CD26 Is Differentially Expressed throughout the Life Cycle of Infantile Hemangiomas and Characterizes the Proliferative Phase
Abstract
1. Introduction
2. Results
2.1. Immunohistochemical Analysis of Infantile Hemangioma
2.2. CD26 and CD133 Expression
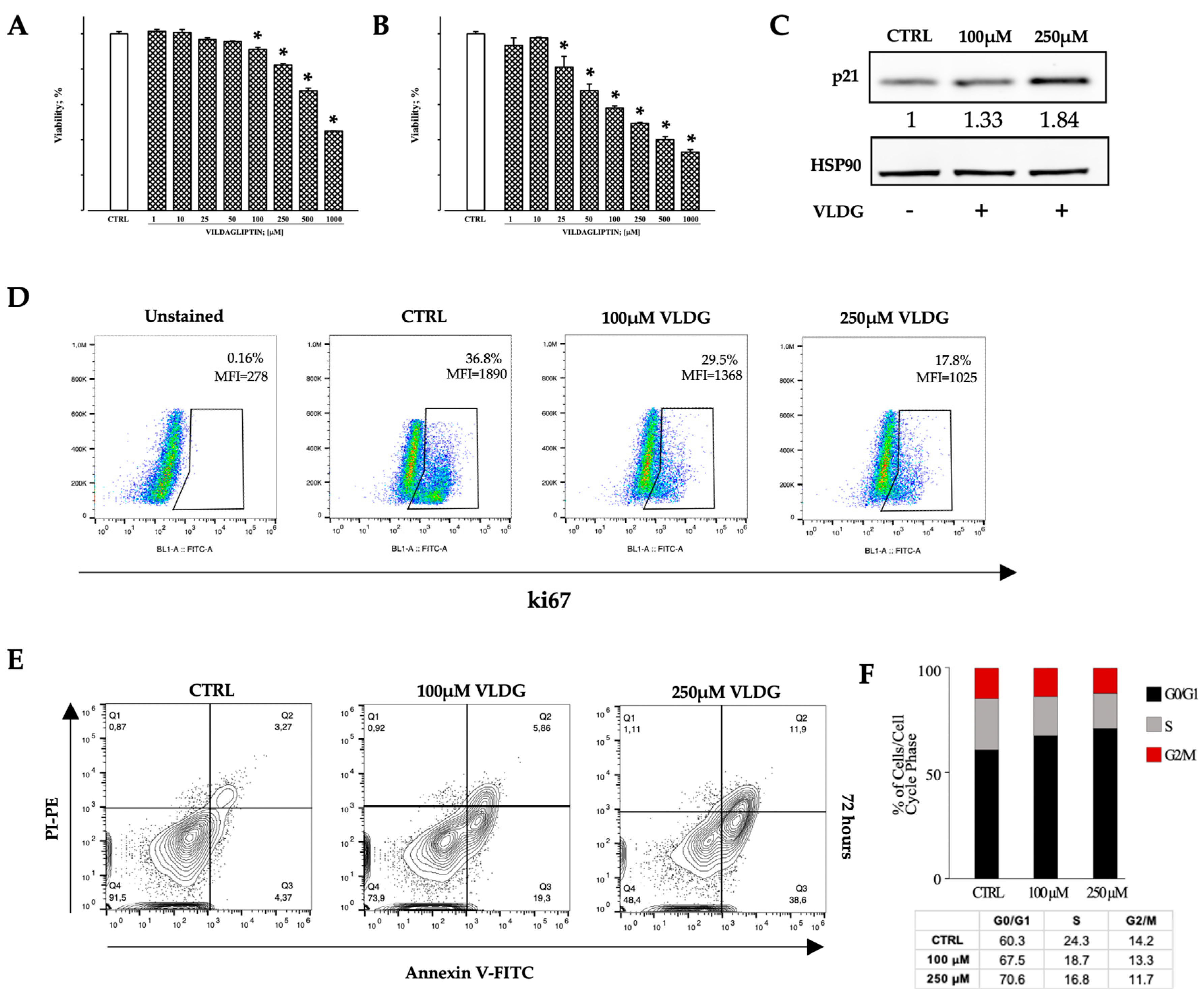
2.3. In Vitro Effects of DPP-IV Pharmacological Inhibition on Hem-ECs
3. Discussion
4. Materials and Methods
4.1. Patient Population and Tissue Sampling
4.2. Immunohistochemical Analysis
4.3. Hemangioma-Derived Endothelial Cell Lines
4.4. Immunofluorescence/Immunocytochemistry
4.5. MTT Assay
4.6. Western Blot Analysis
4.7. Flow Cytometric Analysis
4.8. Apoptosis Assay and Cell Cycle Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenberger, S.; Bischoff, J. Infantile hemangioma-mechanism(s) of drug action on a vascular tumor. Cold Spring Harb. Perspect. Med. 2011, 1, a006460. [Google Scholar] [CrossRef] [PubMed]
- Léauté-Labrèze, C.; Harper, J.I.; Hoeger, P.H. Infantile hemangioma. Lancet 2017, 390, 85–94. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; de la Roque, E.D.; Hubiche, T.; Boralevi, F.; Thambo, J.-B.; Taïeb, A. Propranolol for severe hemangiomas of infancy. N. Engl. J. Med. 2008, 358, 2649–2651. [Google Scholar] [CrossRef]
- Bayart, C.B.; Brandling-Bennett, H.A. Beta-blockers for childhood vascular tumors. Curr. Opin. Pediatr. 2015, 27, 454–459. [Google Scholar] [CrossRef]
- Krowchuk, D.P.; Frieden, I.J.; Mancini, A.J.; Darrow, D.H.; Blei, F.; Greene, A.K.; Annam, A.; Baker, C.N.; Frommelt, P.C.; Hodak, A.; et al. Clinical Practice Guideline for the Management of Infantile Hemangiomas. Pediatrics 2019, 143, e20183475. [Google Scholar] [CrossRef]
- Mulliken, J.; Glowacki, P. Hemangiomas and vascular malformations in infants and children: A classification based on endothelial characteristics. Plast. Reconstr. Surg. 1982, 69, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, S.; Bischoff, J. Pathogenesis of infantile haemangioma. Br. J. Dermatol. 2013, 169, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Itinteang, T.; Marsh, R.; Davis, P.F.; Tan, S.T. Angiotensin II causes cellular proliferation in infantile haemangioma via angiotensin II receptor 2 activation. J. Clin. Pathol. 2015, 68, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Boscolo, E.; Picard, A.; Psutka, S.; Melero-Martin, J.M.; Bartch, T.C.; Mulliken, J.B.; Bischoff, J. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J. Clin. Investig. 2008, 118, 2592–2599. [Google Scholar] [CrossRef]
- Harbi, S.; Wang, R.; Gregory, M.; Hanson, N.; Kobylarz, K.; Ryan, K.; Deng, Y.; Lopez, P.; Chiriboga, L.; Mignatti, P. Infantile hemangioma originates from a dysregulated but not fully transformed multipotent stem cell. Sci. Rep. 2016, 6, 35811. [Google Scholar] [CrossRef]
- Hoeger, P.H.; Colmenero, I. Vascular tumours in infants. Part I: Benign vascular tumours other than infantile haemangioma. Br. J. Dermatol. 2014, 171, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Colmenero, I.; Hoeger, P.H. Vascular tumours of intermediate malignancy [corrected] and malignant tumours. Br. J. Dermatol. 2014, 171, 474–484. [Google Scholar] [CrossRef]
- Gupta, A.; Kozakewich, H. Histopathology of vascular anomalies. Clin. Plast. Surg. 2011, 38, 31–44. [Google Scholar] [CrossRef]
- North, P.E.; Waner, M.; Mizeracki, A.; Mihm, M.C., Jr. GLUT1: A newly discovered immunohistochemical marker for juvenile hemangiomas. Hum. Pathol. 2000, 31, 11–22. [Google Scholar] [CrossRef]
- Al Dhaybi, R.; Powell, J.; McCuaig, C.; Kokta, V. Differentiation of vascular tumors from vascular malformations by expression of Wilms tumor 1 gene: Evaluation of 126 cases. J. Am. Acad. Dermatol. 2010, 63, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Timár, J.; Mészáros, L.; Orosz, Z.; Albini, A.; Rásó, E. WT1 expression in angiogenic tumours of the skin. Histopathology 2005, 47, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Itinteang, T.; Tan, S.T.; Brasch, H.; Day, D.J. Primitive mesodermal cells with a neural crest stem cell phenotype predominate proliferating infantile haemangioma. J. Clin. Pathol. 2010, 63, 771–776. [Google Scholar] [CrossRef]
- Spock, C.L.; Tom, L.K.; Canadas, K.; Sue, G.R.M.; Sawh-Martinez, R.; Maier, C.L.; Pober, J.S.; Galan, A.; Schultz, B.; Waner, M.; et al. Infantile hemangiomas exhibit neural crest and pericyte markers. Ann. Plast. Surg. 2015, 74, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Flint, A.F.; Mulliken, J.B.; Wu, J.K.; Bischoff, J. Endothelial progenitor cells in infantile hemangioma. Blood 2004, 103, 1373–1375. [Google Scholar] [CrossRef]
- Miraglia, S.; Godfrey, W.; Yin, A.H.; Atkins, K.; Warnke, R.; Holden, J.T.; Bray, R.A.; Waller, E.K.; Buck, D.W. A novel five-transmembrane hematopoietic stem cell antigen: Isolation, characterization, and molecular cloning. Blood 1997, 90, 5013–5021. [Google Scholar] [CrossRef]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.T.; Berry, P.A.; Hyde, C.; Stower, M.J.; Maitland, N.J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005, 65, 10946–10951. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, A.; Nagaki, M.; Aoki, H.; Motohashi, T.; Kunisada, T.; Moriwaki, H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem. Biophys. Res. Commun. 2006, 351, 820–824. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. CD133: A stem cell biomarker and beyond. Exp. Hematol. Oncol. 2013, 2, 17. [Google Scholar] [CrossRef]
- Lan, J.; Huang, B.; Liu, R.; Ju, X.; Zhou, Y.; Jiang, J.; Liang, W.; Shen, Y.; Li, F.; Pang, L. Expression of cancer stem cell markers and their correlation with pathogenesis in vascular tumors. Int. J. Clin. Exp. Pathol. 2015, 8, 12621–12633. [Google Scholar]
- Torimoto, Y.; Dang, N.H.; Tanaka, T.; Prado, C.; Schlossman, S.F.; Morimoto, C. Biochemical characterization of CD26 (dipeptidyl peptidase IV): Functional comparison of distinct epitopes recognized by various anti-CD26 monoclonal antibodies. Mol. Immunol. 1992, 29, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Enz, N.; Vliegen, G.; De Meester, I.; Jungraithmayr, W. CD26/DPP4-a potential biomarker and target for cancer therapy. Pharmacol. Ther. 2019, 198, 135–159. [Google Scholar] [CrossRef] [PubMed]
- Klemann, C.; Wagner, L.; Stephan, M.; von Hörsten, S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4’ (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016, 185, 1–21. [Google Scholar] [CrossRef]
- Bailey, S.R.; Nelson, M.H.; Majchrzak, K.; Bowers, J.S.; Wyatt, M.M.; Smith, A.S.; Neal, L.R.; Shirai, K.; Carpenito, C.; June, C.H.; et al. Human CD26high T cell elicit tumor immunity against multiple malignancies via enhanced migration and persistence. Nat. Commun. 2017, 8, 1961. [Google Scholar] [CrossRef]
- Pang, R.; Law, W.L.; Chu, A.C.; Poon, J.T.; Lam, C.S.; Chow, A.K.; Ng, L.; Cheung, L.W.; Lan, X.R.; Lan, H.Y.; et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell 2010, 6, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Naito, M.; Ghani, F.I.; Dang, N.H.; Iwata, S.; Morimoto, C. Characterization of cancer stem cell properties of CD24 and CD26-positive human malignant mesothelioma cells. Biochem. Biophys. Res. Commun. 2012, 419, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Dang, N.H.; Aytac, U.; Sato, K.; O’Brien, S.; Melenhorst, J.; Morimoto, C.; Barrett, A.J.; Molldrem, J.J. T-large granular lymphocyte lymphoproliferative disorder: Expression of CD26 as a marker of clinically aggressive disease and characterization of marrow inhibition. Br. J. Haematol. 2003, 121, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Beckenkamp, A.; Davies, S.; Willig, J.B.; Buffon, A. DPPIV/CD26: A tumor suppressor or a marker of malignancy? Tumor Biol. 2016, 37, 7059–7073. [Google Scholar] [CrossRef]
- Torrence, D.; Antonescu, C.R. The genetics of vascular tumours: An update. Histopathology 2022, 80, 19–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Queisser, A.; Seront, E.; Boon, L.M.; Vikkula, M. Genetic Basis and Therapies for Vascular Anomalies. Circ. Res. 2021, 129, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Itinteang, T.; Tan, S.T.; Brasch, H.D.; Steel, R.; A Best, H.; Vishvanath, A.; Jia, J.; Day, D.J. Infantile haemangioma expresses embryonic stem cell markers. J. Clin. Pathol. 2012, 65, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Kilmister, E.J.; Hansen, L.; Davis, P.F.; Hall, S.R.R.; Tan, S.T. Cell Populations Expressing Stemness-Associated Markers in Vascular Anomalies. Front. Surg. 2021, 7, 610758. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Available online: https://www.issva.org/UserFiles/file/ISSVA-Classification-2018.pdf (accessed on 1 July 2024).
- Kunimoto, K.; Yamamoto, Y.; Jinnin, M. ISSVA Classification of Vascular Anomalies and Molecular Biology. Int. J. Mol. Sci. 2022, 23, 2358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trindade, F.; Tellechea, O.; Torrelo, A.; Requena, L.; Colmenero, I. Wilms tumor 1 expression in vascular neoplasms and vascular malformations. Am. J. Dermatopathol. 2011, 33, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Hagio, M.; Ishiwata, T. Nestin: A novel angiogenesis marker and possible target for tumor angiogenesis. World J. Gastroenterol. 2013, 19, 42–48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hastie, N.D. Wilms’ tumour (WT1) in development, homeostasis and disease. Development 2017, 144, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.; Wagner, N.; Bondke, A.; Nafz, B.; Flemming, B.; Theres, H.; Scholz, H. The Wilms’ tumor suppressor Wt1 is expressed in the coronary vasculature after myocardial infarction. FASEB J. 2002, 16, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-D.; Cherfils-Vicini, J.; Hosen, N.; Hohenstein, P.; Gilson, E.; Hastie, N.D.; Michiels, J.-F.; Wagner, N. The Wilms’ tumour suppressor Wt1 is a major regulator of tumour angiogenesis and progression. Nat. Commun. 2014, 5, 5852. [Google Scholar] [CrossRef] [PubMed]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Mokrý, J.; Cízková, D.; Filip, S.; Ehrmann, J.; Osterreicher, J.; Kolár, Z.; English, D. Nestin expression by newly formed human blood vessels. Stem Cells Dev. 2004, 13, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.D.; Scholz, H.; Kirschner, K.M.; Schedl, A. Intermediate filament protein nestin is expressed in developing kidney and heart and might be regulated by the Wilms’ tumor suppressor Wt1. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R779–R787. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, T.; Yamada, T.; Ohnuma, K.; Kina, S.; Takahashi, N.; Yamochi, T.; Inamoto, S.; Katsuoka, Y.; Hosono, O.; Tanaka, H.; et al. Humanized anti-CD26 monoclonal antibody as a treatment for malignant mesothelioma tumors. Clin. Cancer Res. 2007, 13, 4191–4200. [Google Scholar] [CrossRef] [PubMed]
- Aoe, K.; Amatya, V.J.; Fujimoto, N.; Ohnuma, K.; Hosono, O.; Hiraki, A.; Fujii, M.; Yamada, T.; Dang, N.H.; Takeshima, Y.; et al. CD26 overexpression is associated with prolonged survival and enhanced chemosensitivity in malignant pleural mesothelioma. Clin. Cancer Res. 2012, 18, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Yamazaki, H.; Hatano, R.; Yamada, T.; Kaneko, Y.; Xu, C.W.; Dang, N.H.; Ohnuma, K.; Morimoto, C. Targeting CD26 suppresses proliferation of malignant mesothelioma cell via downmodulation of ubiquitin-specific protease 22. Biochem. Biophys. Res. Commun. 2018, 504, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Nistala, R.; Cao, M.; Pan, Y.; Behrens, M.; Doll, D.; Hammer, R.D.; Nistala, P.; Chang, H.-M.; Yeh, E.T.; et al. Dipeptidylpeptidase 4 promotes survival and stemness of acute myeloid leukemia stem cells. Cell Rep. 2023, 42, 112105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrmann, H.; Sadovnik, I.; Cerny-Reiterer, S.; Rülicke, T.; Stefanzl, G.; Willmann, M.; Hoermann, G.; Bilban, M.; Blatt, K.; Herndlhofer, S.; et al. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood 2014, 123, 3951–3962. [Google Scholar] [CrossRef] [PubMed]
- Grillet, F.; Bayet, E.; Villeronce, O.; Zappia, L.; Lagerqvist, E.L.; Lunke, S.; Charafe-Jauffret, E.; Pham, K.; Molck, C.; Rolland, N.; et al. Circulating tumour cells from patients with colorectal cancer have cancer stem cell hallmarks in ex vivo culture. Gut 2017, 66, 1802–1810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheung, A.H.-K.; Iyer, D.N.; Lam, C.S.-C.; Ng, L.; Wong, S.K.M.; Lee, H.-S.; Wan, T.; Man, J.; Chow, A.K.M.; Poon, R.T.; et al. Emergence of CD26+ Cancer Stem Cells with Metastatic Properties in Colorectal Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, X.; Liu, J.; Li, X.; Tian, R.; Shang, K.; Dong, X.; Cao, B. Angiogenesis is promoted by exosomal DPP4 derived from 5-fluorouracil-resistant colon cancer cells. Cancer Lett. 2021, 497, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Leuven, L.K.U.; Kozlovski, P.; Ag, B.N.P.; Paldánius, P.M.; E Foley, J.; Modgill, V.; Evans, M.; Centre, L.H.D.R.; Serban, C. Clinical Safety and Tolerability of Vildagliptin-Insights from Randomised Trials, Observational Studies and Post-marketing Surveillance. Eur. Endocrinol. 2017, 13, 68–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lorusso, B.; Cerasoli, G.; Falco, A.; Frati, C.; Graiani, G.; Madeddu, D.; Nogara, A.; Corradini, E.; Roti, G.; Cerretani, E.; et al. Β-blockers activate autophagy on infantile hemangioma-derived endothelial cells in vitro. Vasc. Pharmacol. 2022, 146, 107110. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, B.; Falco, A.; Madeddu, D.; Frati, C.; Cavalli, S.; Graiani, G.; Gervasi, A.; Rinaldi, L.; Lagrasta, C.; Maselli, D.; et al. Isolation and Characterization of Human Lung Lymphatic Endothelial Cells. Biomed. Res. Int. 2015, 2015, 747864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
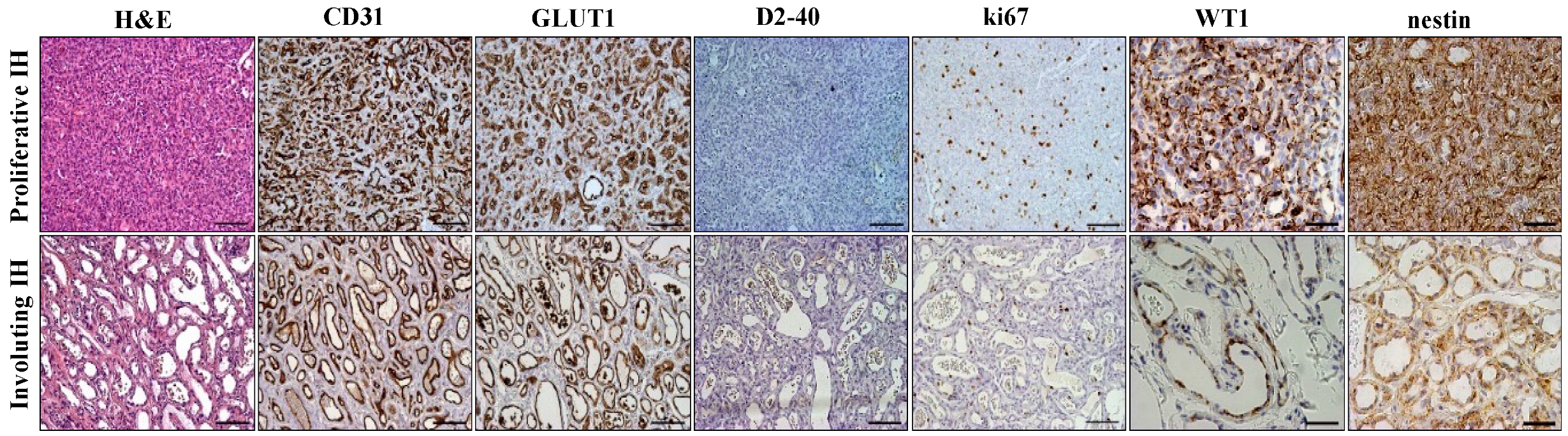
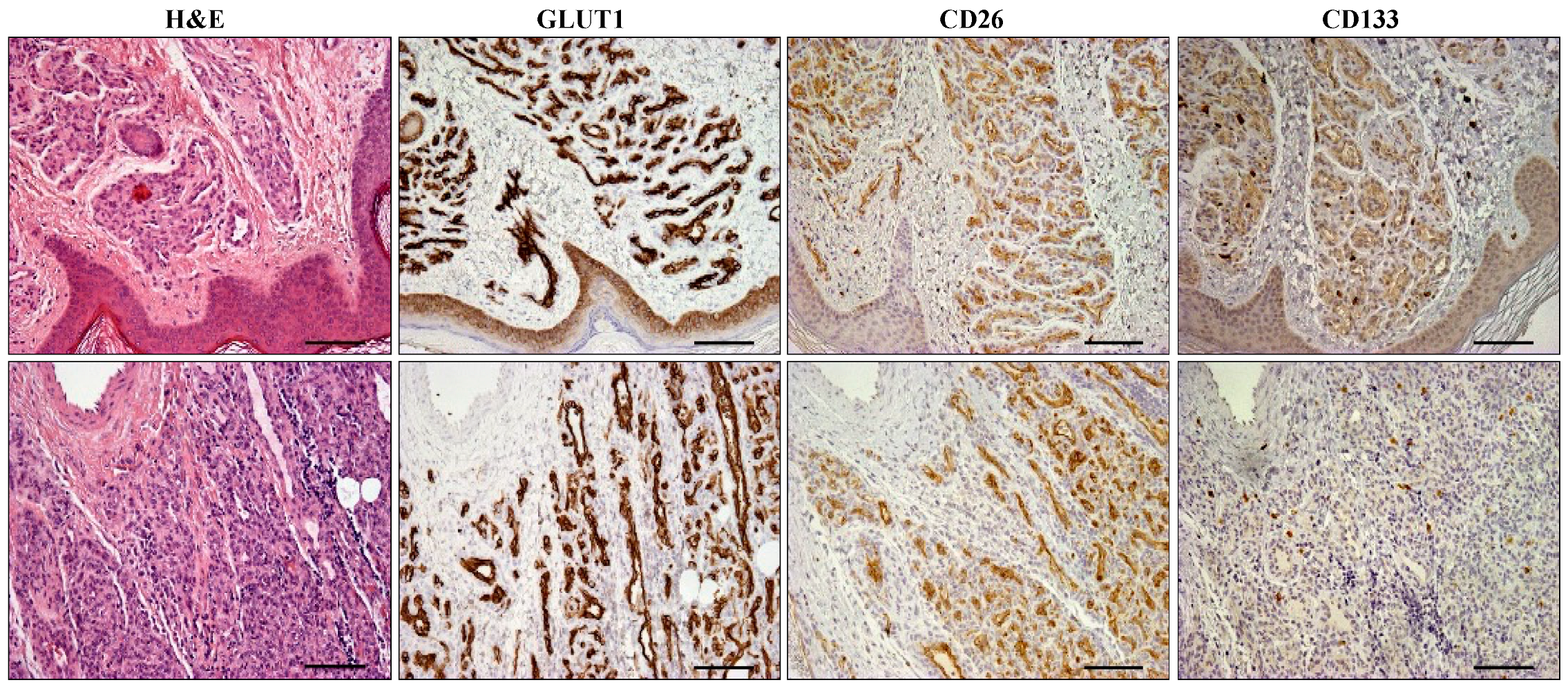

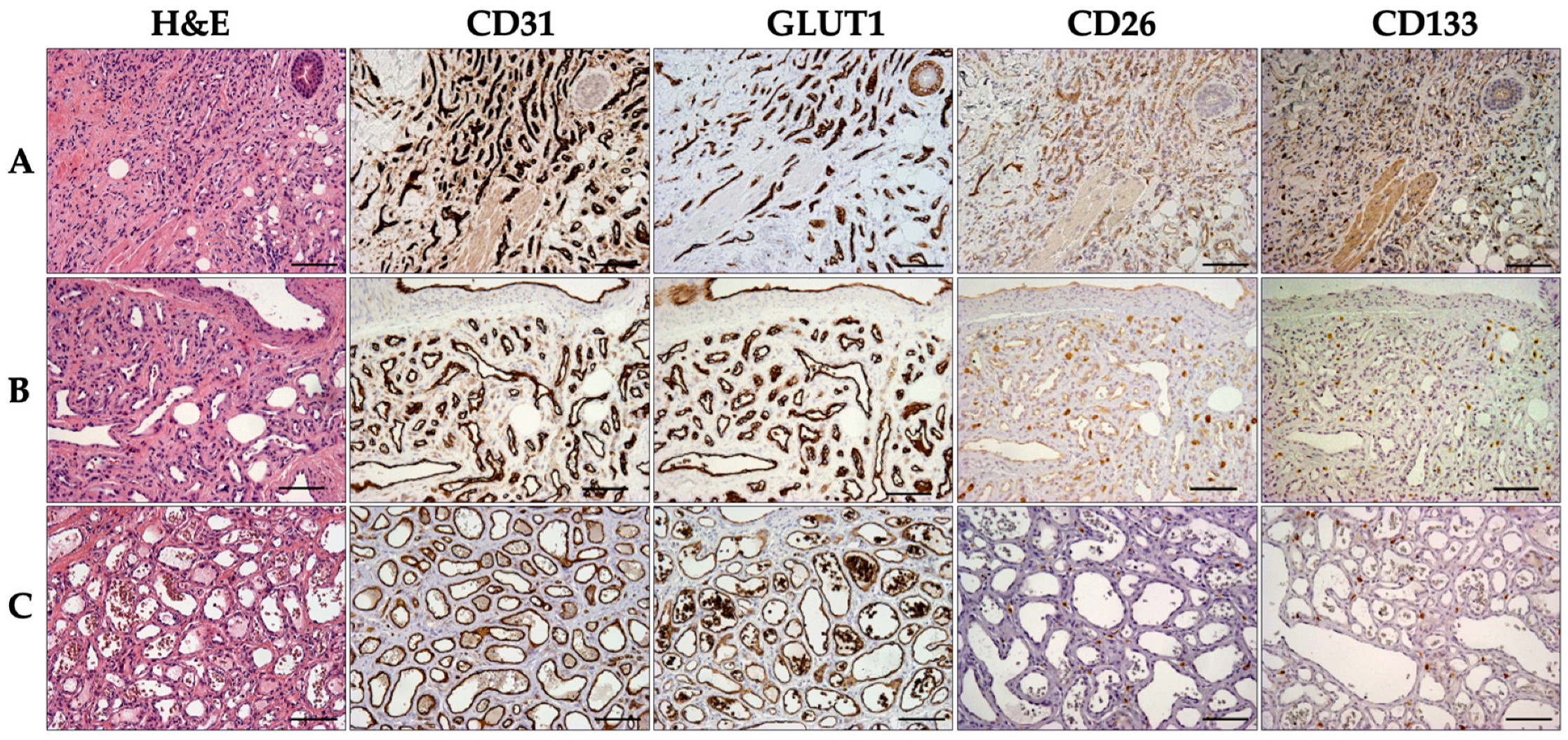

| N Cases | CD26pos % | CD133pos % | |
|---|---|---|---|
| Proliferative IH | 15 | 100 | 66.6 |
| Involuting IH | 12 | 58.3 | 25 |
| w/o mixed phase | 5 | 0 | 0 |
| w mixed phase | 7 | 100 | 42.8 |
| Marker | Source | Company | Antigen Retrieval | Processing System Tool | Dilution | Time (min) | Temp. (°C) | Clone |
|---|---|---|---|---|---|---|---|---|
| CD31 | Ms | Ventana-Roche | 15′ MW | * | ready to use | 28 | 37 | JC70 |
| GLUT1 | Rb | Ventana-Roche | 15′ MW | * | ready to use | 28 | 37 | polyclonal |
| D2-40 | Ms | Cell Marque | 15′ MW | * | ready to use | 28 | 37 | D2-40 |
| ki67 | Ms | DAKO | 15′ MW | * | 1:100 | 28 | 37 | Mib-1 |
| WT1 | Ms | Ventana-Roche | 15′ MW | * | ready to use | 28 | 37 | 6F-H2 |
| Nestin | Rb | Millipore | 15′ MW | ** | 1:1000 | 45 | RT | polyclonal |
| CD26 | Rb | Cell Signaling Technology | 35′ bath 95 °C | ** | 1:50 | o/n | 4 | D6D8K |
| CD133 | Rb | Abcam | 35′ bath 95 °C | ** | 1:100 | 60 | RT | polyclonal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorusso, B.; Nogara, A.; Fioretzaki, R.; Corradini, E.; Bove, R.; Roti, G.; Gherli, A.; Montanaro, A.; Monica, G.; Cavazzini, F.; et al. CD26 Is Differentially Expressed throughout the Life Cycle of Infantile Hemangiomas and Characterizes the Proliferative Phase. Int. J. Mol. Sci. 2024, 25, 9760. https://doi.org/10.3390/ijms25189760
Lorusso B, Nogara A, Fioretzaki R, Corradini E, Bove R, Roti G, Gherli A, Montanaro A, Monica G, Cavazzini F, et al. CD26 Is Differentially Expressed throughout the Life Cycle of Infantile Hemangiomas and Characterizes the Proliferative Phase. International Journal of Molecular Sciences. 2024; 25(18):9760. https://doi.org/10.3390/ijms25189760
Chicago/Turabian StyleLorusso, Bruno, Antonella Nogara, Rodanthi Fioretzaki, Emilia Corradini, Roberta Bove, Giovanni Roti, Andrea Gherli, Anna Montanaro, Gregorio Monica, Filippo Cavazzini, and et al. 2024. "CD26 Is Differentially Expressed throughout the Life Cycle of Infantile Hemangiomas and Characterizes the Proliferative Phase" International Journal of Molecular Sciences 25, no. 18: 9760. https://doi.org/10.3390/ijms25189760
APA StyleLorusso, B., Nogara, A., Fioretzaki, R., Corradini, E., Bove, R., Roti, G., Gherli, A., Montanaro, A., Monica, G., Cavazzini, F., Bonomini, S., Graiani, G., Silini, E. M., Gnetti, L., Pilato, F. P., Cerasoli, G., Quaini, F., & Lagrasta, C. A. M. (2024). CD26 Is Differentially Expressed throughout the Life Cycle of Infantile Hemangiomas and Characterizes the Proliferative Phase. International Journal of Molecular Sciences, 25(18), 9760. https://doi.org/10.3390/ijms25189760








