The Role of the Mu Opioid Receptors of the Medial Prefrontal Cortex in the Modulation of Analgesia Induced by Acute Restraint Stress in Male Mice
Abstract
1. Introduction
2. Results
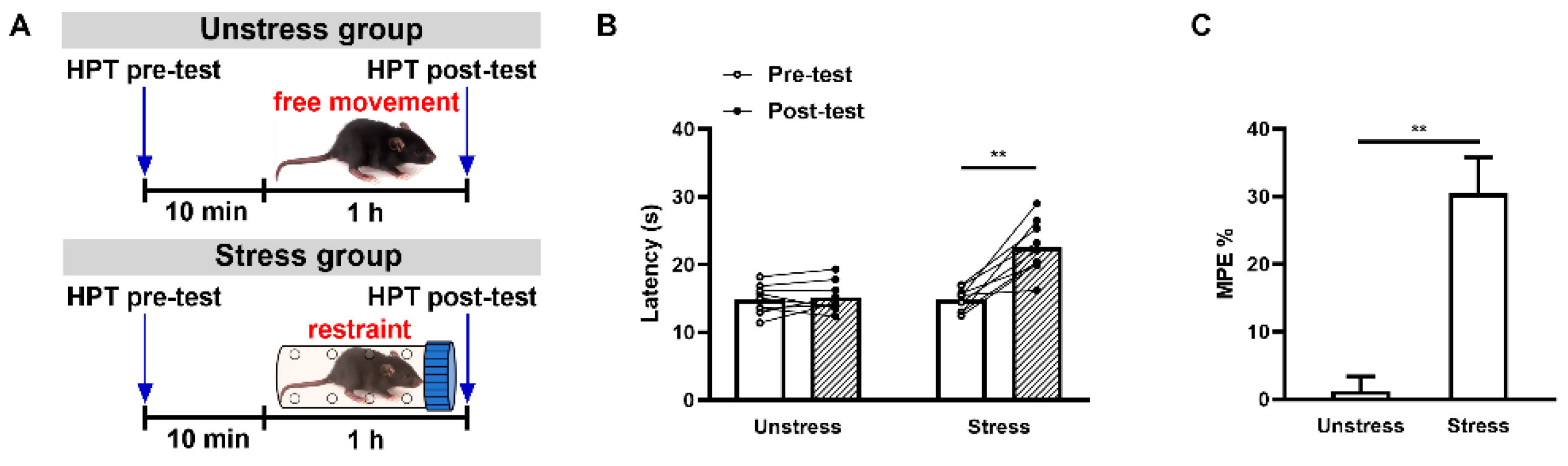
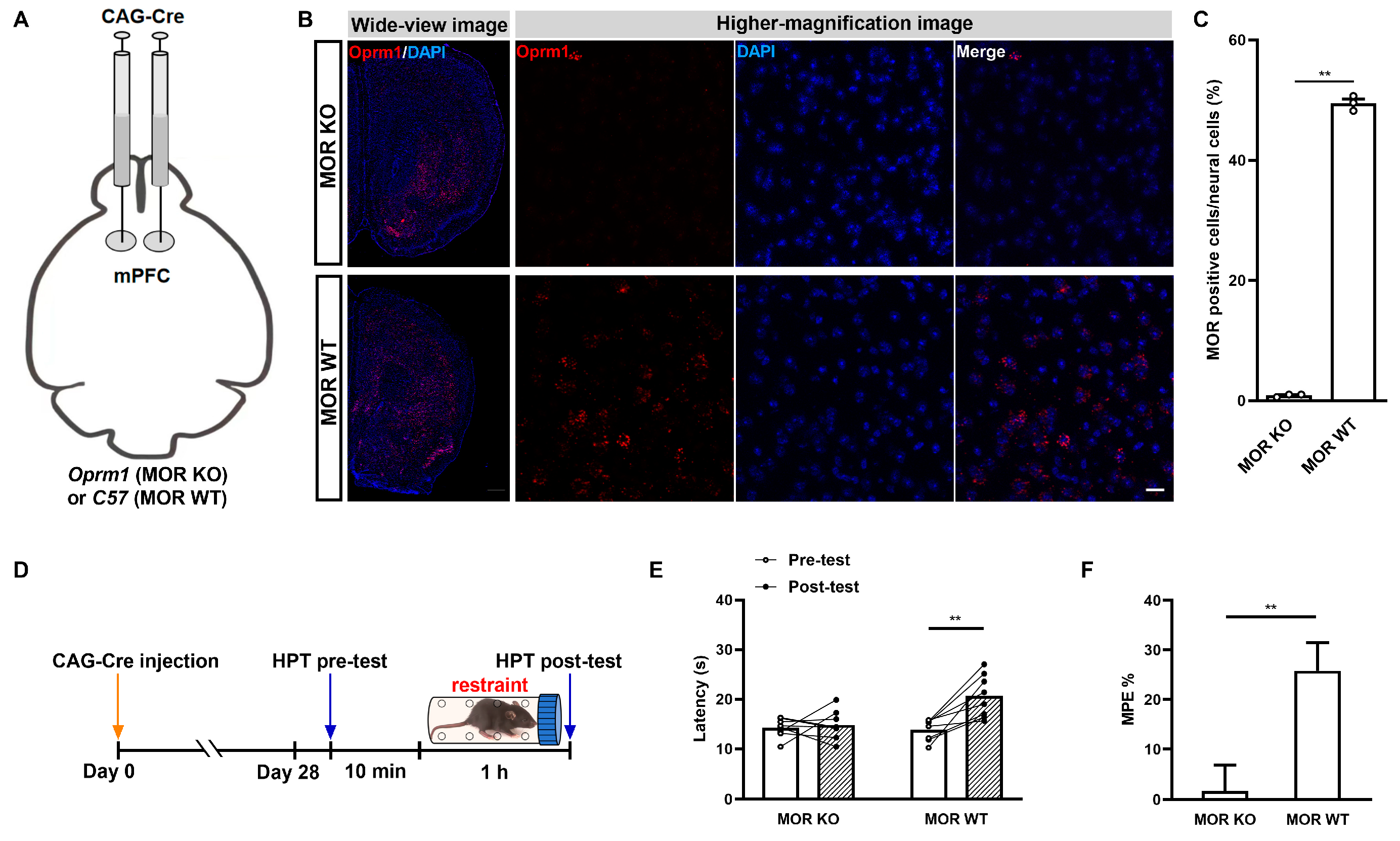
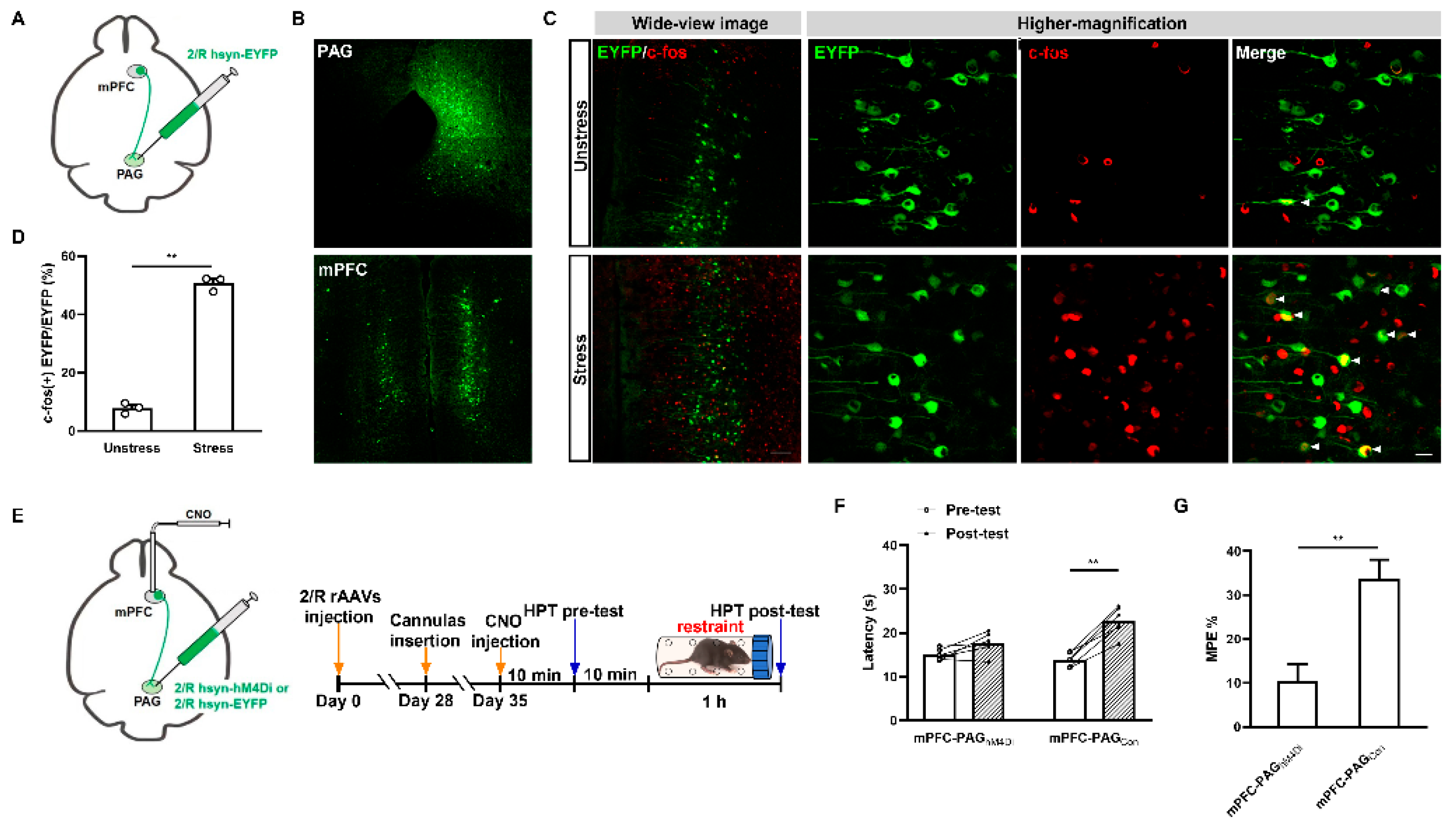
2.1. Acute Restraint Stress Produces mPFC MOR-Dependent SIA
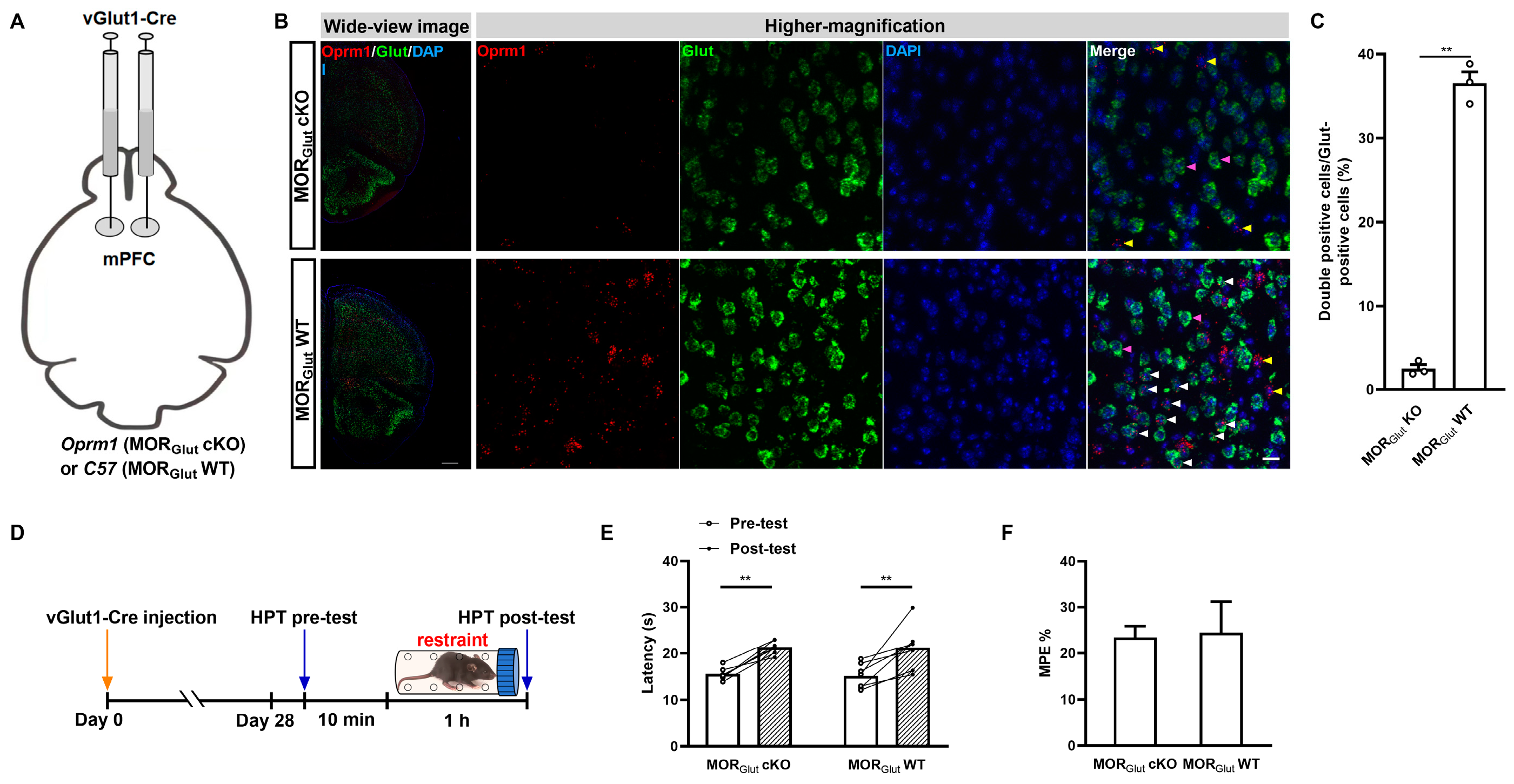
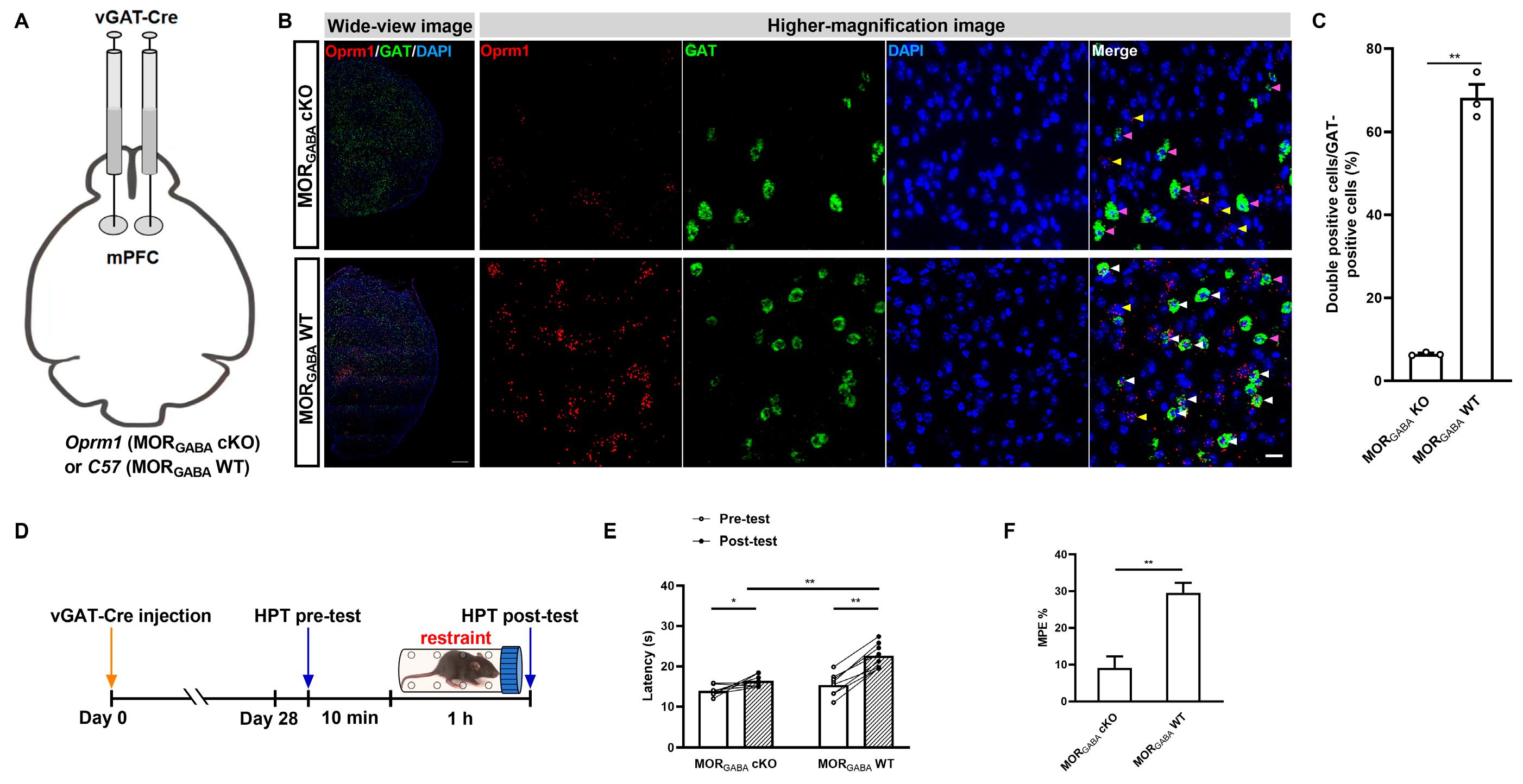
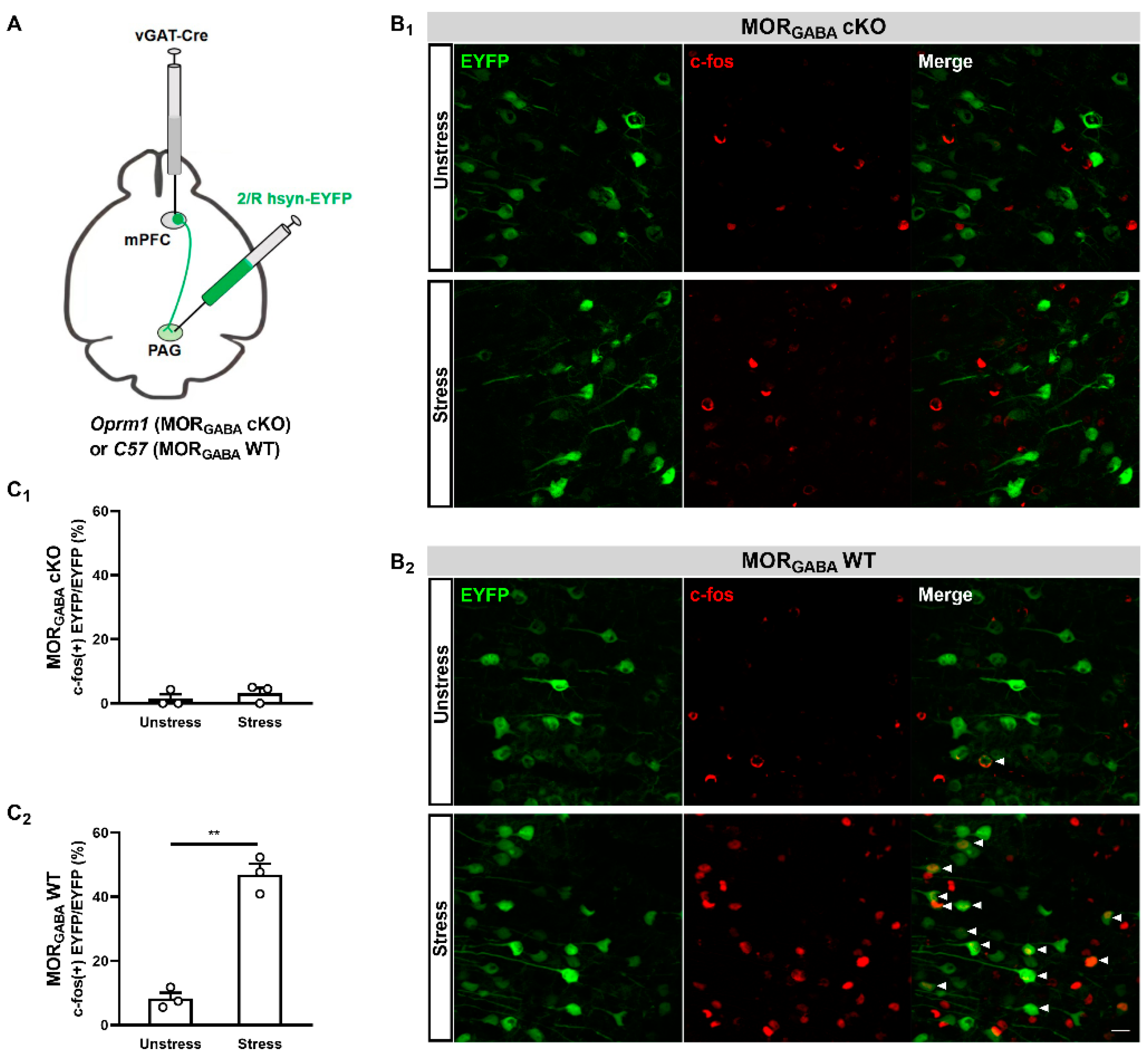
2.2. GABAergic but Not Glutamatergic MORs in the mPFC Modulate MOR-Dependent SIA
2.3. GABAergic MORs in the mPFC Modulate the Activity of mPFC–PAG Projections under the MOR-Dependent SIA Paradigm
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Restraint Stress
4.3. Pain Behavioral Test
4.4. Surgery and Adeno-Associated Virus Injection
4.5. Fluorescence In Situ Hybridization Using an RNAscope
4.6. C-Fos Immunofluorescence
4.7. Intra-mPFC Injection
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, C.P.; Chiang, Y.C.; Tam, W.H.; Chen, Y.J.; Chou, S.H.; Pan, C.L. Neurophysiological basis of stress-induced aversive memory in the nematode Caenorhabditis elegans. Curr. Biol. 2022, 32, 5309–5322.e6. [Google Scholar] [CrossRef]
- Seo, J.S.; Wei, J.; Qin, L.; Kim, Y.; Yan, Z.; Greengard, P. Cellular and molecular basis for stress-induced depression. Mol. Psychiatry 2017, 22, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, L.; Sala, N.; Tornese, P.; Gallivanone, F.; Belloli, S.; Conte, A.; Di Grigoli, G.; Chen, F.; Ikinci, A.; Treccani, G.; et al. Acute Inescapable Stress Rapidly Increases Synaptic Energy Metabolism in Prefrontal Cortex and Alters Working Memory Performance. Cereb. Cortex 2019, 29, 4948–4957. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.K.; Finn, D.P. Stress-induced analgesia. Prog. Neurobiol. 2009, 88, 184–202. [Google Scholar] [CrossRef]
- Ferdousi, M.; Finn, D.P. Stress-induced modulation of pain: Role of the endogenous opioid system. Prog. Brain Res. 2018, 239, 121–177. [Google Scholar]
- Menendez, L.; Andres-Trelles, F.; Hidalgo, A.; Baamonde, A. Opioid footshock-induced analgesia in mice acutely falls by stress prolongation. Physiol. Behav. 1993, 53, 1115–1119. [Google Scholar] [CrossRef]
- Mogil, J.S.; Sternberg, W.F.; Balian, H.; Liebeskind, J.C.; Sadowski, B. Opioid and nonopioid swim stress-induced analgesia: A parametric analysis in mice. Physiol. Behav. 1996, 59, 123–132. [Google Scholar] [CrossRef]
- Miczek, K.A.; Thompson, M.L.; Shuster, L. Naloxone injections into the periaqueductal grey area and arcuate nucleus block analgesia in defeated mice. Psychopharmacology 1985, 87, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Contet, C.; Gavériaux-Ruff, C.; Matifas, A.; Caradec, C.; Champy, M.F.; Kieffer, B.L. Dissociation of analgesic and hormonal responses to forced swim stress using opioid receptor knockout mice. Neuropsychopharmacology 2006, 31, 1733–1744. [Google Scholar] [CrossRef]
- Sherman, J.E.; Strub, H.; Lewis, J.W. Morphine analgesia: Enhancement by shock-associated cues. Behav. Neurosci. 1984, 98, 293–309. [Google Scholar] [CrossRef]
- Lau, B.K.; Vaughan, C.W. Descending modulation of pain: The GABA disinhibition hypothesis of analgesia. Curr. Opin. Neurobiol. 2014, 29, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Bagley, E.E.; Ingram, S.L. Endogenous opioid peptides in the descending pain modulatory circuit. Neuropharmacology 2020, 173, 108131. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, G.; Pan, Y.X. Mu opioid receptors in pain management. Acta Anaesthesiol. Taiwan. 2011, 49, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dou, Y.; Yuan, L.; Li, Q.; Zhu, Y.; Wang, M.; Sun, Y. Different neuronal populations mediate inflammatory pain analgesia by exogenous and endogenous opioids. Elife 2020, 9, e55289. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Borgland, S.L.; Zamponi, G.W. Dopaminergic modulation of pain signals in the medial prefrontal cortex: Challenges and perspectives. Neurosci. Lett. 2019, 702, 71–76. [Google Scholar] [CrossRef]
- Ong, W.Y.; Stohler, C.S.; Herr, D.R. Role of the Prefrontal Cortex in Pain Processing. Mol. Neurobiol. 2019, 56, 1137–1166. [Google Scholar] [CrossRef]
- Bragin, E.O.; Yeliseeva, Z.V.; Vasilenko, G.F.; Meizerov, E.E.; Chuvin, B.T.; Durinyan, R.A. Cortical projections to the periaqueductal grey in the cat: A retrograde horseradish peroxidase study. Neurosci. Lett. 1984, 51, 271–275. [Google Scholar] [CrossRef]
- Cheriyan, J.; Sheets, P.L. Altered Excitability and Local Connectivity of mPFC-PAG Neurons in a Mouse Model of Neuropathic Pain. J. Neurosci. 2018, 38, 4829–4839. [Google Scholar] [CrossRef]
- Yin, J.B.; Liang, S.H.; Li, F.; Zhao, W.J.; Bai, Y.; Sun, Y.; Wu, Z.Y.; Ding, T.; Sun, Y.; Liu, H.X.; et al. dmPFC-vlPAG projection neurons contribute to pain threshold maintenance and antianxiety behaviors. J. Clin. Investig. 2020, 130, 6555–6570. [Google Scholar] [CrossRef]
- Ji, G.; Neugebauer, V. Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. J. Neurophysiol. 2011, 106, 2642–2652. [Google Scholar] [CrossRef]
- Misra, G.; Wang, W.E.; Archer, D.B.; Roy, A.; Coombes, S.A. Automated classification of pain perception using high-density electroencephalography data. J. Neurophysiol. 2017, 117, 786–795. [Google Scholar] [CrossRef]
- Du, Y.; Yu, K.; Yan, C.; Wei, C.; Zheng, Q.; Qiao, Y.; Liu, Y.; Han, J.; Ren, W.; Liu, Z. The Contributions of Mu-Opioid Receptors on Glutamatergic and GABAergic Neurons to Analgesia Induced by Various Stress Intensities. eNeuro 2022, 9, ENEURO.0487-21.2022. [Google Scholar] [CrossRef]
- Calcagnetti, D.J.; Stafinsky, J.L.; Crisp, T. A single restraint stress exposure potentiates analgesia induced by intrathecally administered DAGO. Brain Res. 1992, 592, 305–309. [Google Scholar] [CrossRef]
- Calcagnetti, D.J.; Fleetwood, S.W.; Holtzman, S.G. Pharmacological profile of the potentiation of opioid analgesia by restraint stress. Pharmacol. Biochem. Behav. 1990, 37, 193–199. [Google Scholar] [CrossRef]
- Hough, L.B.; Nalwalk, J.W.; Yang, W.; Ding, X. Significance of neuronal cytochrome P450 activity in opioid-mediated stress-induced analgesia. Brain Res. 2014, 1578, 30–37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atwal, N.; Winters, B.L.; Vaughan, C.W. Endogenous cannabinoid modulation of restraint stress-induced analgesia in thermal nociception. J. Neurochem. 2020, 152, 92–102. [Google Scholar] [CrossRef]
- Al’Absi, M.; Nakajima, M.; Bruehl, S. Stress and pain: Modality-specific opioid mediation of stress-induced analgesia. J. Neural Transm. 2021, 128, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; Tokuyama, S. Stress-Induced Changes in the Endogenous Opioid System Cause Dysfunction of Pain and Emotion Regulation. Int. J. Mol. Sci. 2023, 24, 11713. [Google Scholar] [CrossRef] [PubMed]
- Lev, R.; Granovsky, Y.; Yarnitsky, D. Enhanced pain expectation in migraine: EEG-based evidence for impaired prefrontal function. Headache 2013, 53, 1054–1070. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Kelly, D.D.; Brutus, M.; Glusman, M. Stress-induced analgesia: Neural and hormonal determinants. Neurosci. Biobehav. Rev. 1980, 4, 87–100. [Google Scholar] [CrossRef]
- Zhang, Z.; Gadotti, V.M.; Chen, L.; Souza, I.A.; Stemkowski, P.L.; Zamponi, G.W. Role of Prelimbic GABAergic Circuits in Sensory and Emotional Aspects of Neuropathic Pain. Cell Rep. 2015, 12, 752–759. [Google Scholar] [CrossRef]
- Paul, B.; Sribhashyam, S.; Majumdar, S. Opioid signaling and design of analgesics. Prog. Mol. Biol. Transl. Sci. 2023, 195, 153–176. [Google Scholar] [PubMed]
- Ochandarena, N.E.; Niehaus, J.K.; Tassou, A.; Scherrer, G. Cell-type specific molecular architecture for mu opioid receptor function in pain and addiction circuits. Neuropharmacology 2023, 238, 109597. [Google Scholar] [CrossRef]
- Stein, C. New concepts in opioid analgesia. Expert. Opin. Investig. Drugs 2018, 27, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Euston, D.R.; Gruber, A.J.; McNaughton, B.L. The role of medial prefrontal cortex in memory and decision making. Neuron 2012, 76, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.T.; Sepulveda-Orengo, M.T. Learning-induced intrinsic and synaptic plasticity in the rodent medial prefrontal cortex. Neurobiol. Learn. Mem. 2020, 169, 107117. [Google Scholar] [CrossRef]
- Xu, P.; Chen, A.; Li, Y.; Xing, X.; Lu, H. Medial prefrontal cortex in neurological diseases. Physiol. Genom. 2019, 51, 432–442. [Google Scholar] [CrossRef]
- Corkrum, M.; Rothwell, P.E.; Thomas, M.J.; Kofuji, P.; Araque, A. Opioid-Mediated Astrocyte-Neuron Signaling in the Nucleus Accumbens. Cells 2019, 8, 586. [Google Scholar] [CrossRef]
- Nam, M.H.; Han, K.S.; Lee, J.; Won, W.; Koh, W.; Bae, J.Y.; Woo, J.; Kim, J.; Kwong, E.; Choi, T.Y.; et al. Activation of Astrocytic μ-Opioid Receptor Causes Conditioned Place Preference. Cell Rep. 2019, 28, 1154–1166.e5. [Google Scholar] [CrossRef]
- Takeda, I.; Yoshihara, K.; Cheung, D.L.; Kobayashi, T.; Agetsuma, M.; Tsuda, M.; Eto, K.; Koizumi, S.; Wake, H.; Moorhouse, A.J.; et al. Controlled activation of cortical astrocytes modulates neuropathic pain-like behaviour. Nat. Commun. 2022, 13, 4100. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Xu, C.L.; Yu, J.; Liu, K.; Lin, S.D.; Hu, T.T.; Qu, F.H.; Guo, F.; Lou, G.D.; Nishibori, M.; et al. HMGB1 in the mPFC governs comorbid anxiety in neuropathic pain. J. Headache Pain. 2022, 23, 102. [Google Scholar] [CrossRef]
- Mayer, D.J.; Liebeskind, J.C. Pain reduction by focal electrical stimulation of the brain: An anatomical and behavioral analysis. Brain Res. 1974, 68, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Helmstetter, F.J.; Tershner, S.A. Lesions of the periaqueductal gray and rostral ventromedial medulla disrupt antinociceptive but not cardiovascular aversive conditional responses. J. Neurosci. 1994, 14 Pt 2, 7099–7108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Averitt, D.L.; Eidson, L.N.; Doyle, H.H.; Murphy, A.Z. Neuronal and glial factors contributing to sex differences in opioid modulation of pain. Neuropsychopharmacology 2019, 44, 155–165. [Google Scholar] [CrossRef]
- Shi, M.M.; Fan, K.M.; Qiao, Y.N.; Xu, J.H.; Qiu, L.J.; Li, X.; Liu, Y.; Qian, Z.Q.; Wei, C.L.; Han, J.; et al. Hippocampal µ-opioid receptors on GABAergic neurons mediate stress-induced impairment of memory retrieval. Mol. Psychiatry 2020, 25, 977–992. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Zhao, Y.; Zhang, A.; Li, Z.; Wei, C.; Zheng, Q.; Qiao, Y.; Liu, Y.; Ren, W.; Han, J.; et al. The Role of the Mu Opioid Receptors of the Medial Prefrontal Cortex in the Modulation of Analgesia Induced by Acute Restraint Stress in Male Mice. Int. J. Mol. Sci. 2024, 25, 9774. https://doi.org/10.3390/ijms25189774
Du Y, Zhao Y, Zhang A, Li Z, Wei C, Zheng Q, Qiao Y, Liu Y, Ren W, Han J, et al. The Role of the Mu Opioid Receptors of the Medial Prefrontal Cortex in the Modulation of Analgesia Induced by Acute Restraint Stress in Male Mice. International Journal of Molecular Sciences. 2024; 25(18):9774. https://doi.org/10.3390/ijms25189774
Chicago/Turabian StyleDu, Yinan, Yukui Zhao, Aozhuo Zhang, Zhiwei Li, Chunling Wei, Qiaohua Zheng, Yanning Qiao, Yihui Liu, Wei Ren, Jing Han, and et al. 2024. "The Role of the Mu Opioid Receptors of the Medial Prefrontal Cortex in the Modulation of Analgesia Induced by Acute Restraint Stress in Male Mice" International Journal of Molecular Sciences 25, no. 18: 9774. https://doi.org/10.3390/ijms25189774
APA StyleDu, Y., Zhao, Y., Zhang, A., Li, Z., Wei, C., Zheng, Q., Qiao, Y., Liu, Y., Ren, W., Han, J., Sun, Z., Hu, W., & Liu, Z. (2024). The Role of the Mu Opioid Receptors of the Medial Prefrontal Cortex in the Modulation of Analgesia Induced by Acute Restraint Stress in Male Mice. International Journal of Molecular Sciences, 25(18), 9774. https://doi.org/10.3390/ijms25189774





