± 0.5 | 1.8 ± 0.7 | 2.6 ± 0.6 | 0.7 ± 0.6 |
| 180 | 8.9 ± 1.1 | 4.8 ± 0.9 | 3.1 ± 1.1 | 1.9 ± 1.0 |
| 360 | 11.0 ± 2.4 | 7.1 ± 1.0 | 4.4 ± 1.6 | 2.7 ± 1.5 |
| 1440 | 16.5 ± 3.8 | 15.7 ± 4.2 | 6.7 ± 4.2 | 7.5 ± 3.1 |
| Name | ATCC No | Properties | Protocol of Maintenance | Immune Compatibility with Mouse-Inbred Lines | Peculiarities |
|---|---|---|---|---|---|
| LL/2 (LLC1) | CRL-1642™ | Transplantable lung Lewis adenocarcinoma occurs as three clones Lab, LLCC3 and LLC1. In our study, the clone LLC1 CRL 1642 was used | [27,28] | C57Bl/6 | A traditional model of metastatic growth for the study of resistance to chemotherapeutic agents |
| Ca755 or Bagg-Jackson Adenocarcinoma | - | Transplantable mammary gland adenocarcinoma of C57/Bl mice | [29] | C57Bl/6 | An acknowledged model of triple-negative human breast cancer with a high metastatic potential |
| EMT6 | CRL-2755™ | Metastatic mammary adenocarcinoma | [30,31,32] | Balb/c | https://www.atcc.org/products/crl-2755/ (accessed on 18 July 2023) The rapid growth of the tumour node begins from 12–15 days after grafting |
| 4T1 | CRL-2539™ | Mammary adenocarcinoma | [33] | Balb/c | https://www.atcc.org/products/crl-2539/ (accessed on 18 July 2023) popular model of the human TNM stage IV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozdniakova, N.V.; Lipengolts, A.A.; Skribitsky, V.A.; Shpakova, K.E.; Finogenova, Y.A.; Smirnova, A.V.; Shevelev, A.B.; Grigorieva, E.Y. Transplanted Murine Tumours SPECT Imaging with 99mTc Delivered with an Artificial Recombinant Protein. Int. J. Mol. Sci. 2024, 25, 10197. https://doi.org/10.3390/ijms251810197
Pozdniakova NV, Lipengolts AA, Skribitsky VA, Shpakova KE, Finogenova YA, Smirnova AV, Shevelev AB, Grigorieva EY. Transplanted Murine Tumours SPECT Imaging with 99mTc Delivered with an Artificial Recombinant Protein. International Journal of Molecular Sciences. 2024; 25(18):10197. https://doi.org/10.3390/ijms251810197
Chicago/Turabian StylePozdniakova, Natalia V., Alexey A. Lipengolts, Vsevolod A. Skribitsky, Kristina E. Shpakova, Yulia A. Finogenova, Anna V. Smirnova, Alexei B. Shevelev, and Elena Y. Grigorieva. 2024. "Transplanted Murine Tumours SPECT Imaging with 99mTc Delivered with an Artificial Recombinant Protein" International Journal of Molecular Sciences 25, no. 18: 10197. https://doi.org/10.3390/ijms251810197








