Exploratory Metabolomics and Lipidomics Profiling Contributes to Understanding How Curcumin Improves Quality of Goat Semen Stored at 16 °C in Tropical Areas
Abstract
:1. Introduction
2. Results
2.1. The Impact of Curcumin Supplementation on Sperm Kinetic Parameters
2.2. The Impact of Curcumin on Sperm Quality When Preserved at 16 °C
2.3. Effect of Curcumin on the Antioxidant Properties of Sperm Preserved at 16 °C
2.4. Effect of Curcumin on Mitochondrial Function When Preserved at 16 °C
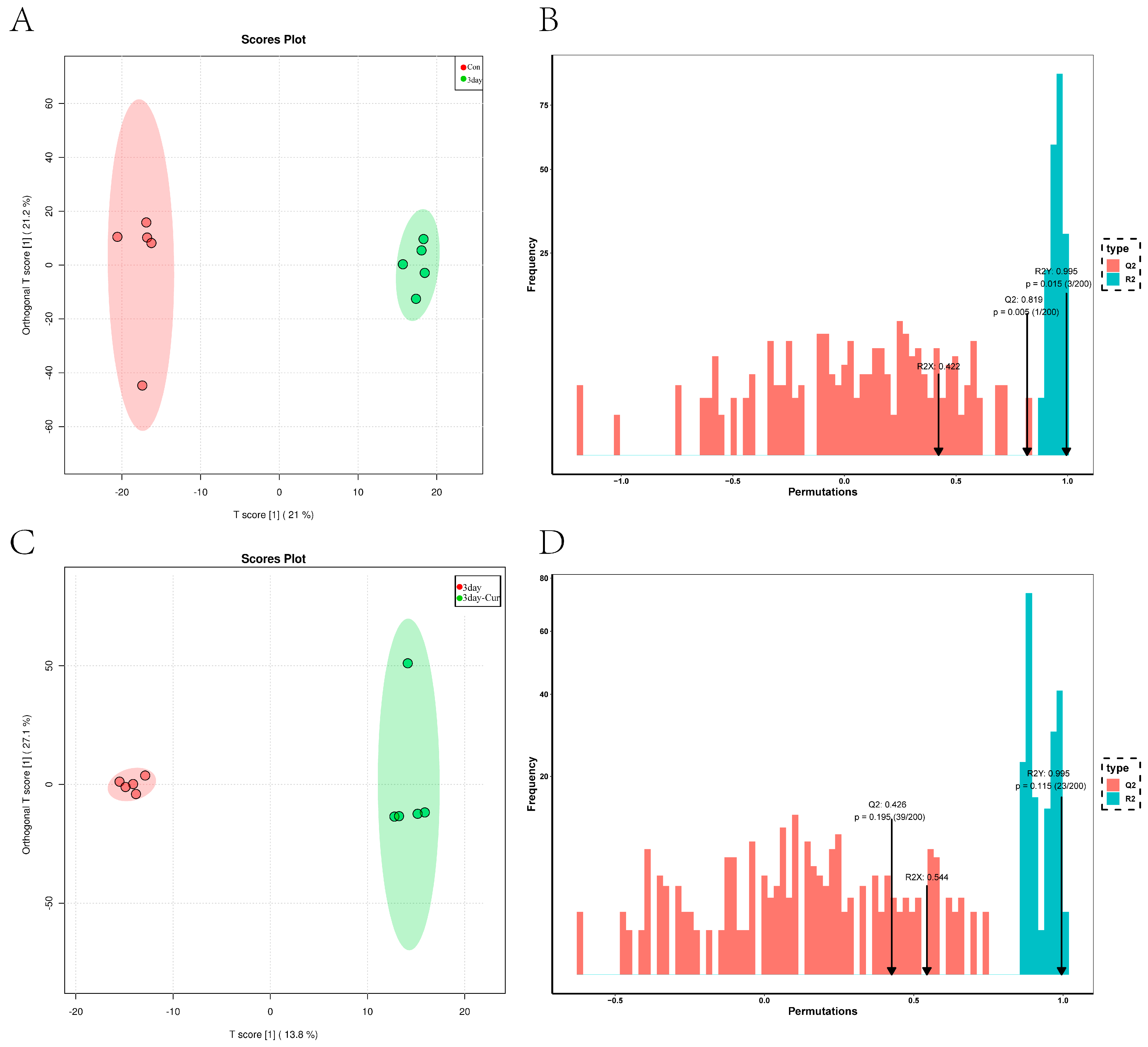
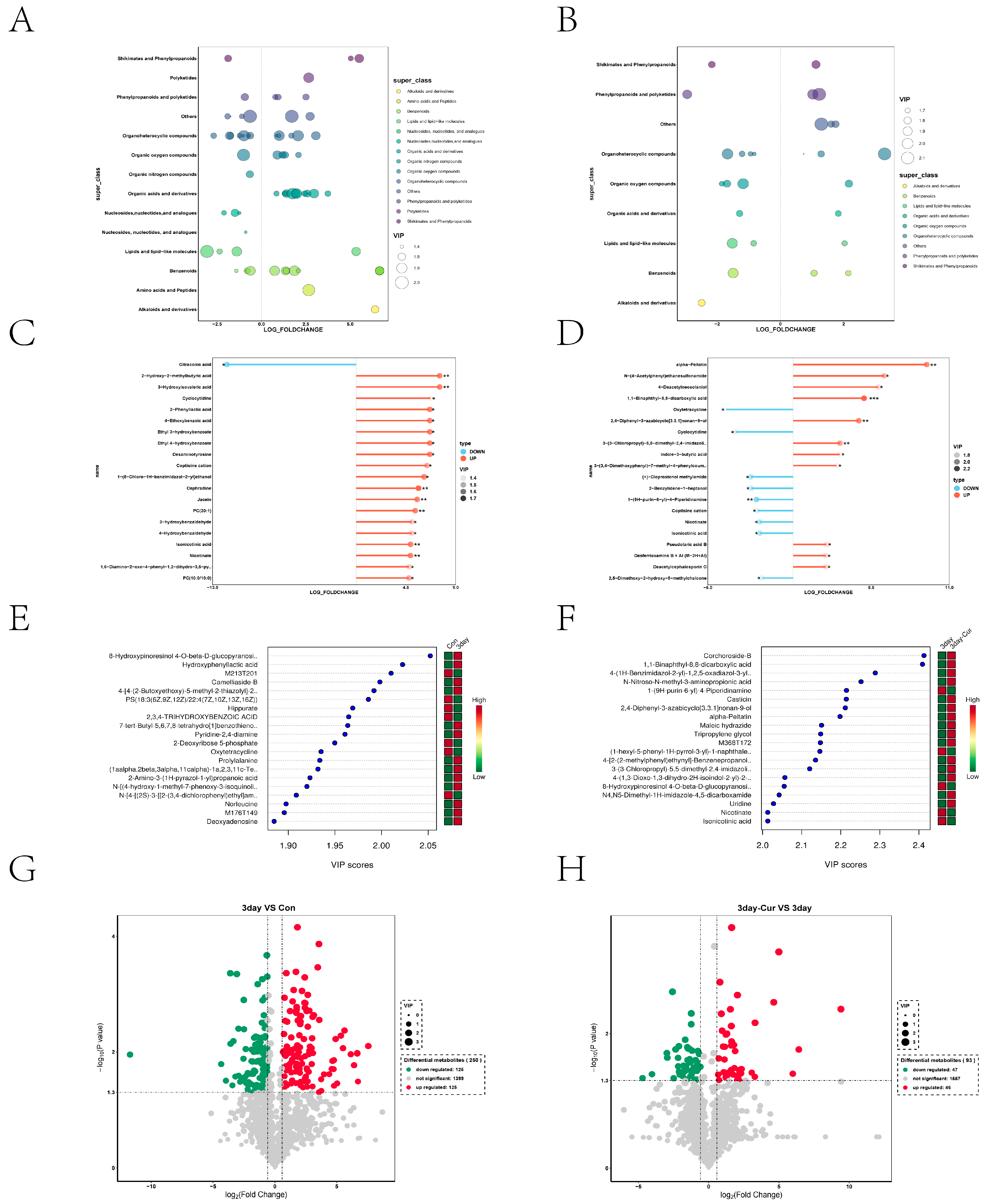
2.5. Metabolite Profiles in Sperm
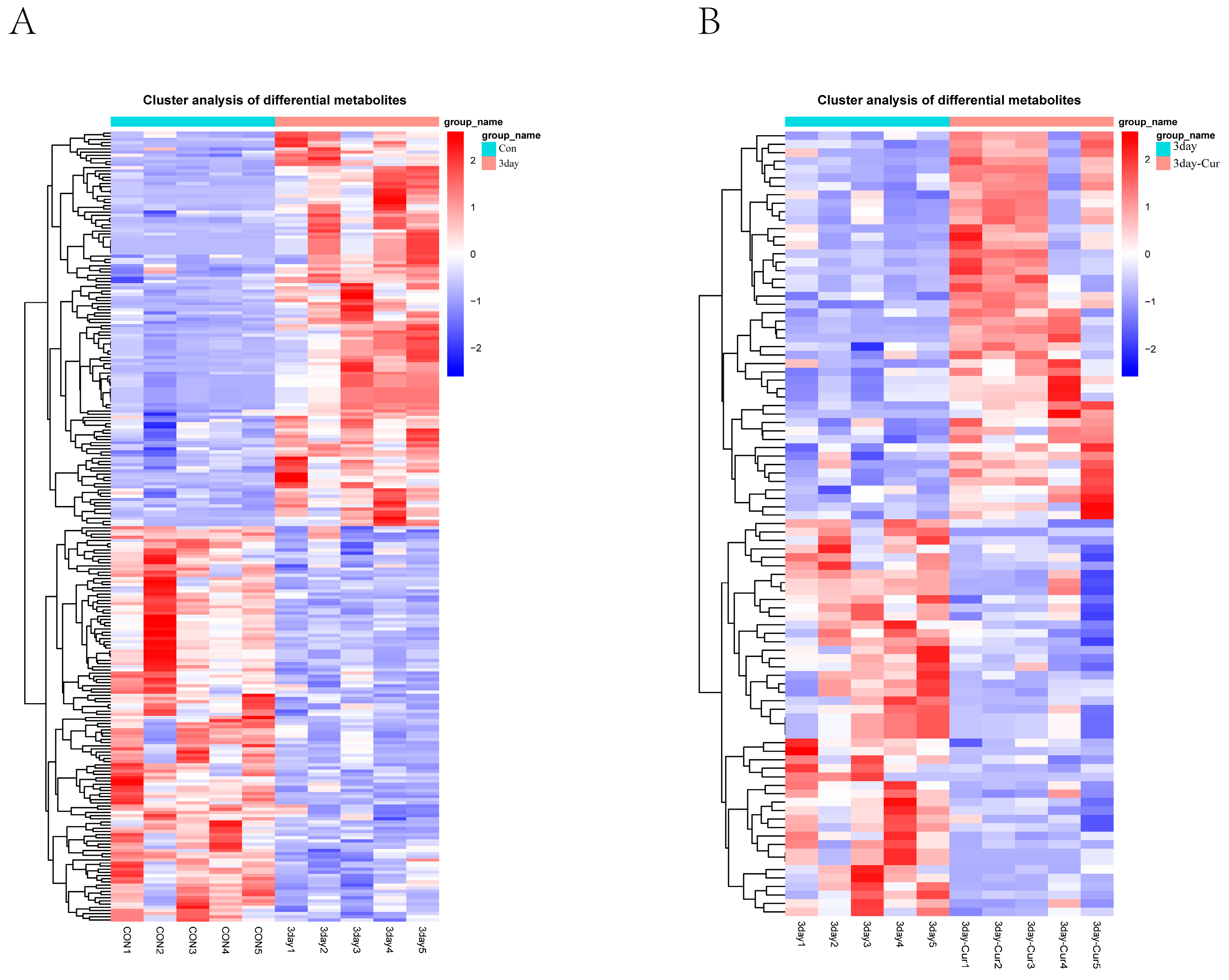
2.6. Cluster Analysis of the Differentially Expressed Metabolites
2.7. Screening and Identification of Differential Metabolites
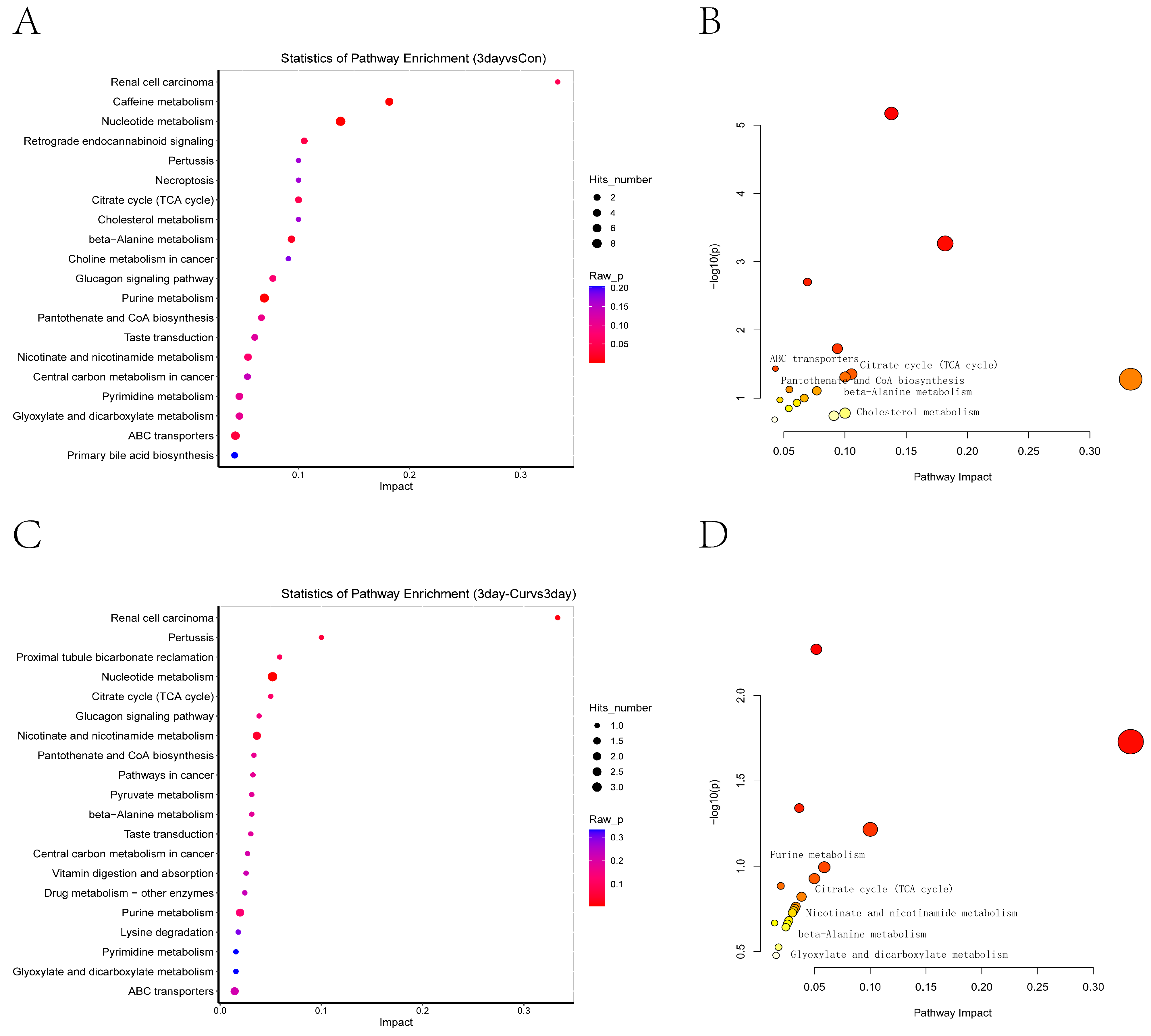
2.8. KEGG Enrichment Analysis of Differential Metabolites
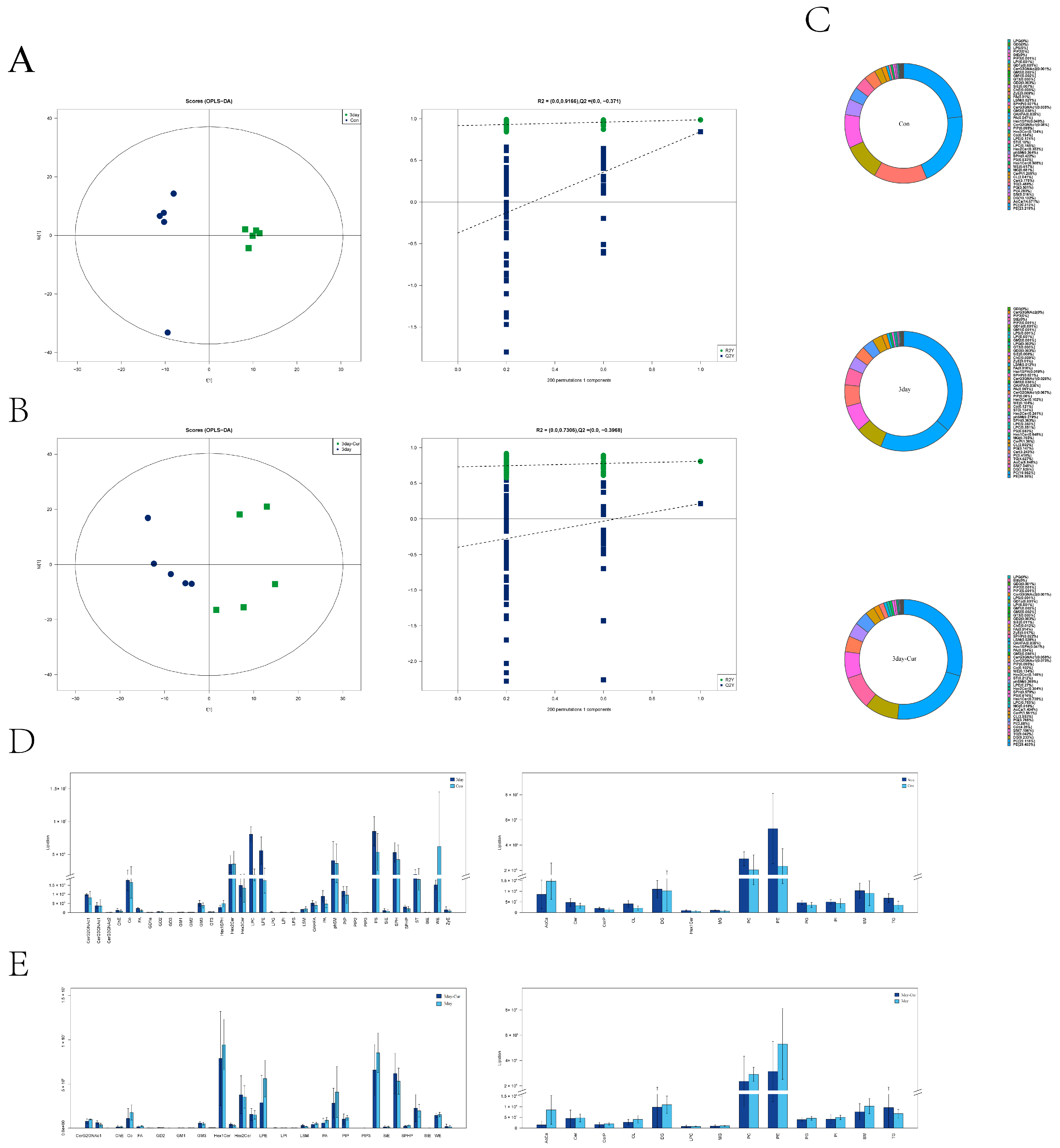
2.9. Identification of Lipid Species and Quantification and Multivariate Statistical Analysis
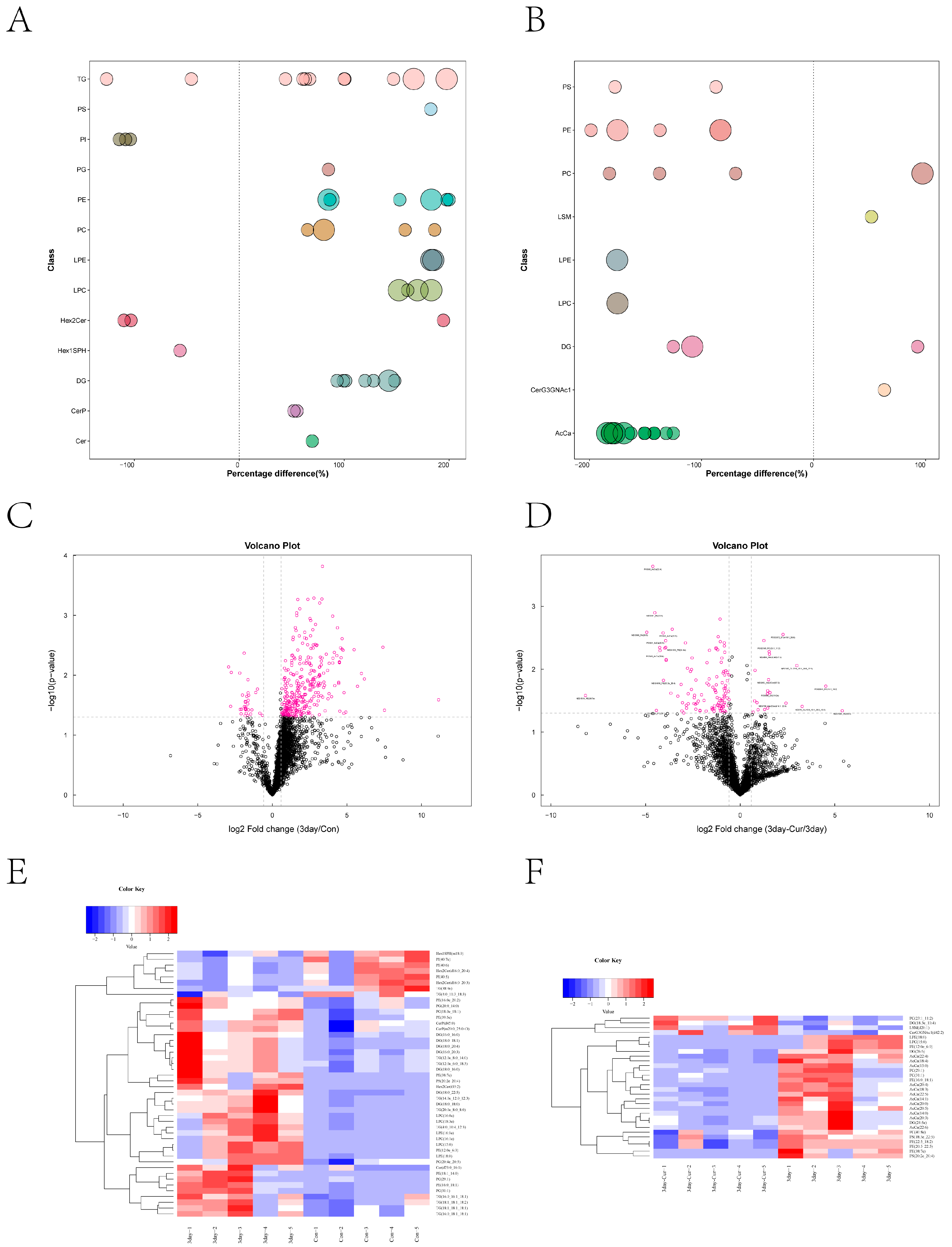
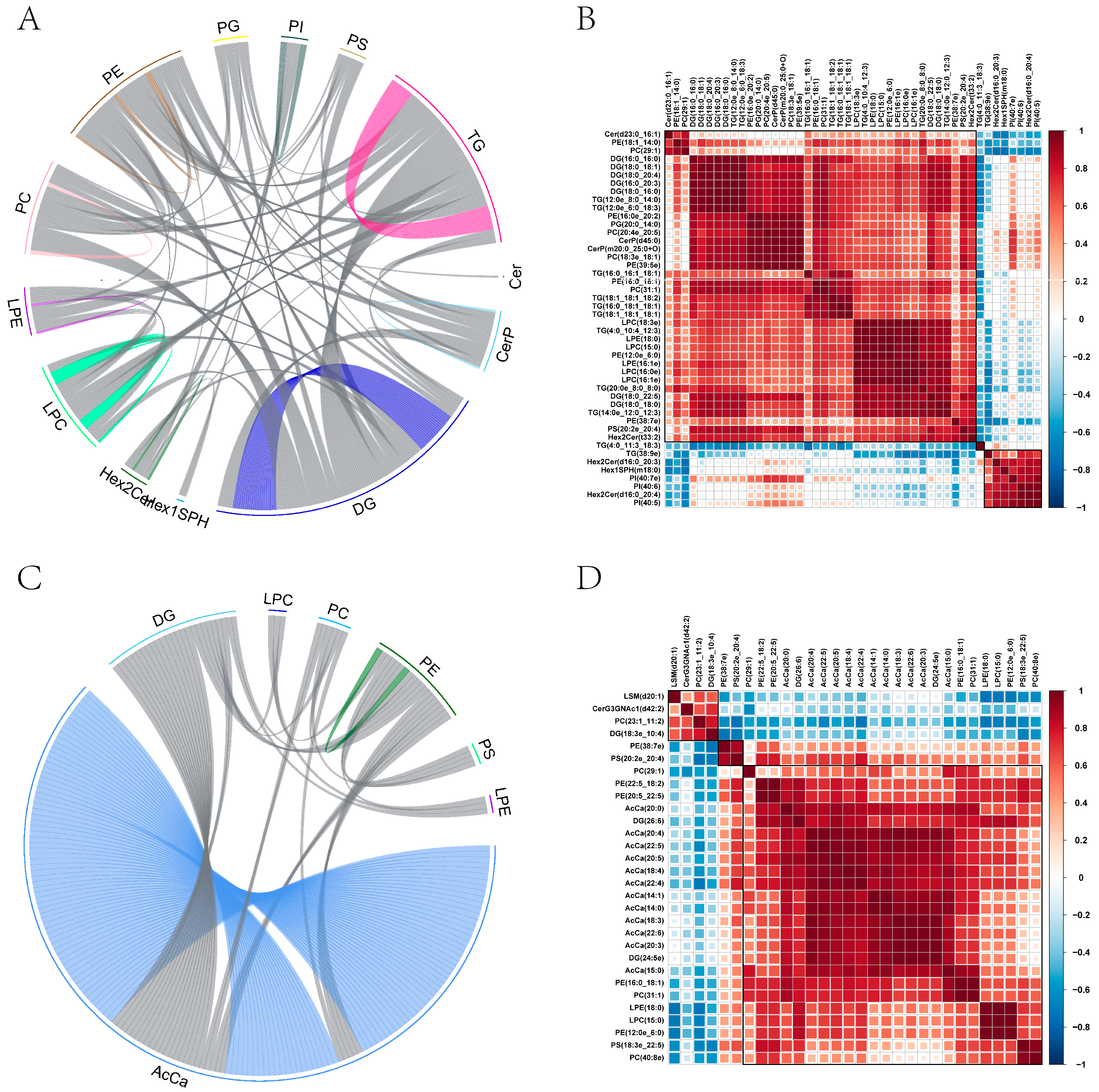
2.10. Screening and Clustering Analysis of Potential Lipid Molecular Markers
2.11. Correlation among Diverse Lipids
3. Discussion
4. Materials and Methods
4.1. Experimental Design
- (1)
- The effect of curcumin on semen quality (sperm motility, sperm plasma membrane and acrosome integrity, and motor performance).
- (2)
- The effect of curcumin on the antioxidant capacity of semen.
- (3)
- Metabolomic sequencing of the effects of curcumin on semen (Con: fresh semen; 3day: semen preserved for three days after adding diluent only; 3day-cur: semen preserved for three days after adding 25 µmol/L Cur).
- (4)
- Lipidomic sequencing of the effects of curcumin on semen (Con: fresh semen; 3day: semen preserved for three days after adding diluent only; 3day-cur: semen preserved for three days after adding 25 µmol/L Cur).
4.2. Preparation of Diluent
4.3. Collection and Dilution of Seminal Fluid
4.4. Detection of Sperm Quality
4.4.1. Sperm Motility Performance
4.4.2. Integrity of the Plasma Membrane
4.4.3. Integrity of the Acrosome
4.5. Detection of the Antioxidant Properties
4.6. Mitochondrial Function Assays
4.7. Statistical Analysis
4.8. Extraction of Metabolites
4.9. LC-MS Detection of the Metabolome
4.10. Metabolome Data Processing and Analysis
4.11. Lipid Extraction
4.12. Lipid Group LC-MS Detection
4.13. Lipidome Data Processing and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| LPO | Lipid peroxidation |
| PUFA | Polyunsaturated fatty acids |
| Cur | Curcumin |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| T-AOC | Total antioxidant capacity |
| ATP | Adenosine triphosphate |
| MMP | Mitochondrial membrane potential. |
| HOST | Hypo-osmotic swelling test |
| VSL | Straight-line velocity |
| VAP | Average path velocity |
| VCL | Curvilinear velocity |
| FC | Fold change |
| LC-MS | Liquid chromatograph mass spectrometer |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| RPTs | Response rank tests |
| VIP | Variable importance projection |
| DAMs | Differential accumulation metabolites |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| TG | Triglycerides |
| DG | Diglycerides |
| PE | Phosphatidylethanolamine |
| AcCa | Acylcarnitine |
| PI | Phosphatidylinositols |
| AA | Arachidonic acid |
References
- Bucak, M.N.; Tekin, N. Protective effect of taurine, glutathione and trehalose on the liquid storage of ram semen. Small Rumin. Res. 2007, 73, 103–108. [Google Scholar] [CrossRef]
- Salamon, S.; Maxwell, W.M.C. Storage of ram semen. Anim. Reprod. Sci. 2000, 62, 77–111. [Google Scholar] [CrossRef] [PubMed]
- Salamon, S.; Maxwell, W.M.C. Frozen storage of ram semen I. Processing, freezing, thawing and fertility after cervical insemination. Anim. Reprod. Sci. 1995, 37, 185–249. [Google Scholar] [CrossRef]
- Pini, T.; Leahy, T.; de Graaf, S.P. Sublethal sperm freezing damage: Manifestations and solutions. Theriogenology 2018, 118, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Salamon, S.; Maxwell, W.M.C. Frozen storage of ram semen II. Causes of low fertility after cervical insemination and methods of improvement. Anim. Reprod. Sci. 1995, 38, 1–36. [Google Scholar] [CrossRef]
- He, B.; Guo, H.; Gong, Y.; Zhao, R. Lipopolysaccharide-induced mitochondrial dysfunction in boar sperm is mediated by activation of oxidative phosphorylation. Theriogenology 2017, 87, 1–8. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Partyka, A.; Babapour, A.; Mikita, M.; Adeniran, S.; Niżański, W. Lipid peroxidation in avian semen. Pol. J. Vet. Sci. 2023, 26, 497–509. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Sohail, T.; Kang, Y.; Niu, H.; Sun, X.; Ji, D.; Li, Y. Effects of Taurine on Sperm Quality during Room Temperature Storage in Hu Sheep. Animals 2021, 11, 2725. [Google Scholar] [CrossRef]
- Gibb, Z.; Lambourne, S.R.; Quadrelli, J.; Smith, N.D.; Aitken, R.J. L-carnitine and pyruvate are prosurvival factors during the storage of stallion spermatozoa at room temperature. Biol. Reprod. 2015, 93, 104. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef] [PubMed]
- Manju, M.; Sherin, T.G.; Rajasekharan, K.N.; Oommen, O.V. Curcumin analogue inhibits lipid peroxidation in a freshwater teleost, Anabas testudineus (Bloch)—An in vitro and in vivo study. Fish Physiol. Biochem. 2009, 35, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Nur Karakus, F.; Bulgurcuoglu Kuran, S.; Solakoglu, S. Effect of curcumin on sperm parameters after the cryopreservation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 161–166. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Colacurci, N.; Rocco, L. Protective Effects of Curcumin on the Outcome of Cryopreservation in Human Sperm. Reprod. Sci. 2021, 28, 2895–2905. [Google Scholar] [CrossRef]
- Ismail, A.A.; Abdel-Khalek, A.E.; Khalil, W.A.; Yousif, A.I.; Saadeldin, I.M.; Abomughaid, M.M.; El-Harairy, M.A. Effects of mint, thyme, and curcumin extract nanoformulations on the sperm quality, apoptosis, chromatin decondensation, enzyme activity, and oxidative status of cryopreserved goat semen. Cryobiology 2020, 97, 144–152. [Google Scholar] [CrossRef]
- Manzoni, C.; Kia, D.A.; Vandrovcova, J.; Hardy, J.; Wood, N.W.; Lewis, P.A.; Ferrari, R. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Brief. Bioinform. 2018, 19, 286–302. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Warth, B.; Spilker, M.E.; Siuzdak, G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat. Biotechnol. 2018, 36, 316–320. [Google Scholar] [CrossRef]
- Evans, H.C.; Dinh, T.T.N.; Hardcastle, M.L.; Gilmore, A.A.; Ugur, M.R.; Hitit, M.; Jousan, F.D.; Nicodemus, M.C.; Memili, E. Advancing Semen Evaluation Using Lipidomics. Front. Vet. Sci. 2021, 8, 601794. [Google Scholar] [CrossRef]
- Leahy, T.; Marti, J.I.; Mendoza, N.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Evans, G.; Maxwell, W.M.C. High pre-freezing dilution improves post-thaw function of ram spermatozoa. Anim. Reprod. Sci. 2010, 119, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. Int. J. Mol. Sci. 2022, 23, 2542. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, M.A.; Yániz, J.L.; Peña, F.J.; Santolaria, P.; Castelló-Ruiz, M. Role of Antioxidants in Cooled Liquid Storage of Mammal Spermatozoa. Antioxidants 2021, 10, 1096. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.J.; Bosch-Morell, F.; Romero, M.J.; Jareño, E.J.; Romero, B.; Marín, N.; Romá, J. Lipid peroxidation products and antioxidants in human disease. Environ. Health Perspect. 1998, 106 (Suppl. 5), 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Smith, T.B.; Lord, T.; Kuczera, L.; Koppers, A.J.; Naumovski, N.; Connaughton, H.; Baker, M.A.; De Iuliis, G.N. On methods for the detection of reactive oxygen species generation by human spermatozoa: Analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology 2013, 1, 192–205. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Brouwers, J.F.H.M.; Gadella, B.M. In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic. Biol. Med. 2003, 35, 1382–1391. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Mitchell, L.A.; Lambourne, S.R.; Connaughton, H.S.; De Iuliis, G.N. Sperm motility is lost in vitro as a consequence of mitochondrial free radical production and the generation of electrophilic aldehydes but can be significantly rescued by the presence of nucleophilic thiols. Biol. Reprod. 2012, 87, 110. [Google Scholar] [CrossRef]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Nanavati, K.; Rutherfurd-Markwick, K.; Lee, S.J.; Bishop, N.C.; Ali, A. Effect of curcumin supplementation on exercise-induced muscle damage: A narrative review. Eur. J. Nutr. 2022, 61, 3835–3855. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kumar, A.; Mahajan, A.; Sharma, P.; Sachan, V.; Aggrawal, J.; Yadav, S.; Saxena, A.; Kumar Swain, D. Curcumin in a tris-based semen extender improves cryosurvival of Hariana bull spermatozoa. Andrologia 2022, 54, e14255. [Google Scholar] [CrossRef] [PubMed]
- Salman, A.; Caamaño, J.N.; Fernández-Alegre, E.; Hidalgo, C.O.; Nadri, T.; Tamargo, C.; Fueyo, C.; Fernández, Á.; Merino, M.J.; Martínez-Pastor, F. Supplementation of the BIOXcell extender with the antioxidants crocin, curcumin and GSH for freezing bull semen. Res. Vet. Sci. 2021, 136, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.; Tavares, R.S.; Baptista, M.; Sousa, M.I.; Silva, A.; Escada-Rebelo, S.; Paiva, C.P.; Ramalho-Santos, J. Mitochondrial Functionality and Chemical Compound Action on Sperm Function. Curr. Med. Chem. 2016, 23, 3575–3606. [Google Scholar] [CrossRef]
- Bilgeri, Y.R.; Winckelmann, A.; Berzin, M.; Lyons, G.; Reinach, S.G. Adenosine triphosphate levels in human spermatozoa. Arch. Androl. 1987, 18, 183–188. [Google Scholar] [CrossRef]
- Dietrich, M.A.; Dietrich, G.J.; Mostek, A.; Ciereszko, A. Motility of carp spermatozoa is associated with profound changes in the sperm proteome. J. Proteom. 2016, 138, 124–135. [Google Scholar] [CrossRef]
- Lv, C.; Larbi, A.; Li, C.; Liang, J.; Wu, G.; Shao, Q.; Quan, Q. Decoding the influence of semen collection processes on goat sperm quality from a perspective of seminal plasma proteomics. J. Proteom. 2024, 298, 105141. [Google Scholar] [CrossRef]
- Martín-Cano, F.E.; Gaitskell-Phillips, G.; Ortiz-Rodríguez, J.M.; Silva-Rodríguez, A.; Román, Á.; Rojo-Domínguez, P.; Alonso-Rodríguez, E.; Tapia, J.A.; Gil, M.C.; Ortega-Ferrusola, C.; et al. Proteomic profiling of stallion spermatozoa suggests changes in sperm metabolism and compromised redox regulation after cryopreservation. J. Proteom. 2020, 221, 103765. [Google Scholar] [CrossRef]
- Akram, M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem. Biophys. 2014, 68, 475–478. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Nazarewicz, R.R.; Bikineyeva, A.; Hilenski, L.; Lassègue, B.; Griendling, K.K.; Harrison, D.G.; Dikalova, A.E. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid. Redox Signal. 2014, 20, 281–294. [Google Scholar] [CrossRef]
- Yamamoto, T.; Jaroenporn, S.; Pan, L.; Azumano, I.; Onda, M.; Nakamura, K.; Watanabe, G.; Taya, K. Effects of pantothenic acid on testicular function in male rats. J. Vet. Med. Sci. 2009, 71, 1427–1432. [Google Scholar] [CrossRef]
- Walczak-Jedrzejowska, R.; Wolski, J.K.; Slowikowska-Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent. Eur. J. Urol. 2013, 66, 60–67. [Google Scholar] [CrossRef]
- Setyawan, E.E.; Cooper, T.G.; Widiasih, D.A.; Junaidi, A.; Yeung, C.H. Effects of cryoprotectant treatments on bovine sperm function and osmolyte content. Asian J. Androl. 2009, 11, 571–581. [Google Scholar] [CrossRef]
- Governini, L.; Ponchia, R.; Artini, P.G.; Casarosa, E.; Marzi, I.; Capaldo, A.; Luddi, A.; Piomboni, P. Respiratory Mitochondrial Efficiency and DNA Oxidation in Human Sperm after In Vitro Myo-Inositol Treatment. J. Clin. Med. 2020, 9, 1638. [Google Scholar] [CrossRef]
- Osman, R.; Lee, S.; Almubarak, A.; Han, J.I.; Yu, I.J.; Jeon, Y. Antioxidant Effects of Myo-Inositol Improve the Function and Fertility of Cryopreserved Boar Semen. Antioxidants 2023, 12, 1673. [Google Scholar] [CrossRef]
- Song, C.; Chang, L.; Wang, B.; Zhang, Z.; Wei, Y.; Dou, Y.; Qi, K.; Yang, F.; Li, X.; Li, X.; et al. Seminal plasma metabolomics analysis of differences in liquid preservation ability of boar sperm. J. Anim. Sci. 2023, 101, skad392. [Google Scholar] [CrossRef]
- Boatman, D.E.; Bavister, B.D.; Cruz, E. Addition of hypotaurine can reactivate immotile golden hamster spermatozoa. J. Androl. 1990, 11, 66–72. [Google Scholar] [CrossRef]
- Brugnon, F.; Ouchchane, L.; Pons-Rejraji, H.; Artonne, C.; Farigoule, M.; Janny, L. Density gradient centrifugation prior to cryopreservation and hypotaurine supplementation improve post-thaw quality of sperm from infertile men with oligoasthenoteratozoospermia. Hum. Reprod. 2013, 28, 2045–2057. [Google Scholar] [CrossRef]
- Flesch, F.M.; Gadella, B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta 2000, 1469, 197–235. [Google Scholar] [CrossRef]
- Hinkovska, V.T.; Dimitrov, G.P.; Koumanov, K.S. Phospholipid composition and phospholipid asymmetry of ram spermatozoa plasma membranes. Int. J. Biochem. 1986, 18, 1115–1121. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Cheng, X.L.; Lin, R.C. Lipidomics applications for discovering biomarkers of diseases in clinical chemistry. Int. Rev. Cell Mol. Biol. 2014, 313, 1–26. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, J.L. Mass-spectrometry-based lipidomics. J. Sep. Sci. 2018, 41, 351–372. [Google Scholar] [CrossRef]
- Lenzi, A.; Picardo, M.; Gandini, L.; Dondero, F. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum. Reprod. Update 1996, 2, 246–256. [Google Scholar] [CrossRef]
- Simpson, A.M.; Swan, M.A.; White, I.G. Susceptibility of epididymal boar sperm to cold shock and protective action of phosphatidylcholine. Gamete Res. 1987, 17, 355–373. [Google Scholar] [CrossRef]
- Lucio, C.F.; Brito, M.M.; Angrimani, D.; Belaz, K.; Morais, D.; Zampieri, D.; Losano, J.; Assumpção, M.; Nichi, M.; Eberlin, M.N.; et al. Lipid composition of the canine sperm plasma membrane as markers of sperm motility. Reprod. Domest. Anim. 2017, 52 (Suppl. 2), 208–213. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, W.; Liu, Y.; Liu, Y.; Liang, H.; Xu, Q.; Liu, Z.; Weng, X. Plasma membrane lipid composition and metabolomics analysis of Yorkshire boar sperms with high and low resistance to cryopreservation. Theriogenology 2023, 206, 28–39. [Google Scholar] [CrossRef]
- Cabrera, T.; Ramires-Neto, C.; Belaz, K.R.A.; Freitas-Dell’aqua, C.P.; Zampieri, D.; Tata, A.; Eberlin, M.N.; Alvarenga, M.A.; Souza, F.F. Influence of spermatozoal lipidomic profile on the cryoresistance of frozen spermatozoa from stallions. Theriogenology 2018, 108, 161–166. [Google Scholar] [CrossRef]
- Arienti, G.; Carlini, E.; Polci, A.; Cosmi, E.V.; Palmerini, C.A. Fatty acid pattern of human prostasome lipid. Arch. Biochem. Biophys. 1998, 358, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Fhaner, C.J.; Liu, S.; Zhou, X.; Reid, G.E. Functional group selective derivatization and gas-phase fragmentation reactions of plasmalogen glycerophospholipids. Mass Spectrom. 2013, 2, S0015. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Daghigh Kia, H.; Mehdipour, M.; Shamsollahi, M.; Miller, D.J. Does fennel extract ameliorate oxidative stress frozen-thawed ram sperm? Cryobiology 2019, 87, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Mehdipour, M.; Daghigh Kia, H.; Najafi, A.; Vaseghi Dodaran, H.; García-Álvarez, O. Effect of green tea (Camellia sinensis) extract and pre-freezing equilibration time on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology 2016, 73, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bhakat, M.; Mohanty, T.K.; Mondal, S.; Yadav, S.K.; Kumar, P.; Kumar, R.; Rahim, A.; Sinha, R.; Shah, N. Effect of Tris-egg Yolk, Soya Milk, and Liposome-based Extenders on Sahiwal (Bos indicus) Sperm Quality during Pre- and Post-Cryopreservation Stages. Cryoletter 2019, 40, 94–102. [Google Scholar]
- Gadella, B.M.; Miller, N.G.; Colenbrander, B.; van Golde, L.M.; Harrison, R.A. Flow cytometric detection of transbilayer movement of fluorescent phospholipid analogues across the boar sperm plasma membrane: Elimination of labeling artifacts. Mol. Reprod. Dev. 1999, 53, 108–125. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; Chen, M.; Xia, Y.; Martin, F.L.; Hang, W.; Shen, H. Urinary metabolome identifies signatures of oligozoospermic infertile men. Fertil. Steril. 2014, 102, 44–53.e12. [Google Scholar] [CrossRef]
- Kohengkul, S.; Tanphaichitr, V.; Muangmun, V.; Tanphaichitr, N. Levels Of L-Carnitine And L-O-Acetylcarnitine In Normal And Infertile Human Semen: A Lower Level Of L-O-Acetylcarnitine In Infertile Semen*. Fertil. Steril. 1977, 28, 1333–1336. [Google Scholar] [CrossRef]
- Masaki, H.; Kim, N.; Nakamura, H.; Kumasawa, K.; Kamata, E.; Hirano, K.I.; Kimura, T. Long-chain fatty acid triglyceride (TG) metabolism disorder impairs male fertility: A study using adipose triglyceride lipase deficient mice. Mol. Hum. Reprod. 2017, 23, 452–460. [Google Scholar] [CrossRef]
- Kuang, W.; Zhang, J.; Lan, Z.; Deepak, R.; Liu, C.; Ma, Z.; Cheng, L.; Zhao, X.; Meng, X.; Wang, W.; et al. SLC22A14 is a mitochondrial riboflavin transporter required for sperm oxidative phosphorylation and male fertility. Cell Rep. 2021, 35, 109025. [Google Scholar] [CrossRef]
- Gill, I.; Valivety, R. Polyunsaturated fatty acids, Part 1: Occurrence, biological activities and applications. Trends Biotechnol. 1997, 15, 401–409. [Google Scholar] [CrossRef]
- Bucak, M.N.; Sarıözkan, S.; Tuncer, P.B.; Sakin, F.; Ateşşahin, A.; Kulaksız, R.; Çevik, M. The effect of antioxidants on post-thawed Angora goat (Capra hircus ancryrensis) sperm parameters, lipid peroxidation and antioxidant activities. Small Rumin. Res. 2010, 89, 24–30. [Google Scholar] [CrossRef]
- Rao, B.; Soufir, J.C.; Martin, M.; David, G. Lipid peroxidation in human spermatozoa as related to midpiece abnormalities and motility. Gamete Res. 1989, 24, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.; Schreiner, T.; Petzoldt, R. Lipid peroxidation in human spermatozoa and maintenance of progressive sperm motility. Andrologia 1999, 31, 17–22. [Google Scholar] [CrossRef]
- Pons-Rejraji, H.; Bailey, J.L.; Leclerc, P. Cryopreservation affects bovine sperm intracellular parameters associated with capacitation and acrosome exocytosis. Reprod. Fertil. Dev. 2009, 21, 525–537. [Google Scholar] [CrossRef]
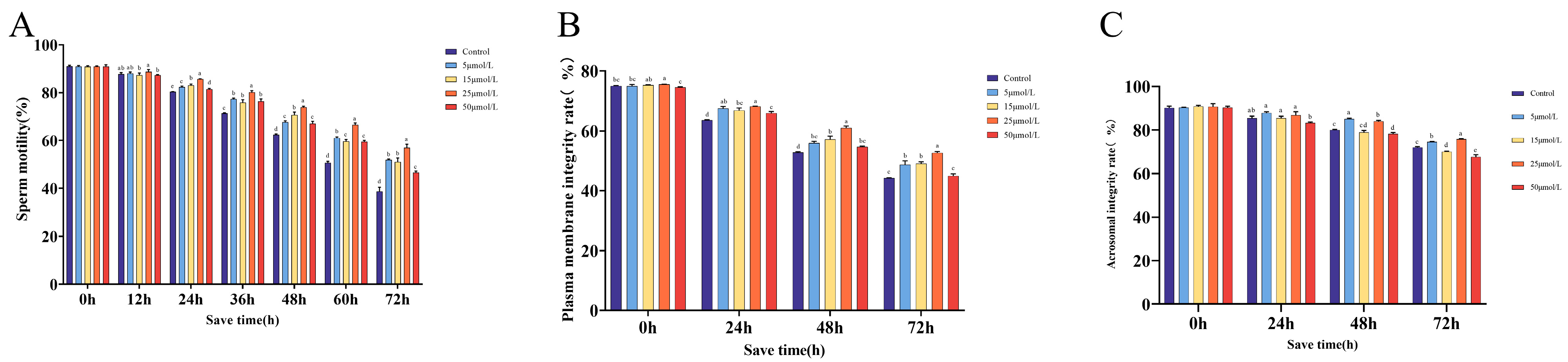

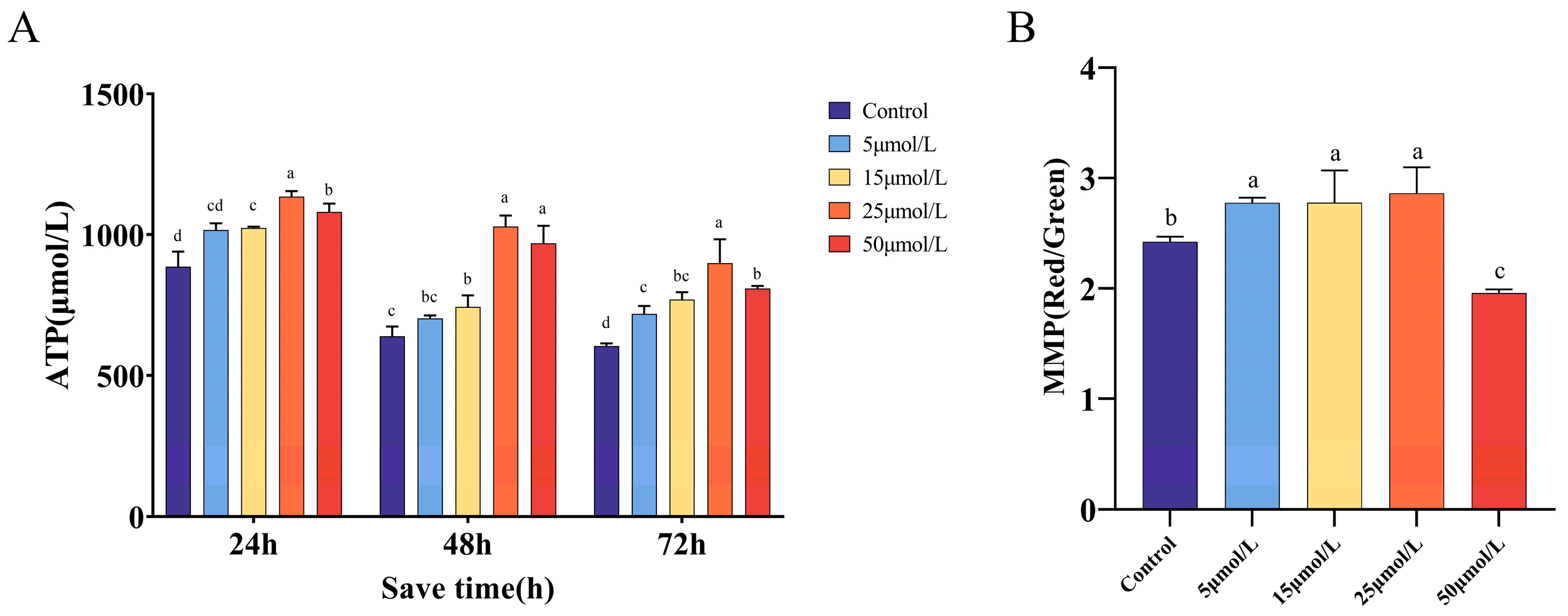
| Index | Time of Storage (h) | Control | 5 μmol/L | 15 μmol/L | 25 μmol/L | 50 μmol/L |
|---|---|---|---|---|---|---|
| VSL (μm/s) | 0 | 30.63 ± 0.48 | 30.55 ± 1.20 | 29.12 ± 0.60 | 29.53 ± 10.38 | 30.12 ± 1.23 |
| 24 | 27.05 ± 1.18 | 26.42 ± 0.39 | 26.85 ± 1.99 | 27.45 ± 1.00 | 26.27 ± 0.37 | |
| 48 | 21.18 ± 1.23 b | 20.82 ± 0.70 b | 21.38 ± 0.68 ab | 22.77 ± 0.43 a | 20.73 ± 0.48 b | |
| 72 | 11.50 ± 0.75 | 11.60 ± 1.08 | 11.25 ± 0.96 | 11.43 ± 1.62 | 11.00 ± 1.13 | |
| VCL (μm/s) | 0 | 32.07 ± 1.33 | 32.35 ± 1.42 | 32.02 ± 1.90 | 32.26 ± 1.45 | 31.63 ± 1.49 |
| 24 | 27.24 ± 0.46 a | 25.66 ± 0.89 b | 25.63 ± 0.46 b | 25.90 ± 0.51 b | 25.07 ± 0.41 b | |
| 48 | 21.45 ± 1.19 | 20.80 ± 0.73 | 20.81 ± 0.65 | 21.63 ± 0.41 | 20.50 ± 1.09 | |
| 72 | 15.70 ± 0.40 ab | 15.96 ± 0.47 ab | 16.06 ± 0.94 b | 16.66 ± 0.99 a | 15.18 ± 0.70 a | |
| VAP (μm/s) | 0 | 46.91 ± 1.36 | 47.20 ± 1.61 | 47.11 ± 1.11 | 47.27 ± 2.48 | 47.04 ± 1.30 |
| 24 | 44.75 ± 0.74 | 45.39 ± 0.96 | 44.53 ± 0.43 | 44.75 ± 0.96 | 45.24 ± 1.01 | |
| 48 | 40.13 ± 0.61 | 39.72 ± 1.21 | 39.76 ± 0.56 | 40.24 ± 0.61 | 39.43 ± 0.77 | |
| 72 | 37.57 ± 0.67 ab | 37.48 ± 0.93 ab | 36.72 ± 0.48 b | 38.17 ± 0.31 a | 36.43 ± 0.85 b |
| Lipid Ion | Lipid Group | Ion Formula | FC | p-Value | VIP |
|---|---|---|---|---|---|
| PE(18:1_14:0) - H | PE(32:1) - H | C37 H71 O8 N1 P1 | 183.51 | 0.038 | 2.12 |
| PE(16:0_18:1) - H | PE(34:1) - H | C39 H75 O8 N1 P1 | 7.49 | 0.019 | 1.34 |
| PE(38:7e) - H | PE(38:7e) - H | C43 H73 O7 N1 P1 | 2262.42 | 0.026 | 8.03 |
| PS(20:2e_20:4) - H | PS(40:6e) - H | C46 H79 O9 N1 P1 | 21.89 | 0.014 | 1.27 |
| Hex2Cer(d16:0_20:3) + HCOO | Hex2Cer(d36:3) + HCOO | C49 H88 O15 N1 | 0.29 | 0.036 | 1.09 |
| Hex2Cer(d16:0_20:4) + HCOO | Hex2Cer(d36:4) + HCOO | C49 H86 O15 N1 | 0.32 | 0.030 | 2.59 |
| Hex2Cer(t33:2) + HCOO | Hex2Cer(t33:2) + HCOO | C46 H84 O16 N1 | 72.23 | 0.012 | 1.36 |
| LPC(16:0e) + HCOO | LPC(16:0e) + HCOO | C25 H53 O8 N1 P1 | 7.35 | 0.003 | 1.82 |
| LPC(16:1e) + HCOO | LPC(16:1e) + HCOO | C25 H51 O8 N1 P1 | 9.11 | 0.010 | 3.33 |
| LPE(16:1e) - H | LPE(16:1e) - H | C21 H43 O6 N1 P1 | 22.55 | 0.006 | 2.32 |
| LPE(18:0) - H | LPE(18:0) - H | C23 H47 O7 N1 P1 | 25.48 | 0.004 | 1.25 |
| DG(18:0_16:0) + NH4 | DG(34:0) + NH4 | C37 H76 O5 N1 | 2.97 | 0.028 | 1.93 |
| DG(18:0_18:0) + NH4 | DG(36:0) + NH4 | C39 H80 O5 N1 | 6.70 | 0.011 | 3.16 |
| DG(18:0_18:1) + NH4 | DG(36:1) + NH4 | C39 H78 O5 N1 | 3.98 | 0.023 | 1.55 |
| DG(16:0_20:3) + H | DG(36:3) + H | C39 H71 O5 | 3.05 | 0.020 | 1.93 |
| DG(18:0_20:4) + H | DG(38:4) + H | C41 H73 O5 | 4.55 | 0.016 | 1.69 |
| DG(18:0_22:5) + NH4 | DG(40:5) + NH4 | C43 H78 O5 N1 | 5.96 | 0.001 | 1.05 |
| Hex1SPH(m18:0) + H - H2O | Hex1SPH(m18:0) + H - H2O | C24 H48 O5 N1 | 0.56 | 0.043 | 2.00 |
| LPC(15:0) + H | LPC(15:0) + H | C23 H49 O7 N1 P1 | 22.55 | 0.006 | 1.50 |
| LPC(18:3e) + H | LPC(18:3e) + H | C26 H51 O6 N1 P1 | 12.27 | 0.004 | 3.04 |
| PC(29:1) + H | PC(29:1) + H | C37 H73 O8 N1 P1 | 27.90 | 0.043 | 1.90 |
| PC(31:1) + H | PC(31:1) + H | C39 H77 O8 N1 P1 | 8.52 | 0.023 | 1.92 |
| PC(18:3e_18:1) + H | PC(36:4e) + H | C44 H83 O7 N1 P1 | 2.35 | 0.009 | 1.64 |
| PC(20:4e_20:5) + H | PC(40:9e) + H | C48 H81 O7 N1 P1 | 1.96 | 0.038 | 1.45 |
| PE(12:0e_6:0) + H | PE(18:0e) + H | C23 H49 O7 N1 P1 | 22.58 | 0.006 | 1.49 |
| PE(16:0e_20:2) + Na | PE(36:2e) + Na | C41 H80 O7 N1 P1 Na1 | 2.52 | 0.033 | 1.55 |
| PE(39:5e) + H | PE(39:5e) + H | C44 H81 O7 N1 P1 | 2.48 | 0.006 | 4.78 |
| Cer(d23:0_16:1) + H | Cer(d39:1) + H | C39 H78 O3 N1 | 2.06 | 0.043 | 1.36 |
| PG(20:0_14:0) + H | PG(34:0) + H | C40 H80 O10 N0 P1 | 2.48 | 0.036 | 2.92 |
| PI(40:5) + NH4 | PI(40:5) + NH4 | C49 H89 O13 N1 P1 | 0.32 | 0.047 | 3.90 |
| PI(40:6) + NH4 | PI(40:6) + NH4 | C49 H87 O13 N1 P1 | 0.27 | 0.033 | 1.06 |
| PI(40:7e) + NH4 | PI(40:7e) + NH4 | C49 H87 O12 N1 P1 | 0.30 | 0.043 | 2.69 |
| TG(4:0_10:4_12:3) + NH4 | TG(26:7) + NH4 | C29 H44 O6 N1 | 10.85 | 0.009 | 4.93 |
| TG(4:0_11:3_18:3) + Na | TG(33:6) + Na | C36 H56 O6 Na1 | 0.63 | 0.047 | 1.15 |
| TG(12:0e_8:0_14:0) + Na | TG(34:0e) + Na | C37 H72 O5 Na1 | 3.02 | 0.029 | 1.82 |
| TG(20:0e_8:0_8:0) + Na | TG(36:0e) + Na | C39 H76 O5 Na1 | 170.56 | 0.003 | 3.58 |
| TG(12:0e_6:0_18:3) + H | TG(36:3e) + H | C39 H71 O5 | 3.00 | 0.028 | 1.82 |
| TG(14:0e_12:0_12:3) + H | TG(38:3e) + H | C41 H75 O5 | 6.54 | 0.012 | 2.87 |
| TG(38:9e) + Na | TG(38:9e) + Na | C41 H62 O5 Na1 | 0.23 | 0.037 | 1.37 |
| TG(16:0_16:1_18:1) + NH4 | TG(50:2) + NH4 | C53 H102 O6 N1 | 1.56 | 0.047 | 2.09 |
| TG(16:0_18:1_18:1) + NH4 | TG(52:2) + NH4 | C55 H106 O6 N1 | 2.01 | 0.029 | 1.58 |
| TG(18:1_18:1_18:1) + NH4 | TG(54:3) + NH4 | C57 H108 O6 N1 | 1.92 | 0.050 | 1.20 |
| TG(18:1_18:1_18:2) + NH4 | TG(54:4) + NH4 | C57 H106 O6 N1 | 1.87 | 0.040 | 1.11 |
| CerP(d45:0) + H | CerP(d45:0) + H | C45 H93 O6 N1 P1 | 1.70 | 0.047 | 2.07 |
| CerP(m20:0_25:0+O) + H | CerP(m45:0+O) + H | C45 H93 O6 N1 P1 | 1.76 | 0.040 | 2.23 |
| DG(16:0_16:0) + NH4 | DG(32:0) + NH4 | C35 H72 O5 N1 | 2.74 | 0.050 | 2.67 |
| Lipid Ion | Lipid Group | Ion Formula | FC | p-Value | VIP |
|---|---|---|---|---|---|
| PE(16:0_18:1) - H | PE(34:1) - H | C39 H75 O8 N1 P1 | 0.19 | 0.028 | 1.34 |
| PE(38:7e) - H | PE(38:7e) - H | C43 H73 O7 N1 P1 | 0.00 | 0.026 | 8.73 |
| PS(20:2e_20:4) - H | PS(40:6e) - H | C46 H79 O9 N1 P1 | 0.06 | 0.015 | 1.34 |
| PS(18:3e_22:5) - H | PS(40:8e) - H | C46 H75 O9 N1 P1 | 0.39 | 0.042 | 1.36 |
| LPE(18:0) - H | LPE(18:0) - H | C23 H47 O7 N1 P1 | 0.07 | 0.004 | 1.36 |
| AcCa(14:1) + H | AcCa(14:1) + H | C21 H40 O4 N1 | 0.14 | 0.037 | 1.47 |
| AcCa(15:0) + H | AcCa(15:0) + H | C22 H44 O4 N1 | 0.17 | 0.021 | 1.06 |
| LPC(15:0) + H | LPC(15:0) + H | C23 H49 O7 N1 P1 | 0.07 | 0.007 | 1.64 |
| LSM(d20:1) + H | LSM(d20:1) + H | C25 H54 O5 N2 P1 | 1.70 | 0.032 | 1.01 |
| PC(29:1) + H | PC(29:1) + H | C37 H73 O8 N1 P1 | 0.05 | 0.045 | 3.23 |
| PC(31:1) + H | PC(31:1) + H | C39 H77 O8 N1 P1 | 0.19 | 0.035 | 1.89 |
| PC(23:1_11:2) + H | PC(34:3) + H | C42 H79 O8 N1 P1 | 2.90 | 0.005 | 2.15 |
| PC(40:8e) + H | PC(40:8e) + H | C48 H83 O7 N1 P1 | 0.48 | 0.038 | 5.30 |
| PE(12:0e_6:0) + H | PE(18:0e) + H | C23 H49 O7 N1 P1 | 0.07 | 0.007 | 1.63 |
| PE(22:5_18:2) + Na | PE(40:7) + Na | C45 H76 O8 N1 P1 Na1 | 0.41 | 0.005 | 1.41 |
| PE(20:5_22:5) + H | PE(42:10) + H | C47 H75 O8 N1 P1 | 0.41 | 0.005 | 1.40 |
| AcCa(18:3) + H | AcCa(18:3) + H | C25 H44 O4 N1 | 0.17 | 0.010 | 1.29 |
| AcCa(18:4) + H | AcCa(18:4) + H | C25 H42 O4 N1 | 0.08 | 0.002 | 1.19 |
| AcCa(20:0) + H | AcCa(20:0) + H | C27 H54 O4 N1 | 0.21 | 0.035 | 1.64 |
| AcCa(20:3) + H | AcCa(20:3) + H | C27 H48 O4 N1 | 0.23 | 0.046 | 2.24 |
| AcCa(20:4) + H | AcCa(20:4) + H | C27 H46 O4 N1 | 0.05 | 0.005 | 6.09 |
| AcCa(20:5) + H | AcCa(20:5) + H | C27 H44 O4 N1 | 0.05 | 0.005 | 6.11 |
| CerG3GNAc1(d42:2) + H | CerG3GNAc1(d42:2) + H | C68 H125 O23 N2 | 1.92 | 0.044 | 1.01 |
| AcCa(22:4) + H | AcCa(22:4) + H | C29 H50 O4 N1 | 0.04 | 0.000 | 2.21 |
| AcCa(22:5) + H | AcCa(22:5) + H | C29 H48 O4 N1 | 0.06 | 0.003 | 8.41 |
| AcCa(22:6) + H | AcCa(22:6) + H | C29 H46 O4 N1 | 0.14 | 0.042 | 7.85 |
| AcCa(14:0) + H | AcCa(14:0) + H | C21 H42 O4 N1 | 0.10 | 0.027 | 7.06 |
| DG(24:5e) + NH4 | DG(24:5e) + NH4 | C27 H48 O4 N1 | 0.23 | 0.045 | 2.24 |
| DG(26:6) + NH4 | DG(26:6) + NH4 | C29 H48 O5 N1 | 0.30 | 0.008 | 1.14 |
| DG(18:3e_10:4) + NH4 | DG(28:7e) + NH4 | C31 H52 O4 N1 | 2.73 | 0.041 | 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Z.; Shi, L.; Zhou, H.; Hou, G.; Xun, W. Exploratory Metabolomics and Lipidomics Profiling Contributes to Understanding How Curcumin Improves Quality of Goat Semen Stored at 16 °C in Tropical Areas. Int. J. Mol. Sci. 2024, 25, 10200. https://doi.org/10.3390/ijms251810200
An Z, Shi L, Zhou H, Hou G, Xun W. Exploratory Metabolomics and Lipidomics Profiling Contributes to Understanding How Curcumin Improves Quality of Goat Semen Stored at 16 °C in Tropical Areas. International Journal of Molecular Sciences. 2024; 25(18):10200. https://doi.org/10.3390/ijms251810200
Chicago/Turabian StyleAn, Zhaoxiang, Liguang Shi, Hanlin Zhou, Guanyu Hou, and Wenjuan Xun. 2024. "Exploratory Metabolomics and Lipidomics Profiling Contributes to Understanding How Curcumin Improves Quality of Goat Semen Stored at 16 °C in Tropical Areas" International Journal of Molecular Sciences 25, no. 18: 10200. https://doi.org/10.3390/ijms251810200






