Pathophysiology of Arginases in Cancer and Efforts in Their Pharmacological Inhibition
Abstract
:1. Introduction
2. Arginases as Crucial Players in Physiology and Cancer Pathophysiology
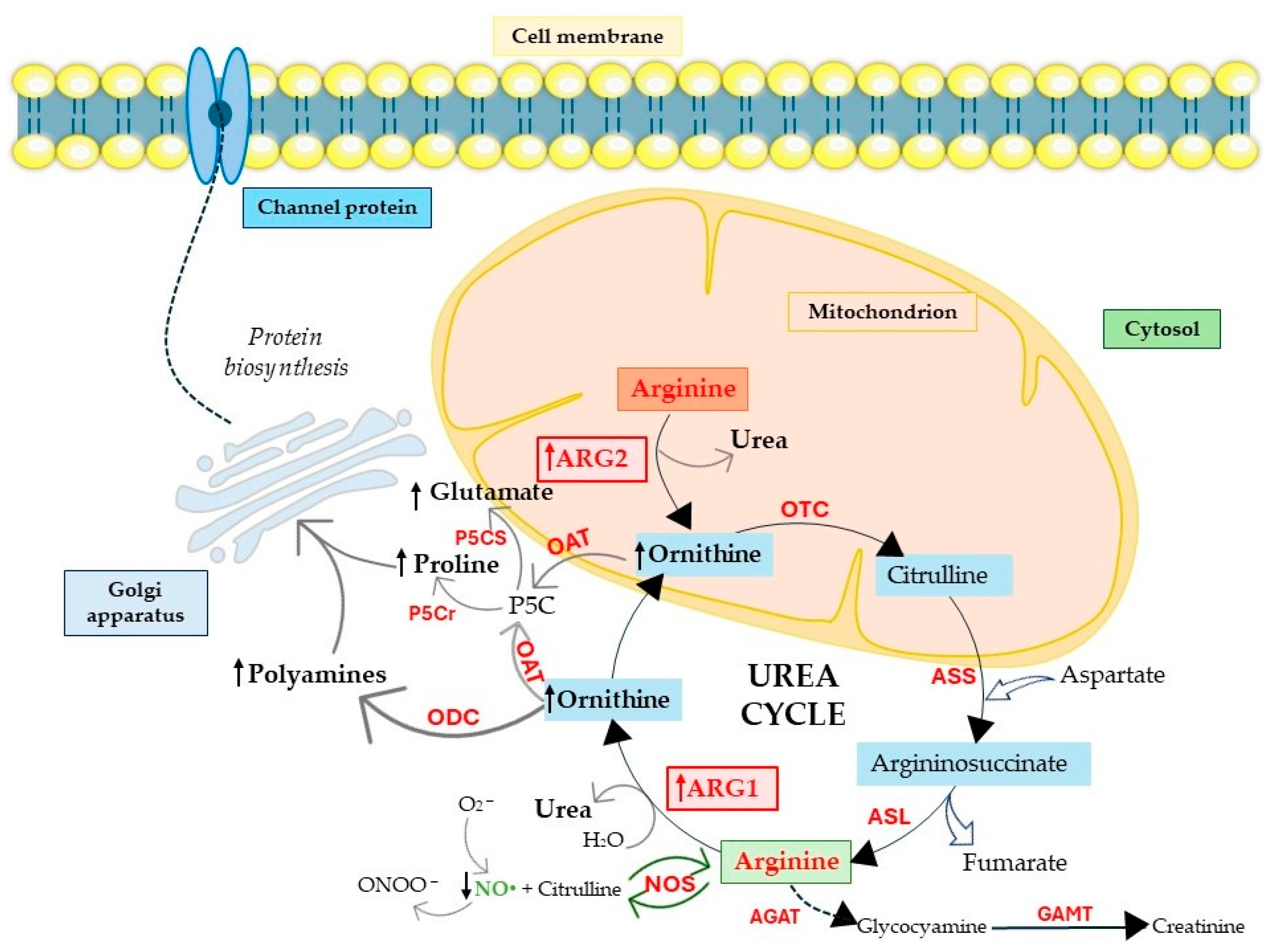
2.1. Arginine Biosynthesis and Polyamine Production
2.2. Arginases and Proline and Glutamate Production
2.3. Arginases Control NO Production
3. Cellular Consequences of Induced Arginase Expression
3.1. Apoptosis
3.2. Cell Senescence
3.3. Autophagy
3.4. Vascularization
3.5. Immune Response and Inflammation
4. Arginases Regulate Metabolic and Mitochondria Machinery
4.1. ARG2 Regulates Oxidative Metabolism
4.2. ARG2’s Specific Role in Mitochondrial Function
5. Significance of Arginases in Non-Cancer Diseases
6. Clinical Perspectives of Arginase Inhibitors
6.1. Arginase Vaccines and Pegylated rhARG in Clinical Trials
6.2. Naturally Occurring Arginase Inhibitors
6.3. Synthetic Arginase Inhibitors
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Niu, F.; Yu, Y.; Li, Z.; Ren, Y.; Li, Z.; Ye, Q.; Liu, P.; Ji, C.; Qian, L.; Xiong, Y. Arginase: An emerging and promising therapeutic target for cancer treatment. Biomed. Pharmacother. 2022, 149, 112840. [Google Scholar] [CrossRef]
- Toque, H.A.; Narayanan, S.P.; Caldwell, R.W. Arginase: An old enzyme with new tricks. Trends Pharmacol. Sci. 2015, 36, 395–405. [Google Scholar] [CrossRef]
- Das, P.; Lahiri, A.; Lahiri, A.; Chakravortty, D. Modulation of the Arginase Pathway in the Context of Microbial Pathogenesis: A Metabolic Enzyme Moonlighting as an Immune Modulator. PLoS Pathog. 2010, 6, e1000899. [Google Scholar] [CrossRef] [PubMed]
- Ash, D.E. Structure and Function of Arginases 1,2. J. Nutr. 2004, 134, 2760S–2764S. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336 Pt 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.P.; Grody, W.W.; Cederbaum, S.D. Comparative properties of arginases. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1996, 114, 107–132. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, S.D.; Yu, H.; Grody, W.W.; Kern, R.M.; Yoo, P.; Iyer, R.K. Arginases I and II: Do their functions overlap? Mol. Genet. Metab. 2004, 81 (Suppl 1), S38–S44. [Google Scholar] [CrossRef]
- Bhatta, A.; Yao, L.; Xu, Z.; Toque, H.A.; Chen, J.; Atawia, R.T.; Fouda, A.Y.; Bagi, Z.; Lucas, R.; Caldwell, R.B.; et al. Obesity-induced vascular dysfunction and arterial stiffening requires endothelial cell arginase 1. Cardiovasc. Res. 2017, 113, 1664–1676. [Google Scholar] [CrossRef]
- Savelieva, O.N.; Karunas, A.S.; Fedorova, Y.Y.; Murzina, R.R.; Savelieva, A.N.; Gatiyatullin, R.F.; Etkina, E.I.; Khusnutdinova, E.K. The role of polymorphic variants of arginase genes (ARG1, ARG2) involved in beta-2-agonist metabolism in the development and course of asthma. Vavilov J. Genet. Breed. 2020, 24, 391–398. [Google Scholar] [CrossRef]
- Marselli, L.; Bosi, E.; De Luca, C.; Del Guerra, S.; Tesi, M.; Suleiman, M.; Marchetti, P. Arginase 2 and Polyamines in Human Pancreatic Beta Cells: Possible Role in the Pathogenesis of Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12099. [Google Scholar] [CrossRef]
- Tian, Y.; Du, W.; Cao, S.; Wu, Y.; Dong, N.; Wang, Y.; Xu, Y. Systematic analyses of glutamine and glutamate metabolisms across different cancer types. Chin. J. Cancer 2017, 36, 88. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yepuri, G.; Forbiteh, M.; Yu, Y.; Montani, J.-P.; Yang, Z.; Ming, X.-F. ARG2 impairs endothelial autophagy through regulation of MTOR and PRKAA/AMPK signaling in advanced atherosclerosis. Autophagy 2014, 10, 2223–2238. [Google Scholar] [CrossRef] [PubMed]
- Yepuri, G.; Velagapudi, S.; Xiong, Y.; Rajapakse, A.G.; Montani, J.; Ming, X.; Yang, Z. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell 2012, 11, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yu, Y.; Montani, J.-P.; Yang, Z.; Ming, X.-F. Arginase-II Induces Vascular Smooth Muscle Cell Senescence and Apoptosis Through p66Shc and p53 Independently of Its l -Arginine Ureahydrolase Activity: Implications for Atherosclerotic Plaque Vulnerability. J. Am. Hear. Assoc. 2013, 2, e000096. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Buck, M.D.; Flamar, A.L.; Saenz, S.A.; Wojno, E.D.T.; Yudanin, N.A.; Osborne, L.C.; Hepworth, M.R.; Tran, S.V.; Rodewald, H.R.; et al. Arginase 1 is an innate lymphoid-cell-intrinsic metabolic checkpoint controlling type 2 inflammation. Nat. Immunol. 2016, 17, 656–665. [Google Scholar] [CrossRef]
- Ming, X.; Rajapakse, A.G.; Yepuri, G.; Xiong, Y.; Carvas, J.M.; Ruffieux, J.; Scerri, I.; Wu, Z.; Popp, K.; Li, J.; et al. Arginase II Promotes Macrophage Inflammatory Responses through Mitochondrial Reactive Oxygen Species, Contributing to Insulin Resistance and Atherogenesis. J. Am. Hear. Assoc. 2012, 1, e000992. [Google Scholar] [CrossRef]
- Grzybowski, M.M.; Stańczak, P.S.; Pomper, P.; Błaszczyk, R.; Borek, B.; Gzik, A.; Nowicka, J.; Jędrzejczak, K.; Brzezińska, J.; Rejczak, T.; et al. OATD-02 Validates the Benefits of Pharmacological Inhibition of Arginase 1 and 2 in Cancer. Cancers 2022, 14, 3967. [Google Scholar] [CrossRef]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2018, 120, 16–25. [Google Scholar] [CrossRef]
- Sullivan, M.R.; Danai, L.V.; Lewis, C.A.; Chan, S.H.; Gui, D.Y.; Kunchok, T.; Dennstedt, E.A.; Vander Heiden, M.G.; Muir, A. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. eLife 2019, 8, e44235. [Google Scholar] [CrossRef]
- Lowe, M.M.; Boothby, I.; Clancy, S.; Ahn, R.S.; Liao, W.; Nguyen, D.N.; Schumann, K.; Marson, A.; Mahuron, K.M.; Kingsbury, G.A.; et al. Regulatory T cells use arginase 2 to enhance their metabolic fitness in tissues. J. Clin. Investig. 2019, 4. [Google Scholar] [CrossRef]
- Líndez, A.-A.M.; Dunand-Sauthier, I.; Conti, M.; Gobet, F.; Núñez, N.; Hannich, J.T.; Riezman, H.; Geiger, R.; Piersigilli, A.; Hahn, K.; et al. Mitochondrial arginase-2 is a cell-autonomous regulator of CD8+ T cell function and antitumor efficacy. J. Clin. Investig. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, D.E.; White, R.; Li, D.; Minhas, K.M.; Cernetich, A.; Kim, S.; Burke, S.; Shoukas, A.A.; Nyhan, D.; Champion, H.C.; et al. Arginase Reciprocally Regulates Nitric Oxide Synthase Activity and Contributes to Endothelial Dysfunction in Aging Blood Vessels. Circulation 2003, 108, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Wiesinger, H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog. Neurobiol. 2001, 64, 365–391. [Google Scholar] [CrossRef]
- Liu, P.; Fleete, M.S.; Jing, Y.; Collie, N.D.; Curtis, M.A.; Waldvogel, H.J.; Faull RAbraham, W.C.; Zhang, H. Altered arginine metabolism in Alzheimer’s disease brains. Neurobiol. Aging 2014, 35, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Grzywa, T.M.; Sosnowska, A.; Matryba, P.; Rydzynska, Z.; Jasinski, M.; Nowis, D.; Golab, J. Myeloid Cell-Derived Arginase in Cancer Immune Response. Front. Immunol. 2020, 11, 938. [Google Scholar] [CrossRef] [PubMed]
- Polis, B.; Samson, A.O. Arginase as a Potential Target in the Treatment of Alzheimer’s Disease. Adv. Alzheimer’s Dis. 2018, 7, 119–140. [Google Scholar] [CrossRef]
- Roci, I.; Watrous, J.D.; Lagerborg, K.A.; Lafranchi, L.; Lindqvist, A.; Jain, M.; Nilsson, R. Mapping Metabolic Events in the Cancer Cell Cycle Reveals Arginine Catabolism in the Committed SG2M Phase. Cell Rep. 2019, 26, 1691–1700.e5. [Google Scholar] [CrossRef]
- Wu, C.W.; Chi, C.W.; Lin, E.C.; Lui, W.Y.; P’eng, F.K.; Wang, S.R. Serum arginase level in patients with gastric cancer. J. Clin. Gastroenterol. 1994, 18, 84–85. [Google Scholar] [CrossRef]
- Leu, S.Y.; Wang, S.R. Clinical significance of arginase in colorectal cancer. Cancer 1992, 70, 733–736. [Google Scholar] [CrossRef]
- Sovová, V.; Sloncová; Fric, P. Differences of alkaline phosphatase and arginase activities in human colorectal carcinoma cell lines. Folia Biol. 1997, 43, 101–104. [Google Scholar]
- Chrzanowska, A.; Graboń, W.; Mielczarek-Puta, M.; Barańczyk-Kuźma, A. Significance of arginase determination in body fluids of patients with hepatocellular carcinoma and liver cirrhosis before and after surgical treatment. Clin. Biochem. 2014, 47, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Clemente, G.S.; van Waarde, A.; Antunes, I.F.; Dömling, A.; Elsinga, P.H. Arginase as a Potential Biomarker of Disease Progression: A Molecular Imaging Perspective. Int. J. Mol. Sci. 2020, 21, 5291. [Google Scholar] [CrossRef] [PubMed]
- Steggerda, S.M.; Bennett, M.K.; Chen, J.; Emberley, E.; Huang, T.; Janes, J.R.; Li, W.; MacKinnon, A.L.; Makkouk, A.; Marguier, G.; et al. Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J. Immunother. Cancer 2017, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Dunddel, D.; Pan, F.; Zeng, Q.; Gorbounov, M.; Albesiano, E.; Fu, J.; Blosser, R.L.; Tam, A.J.; Bruno, T.; Zhang, H.; et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J. Clin. Investig. 2013, 123, 1580–1589. [Google Scholar] [CrossRef]
- Toque, H.A.; Romero, M.J.; Tostes, R.C.; Shatanawi, A.; Chandra, S.; Carneiro, Z.N.; Inscho, E.W.; Webb, R.C.; Caldwell, R.B.; Caldwell, R.W. p38 Mitogen-Activated Protein Kinase (MAPK) Increases Arginase Activity and Contributes to Endothelial Dysfunction in Corpora Cavernosa from Angiotensin-II-Treated Mice. J. Sex. Med. 2010, 7, 3857–3867. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Ren, Y.; Huang, Y.; Liu, W.; Lv, Z.; Qian, L.; Yu, Y.; Xiong, Y. Arginase: Shedding light on the mechanisms and opportunities in cardiovascular diseases. Cell Death Discov. 2022, 8, 1–14. [Google Scholar] [CrossRef]
- Caldwell, R.W.; Rodriguez, P.C.; Toque, H.A.; Narayanan, S.P. Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol. Rev. 2018, 98, 641–665. [Google Scholar] [CrossRef]
- Mielczarek-Puta, M.; Chrzanowska, A.; Graboń, W.; Barańczyk-Kuźma, A. Nowe oblicza arginazy. Część II. Rola w fizjologii i patologii. Postepy Hig. Med. Dosw. 2008, 62, 214–221. [Google Scholar]
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious Modulators of Cellular Functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef]
- Pegg, A.E.; McCann, P.P. Polyamine metabolism and function. Am. J. Physiol. 1982, 243, C212–C221. [Google Scholar] [CrossRef]
- Satriano, J. Arginine pathways and the inflammatory response: Interregulation of nitric oxide and polyamines: Review article. Amino Acids 2004, 26, 321–329. [Google Scholar] [CrossRef]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential factors for growth and survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Dhara, M.; Matta, J.A.; Lei, M.; Knowland, D.; Yu, H.; Gu, S.; Bredt, D.S. Polyamine regulation of ion channel assembly and implications for nicotinic acetylcholine receptor pharmacology. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Delage, B.; Fennell, D.A.; Nicholson, L.; McNeish, I.; Lemoine, N.R.; Crook, T.; Szlosarek, P.W. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int. J. Cancer 2010, 126, 2762–2772. [Google Scholar] [CrossRef]
- Cervelli, M.; Pietropaoli, S.; Signore, F.; Amendola, R.; Mariottini, P. Polyamines metabolism and breast cancer: State of the art and perspectives. Breast Cancer Res. Treat. 2014, 148, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Gerner, E.W.; Bruckheimer, E.; Cohen, A. Cancer pharmacoprevention: Targeting polyamine metabolism to manage risk factors for colon cancer. J. Biol. Chem. 2018, 293, 18770–18778. [Google Scholar] [CrossRef]
- López-Contreras, F.; Muñoz-Uribe, M.; Pérez-Laines, J.; Ascencio-Leal, L.; Rivera-Dictter, A.; Martin-Martin, A.; Burgos, R.A.; Alarcon, P.; López-Muñoz, R. Searching for Drug Synergy Against Cancer Through Polyamine Metabolism Impairment: Insight into the Metabolic Effect of Indomethacin on Lung Cancer Cells. Front. Pharmacol. 2020, 10, 1670. [Google Scholar] [CrossRef]
- Srivastava, S.; Ghosh, S.K. Modulation of L-Arginine-Arginase Metabolic Pathway Enzymes: Immunocytochemistry and mRNA Expression in Peripheral Blood and Tissue Levels in Head and Neck Squamous Cell Carcinomas in North East India. Asian Pac. J. Cancer Prev. 2015, 16, 7031–7038. [Google Scholar] [CrossRef]
- Avtandilyan, N.; Javrushyan, H.; Mamikonyan, A.; Grigoryan, A.; Trchounian, A. The potential therapeutic effect of NG-hydroxy-nor-L-arginine in 7,12-dimethylbenz(a)anthracene-induced breast cancer in rats. Exp. Mol. Pathol. 2019, 111, 104316. [Google Scholar] [CrossRef]
- Caldovic, L.; Tuchman, M. N-Acetylglutamate and its changing role through evolution. Biochem. J. 2003, 372, 279–290. [Google Scholar] [CrossRef]
- Scibior, D.; Czeczot, H. Arginina—Metabolizm i funkcje w organizmie człowieka. Post. Hig. Med. Dośw. 2004, 58, 321–332. [Google Scholar]
- Romero, M.J.; Platt, D.H.; Tawfik, H.E.; Labazi, M.; El-Remessy, A.B.; Bartoli, M.; Caldwell, R.B.; Caldwell, R.W. Diabetes-induced Coronary Vascular Dysfunction Involves Increased Arginase Activity. Circ. Res. 2008, 102, 95–102. [Google Scholar] [CrossRef] [PubMed]
- White, A.R.; Ryoo, S.; Li, D.; Champion, H.C.; Steppan, J.; Wang, D.; Nyhan, D.; Shoukas, A.A.; Hare, J.M.; Berkowitz, D.E. Knockdown of Arginase I Restores NO Signaling in the Vasculature of Old Rats. Hypertension 2006, 47, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef]
- Katusic, Z.S.; d’Uscio, L.; Nath, K.A. Vascular Protection by Tetrahydrobiopterin: Progress and Therapeutic Prospects. Trends Pharmacol. Sci. 2009, 30, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Di Costanzo, L.; Sabio, G.; Mora, A.; Rodriguez, P.C.; Ochoa, A.C.; Centeno, F.; Christianson, D.W. Crystal structure of human arginase I at 1.29-A resolution and exploration of inhibition in the immune response. Proc. Natl. Acad. Sci. USA 2005, 102, 13058–13063. [Google Scholar] [CrossRef]
- Gerber, N.C.; Nishida, C.R.; de Montellano, P.R.O. Characterization of Human Liver Inducible Nitric Oxide Synthase Expressed inEscherichia coli. Arch. Biochem. Biophys. 1997, 343, 249–253. [Google Scholar] [CrossRef]
- Munder, M. Arginase: An emerging key player in the mammalian immune system. Br. J. Pharmacol. 2009, 158, 638–651. [Google Scholar] [CrossRef]
- Knowles, R.G.; Moncada, S. Nitric oxide synthases in mammals. Biochem. J. 1994, 298, 249–258. [Google Scholar] [CrossRef]
- Lechner, M.; Lirk, P.; Rieder, J. Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin. Cancer Biol. 2005, 15, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Siddhanta, U.; Wu, C.; Abu-Soud, H.M.; Zhang, J.; Ghosh, D.K.; Stuehr, D.J. Heme Iron Reduction and Catalysis by a Nitric Oxide Synthase Heterodimer Containing One Reductase and Two Oxygenase Domains. J. Biol. Chem. 1996, 271, 7309–7312. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Kwon, N.S.; Nathan, C.F.; Griffith, O.W.; Feldman, P.L.; Wiseman, J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J. Biol. Chem. 1991, 266, 6259–6263. [Google Scholar] [CrossRef] [PubMed]
- Liew, F.Y. Regulation of nitric oxide synthesis in infectious and autoimmune diseases. Immunol. Lett. 1994, 43, 95–98. [Google Scholar] [CrossRef]
- Morris, S.M.; Billiar, T.R. New insights into the regulation of inducible nitric oxide synthesis. Am. J. Physiol. Metab. 1994, 266, E829–E839. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, H.; Hirata, K.; Yoshikawa, J. Role of endogenous nitric oxide in exercise-induced airway narrowing in patients with bronchial asthma. J. Allergy Clin. Immunol. 2000, 106, 1081–1087. [Google Scholar] [CrossRef]
- Romero-Grimaldi, C.; Gheusi, G.; Lledo, P.-M.; Estrada, C. Chronic inhibition of nitric oxide synthesis enhances both subventricular zone neurogenesis and olfactory learning in adult mice. Eur. J. Neurosci. 2006, 24, 2461–2470. [Google Scholar] [CrossRef]
- Rao, C.V. Nitric oxide signaling in colon cancer chemoprevention. Mutat. Res. 2004, 555, 107–119. [Google Scholar] [CrossRef]
- Heller, A. Apoptosis-inducing high (.)NO concentrations are not sustained either in nascent or in developed cancers. ChemMedChem 2008, 3, 1493–1499. [Google Scholar] [CrossRef]
- Vahora, H.; Khan, M.A.; Alalami, U.; Hussain, A. The Potential Role of Nitric Oxide in Halting Cancer Progression Through Chemoprevention. J. Cancer Prev. 2016, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Press, M.; Eason, A. Polyamines in Relation to Cell Division and Xylogenesis in Cultured Explants ofHelianthus tuberosus: Lack of Evidence for Growth-Regulatory Action. J. Exp. Bot. 1987, 38, 164–172. [Google Scholar] [CrossRef]
- Ferguson, L.P.; Diaz, E.; Reya, T. The Role of the Microenvironment and Immune System in Regulating Stem Cell Fate in Cancer. Trends Cancer 2021, 7, 624–634. [Google Scholar] [CrossRef]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immuno-editing and its three component phases—Elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Chokshi, S. Immune checkpoint receptors: Homeostatic regulators of immunity. Hepatol. Int. 2018, 12, 223–236. [Google Scholar] [CrossRef]
- Munder, M.; Engelhardt, M.; Knies, D.; Medenhoff, S.; Wabnitz, G.; Luckner-Minden, C.; Feldmeyer, N.; Voss, R.-H.; Kropf, P.; Müller, I.; et al. Cytotoxicity of Tumor Antigen Specific Human T Cells Is Unimpaired by Arginine Depletion. PLoS ONE 2013, 8, e63521. [Google Scholar] [CrossRef]
- Tate, D.J., Jr.; Vonderhaar, D.J.; Caldas, Y.A.; Metoyer, T.; Patterson, J.R.; Aviles, D.H.; Zea, A.H. Effect of arginase II on L-arginine depletion and cell growth in murine cell lines of renal cell carcinoma. J. Hematol. Oncol. 2008, 25, 14. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-Derived Suppressor Cells: Linking Inflammation and Cancer. J. Immunol. 2009, 182, 4499–4506. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Chen, B.; Xue, J.; Meng, X.; Slutzky, J.L.; Calvert, A.E.; Chicoine, L.G. Resveratrol prevents hypoxia-induced arginase II expression and proliferation of human pulmonary artery smooth muscle cells via Akt-dependent signaling. Am. J. Physiol. Cell. Mol. Physiol. 2014, 307, L317–L325. [Google Scholar] [CrossRef]
- Bjornsti, M.-A.; Houghton, P.J. The tor pathway: A target for cancer therapy. Nat. Rev. Cancer 2004, 4, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Dange, P.; Kailaje, V.; Vaidya, M.M.; Ramchandani, A.G.; Maru, G.B. Polymeric black tea polyphenols modulate the localization and activity of 12-O-tetradecanoylphorbol-13-acetate-mediated kinases in mouse skin: Mechanisms of their anti-tumor-promoting action. Free. Radic. Biol. Med. 2012, 53, 1358–1370. [Google Scholar] [CrossRef]
- Yu, Y.; Xiong, Y.; Montani, J.-P.; Yang, Z.; Ming, X.-F. Arginase-II activates mTORC1 through myosin-1b in vascular cell senescence and apoptosis. Cell Death Dis. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.S.A.; Latini, F.R.M.; Monteiro, H.P.; Cerutti, J.M. Arginase 2 and nitric oxide synthase: Pathways associated with the pathogenesis of thyroid tumors. Free Radic. Biol. Med. 2010, 49, 997–1007. [Google Scholar] [CrossRef]
- Hackett, C.S.; Quigley, D.A.; Wong, R.A.; Chen, J.; Cheng, C.; Song, Y.K.; Wei, J.S.; Pawlikowska, L.; Goldenberg, D.D.; Nguyen, K.; et al. Expression quantitative trait loci and receptor pharmacology implicate Arg1 and the GABA-A receptor as therapeutic targets in neuroblastoma. Cell Rep. 2014, 9, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.Y.; Zhao, Y.L.; Lv, Y.P.; Teng, Y.S.; Kong, H.; Liu, Y.G.; Wu, X.L.; Hao, C.J.; Chen, W.; Duan, M.B.; et al. CD45+CD33lowCD11bdim myeloid-derived suppressor cells suppress CD8+ T cell activity via the IL-6/IL-8-arginase I axis in human gastric cancer. Cell Death Dis. 2018, 9, 763. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, Y.; Liu, C.; Xiong, Y.; Montani, J.P.; Yang, Z.; Ming, X.F. Role of p38 mito-gen-activated protein kinase in vascular endothelial aging: Interaction with Arginase-II and S6K1 signaling pathway. Aging 2015, 7, 70–81. [Google Scholar] [CrossRef]
- Shahmarvand, N.; Nagy, A.; Shahryari, J.; Ohgami, R.S. Mutations in the signal transducer and activator of transcription family of genes in cancer. Cancer Sci. 2018, 109, 926–933. [Google Scholar] [CrossRef]
- Zhang, Q.; Hossain, D.M.S.; Duttagupta, P.; Moreira, D.; Zhao, X.; Won, H.; Buettner, R.; Nechaev, S.; Majka, M.; Zhang, B.; et al. Serum-resistant CpG-STAT3 decoy for targeting survival and immune checkpoint signaling in acute myeloid leukemia. Blood 2016, 127, 1687–1700. [Google Scholar] [CrossRef]
- Yu, Y.; Ladeiras, D.; Xiong, Y.; Boligan, K.F.; Liang, X.; von Gunten, S.; Hunger, R.E.; Ming, X.; Yang, Z. Arginase-II promotes melanoma migration and adhesion through enhancing hydrogen peroxide production and STAT3 signaling. J. Cell. Physiol. 2020, 235, 9997–10011. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Li, H.; Xu, X. miR-495 reduces neuronal cell apoptosis and relieves acute spinal cord injury through inhibiting PRDM5. Histochem. J. 2021, 52, 385–396. [Google Scholar] [CrossRef]
- Métayer, L.E.; Brown, R.D.; Carlebur, S.; Burke, G.A.A.; Brown, G.C. Mechanisms of cell death induced by arginase and asparaginase in precursor B-cell lymphoblasts. Apoptosis 2018, 24, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lam, S.K.; Cheng, P.N.M.; Ho, J.C.M. Recombinant human arginase induces apoptosis through oxidative stress and cell cycle arrest in small cell lung cancer. Cancer Sci. 2018, 109, 3471–3482. [Google Scholar] [CrossRef] [PubMed]
- Talavera, M.M.; Nuthakki, S.; Cui, H.; Jin, Y.; Liu, Y.; Nelin, L.D. Immunostimulated arginase II expression in intestinal epithelial cells reduces nitric oxide production and apoptosis. Front. Cell Dev. Biol. 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.O.; Lee, Y.K.; Kim, J.M.; Yoon, G. From cell senescence to age-related diseases: Differential mechanisms of action of senescence-associated secretory phenotypes. BMB Rep. 2016, 49, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yu, Y.; Montani, J.P.; Ming, X.F.; Yang, Z. Arginase-I enhances vascular endothelial inflammation and senescence through eNOS-uncoupling. BMC Res. Notes 2017, 10, 82. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, S.H.; Yi, N.; Lee, T.R.; Lee, A.Y. Arginase-2, a miR-1299 target, enhances pigmentation in melasma by reducing melanosome degradation via senescence-induced autophagy inhibition. Pigment Cell Melanoma Res. 2017, 30, 521–530. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the Pathogenesis of Disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, P.; Li, W.; Wang, D.; Ke, C.; Liu, Y.; Ho, J.C.-M.; Cheng, P.N.-M.; Xu, S. Pegylated Recombinant Human Arginase 1 Induces Autophagy and Apoptosis via the ROS-Activated AKT/mTOR Pathway in Bladder Cancer Cells. Oxidative Med. Cell. Longev. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Wang, Z.; Song, P.; Li, Y.; Wang, S.; Fan, J.; Zhang, X.; Luan, J.; Chen, W.; Wang, Y.; Liu, P.; et al. Recombinant human arginase I elicited immunosuppression in activated macrophages through inhibiting autophagy. Appl. Microbiol. Biotechnol. 2019, 103, 4825–4838. [Google Scholar] [CrossRef]
- Wei, L.H.; Wu, G.; Morris, S.M., Jr.; Ignarro, L.J. Elevated arginase I expression in rat aortic smooth muscle cells increases cell proliferation. Proc. Natl. Acad. Sci. USA 2001, 98, 9260–9264. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, L.; Song, M.; Li, X.; He, F.; Wang, C.; Chen, M.; Zhou, J.; Mei, C. The role of the complement factor B—Arginase—Polyamine molecular axis in uremia-induced cardiac remodeling in mice. Eur. J. Immunol. 2019, 50, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meininger, C.J.; Kelly, K.A.; Hawker, J.J.R.; Morris, S.M.; Wu, G. Activities of arginase I and II are limiting for endothelial cell proliferation. Am. J. Physiol. Integr. Comp. Physiol. 2002, 282, R64–R69. [Google Scholar] [CrossRef]
- Pool, C.M.; Jin, Y.; Chen, B.; Liu, Y.; Nelin, L.D. Hypoxic-induction of arginase II requires EGF-mediated EGFR activation in human pulmonary microvascular endothelial cells. Physiol. Rep. 2018, 6, e13693. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, H.; Xu, F.; Yang, H.; Li, P.; Sheng, Y.; Liu, P.; Kong, W.; Liu, X.; Yang, L.; et al. Hepatic Ischemia-Reperfusion Impairs Blood-Brain Barrier Partly Due to Release of Arginase from Injured Liver. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Tasneem, S.; Liu, B.; Li, B.; Choudhary, M.I.; Wang, W. Molecular pharmacology of inflammation: Medicinal plants as anti-inflammatory agents. Pharmacol. Res. 2019, 139, 126–140. [Google Scholar] [CrossRef]
- Hannemann, N.; Cao, S.; Eriksson, D.; Schnelzer, A.; Jordan, J.; Eberhardt, M.; Schleicher, U.; Rech, J.; Ramming, A.; Uebe, S.; et al. Transcription factor Fra-1 targets arginase-1 to enhance macrophage-mediated inflammation in arthritis. J. Clin. Investig. 2019, 129, 2669–2684. [Google Scholar] [CrossRef]
- Sosnowska, A.; Czystowska-Kuzmicz, M.; Golab, J. Extracellular vesicles released by ovarian carcinoma contain arginase 1 that mitigates antitumor immune response. OncoImmunology 2019, 8, e1655370. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, C.; Ming, X.-F.; Yang, Z. Inhibition of p38mapk Reduces Adipose Tissue Inflammation in Aging Mediated by Arginase-II. Pharmacology 2020, 105, 491–504. [Google Scholar] [CrossRef]
- Xiong, Y.; Yepuri, G.; Necetin, S.; Montani, J.-P.; Ming, X.-F.; Yang, Z. Arginase-II Promotes Tumor Necrosis Factor-α Release from Pancreatic Acinar Cells Causing β-Cell Apoptosis in Aging. Diabetes 2017, 66, 1636–1649. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rajapakse, A.G.; Riedo, E.; Fellay, B.; Bernhard, M.-C.; Montani, J.-P.; Yang, Z.; Ming, X.-F. Targeting arginase-II protects mice from high-fat-diet-induced hepatic steatosis through suppression of macrophage inflammation. Sci. Rep. 2016, 6, 20405. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Ochoa, A.C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 2007, 109, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Orr, A.L.; Perevoshchikova, I.V.; Quinlan, C.L. The role of mitochondrial function and cellular bioenergetics in ageing and disease. Br. J. Dermatol. 2013, 169 (Suppl. S2), 1–8. [Google Scholar] [CrossRef]
- Ding, Q.; Qi, Y.; Tsang, S.-Y. Mitochondrial Biogenesis, Mitochondrial Dynamics, and Mitophagy in the Maturation of Cardiomyocytes. Cells 2021, 10, 2463. [Google Scholar] [CrossRef]
- Touyz, R.M. Linking LOX-1 and Arginase II Through Mitochondria. Circ. Res. 2014, 115, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Gakh, O.; Cavadini, P.; Isaya, G. Mitochondrial processing peptidases. Biochim. Biophys. Acta 2002, 1592, 63–77. [Google Scholar] [CrossRef]
- Chandra, S.; Romero, M.J.; Shatanawi, A.; Alkilany, A.M.; Caldwell, R.B.; Caldwell, R.W. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br. J. Pharmacol. 2012, 165, 506–519. [Google Scholar] [CrossRef]
- Matthiesen, S.; Lindemann, D.; Warnken, M.; Juergens, U.R.; Racké, K. Inhibition of NADPH oxidase by apocynin inhibits lipopolysaccharide (LPS) induced up-regulation of arginase in rat alveolar macrophages. Eur. J. Pharmacol. 2008, 579, 403–410. [Google Scholar] [CrossRef]
- Ryoo, S.; Lemmon, C.A.; Soucy, K.G.; Gupta, G.; White, A.R.; Nyhan, D.; Shoukas, A.; Romer, L.H.; Berkowitz, D.E. Oxidized Low-Density Lipoprotein–Dependent Endothelial Arginase II Activation Contributes to Impaired Nitric Oxide Signaling. Circ. Res. 2006, 99, 951–960. [Google Scholar] [CrossRef]
- Pandey, D.; Bhunia, A.; Oh, Y.J.; Chang, F.; Bergman, Y.; Kim, J.H.; Serbo, J.; Boronina, T.N.; Cole, R.N.; Van Eyk, J.; et al. OxLDL Triggers Retrograde Translocation of Arginase2 in Aortic Endothelial Cells via ROCK and Mitochondrial Processing Peptidase. Circ. Res. 2014, 115, 450–459. [Google Scholar] [CrossRef]
- Koo, B.H.; Hwang, H.M.; Yi, B.G.; Lim, H.K.; Jeon, B.H.; Hoe, K.L.; Kwon, Y.G.; Won, M.H.; Kim, Y.M.; Berkowitz, D.E.; et al. Arginase II Contributes to the Ca2+/CaMKII/eNOS Axis by Regulating Ca2+ Concentration Between the Cytosol and Mitochondria in a p32-Dependent Manner. J. Am. Heart Assoc. 2018, 7, e009579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Wasserman, H.D.; Adams, J.A.; Higgins, C.B.; Kelly, S.C.; Lantier, L.; DeBosch, B.J. A Structure-function Analysis of Hepatocyte Arginase 2 Reveals Mitochondrial Ureahydrolysis as a Determinant of Glucose Oxidation. Cell. Mol. Gastroenterol. Hepatol. 2024, 17, 801–820. [Google Scholar] [CrossRef]
- Lim, H.K.; Ryoo, S.; Benjo, A.; Shuleri, K.; Miriel, V.; Baraban, E.; Camara, A.; Soucy, K.; Nyhan, D.; Shoukas, A.; et al. Mitochondrial arginase II constrains endothelial NOS-3 activity. Am. J. Physiol. Circ. Physiol. 2007, 293, H3317–H3324. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef]
- Uchida, Y.; Torisu, K.; Aihara, S.; Imazu, N.; Ooboshi, H.; Kitazono, T.; Nakano, T. Arginase 2 Promotes Cisplatin-Induced Acute Kidney Injury by the Inflammatory Response of Macrophages. Mod. Pathol. 2023, 103, 100227. [Google Scholar] [CrossRef]
- Zaytouni, T.; Tsai, P.-Y.; Hitchcock, D.S.; DuBois, C.D.; Freinkman, E.; Lin, L.; Morales-Oyarvide, V.; Lenehan, P.J.; Wolpin, B.M.; Mino-Kenudson, M.; et al. Critical role for arginase 2 in obesity-associated pancreatic cancer. Nat. Commun. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hardbower, D.M.; Asim, M.; Murray-Stewart, T.; Casero, R.A.; Verriere, T.; Lewis, N.D.; Chaturvedi, R.; Piazuelo, M.B.; Wilson, K.T. Arginase 2 deletion leads to enhanced M1 macrophage activation and upregulated polyamine metabolism in response to Helicobacter pylori infection. Amino Acids 2016, 48, 2375–2388. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.K.; Afzal, R.; Gearing, L.J.; Cervantes-Silva, M.P.; Annett, S.; Davis, G.M.; De Santi, C.; Assmann, N.; Dettmer, K.; Gough, D.J.; et al. Mitochondrial arginase-2 is essential for IL-10 metabolic reprogramming of inflammatory macrophages. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Koshland, D.E. The molecule of the year. Science 1992, 258, 1861. [Google Scholar] [CrossRef]
- Toque, H.A.; Caldwell, R.W. New approaches to the design and discovery of therapies to prevent erectile dysfunction. Expert Opin. Drug Discov. 2014, 9, 1447–1469. [Google Scholar] [CrossRef]
- Bagnost, T.; Ma, L.; da Silva, R.F.; Rezakhaniha, R.; Houdayer, C.; Stergiopulos, N.; André, C.; Guillaume, Y.; Berthelot, A.; Demougeot, C. Cardiovascular effects of arginase inhibition in spontaneously hypertensive rats with fully developed hypertension. Cardiovasc. Res. 2010, 87, 569–577. [Google Scholar] [CrossRef]
- Chen, B.; Calvert, A.E.; Cui, H.; Nelin, L.D. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am. J. Physiol. Cell. Mol. Physiol. 2009, 297, L1151–L1159. [Google Scholar] [CrossRef]
- Watts, J.A.; Gellar, M.A.; Fulkerson, M.-B.K.; Das, S.K.; Kline, J.A. Arginase depletes plasma l-arginine and decreases pulmonary vascular reserve during experimental pulmonary embolism. Pulm. Pharmacol. Ther. 2012, 25, 48–54. [Google Scholar] [CrossRef]
- Potoka, K.P.; Gladwin, M.T. Vasculopathy and pulmonary hypertension in sickle cell disease. Am. J. Physiol. Cell. Mol. Physiol. 2015, 308, L314–L324. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, N.; Morris, C.R. The role of the arginine metabolome in pain: Implications for sickle cell disease. J. Pain Res. 2016, 9, 167–175. [Google Scholar]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Investig. 2017, 127, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Fulton, D.J.; Caldwell, R.W.; Toque, H.A. Hyperglycemia-impaired aortic vasorelaxation mediated through arginase elevation: Role of stress kinase pathways. Eur. J. Pharmacol. 2018, 844, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Mahdi, A.; Tratsiakovich, Y.; Zahorán, S.; Kövamees, O.; Nordin, F.; Uribe Gonzalez, A.E.; Alvarsson, M.; Östenson, C.-G.; Andersson, D.C.; et al. Erythrocytes from Patients with Type 2 Diabetes Induce Endothelial Dysfunction Via Arginase I. J. Am. Coll. Cardiol. 2018, 72, 769–780. [Google Scholar] [CrossRef]
- Yu, H.; Iyer, R.K.; Kern, R.M.; Rodriguez, W.I.; Grody, W.W.; Cederbaum, S.D. Expression of arginase isozymes in mouse brain. J. Neurosci. Res. 2001, 66, 406–422. [Google Scholar] [CrossRef]
- Lee, J.; Ryu, H.; Ferrante, R.J.; Morris, S.M.; Ratan, R.R. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc. Natl. Acad. Sci. USA 2003, 100, 4843–4848. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, N.; King, N.E.; Laporte, J.; Yang, M.; Mishra, A.; Pope, S.M.; Muntel, E.E.; Witte, D.P.; Pegg, A.A.; Foster, P.S.; et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J. Clin. Investig. 2003, 111, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, E.; Aksoy, N.; Gencer, M.; Vural, H.; Keles, H.; Selek, S. Evaluation of oxidative–antioxidative status and the l-arginine–nitric oxide pathway in asthmatic patients. Respir. Med. 2005, 99, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Lewandowicz, A.M.; Pawliczak, R. Znaczenie metabolizmu argininy w astmie oskrzelowej. Postepy Hig. Med. Dosw. 2007, 61, 156–166. [Google Scholar]
- Benson, R.C.; Hardy, K.A.; Morris, C.R. Arginase and Arginine Dysregulation in Asthma. J. Allergy 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Ballantyne, L.L.; Sin, Y.Y.; Al-Dirbashi, O.Y.; Li, X.; Hurlbut, D.J.; Funk, C.D. Liver-specific knockout of arginase-1 leads to a profound phenotype similar to inducible whole body arginase-1 deficiency. Mol. Genet. Metab. Rep. 2016, 9, 54–60. [Google Scholar] [CrossRef]
- Ballantyne, L.L.; Sin, Y.Y.; Amand, T.S.; Si, J.; Goossens, S.; Haenebalcke, L.; Haigh, J.J.; Kyriakopoulou, L.; Schulze, A.; Funk, C.D. Strategies to Rescue the Consequences of Inducible Arginase-1 Deficiency in Mice. PLoS ONE 2015, 10, e0125967. [Google Scholar] [CrossRef]
- Ochoa, J.B.; Bernard, A.C.; Mistry, S.K.; Morris, S.M., Jr.; Figert, P.L.; Maley, M.E.; Tsuei, B.J.; Boulanger, B.R.; Kearney, P.A. Trauma increases extrahepatic arginase activity. Surgery 2000, 127, 419–426. [Google Scholar] [CrossRef]
- Martinenaite, E.; Ahmad, S.M.; Svane, I.M.; Andersen, M.H. Peripheral memory T cells specific for Arginase-1. Cell. Mol. Immunol. 2019, 16, 718–719. [Google Scholar] [CrossRef]
- Martinenaite, E.; Ahmad, S.M.; Bendtsen, S.K.; Jørgensen, M.A.; Weis-Banke, S.E.; Svane, I.M.; Andersen, M.H. Arginase-1-based vaccination against the tumor microenvironment: The identification of an optimal T-cell epitope. Cancer Immunol. Immunother. 2019, 68, 1901–1907. [Google Scholar] [CrossRef]
- Martinenaite, E.; Mortensen, R.E.J.; Hansen, M.; Holmström, M.O.; Ahmad, S.M.; Jørgensen, N.G.D.; Met, O.; Donia, M.; Svane, I.M.; Andersen, M.H. Frequent adaptive immune responses against arginase-1. OncoImmunology 2017, 7, e1404215. [Google Scholar] [CrossRef]
- Andersen, M.H. The targeting of tumor-associated macrophages by vaccination. Cell Stress 2019, 3, 139–140. [Google Scholar] [CrossRef]
- Jørgensen, M.A.; Ugel, S.; Hübbe, M.L.; Carretta, M.; Perez-Penco, M.; Weis-Banke, S.E.; Martinenaite, E.; Kopp, K.; Chapellier, M.; Adamo, A.; et al. Arginase 1–Based Immune Modulatory Vaccines Induce Anticancer Immunity and Synergize with Anti–PD-1 Checkpoint Blockade. Cancer Immunol. Res. 2021, 9, 1316–1326. [Google Scholar] [CrossRef]
- Weis-Banke, S.E.; Hübbe, M.L.; Holmström, M.O.; Jørgensen, M.A.; Bendtsen, S.K.; Martinenaite, E.; Carretta, M.; Svane, I.M.; Ødum, N.; Pedersen, A.W.; et al. The metabolic enzyme arginase-2 is a potential target for novel immune modulatory vaccines. OncoImmunology 2020, 9, 1771142. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Cheng, P.N.M.; Liu, A.M.; Chan, L.L.; Li, L.; Chu, C.M.; Chong, C.C.N.; Lau, Y.M.; Yeo, W.; Ng, K.K.C.; et al. A phase II clinical study on the efficacy and predictive biomarker of pegylated recombinant arginase on hepatocellular carcinoma. Investig. New Drugs 2021, 39, 1375–1382. [Google Scholar] [CrossRef]
- Seyed, M.A.; Jantan, I.; Bukhari, S.N.A.; Vijayaraghavan, K. A Comprehensive Review on the Chemotherapeutic Potential of Piceatannol for Cancer Treatment, with Mechanistic Insights. J. Agric. Food Chem. 2016, 64, 725–737. [Google Scholar] [CrossRef]
- Bordage, S.; Pham, T.-N.; Zedet, A.; Gugglielmetti, A.-S.; Nappey, M.; Demougeot, C.; Girard-Thernier, C. Investigation of Mammal Arginase Inhibitory Properties of Natural Ubiquitous Polyphenols by Using an Optimized Colorimetric Microplate Assay. Planta Medica 2016, 83, 647–653. [Google Scholar] [CrossRef]
- Lucas, J.; Hsieh, T.C.; Halicka, H.D.; Darzynkiewicz, Z.; Wu, J.M. Upregulation of PD-L1 expression by resveratrol and piceatannol in breast and colorectal cancer cells occurs via HDAC3/p300-mediated NF-κB signaling. Int. J. Oncol. 2018, 53, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Sáez, V.; Pastene, E.; Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Gómez, M.V.; Theoduloz, C.; Riquelme, S.; von Baer, D. Oligostilbenoids in Vitis vinifera L. Pinot Noir grape cane extract: Isolation, characterization, in vitro antioxidant capacity and anti-proliferative effect on cancer cells. Food Chem. 2018, 265, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, J.; Ravindrran, M.B. Chitosan/poly (lactic acid)-coated piceatannol nanoparticles exert an in vitro apoptosis activity on liver, lung and breast cancer cell lines. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 274–282. [Google Scholar] [CrossRef]
- Kimura, Y.; Baba, K.; Okuda, H. Inhibitory effects of active substances isolated from Cassia garrettiana heartwood on tumor growth and lung metastasis in Lewis lung carcinoma-bearing mice (Part 2). Anticancer Res. 2000, 20, 2923–2930. [Google Scholar]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.L.; Xue, N.N.; Li, C.; Guo, H.H.; Ren, T.K.; Zhan, Y.; Li, W.B.; Zhang, J.; Chen, X.G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef] [PubMed]
- Samardzic, K.; Rodgers, K.J. Cytotoxicity and mitochondrial dysfunction caused by the dietary supplement l-norvaline. Toxicol. In Vitro 2019, 56, 163–171. [Google Scholar] [CrossRef]
- Qu, N.; Ignatenko, N.A.; Yamauchi, P.; Stringer, D.E.; Levenson, C.; Shannon, P.; Perrin, S.; Gerner, E.W. Inhibition of human ornithine decarboxylase activity by enantiomers of difluoromethylornithine. Biochem. J. 2003, 375, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, L.; Ilies, M.; Thorn, K.J.; Christianson, D.W. Inhibition of human arginase I by substrate and product analogues. Arch. Biochem. Biophys. 2010, 496, 101–108. [Google Scholar] [CrossRef]
- Colleluori, D.M.; Ash, D.E. Classical and slow-binding inhibitors of human type II arginase. Biochemistry 2001, 40, 9356–9362. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, A.J.; Ye, S.-K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019, 52, 415–423. [Google Scholar] [CrossRef]
- Buga, G.M.; Wei, L.H.; Bauer, P.M.; Fukuto, J.M.; Ignarro, L.J. NG-hydroxy-l-arginine and nitric oxide inhibit Caco-2 tumor cell proliferation by distinct mechanisms. Am. J. Physiol. Integr. Comp. Physiol. 1998, 275, R1256–R1264. [Google Scholar] [CrossRef]
- Li, X.; Zhu, F.; He, Y.; Luo, F. Arginase inhibitor nor-NOHA induces apoptosis and inhibits invasion and migration of HepG2 cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2017, 33, 477–482. [Google Scholar] [PubMed]
- Azambuja, J.H.; Ludwig, N.; Yerneni, S.S.; Braganhol, E.; Whiteside, T.L. Arginase-1+ Exosomes from Reprogrammed Macrophages Promote Glioblastoma Progression. Int. J. Mol. Sci. 2020, 21, 3990. [Google Scholar] [CrossRef] [PubMed]
- Kövamees, O.; Shemyakin, A.; Checa, A.; Wheelock, C.E.; Lundberg, J.O.; Östenson, C.-G.; Pernow, J. Arginase Inhibition Improves Microvascular Endothelial Function in Patients with Type 2 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2016, 101, 3952–3958. [Google Scholar] [CrossRef] [PubMed]
- Kövamees, O.; Shemyakin, A.; Pernow, J. Effect of Arginase Inhibition on Ischemia-Reperfusion Injury in Patients with Coronary Artery Disease with and without Diabetes Mellitus. PLoS ONE 2014, 9, e103260. [Google Scholar] [CrossRef]
- Olivon, V.C.; Fraga-Silva, R.A.; Segers, D.; Demougeot, C.; de Oliveira, A.M.; Savergnini, S.S.; Berthelot, A.; de Crom, R.; Krams, R.; Stergiopulos, N.; et al. Arginase inhibition prevents the low shear stress-induced development of vulnerable atherosclerotic plaques in ApoE-/- mice. Atherosclerosis 2013, 227, 236–243. [Google Scholar] [CrossRef]
- Van Zandt, M.C.; Jagdmann, G.E.; Whitehouse, D.L.; Ji, M.; Savoy, J.; Potapova, O.; Cousido-Siah, A.; Mitschler, A.; Howard, E.I.; Pyle, A.M.; et al. Discovery of N-Substituted 3-Amino-4-(3-boronopropyl)pyrrolidine-3-carboxylic Acids as Highly Potent Third-Generation Inhibitors of Human Arginase I and II. J. Med. Chem. 2019, 62, 8164–8177. [Google Scholar] [CrossRef]
- Cama, E.; Colleluori, D.M.; Emig, F.A.; Shin, H.; Kim, S.W.; Kim, N.N.; Traish, A.M.; Ash, D.E.; Christianson, D.W. Human arginase II: Crystal structure and physiological role in male and female sexual arousal. Biochemistry 2003, 42, 8445–8451. [Google Scholar] [CrossRef]
- Cox, J.D.; Kim, N.N.; Traish, A.M.; Christianson, D.W. Arginase-boronic acid complex highlights a physiological role in erectile function. Nat. Struct. Biol. 1999, 6, 1043–1047. [Google Scholar]
- Pudlo, M.; Demougeot, C.; Girard-Thernier, C. Arginase Inhibitors: A Rational Approach Over One Century. Med. Res. Rev. 2016, 37, 475–513. [Google Scholar] [CrossRef]
- Golebiowski, A.; Beckett, R.P.; Van Zandt, M.; Ji, M.K.; Whitehouse, D.; Ryder, T.R.; Jagdmann, E.; Andreoli, M.; Mazur, A.; Padmanilayam, M.; et al. 2-Substituted-2-amino-6-boronohexanoic acids as arginase inhibitors. Bioorganic Med. Chem. Lett. 2013, 23, 2027–2030. [Google Scholar] [CrossRef]
- Abdelkawy, K.S.; Lack, K.; Elbarbry, F. Pharmacokinetics and Pharmacodynamics of Promising Arginase Inhibitors. Eur. J. Drug Metab. Pharmacokinet. 2016, 42, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Ivanenkov, Y.A.; Chufarova, N.V. Small-molecule arginase inhibitors. Pat. Anal. 2014, 3, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.M.G.; García-Clemente, M.; Diab-Cáceres, L.; Martínez-Vergara, A.; Martínez-García, M.; Gómez-Punter, R.M. Treatment of Pulmonary Disease of Cystic Fibrosis: A Comprehensive Review. Antibiotics 2021, 10, 486. [Google Scholar] [CrossRef]
- Blaszczyk, R.; Brzezinska, J.; Dymek, B.; Stanczak, P.S.; Mazurkiewicz, M.; Olczak, J.; Nowicka, J.; Dzwonek, K.; Zagozdzon, A.; Golab, J.; et al. Discovery and Pharmacokinetics of Sulfamides and Guanidines as Potent Human Arginase 1 Inhibitors. ACS Med. Chem. Lett. 2020, 11, 433–438. [Google Scholar] [CrossRef]
- Works, M.; Bennett, M.; Chen, J.; Emberley, E.; Huang, T.; Janes, J.; Li, W.; Mackinnon, A.; Marguier, G.; Neou, S.; et al. Abstract 552: Immuno-oncology agent CB-1158 is a potent and selective arginase inhibitor and causes an immune-mediated anti-tumor response. Cancer Res. 2016, 76, 552. [Google Scholar] [CrossRef]
- Javle, M.M.; Bridgewater, J.A.; Gbolahan, O.B.; Jungels, C.; Cho, M.T.; Papadopoulos, K.P.; Thistlethwaite, F.C.; Canon, J.-L.R.; Cheng, L.; Ioannidis, S.; et al. A phase I/II study of safety and efficacy of the arginase inhibitor INCB001158 plus chemotherapy in patients (Pts) with advanced biliary tract cancers. J. Clin. Oncol. 2021, 39, 311. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I Production in the Tumor Microenvironment by Mature Myeloid Cells Inhibits T-Cell Receptor Expression and Antigen-Specific T-Cell Responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Tsai, F.Y.-C.; Bauer, T.M.; Muigai, L.; Liang, Y.; Bennett, M.K.; Orford, K.W.; Fu, S. CX-1158-101: A first-in-human phase 1 study of CB-1158, a small molecule inhibitor of arginase, as monotherapy and in combination with an anti-PD-1 checkpoint inhibitor in patients (pts) with solid tumors. J. Clin. Oncol. 2017, 35, 3005. [Google Scholar] [CrossRef]
- Borek, B.; Gajda, T.; Golebiowski, A.; Blaszczyk, R. Boronic acid-based arginase inhibitors in cancer immunotherapy. Bioorganic Med. Chem. 2020, 28, 115658. [Google Scholar] [CrossRef]
- Sosnowska, A.; Chlebowska-Tuz, J.; Matryba, P.; Pilch, Z.; Greig, A.; Wolny, A.; Grzywa, T.M.; Rydzynska, Z.; Sokolowska, O.; Rygiel, T.P.; et al. Inhibition of arginase modulates T-cell response in the tumor microenvironment of lung carcinoma. OncoImmunology 2021, 10, 1956143. [Google Scholar] [CrossRef]
- Pilanc, P.; Wojnicki, K.; Roura, A.J.; Cyranowski, S.; Ellert-Miklaszewska, A.; Ochocka, N.; Gielniewski, B.; Grzybowski, M.M.; Błaszczyk, R.; Stańczak, P.S.; et al. A Novel Oral Arginase 1/2 Inhibitor Enhances the Antitumor Effect of PD-1 Inhibition in Murine Experimental Gliomas by Altering the Immunosuppressive Environment. Front. Oncol. 2021, 11, 703465. [Google Scholar] [CrossRef] [PubMed]
- Czystowska-Kuzmicz, M.; Sosnowska, A.; Nowis, D.; Ramji, K.; Szajnik, M.; Chlebowska-Tuz, J.; Wolinska, E.; Gaj, P.; Grazul, M.; Pilch, Z.; et al. Small extracellular vesicles containing arginase-1 suppress T-cell responses and promote tumor growth in ovarian carcinoma. Nat. Commun. 2019, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Mussai, F.; De Santo, C.; Abu-Dayyeh, I.; Booth, S.; Quek, L.; McEwen-Smith, R.M.; Qureshi, A.; Dazzi, F.; Vyas, P.; Cerundolo, V. Acute myeloid leukemia creates an arginase-dependent immunosuppressive microenvironment. Blood J. Am. Soc. Hematol. 2013, 122, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.P.; Manjeri, A.; Lee, L.M.; Chan, Z.E.; Tan, C.Y.; Tan, Q.D.; Majeed, A.; Lee, K.L.; Chuah, C.; Suda, T.; et al. The arginase inhibitor Nω-hydroxy-nor-arginine (nor-NOHA) induces apoptosis in leukemic cells specifically under hypoxic conditions but CRISPR/Cas9 excludes arginase 2 (ARG2) as the functional target. PLoS ONE. 2018, 13, e0205254. [Google Scholar] [CrossRef] [PubMed]
- Ino, Y.; Yamazaki-Itoh, R.; Oguro, S.; Shimada, K.; Kosuge, T.; Zavada, J.; Kanai, Y.; Hiraoka, N. Arginase II Expressed in Cancer-Associated Fibroblasts Indicates Tissue Hypoxia and Predicts Poor Outcome in Patients with Pancreatic Cancer. PLoS ONE 2013, 8, e55146. [Google Scholar] [CrossRef] [PubMed]


| Arginase Isoform | Substrate | Mediator | Process |
|---|---|---|---|
| ARG1 | L-arginine | NO | Apoptosis |
| ROS | Cell senescence, apoptosis, inflammation | ||
| IL-6 IL-8 | Suppression of T cells | ||
| ARG2 | NO | Apoptosis | |
| ROS | Cell senescence, apoptosis, inflammation | ||
| IL-6 IL-8 TNF-α | Inflammation |
| Compound | Clinical Trial ID | Phase of Clinical Trial | Condition or Disease |
|---|---|---|---|
| N(ω)-hydroxy-nor-L-arginine (nor-NOHA) 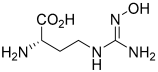 | NCT02687152 | Phase I (completed) | Type 2 Diabetes Mellitus |
| N(ω)-hydroxy-nor-L-arginine (nor-NOHA) 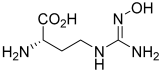 | NCT02009527 | Phase I (completed) | Ischemia–Reperfusion Injury |
Numidargistat (CB-1158)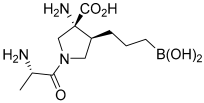 | INCB001158 | Phase II (completed) | Metastatic Cancer; Colorectal Cancer; Lung Cancer; Solid Tumors; Gastric Cancer; Head and Neck Cancer; Bladder Cancer; Mesothelioma; Renal Cell Carcinoma; Urothelial Cancer |
| CB-280 (chemical structure of the compound undisclosed) | NCT04279769 | Phase I (completed) | Cystic Fibrosis |
OATD-02 | NCT05759923 | Phase I (active) | Advanced/Metastatic Ovarian Carcinoma; Advanced/Metastatic Colorectal Carcinoma; Advanced/Metastatic Renal Cell Carcinoma; Advanced /Metastatic Pancreatic Carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzęta-Assas, P.; Jacenik, D.; Zasłona, Z. Pathophysiology of Arginases in Cancer and Efforts in Their Pharmacological Inhibition. Int. J. Mol. Sci. 2024, 25, 9782. https://doi.org/10.3390/ijms25189782
Marzęta-Assas P, Jacenik D, Zasłona Z. Pathophysiology of Arginases in Cancer and Efforts in Their Pharmacological Inhibition. International Journal of Molecular Sciences. 2024; 25(18):9782. https://doi.org/10.3390/ijms25189782
Chicago/Turabian StyleMarzęta-Assas, Patrycja, Damian Jacenik, and Zbigniew Zasłona. 2024. "Pathophysiology of Arginases in Cancer and Efforts in Their Pharmacological Inhibition" International Journal of Molecular Sciences 25, no. 18: 9782. https://doi.org/10.3390/ijms25189782







