Zinc and Ferritin Levels and Their Associations with Functional Disorders and/or Thyroid Autoimmunity: A Population-Based Case–Control Study
Abstract
1. Introduction
2. Results
2.1. Participants Included in the Study
2.2. Differences between Baseline Characteristics
2.3. Ferritin and Zinc Levels and Their Associations with Alterations in Thyroid Function
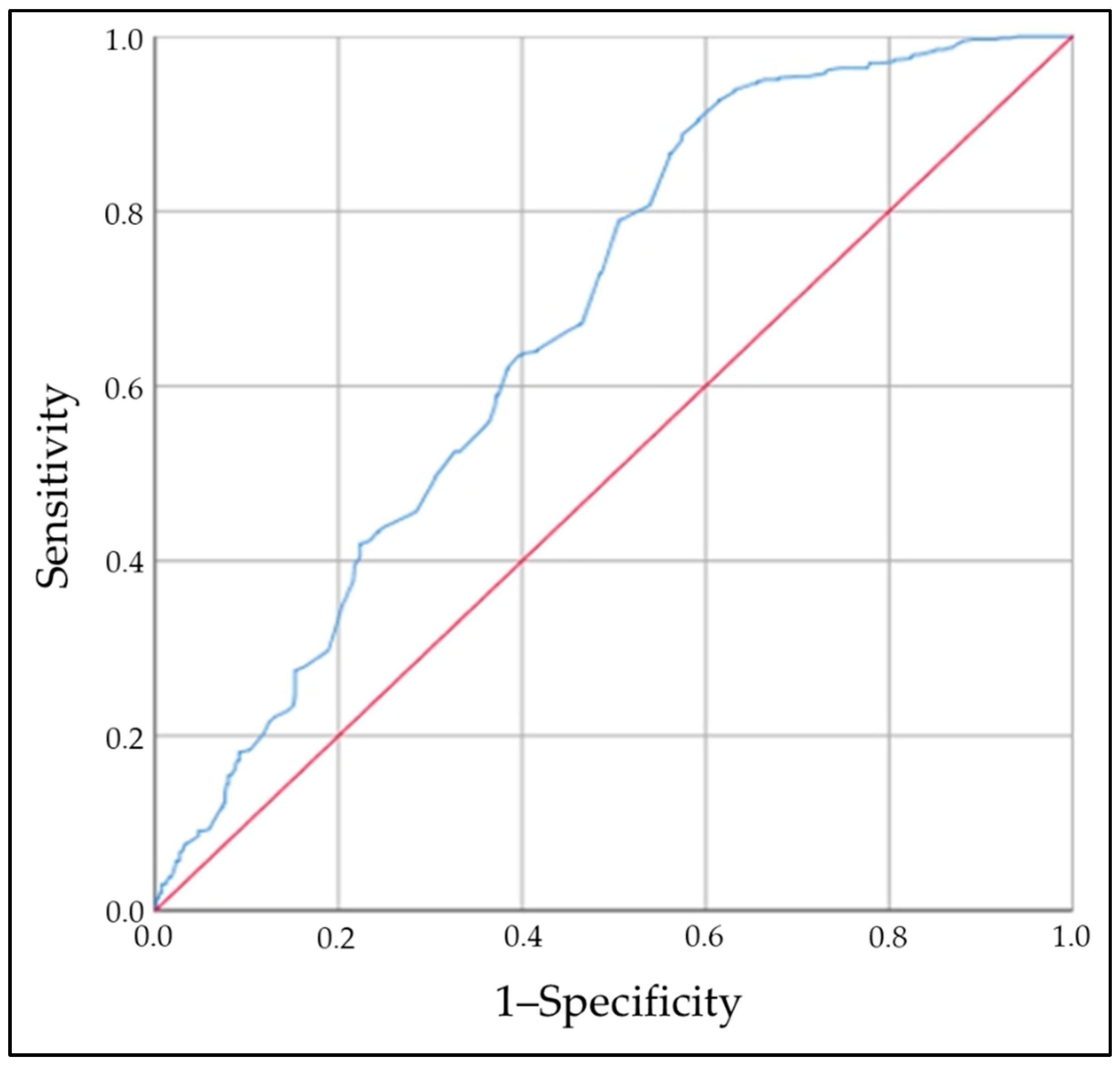
2.4. Zinc Levels and Risk of Thyroid Autoimmunity
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Design
4.3. Definition of AITD and Normal or Abnormal Thyroid Function
4.4. Exclusion Criteria
4.5. Anthropometric and Laboratory Measurements
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bogusławska, J.; Godlewska, M.; Gajda, E.; Piekiełko-Witkowska, A. Cellular and molecular basis of thyroid autoimmunity. Eur. Thyroid. J. 2022, 11, e210024. [Google Scholar] [CrossRef] [PubMed]
- Petranović Ovčariček, P.; Görges, R.; Giovanella, L. Autoimmune Thyroid Diseases. Semin. Nucl. Med. 2024, 54, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.Y.; Lee, E.; Lee, M.K.; Lee, J.H. The Association of Overt and Subclinical Hyperthyroidism with the Risk of Cardiovascular Events and Cardiovascular Mortality: Meta-Analysis and Systematic Review of Cohort Studies. Endocrinol. Metab. 2020, 35, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.Y.; Seo, G.H.; Chung, J.H. Risk of All-Cause Mortality in Levothyroxine-Treated Hypothyroid Patients: A Nationwide Korean Cohort Study. Front. Endocrinol. 2021, 12, 680647. [Google Scholar] [CrossRef]
- Ning, Y.; Cheng, Y.J.; Liu, L.J.; Sara, J.D.; Cao, Z.Y.; Zheng, W.P.; Zhang, T.S.; Han, H.J.; Yang, Z.Y.; Zhang, Y.; et al. What is the association of hypothyroidism with risks of cardiovascular events and mortality? A meta-analysis of 55 cohort studies involving 1,898,314 participants. BMC Med. 2017, 15, 21. [Google Scholar] [CrossRef]
- Shulhai, A.-M.; Rotondo, R.; Petraroli, M.; Patianna, V.; Predieri, B.; Iughetti, L.; Esposito, S.; Street, M.E. The Role of Nutrition on Thyroid Function. Nutrients 2024, 16, 2496. [Google Scholar] [CrossRef]
- O’Kane, S.M.; Mulhern, M.S.; Pourshahidi, L.K.; Strain, J.J.; Yeates, A.J. Micronutrients, iodine status and concentrations of thyroid hormones: A systematic review. Nutr. Rev. 2018, 76, 418–431. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace elements and the thyroid. Front. Endocrinol. 2022, 13, 904889. [Google Scholar] [CrossRef]
- Severo, J.S.; Morais, J.B.S.; de Freitas, T.E.C.; Andrade, A.L.P.; Feitosa, M.M.; Fontenelle, L.C.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D. The Role of Zinc in Thyroid Hormones Metabolism. Int. J. Vitam. Nutr. Res. 2019, 89, 80–88. [Google Scholar] [CrossRef]
- Stiles, L.I.; Ferrao, K.; Mehta, K.J. Role of zinc in health and disease. Clin. Exp. Med. 2024, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Wróblewski, M.; Wróblewska, J.; Nuszkiewicz, J.; Pawłowska, M.; Wesołowski, R.; Woźniak, A. The Role of Selected Trace Elements in Oxidoreductive Homeostasis in Patients with Thyroid Diseases. Int. J. Mol. Sci. 2023, 24, 4840. [Google Scholar] [CrossRef] [PubMed]
- Ihnatowicz, P.; Drywień, M.; Wątor, P.; Wojsiat, J. The importance of nutritional factors and dietary management of Hashimoto’s thyroiditis. Ann. Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef]
- Szklarz, M.; Gontarz-Nowak, K.; Matuszewski, W.; Bandurska-Stankiewicz, E. Iron: Not Just a Passive Bystander in AITD. Nutrients 2022, 14, 4682. [Google Scholar] [CrossRef]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef]
- Garofalo, V.; Condorelli, R.A.; Cannarella, R.; Aversa, A.; Calogero, A.E.; La Vignera, S. Relationship between Iron Deficiency and Thyroid Function: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4790. [Google Scholar] [CrossRef]
- Wan, S.; Jin, B.; Ren, B.; Boah, M.; Shen, H. Relationship between mild iodine deficiency in pregnant women and thyroid function: A meta-analysis. J. Trace Elements Med. Biol. 2023, 78, 127197. [Google Scholar] [CrossRef]
- Luo, J.; Wang, X.; Yuan, L.; Guo, L. Iron Deficiency, a Risk Factor of Thyroid Disorders in Reproductive-Age and Pregnant Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 629831. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Mera-Mamian, A.; Bastidas-Sanchez, B.; Pinzon-Fernandez, M.; Murillo-Palacios, J.; Ramirez-Bejarano, L. Population Status of Iodine and Its Potential Effects on Thyroid Function and Autoimmunity in Southwestern Colombia. J. Clin. Med. Res. 2022, 14, 126–135. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Bastidas, B.; Pinzón, M.V. Population status of selenium in Colombia and associated factors: A cross-sectional study. Horm. Mol. Biol. Clin. Investig. 2022, 44, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ertek, S.; Cicero, A.; Caglar, O.; Erdogan, G. Relationship between serum zinc levels, thyroid hormones and thyroid volume following successful iodine supplementation. Hormones 2010, 9, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Al-Bazi, M.M.; Kumosani, T.A.; Al-Malki, A.L.; Kannan, K.; Moselhy, S.S. Association of trace elements abnormalities with thyroid dysfunction. Afr. Health Sci. 2021, 21, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Beserra, J.B.; Morais, J.B.S.; Severo, J.S.; Cruz, K.J.C.; de Oliveira, A.R.S.; Henriques, G.S.; do Nascimento Marreiro, D. Relation Between Zinc and Thyroid Hormones in Humans: A Systematic Review. Biol. Trace Element Res. 2021, 199, 4092–4100. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Thyroid function and serum copper, selenium, and zinc in general U.S. population. Biol. Trace Element Res. 2014, 159, 87–98. [Google Scholar] [CrossRef]
- Babić Leko, M.; Gunjača, I.; Pleić, N.; Zemunik, T. Environmental Factors Affecting Thyroid-Stimulating Hormone and Thyroid Hormone Levels. Int. J. Mol. Sci. 2021, 22, 6521. [Google Scholar] [CrossRef]
- Pekary, A.; Lukaski, H.C.; Mena, I.; Hershman, J.M. Processing of TRH precursor peptides in rat brain and pituitary is zinc dependent. Peptides 1991, 12, 1025–1032. [Google Scholar] [CrossRef]
- Osterman, A.L.; Grishin, N.V.; Smulevitch, S.V.; Matz, M.V.; Zagnitko, O.P.; Revina, L.P.; Stepanov, V.M. Primary structure of carboxypeptidase T: Delineation of functionally relevant features in Zn-carboxypeptidase family. Protein J. 1992, 11, 561–570. [Google Scholar] [CrossRef]
- Kralik, A.; Eder, K.; Kirchgessner, M. Influence of zinc and selenium deficiency on parameters relating to thyroid hormone metabolism. Horm. Metab. Res. 1996, 28, 223–226. [Google Scholar] [CrossRef]
- Freake, H.C.; Govoni, K.E.; Guda, K.; Huang, C.; Zinn, S.A. Actions and interactions of thyroid hormone and zinc status in growing rats. J. Nutr. 2001, 131, 1135–1141. [Google Scholar] [CrossRef]
- Burke, L.; Zhang, R.; Lutz, M.; Renkawitz, R. The thyroid hormone receptor and the insulator protein CTCF: Two different factors with overlapping functions. J. Steroid Biochem. Mol. Biol. 2002, 83, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Iitaka, M.; Kakinuma, S.; Fujimaki, S.; Oosuga, I.; Fujita, T.; Yamanaka, K.; Wada, S.; Katayama, S. Induction of apoptosis and necrosis by zinc in human thyroid cancer cell lines. J. Endocrinol. 2001, 169, 417–424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rakhra, G.; Rakhra, G. Zinc finger proteins: Insights into the transcriptional and post transcriptional regulation of immune response. Mol. Biol. Rep. 2021, 48, 5735–5743. [Google Scholar] [CrossRef] [PubMed]
- Baltaci, A.K.; Mogulkoc RLeptin, N.P.Y. Melatonin and Zinc Levels in Experimental Hypothyroidism and Hyperthyroidism: The Relation to Zinc. Biochem. Genet. 2017, 55, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kiso, Y.; Watanabe, T.; Kaise, K.; Kaise, N.; Itagaki, Y.; Yamamoto, M.; Sakurada, T.; Yoshinaga, K. Erythrocyte zinc in hyperthyroidism: Reflection of integrated thyroid hormone levels over the previous few months. Metabolism 1990, 39, 182–186. [Google Scholar] [CrossRef]
- Liu, M.-J.; Bao, S.; Bolin, E.R.; Burris, D.L.; Xu, X.; Sun, Q.; Killilea, D.W.; Shen, Q.; Ziouzenkova, O.; Belury, M.A.; et al. Zinc deficiency augments leptin production and exacerbates macrophage infiltration into adipose tissue in mice fed a high-fat diet. J. Nutr. 2013, 143, 1036–1045. [Google Scholar] [CrossRef]
- Keikhaei, N.; Heidari, Z. Alterations of Serum Leptin Levels in Patients with Autoimmune Thyroid Disorders. Med. J. Islam. Repub. Iran 2021, 35, 166. [Google Scholar] [CrossRef]
- Khorshidi, M.; Zarezadeh, M.; Sadeghi, A.; Teymouri, A.; Emami, M.R.; Kord-Varkaneh, H.; Aryaeian, N.; Rahmani, J.; Mousavi, S.M. The Effect of Zinc Supplementation on Serum Leptin Levels: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2019, 51, 503–510. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R. Leptin and zinc relation: In regulation of food intake and immunity. Indian J. Endocrinol. Metab. 2012, 16 (Supp. S3), S611–S616. [Google Scholar] [CrossRef]
- Teixeira, P.d.F.d.S.; Dos Santos, P.B.; Pazos-Moura, C.C. The role of thyroid hormone in metabolism and metabolic syndrome. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820917869. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Wu, R.; Sun, X.; Yang, B.; Wang, Y.; Xu, Y. Changes in profile of lipids and adipokines in patients with newly diagnosed hypothyroidism and hyperthyroidism. Sci. Rep. 2016, 6, 26174. [Google Scholar] [CrossRef] [PubMed]
- Simsek, G.; Andican, G.; Ünal, E.; Hatemi, H.; Yigit, G.; Candan, G. Calcium, magnesium, and zinc status in experimental hyperthyroidism. Biol. Trace Element Res. 1997, 57, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, H.; Morita, Y.; Hamada, I.; Ohta, Y.; Fukui, N.; Makino, N.; Ohata, E.; Naito, T. Demographic and clinical characteristics of patients with zinc deficiency: Analysis of a nationwide Japanese medical claims database. Sci. Rep. 2024, 14, 2791. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, R.R.; Chinnaiah Govindareddy, D.; Sahoo, J.; Bobby, Z.; Chinnakali, P. Effect of daily zinc supplementation for 12 weeks on serum thyroid auto-antibody levels in children and adolescents with autoimmune thyroiditis—A randomized controlled trial. J. Pediatr. Endocrinol. Metab. 2024, 37, 137–143. [Google Scholar] [CrossRef]
- Lu, L.; Huang, Z.; Wang, X.; Chen, J. Interaction Between Dietary Selenium and Zinc Intakes on Hypothyroidism. Biol. Trace Elem. Res. 2023, 201, 4667–4676. [Google Scholar] [CrossRef]
- Rabbani, E.; Golgiri, F.; Janani, L.; Moradi, N.; Fallah, S.; Abiri, B.; Vafa, M. Randomized Study of the Effects of Zinc, Vitamin A, and Magnesium Co-supplementation on Thyroid Function, Oxidative Stress, and hs-CRP in Patients with Hypothyroidism. Biol. Trace Elem. Res. 2021, 199, 4074–4083. [Google Scholar] [CrossRef]
- Gobierno de Colombia. Encuesta Nacional de Situación Nutricional ENSIN 2015; Instituto Colombiano de Bienestar Familiar, Instituto Nacional de Salud, Universidad Nacional de Colombia: Bogotá, Colombia, 2015; ISBN 958-623-087-2. [Google Scholar]
- Dwivedi, S.N.; Kalaria, T.; Buch, H. Thyroid autoantibodies. J. Clin. Pathol. 2023, 76, 19–28. [Google Scholar] [CrossRef]
- Campi, I.; Covelli, D.; Moran, C.; Fugazzola, L.; Cacciatore, C.; Orlandi, F.; Gallone, G.; Chatterjee, K.; Beck-Peccoz, P.; Persani, L. The Differential Diagnosis of Discrepant Thyroid Function Tests: Insistent Pitfalls and Updated Flow-Chart Based on a Long-Standing Experience. Front. Endocrinol. 2020, 11, 432. [Google Scholar] [CrossRef]
- World Health Organization-WHO. International Council for Control of Iodine Deficiency Disorders. In Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. A Guide Programmed Managers, 2nd ed.; WHO/NHD/01.1; WHO, Department of Nutrition for Health and Development: Geneva, Switzerland, 2001. [Google Scholar]
- Vargas-Uricoechea, H.; Agredo-Delgado, V.; Vargas-Sierra, H.D.; Pinzón-Fernández, M.V. Prevalence of Functional Alterations and the Effects of Thyroid Autoimmunity on the Levels of TSH in an Urban Population of Colombia: A Population-Based Study. Endocr. Metab. Immune. Disord. Drug. Targets. 2023, 23, 857–866. [Google Scholar] [CrossRef]
- Butrimovitz, G.P.; Purdy, W.C. The determination of zinc in blood plasma by atomic absorption spectrometry. Anal. Chim. Acta. 1977, 94, 63–73. [Google Scholar] [CrossRef]
- Hamwi, A.; Endler, G.; Rubi, K.; Wagner, O.; Endler, A.T. Reference values for a heterogeneous ferritin assay and traceability to the 3rd international recombinant standard for ferritin (NIBSC Code 94/572). Clin. Chem. Lab. Med. 2002, 40, 365–370. [Google Scholar] [CrossRef]

| Characteristics | Cases (n = 524) | Controls (n = 524) | p-Value |
|---|---|---|---|
| Age, median, and IQR | 47.6 (33–64) | 47.1 (34–63) | 1.00 |
| Female (%) | 63.7 | 63.7 | 1.00 |
| BMI, median, and IQR | 27 (24.2–30.0) | 27.5 (24.3–30.9) | 0.387 |
| Low–middle SES (%) | 34.5 | 34.5 | 1.00 |
| High SES (%) | 65.5 | 65.5 | 1.00 |
| Origin (urban/rural) | 59.9/40.1 | 59.1/40.1 | 1.00 |
| Systolic blood pressure (mmHg), median, and IQR | 128 (111–147) | 125 (109–143) | 0.40 |
| Diastolic blood pressure (mmHg), median, and IQR | 79 (72–85) | 77 (70–82) | 0.39 |
| Prevalence (%) of goiter (F/M) | 60.9 (63.8/55.8) | 12 (58.7/41.26) | 0.000 |
| Prevalence (%) of normal thyroid function | 39.5 | 100 | 0.000 |
| Prevalence (%) of subclinical/overt hypothyroidism (F/M) | 33.6/20.8 | NA | 0.001 |
| Prevalence (%) of subclinical/overt hyperthyroidism (F/M) | 5.0/1.1 | NA | 0.000 |
| Prevalence (%) of TPOAb positivity (F/M) | 73.9 (73.1/75.3) | NA | 0.41 |
| Prevalence (%) of TgAb positivity (F/M) | 49.6 (50.0/48.9) | NA | 0.46 |
| Prevalence (%) of TRAb positivity (F/M) | 17.9 (19.2/15.8) | NA | 0.38 |
| Zinc Values (µg/dL) | p-Value | Ferritin Values (ng/mL) | p-Value | |||
|---|---|---|---|---|---|---|
| Cases (n = 524) | Controls (n = 524) | Cases (n = 524) | Controls (n = 524) | |||
| Median (IQR) | 78 (61–91) | 88 (78–103) | 0.000 | 73.5 (32–125) | 76 (39–148) | 0.278 |
| Low values (% participants) | 34.5 | 5.3 | 0.001 | 20.6 | 18.1 | 0.31 |
| Normal values (% participants) | 63 | 88.9 | 0.001 | 76 | 79.4 | 0.12 |
| High values (% participants) | 2.5 | 5.7 | 0.08 | 3.4 | 2.5 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Uricoechea, H.; Urrego-Noguera, K.; Vargas-Sierra, H.; Pinzón-Fernández, M. Zinc and Ferritin Levels and Their Associations with Functional Disorders and/or Thyroid Autoimmunity: A Population-Based Case–Control Study. Int. J. Mol. Sci. 2024, 25, 10217. https://doi.org/10.3390/ijms251810217
Vargas-Uricoechea H, Urrego-Noguera K, Vargas-Sierra H, Pinzón-Fernández M. Zinc and Ferritin Levels and Their Associations with Functional Disorders and/or Thyroid Autoimmunity: A Population-Based Case–Control Study. International Journal of Molecular Sciences. 2024; 25(18):10217. https://doi.org/10.3390/ijms251810217
Chicago/Turabian StyleVargas-Uricoechea, Hernando, Karen Urrego-Noguera, Hernando Vargas-Sierra, and María Pinzón-Fernández. 2024. "Zinc and Ferritin Levels and Their Associations with Functional Disorders and/or Thyroid Autoimmunity: A Population-Based Case–Control Study" International Journal of Molecular Sciences 25, no. 18: 10217. https://doi.org/10.3390/ijms251810217
APA StyleVargas-Uricoechea, H., Urrego-Noguera, K., Vargas-Sierra, H., & Pinzón-Fernández, M. (2024). Zinc and Ferritin Levels and Their Associations with Functional Disorders and/or Thyroid Autoimmunity: A Population-Based Case–Control Study. International Journal of Molecular Sciences, 25(18), 10217. https://doi.org/10.3390/ijms251810217





