IGF-1 and Glucocorticoid Receptors Are Potential Target Proteins for the NGF-Mimic Effect of β-Cyclocitral from Lavandula angustifolia Mill. in PC12 Cells
Abstract
1. Introduction
2. Results
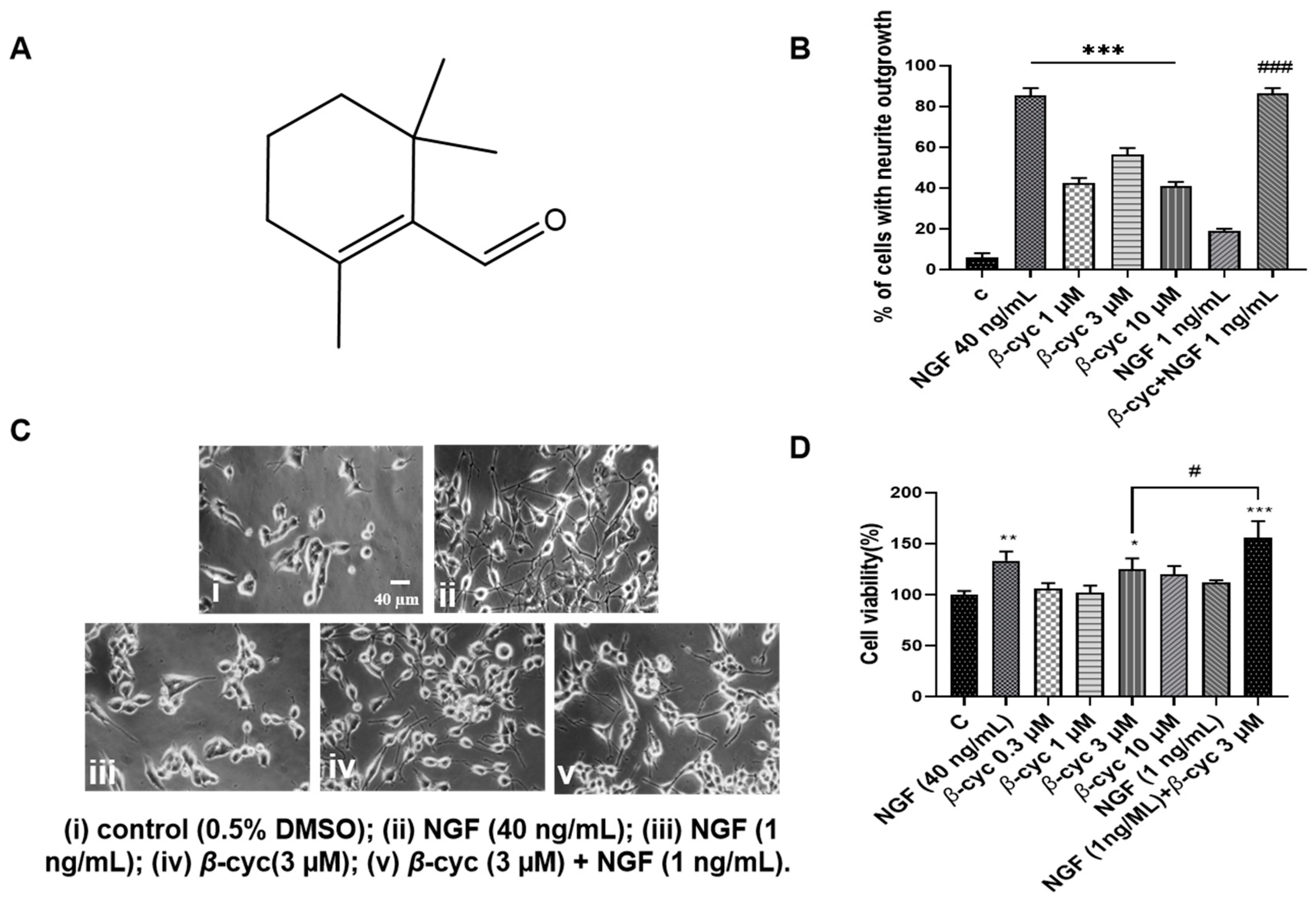
2.1. β-cyc Exhibits NGF-Mimic and NGF-Enhancing Effects on PC12 Cells
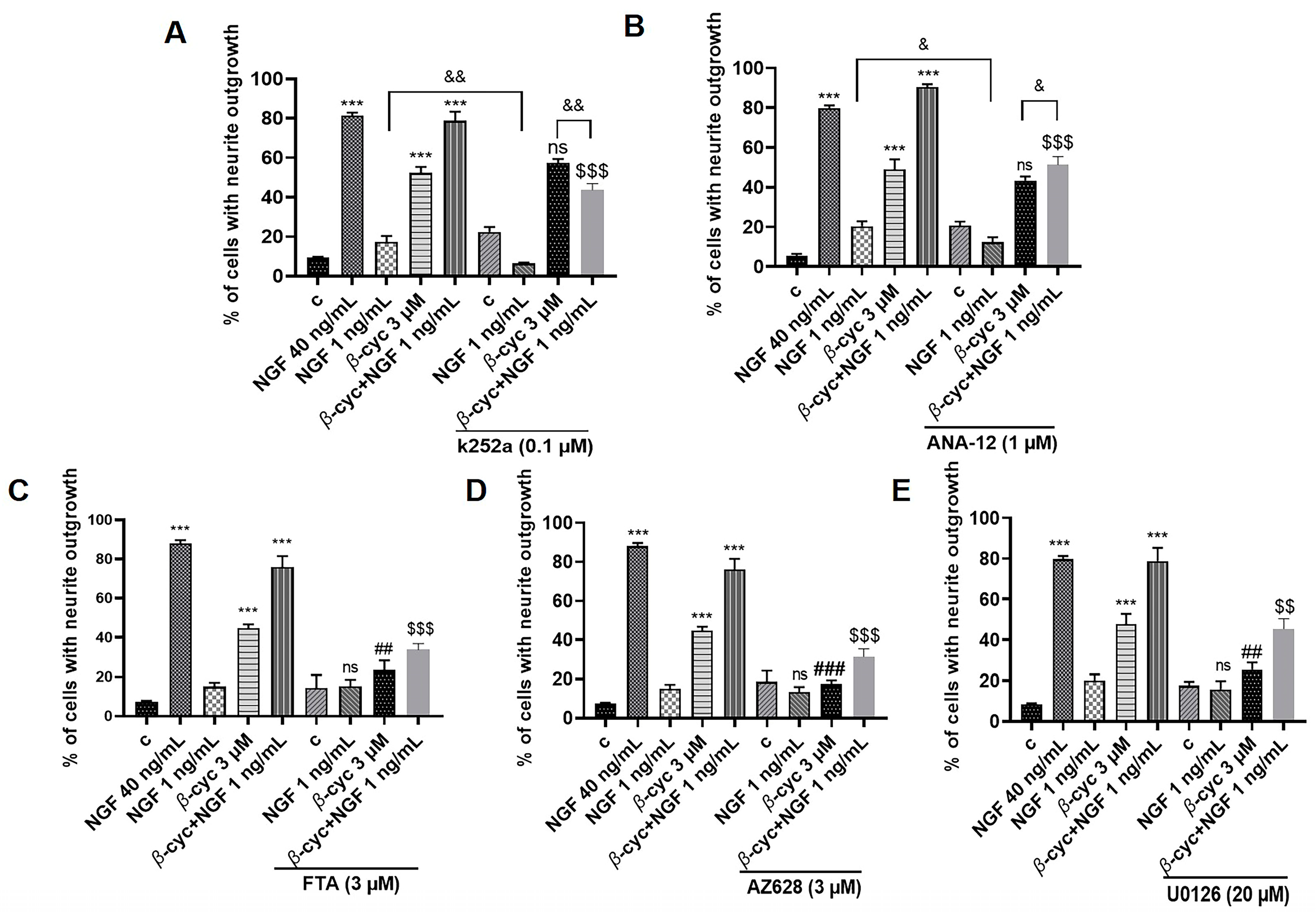
2.2. Ras/Raf/MEK/ERK Signaling Pathway Involves in NGF-Mimic and NGF–Enhancing Effect of β-cyc
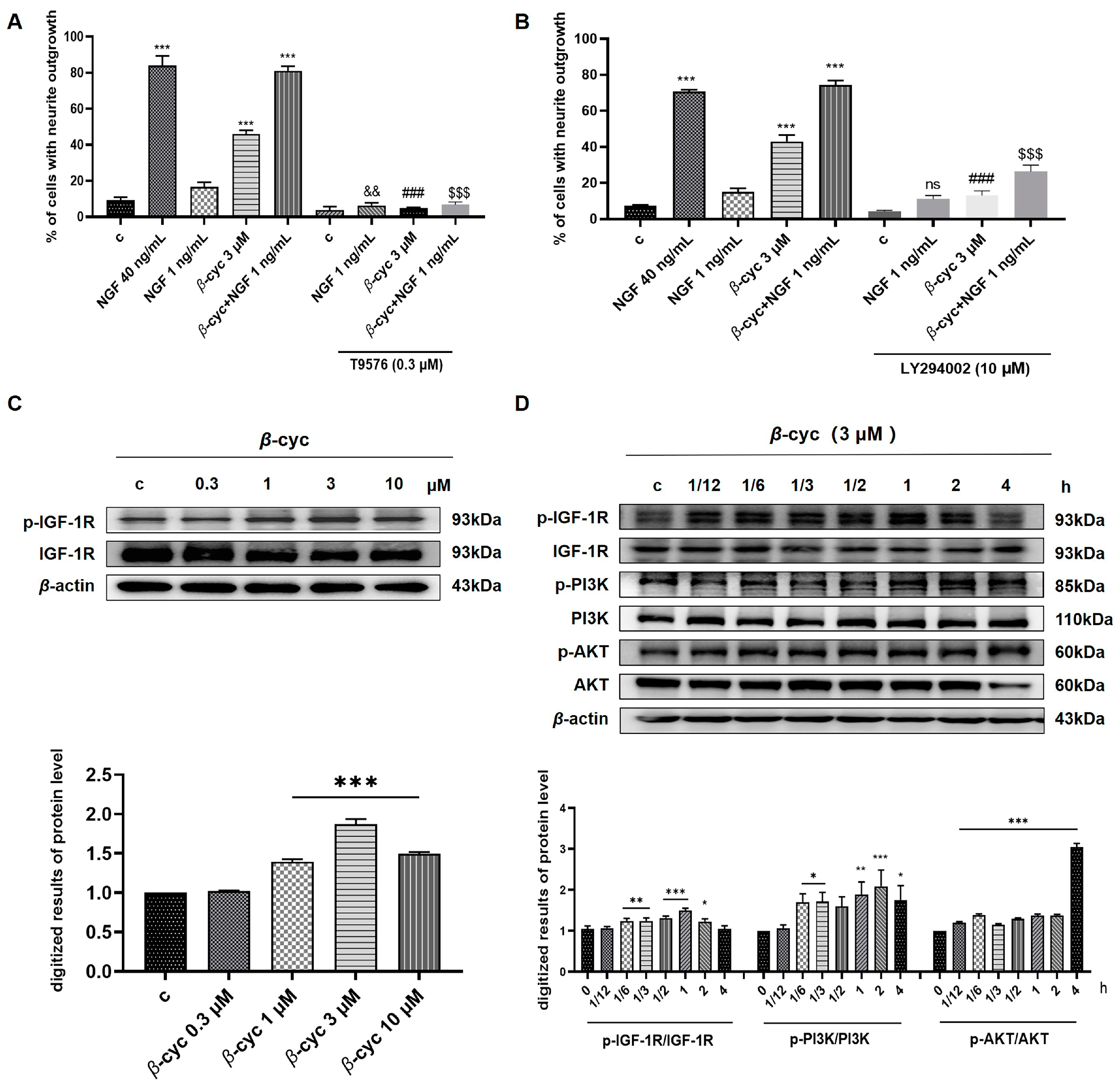
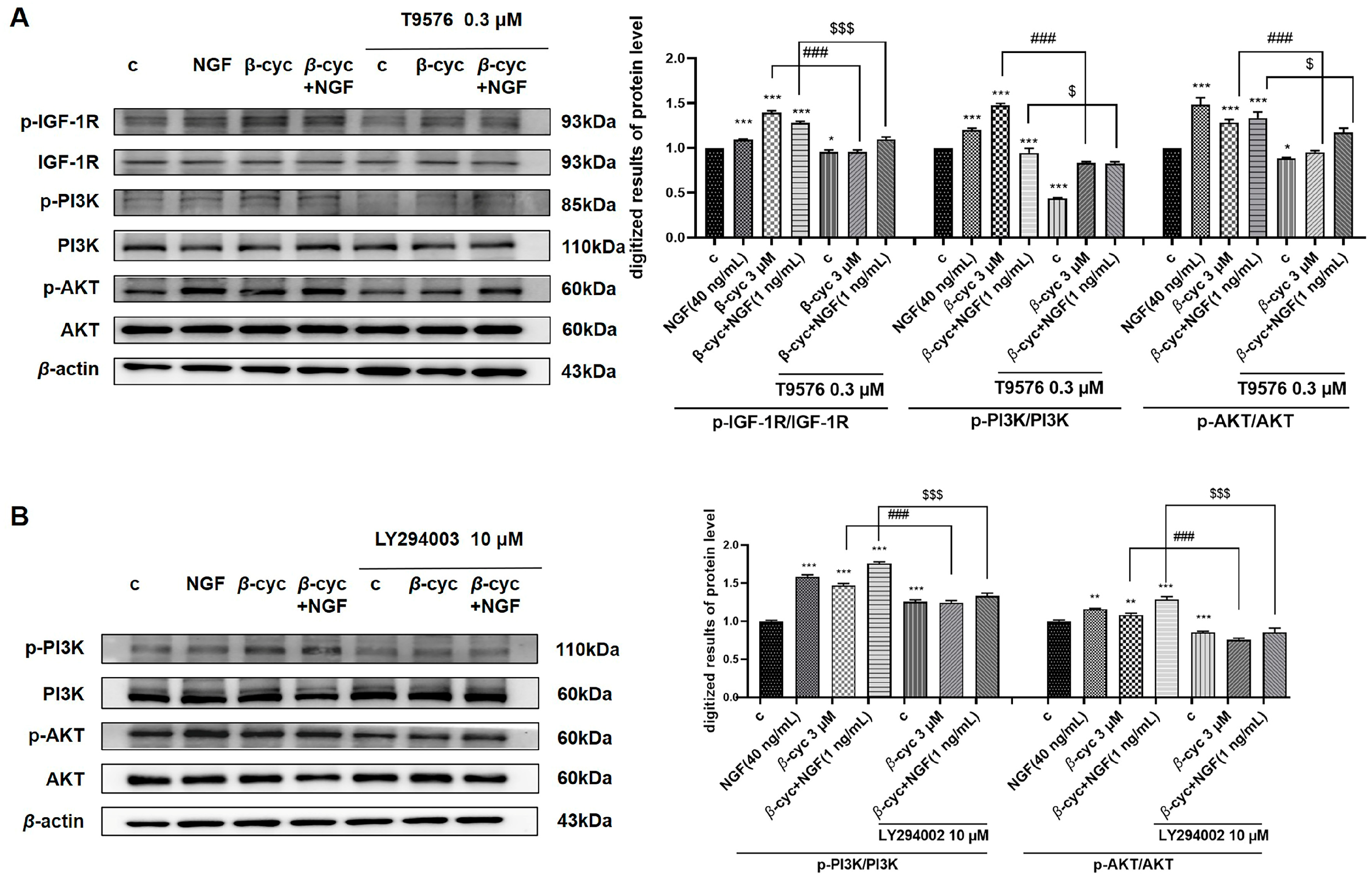
2.3. IGF-1R/PI3K/AKT Signaling Pathway Takes an Important Role in NGF-Mimic Effect of β-cyc
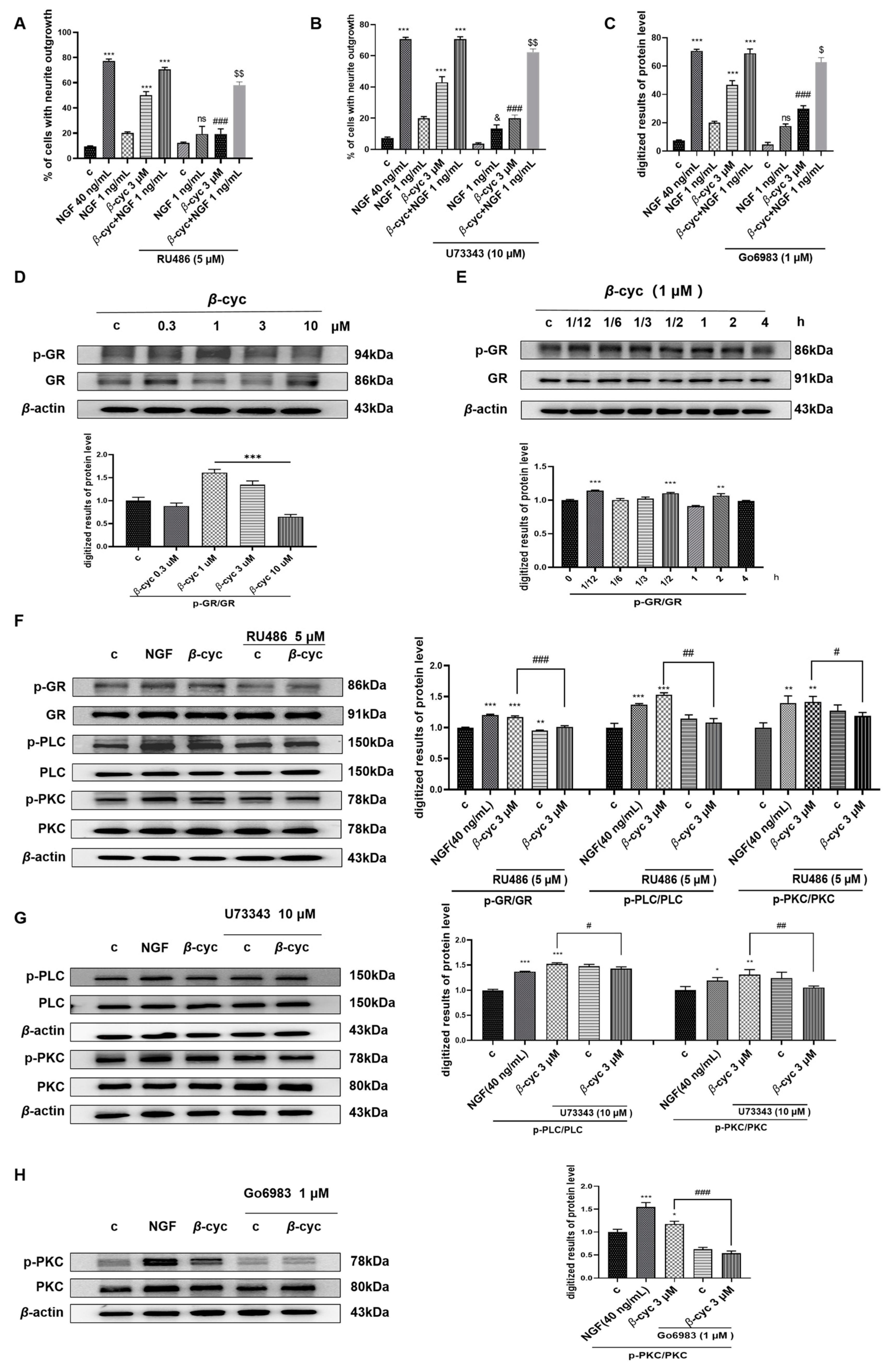
2.4. GR/PLC/PKC Signaling Pathway Participates in the NGF-Mimic Effect of β-cyc
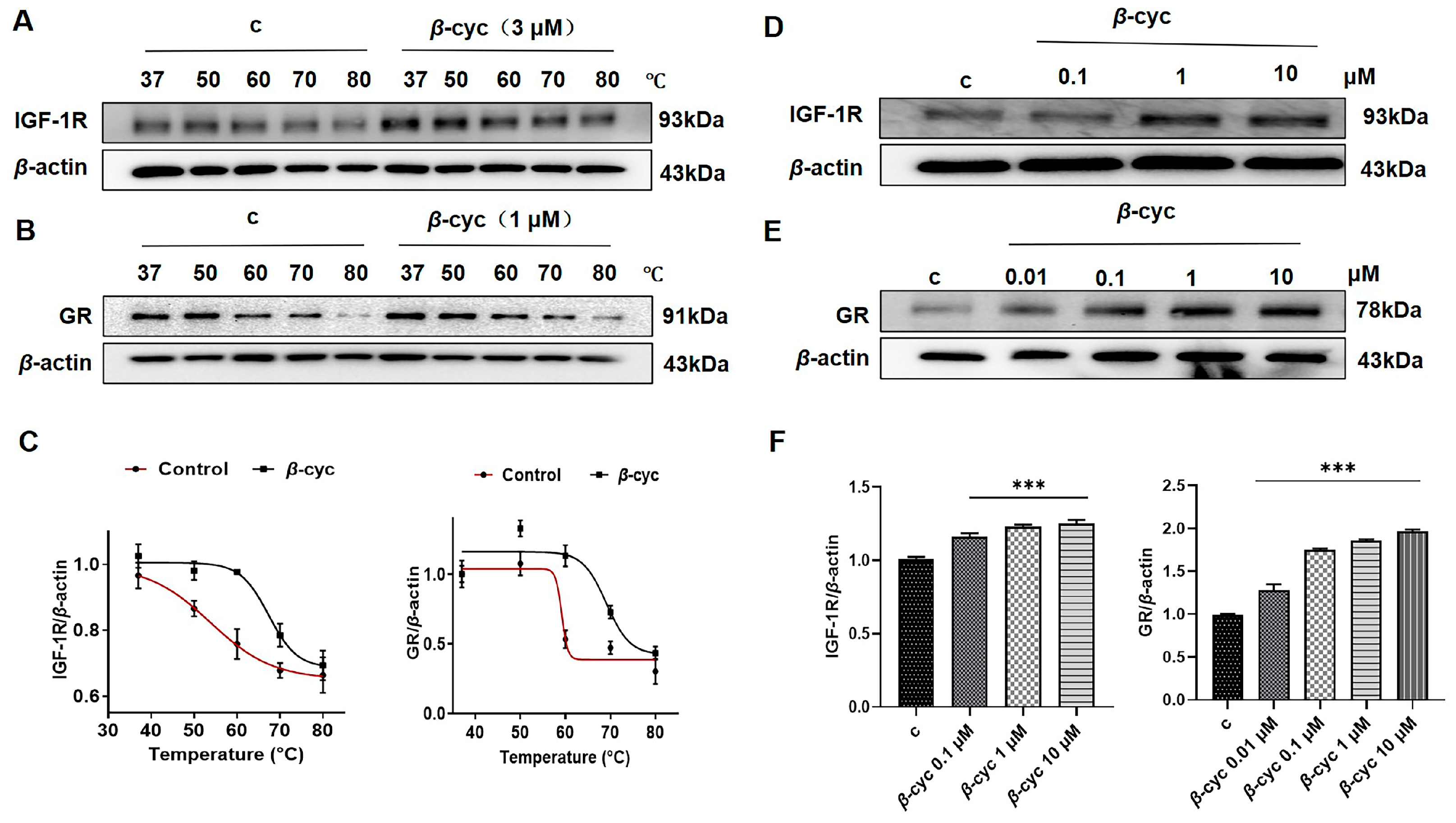
2.5. β-cyc Increases the Thermal Stable Ability of IGF-1R in CETSA
2.6. β-cyc Enhances the Stability of IGF-1R and GR Proteins for Pronase E During DARTS Analysis
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Extraction and Isolation
4.3. Evaluation of the Neuritogenic Activity of PC12 Cells
4.4. Cell Viability by MTT Bioassay
4.5. Western Blot Analysis
4.6. CETSA
4.7. DARTS
4.8. Quantification and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Alzheimer’s Disease International. World Alzheimer Report 2023: Reducing Dementia Risk: Never Too Early, Never Too Late. 2023. Available online: https://www.alzint.org/resource/world-alzheimer-report-2023/ (accessed on 21 September 2023).
- Milà-Alomà, M.; Ashton, N.J.; Shekari, M.; Salvadó, G.; Ortiz-Romero, P.; Montoliu-Gaya, L.; Benedet, A.L.; Karikari, T.K.; Lantero-Rodriguez, J.; Vanmechelen, E.; et al. Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer’s disease. Nat. Med. 2022, 28, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef] [PubMed]
- Tucker, S.; Möller, C.; Tegerstedt, K.; Lord, A.; Laudon, H.; Sjödahl, J.; Söderberg, L.; Spens, E.; Sahlin, C.; Waara, E.R.; et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J. Alzheimers Dis. 2015, 43, 575–588. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Lest’anová, Z.; Bacová, Z.; Havránek, T.; Bakos, J. Mechanizmy zmien dĺzky axónov a dendritov neurónu Mechanisms of growth of neuronal axons and dendrites. Cesk Fysiol. 2013, 62, 47–53. [Google Scholar]
- Akram, R.; Anwar, H.; Javed, M.S.; Rasul, A.; Imran, A.; Malik, S.A.; Raza, C.; Khan, I.U.; Sajid, F.; Iman, T.; et al. Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets. Biomedicines 2022, 10, 3186. [Google Scholar] [CrossRef]
- Makin, S. The amyloid hypothesis on trial. Nature 2018, 559, S4–S7. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The cellular phase of Alzheimer’s disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Loy, R.; Taglialatela, G.; Angelucci, L.; Heyer, D.; Perez-Polo, R. Regional CNS uptake of blood-borne nerve growth factor. J. Neurosci. Res. 1994, 39, 339–346. [Google Scholar] [CrossRef]
- Xu, C.J.; Wang, J.L.; Jin, W.L. The emerging therapeutic role of NGF in Alzheimer’s disease. Neurochem. Res. 2016, 41, 1211–1218. [Google Scholar] [CrossRef]
- Mitra, S.; Behbahani, H.; Eriksdotter, M. Innovative therapy for Alzheimer’s disease-with focus on biodelivery of NGF. Front. Neurosci. 2019, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, K.; Muroi, M.; Gao, L.; Chang, Y.; Osada, H.; Xiang, L.; Qi, J. Cucurbitacin B induces neurogenesis in PC12 cells and protects memory in APP/PS1 mice. J. Cell Mol. Med. 2019, 23, 6283–6294. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Osada, H.; Xing, T.; Yoshida, M.; Xiang, L.; Qi, J. The Insulin receptor: A potential target of amarogentin isolated from Gentiana rigescens Franch that induces neurogenesis in PC12 Cells. Biomedicines 2021, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ye, Y.; Xiang, L.; Osada, H.; Qi, J. Lindersin B from Lindernia crustacea induces neuritogenesis by activation of tyrosine kinase A/phosphatidylinositol 3 kinase/extracellular signal-regulated kinase signaling pathway. Phytomedicine 2017, 24, 31–38. [Google Scholar] [CrossRef]
- Tsai, S.J.; Liu, W.H.; Yin, M.C. Trans Fatty Acids Enhanced Beta-Amyloid Induced Oxidative Stress in Nerve Growth Factor Differentiated PC12 Cells. Springer 2012, 37, 786–794. [Google Scholar] [CrossRef]
- El Abdali, Y.; Agour, A.; Allali, A.; Bourhia, M.; El Moussaoui, A.; Eloutassi, N.; Salamatullah, A.M.; Alzahrani, A.; Ouahmane, L.; Aboul-Soud, M.A.M.; et al. Lavandula dentata L.: Phytochemical Analysis, Antioxidant, Antifungal and Insecticidal Activities of Its Essential Oil. Plants 2022, 11, 311. [Google Scholar] [CrossRef]
- Zheng, T.F.; Zhou, M.; Yang, L.; Wang, Y.Y.; Meng, Y.Y.; Liu, J.L.; Zuo, A.J. Effects of high light and temperature on Microcystis aeruginosa cell growth and β-cyclocitral emission. Ecotoxicol. Environ. Saf. 2020, 192, 110313. [Google Scholar] [CrossRef]
- O’Neill, C.; Kiely, A.P.; Coakley, M.F.; Manning, S.; Long-Smith, C.M. Insulin and IGF-1 signaling: Longevity, protein homoeostasis and Alzheimer’s disease. Biochem. Soc. Trans. 2012, 40, 721–727. [Google Scholar] [CrossRef]
- Lejri, I.; Grimm, A.; Eckert, A. Ginkgo biloba extract increases neurite outgrowth and activates the AKT/mTOR pathway. PLoS ONE 2019, 14, e0225761. [Google Scholar] [CrossRef]
- O’ Neill, C. PI3-kinase/AKT/mTOR signaling: Impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp. Gerontol. 2013, 48, 647–653. [Google Scholar] [CrossRef]
- Terada, K.; Kojima, Y.; Watanabe, T.; Izumo, N.; Chiba, K.; Karube, Y. Inhibition of nerve growth factor-induced neurite outgrowth from PC12 cells by dexamethasone: Signaling pathways through the glucocorticoid receptor and phosphorylated AKT and ERK1/2. PLoS ONE 2014, 9, e93223. [Google Scholar] [CrossRef] [PubMed]
- Ebendal, T. Function and evolution in the NGF family and its receptors. J. Neurosci. Res. 1992, 32, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. NGF, BDNF, NT3, and NT4. Handb. Exp. Pharmacol. 2014, 220, 3–15. [Google Scholar] [CrossRef]
- Vaudry, D.; Stork, P.J.S.; Lazarovici, P.; Eiden, L.E. Signaling pathways for PC12 cell differentiation: Making the right connections. Science 2002, 296, 1648–1649. [Google Scholar] [CrossRef]
- Li, W.; Miller, W.T. Role of the activation loop tyrosines in regulation of the Insulin-like growth factor I receptor-tyrosine Kinase. J. Biol. Chem. 2006, 281, 23785–23791. [Google Scholar] [CrossRef]
- Polman, J.A.; Welten, J.E.; Bosch, D.S.; de Jonge, R.T.; Balog, J.; van der Maarel, S.M.; de Kloet, E.R.; Datson, N.A. A genome-wide signature of glucocorticoid receptor binding in neuronal PC12 cells. BMC Neurosci. 2012, 13, 118. [Google Scholar] [CrossRef]
- Jozic, I.; Vukelic, S.; Stojadinovic, O.; Liang, L.; Ramirez, H.A.; Pastar, I.; Tomic Canic, M. Stress Signals, Mediated by Membranous Glucocorticoid Receptor, Activate PLC/PKC/GSK-3β/β-catenin Pathway to Inhibit Wound Closure. J. Investig. Dermatol. 2017, 137, 1144–1154. [Google Scholar] [CrossRef]
- Kashani, M.S.; Tavirani, M.R.; Talaei, S.A.; Salami, M. Aqueous extract of lavender (Lavandula angustifolia) improves the spatial performance of a rat model of Alzheimer’s disease. Neurosci. Bull. 2011, 27, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yuan, X.; Liu, T.; Liu, L.; Hu, Y.; Wang, Z.; Zheng, Q. Neuroprotective activity of lavender oil on transient focal cerebral ischemia in mice. Molecules 2012, 17, 9803–9817. [Google Scholar] [CrossRef]
- Jimbo, D.; Kimura, Y.; Taniguchi, M.; Inoue, M.; Urakami, K. Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics 2009, 9, 173–179. [Google Scholar] [CrossRef]
- Degel, J.; Köster, E.P. Odors: Implicit memory and performance effects. Chem. Senses. 1999, 24, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, S.; Mehan, S.; Khan, A.; Rehman, M.U. Protective role of IGF-1 and GLP-1 signaling activation in neurological dysfunctions. Neurosci. Biobehav. Rev. 2022, 142, 104896. [Google Scholar] [CrossRef] [PubMed]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Xu, Y.; Chen, Y.; Zhang, Y.; Ni, X. Corticotropin-releasing hormone stimulates mitotic kinesin-like protein 1 expression via a PLC/PKC-dependent signaling pathway in hippocampal neurons. Mol. Cell Endocrinol. 2012, 362, 157–164. [Google Scholar] [CrossRef]
- Pulte, E.D.; Vallejo, J.; Przepiorka, D.; Nie, L.; Farrell, A.T.; Goldberg, K.B.; McKee, A.E.; Pazdur, R. FDA Supplemental Approval: Blinatumomab for Treatment of Relapsed and Refractory Precursor B-Cell Acute Lymphoblastic Leukemia. Oncologist 2018, 23, 1366–1371. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Zhang, Q.; Ouyang, Q.; Zhang, Y.; Liu, Q.; Sun, T.; Ye, F.; Zhang, B.; Xia, S.; et al. The anti-PD-L1/CTLA-4 bispecific antibody KN046 in combination with nab-paclitaxel in first-line treatment of metastatic triple-negative breast cancer: A multicenter phase II trial. Nat. Commun. 2024, 15, 1015. [Google Scholar] [CrossRef]
- Gao, X.; Xu, N.; Li, Z.; Shen, L.; Ji, K.; Zheng, Z.; Liu, D.; Lou, H.; Bai, L.; Liu, T.; et al. Safety and antitumour activity of cadonilimab, an anti-PD-1/CTLA-4 bispecific antibody, for patients with advanced solid tumours (COMPASSION-03): A multicentre, open-label, phase 1b/2 trial. Lancet Oncol. 2023, 24, 1134–1146. [Google Scholar] [CrossRef]
- Zha, G.; Fang, W.; Leng, J.; Qin, H. A simple, mild and general oxidation of alcohols to aldehydes or ketones by SO2F2/K2CO3 using DMSO as solvent and oxidant. Adv. Synth. Catal. 2019, 361, 2262–2267. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, C.; Gao, L.; Xiang, L.; Qi, J. IGF-1 and Glucocorticoid Receptors Are Potential Target Proteins for the NGF-Mimic Effect of β-Cyclocitral from Lavandula angustifolia Mill. in PC12 Cells. Int. J. Mol. Sci. 2024, 25, 9763. https://doi.org/10.3390/ijms25189763
An C, Gao L, Xiang L, Qi J. IGF-1 and Glucocorticoid Receptors Are Potential Target Proteins for the NGF-Mimic Effect of β-Cyclocitral from Lavandula angustifolia Mill. in PC12 Cells. International Journal of Molecular Sciences. 2024; 25(18):9763. https://doi.org/10.3390/ijms25189763
Chicago/Turabian StyleAn, Chenyue, Lijuan Gao, Lan Xiang, and Jianhua Qi. 2024. "IGF-1 and Glucocorticoid Receptors Are Potential Target Proteins for the NGF-Mimic Effect of β-Cyclocitral from Lavandula angustifolia Mill. in PC12 Cells" International Journal of Molecular Sciences 25, no. 18: 9763. https://doi.org/10.3390/ijms25189763
APA StyleAn, C., Gao, L., Xiang, L., & Qi, J. (2024). IGF-1 and Glucocorticoid Receptors Are Potential Target Proteins for the NGF-Mimic Effect of β-Cyclocitral from Lavandula angustifolia Mill. in PC12 Cells. International Journal of Molecular Sciences, 25(18), 9763. https://doi.org/10.3390/ijms25189763






