Calcium Role in Gap Junction Channel Gating: Direct Electrostatic or Calmodulin-Mediated?
Abstract
1. Introduction
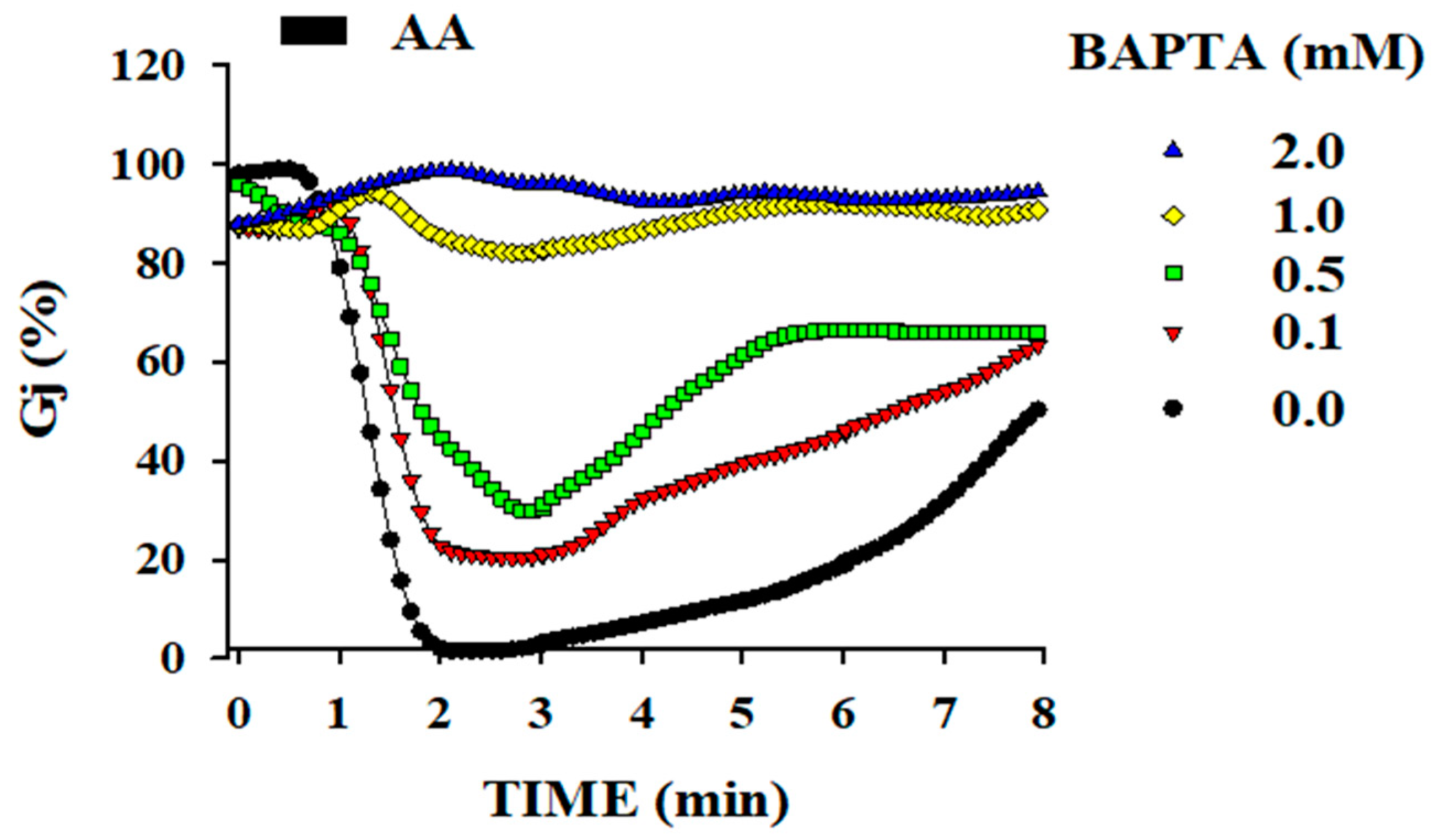
2. Cytosolic Calcium (Ca2+i) and Gap Junction Channel Regulation
[Ca2+]i Affecting Gating of Gap Junction Channel
3. Does Calcium Act Directly on Gap Junction Channel Gating?
4. Calmodulin Role in Channel Gating
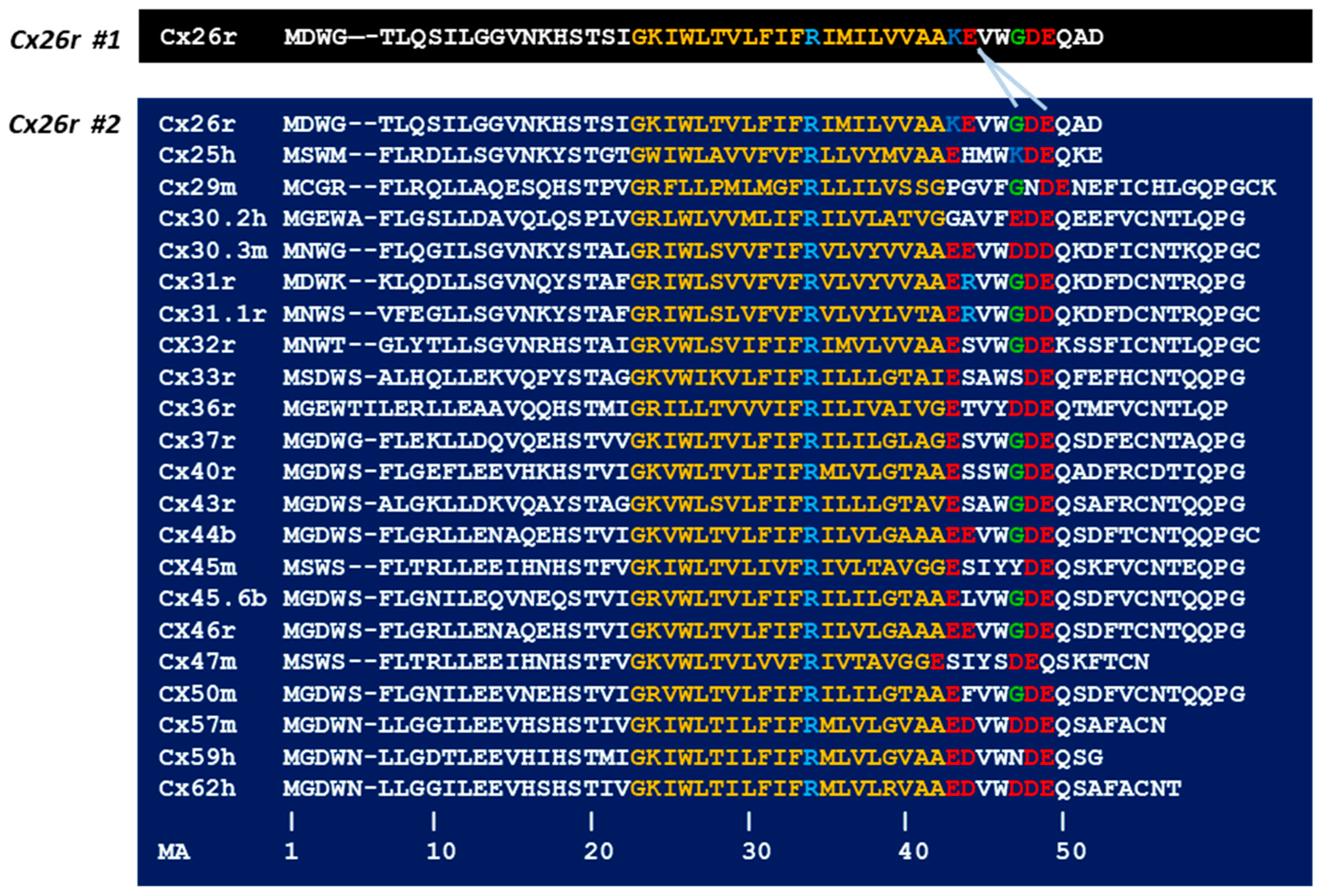
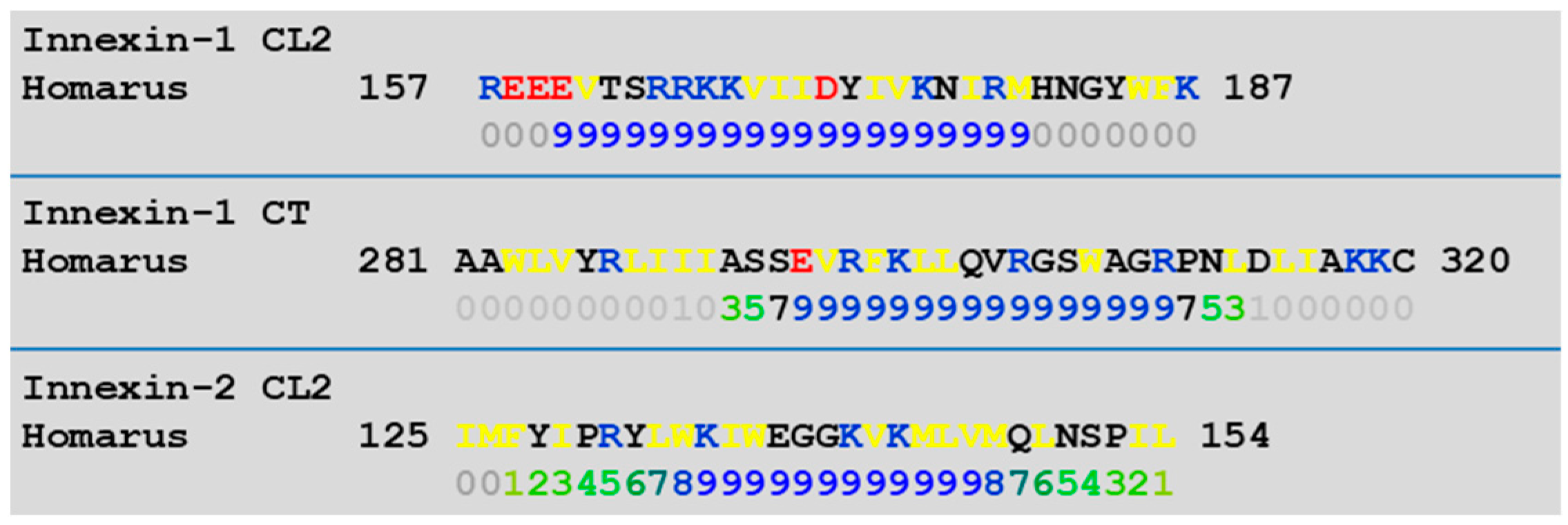
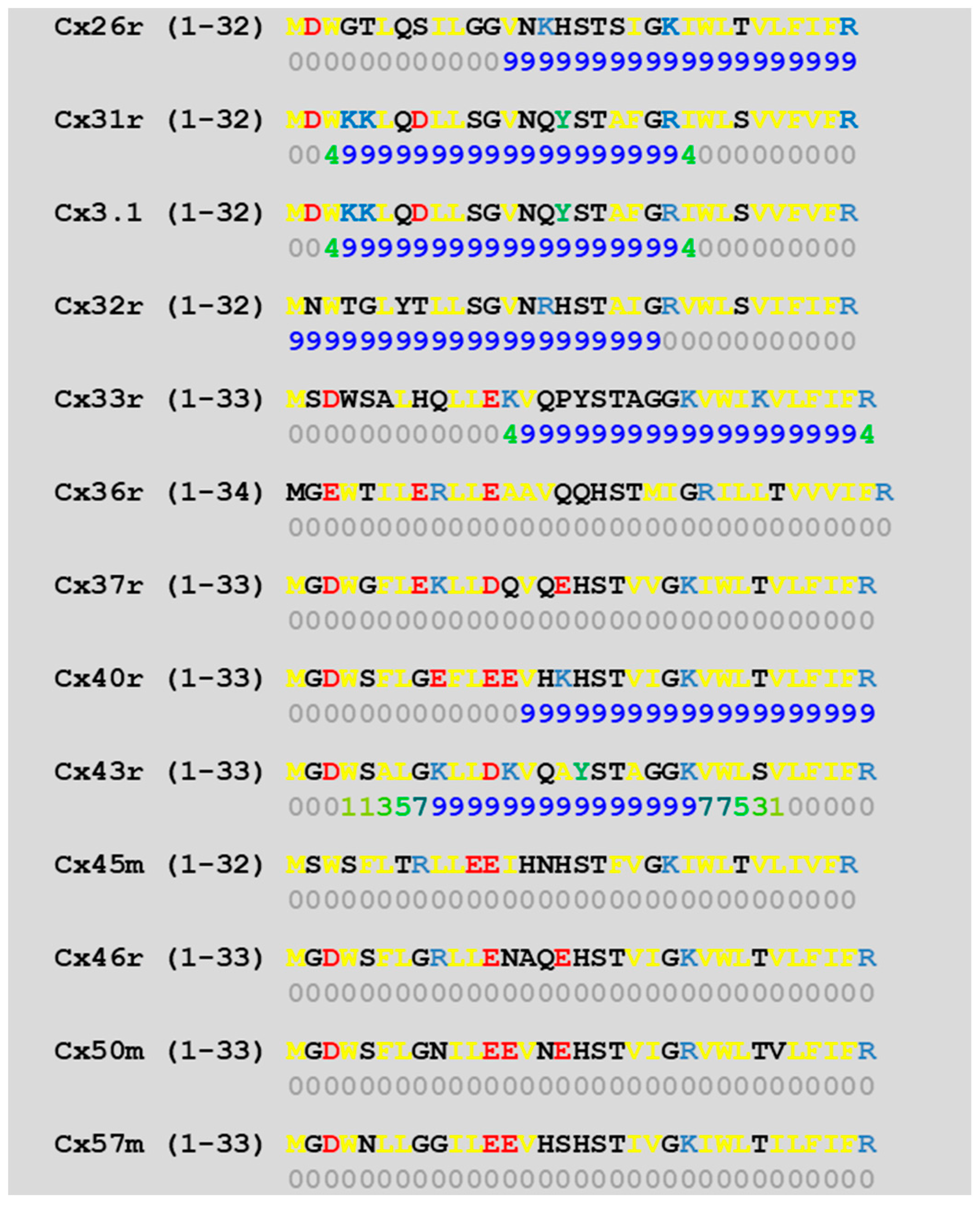
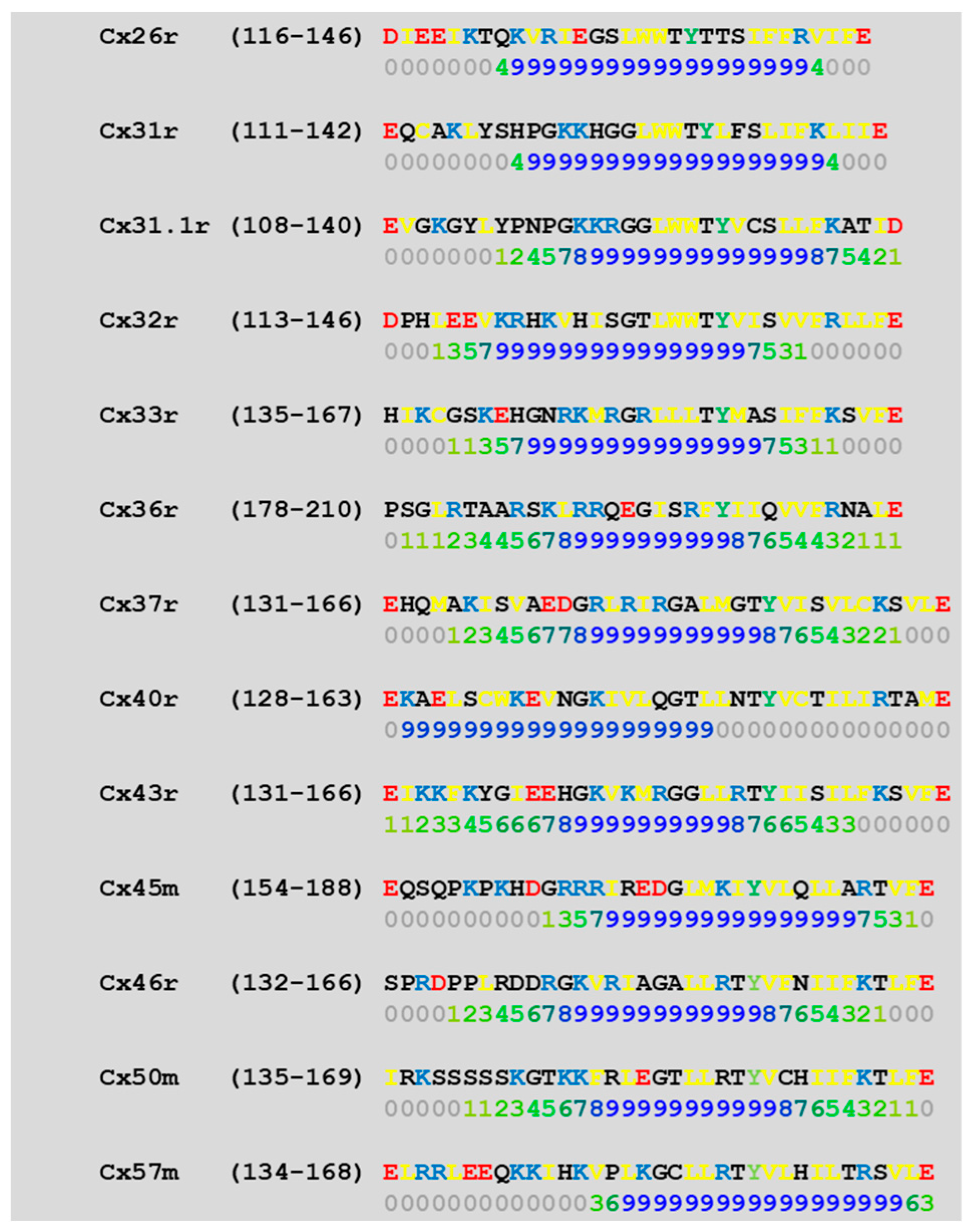
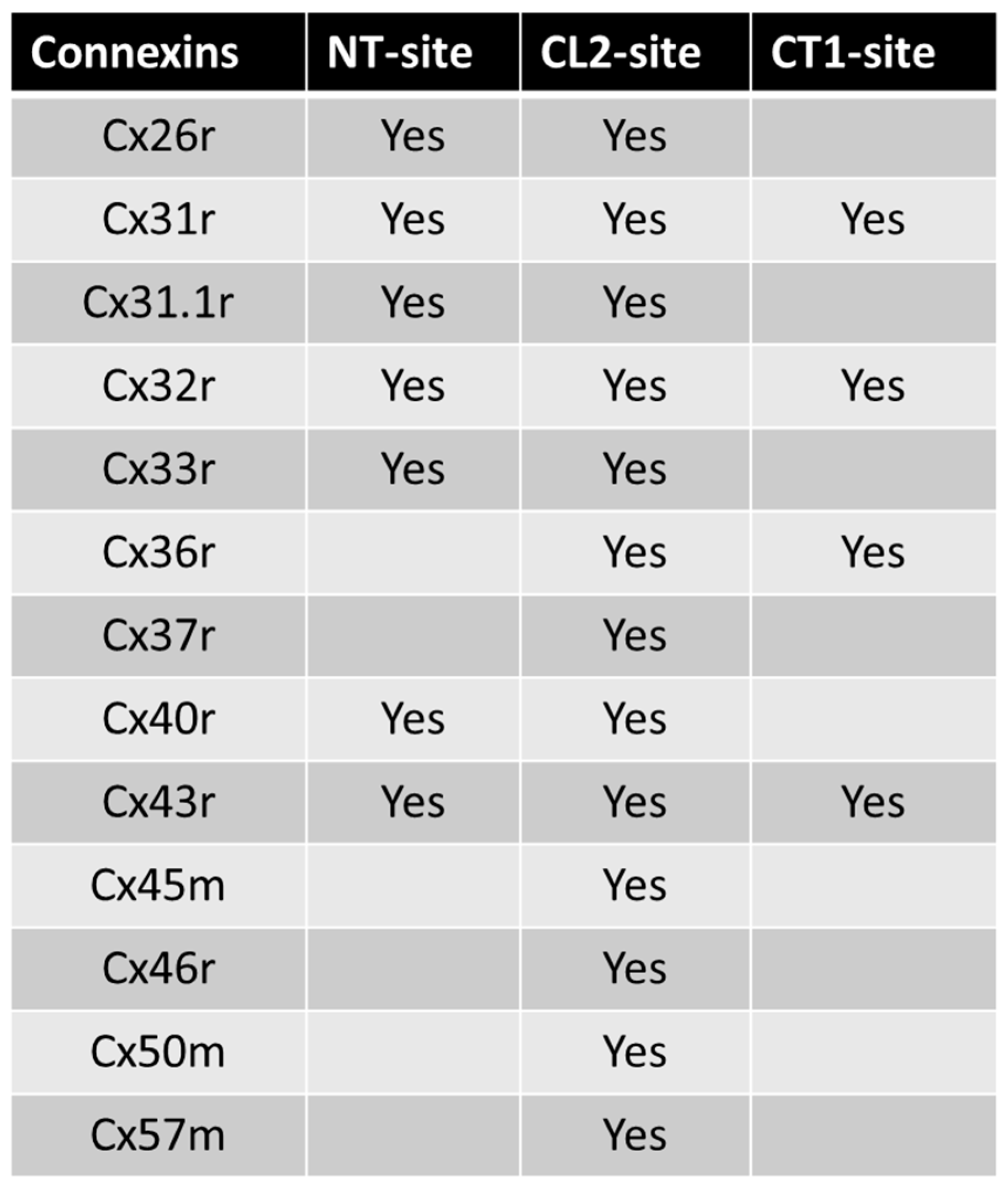
4.1. Calmodulin Binding Domains in Connexins
4.2. CaM Is Linked to Connexins at Resting [Ca2+]i
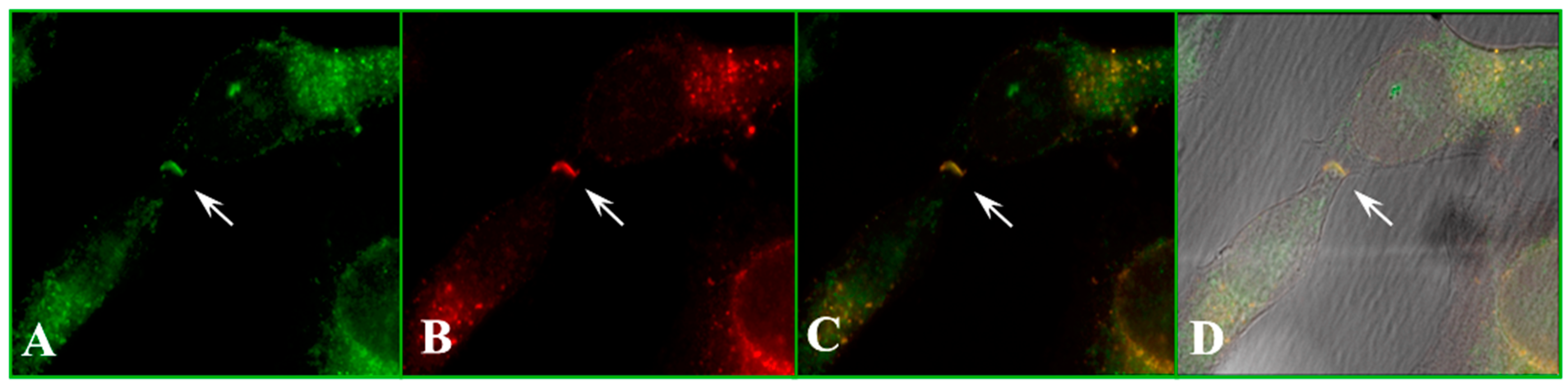
4.3. CaM–Connexin Co-Localization
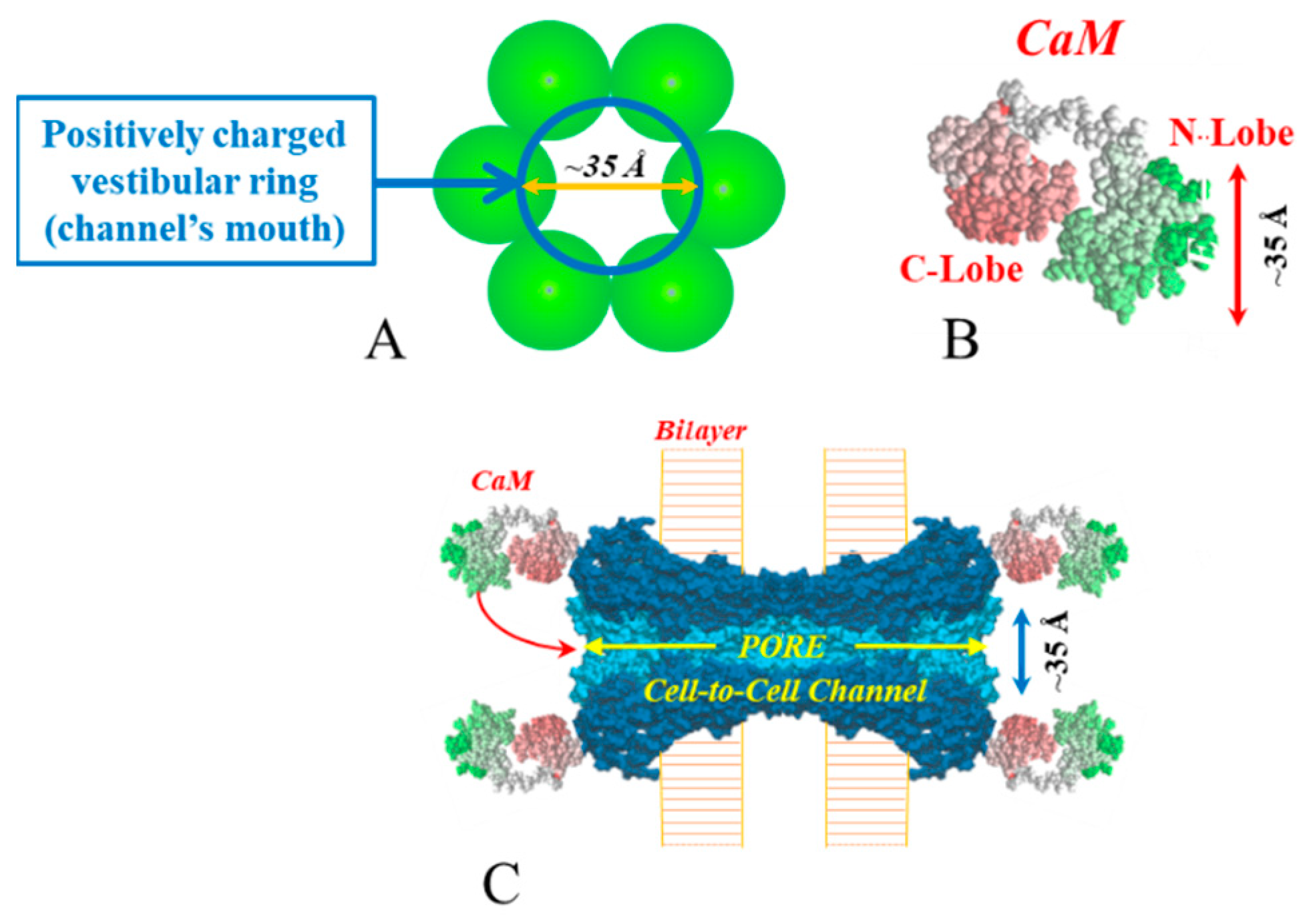
5. Cork-Gating Model
6. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Engelmann, T.W. Vergleichende Untersuchungen zur Lehre von der Muskel und Nervenelektricität. Pflüg. Arch. 1877, 15, 116–148. [Google Scholar] [CrossRef]
- Engelmann, T.W. Hüber die leitung der erregung im herzmuskel. Pflüg. Arch. 1875, 11, 465–480. [Google Scholar] [CrossRef][Green Version]
- Rothschuh, K.E. Über den aufbau des herzmuskels aus “elektrophysiologischen elementen”. Verhandlungen Dtsch. Ges. Pathol. 1950, 16, 226–231. [Google Scholar]
- Rothschuh, K.E. Über den funktionellen aufbaudes herzens aus elektrophysiologischen Elementen und üiber den mechanismus der erregungsleitung im herzen. Pflüg. Arch. 1951, 253, 238–251. [Google Scholar] [CrossRef]
- Weidmann, S. The electrical constants of Purkinje fibres. J. Physiol. 1952, 118, 348–360. [Google Scholar] [CrossRef]
- De Mello, W.C.; Motta, G.E.; Chapeau, M. A study on the healing-over of myocardial cells of toads. Circ. Res. 1969, 24, 475–487. [Google Scholar] [CrossRef] [PubMed]
- De Mello, W.C. Effect of intracellular injection of calcium and strontium on cell communication in heart. J. Physiol. 1975, 250, 231–245. [Google Scholar] [CrossRef]
- Loewenstein, W.R. Permeable junctions. Cold Spring Harb. Symp. Quant. Biol. 1975, 40, 49–63. [Google Scholar] [CrossRef]
- Loewenstein, W.R. Junctional intercellular communication: The cell-to-cell membrane channel. Physiol. Rev. 1981, 61, 829–913. [Google Scholar] [CrossRef]
- Jagielnicki, M.; Kucharska, I.; Bennett, B.C.; Harris, A.L.; Yeager, M. Connexin Gap Junction Channels and Hemichannels: Insights from High-Resolution Structures. Biology 2024, 13, 298. [Google Scholar] [CrossRef]
- Bennett, B.C.; Purdy, M.D.; Baker, K.A.; Acharya, C.; McIntire, W.E.; Stevens, R.C.; Zhang, Q.; Harris, A.L.; Abagyan, R.; Yeager, M. An electrostatic mechanism for Ca2+-mediated regulation of gap junction channels. Nat. Commun. 2016, 7, 8770. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Salarian, M.; Chen, Y.; Veenstra, R.; Louis, C.F.; Yang, J.J. Gap junction regulation by calmodulin. FEBS Lett. 2014, 588, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Calmodulin-Mediated Regulation of Gap Junction Channels. Int. J. Mol. Sci. 2020, 21, 485. [Google Scholar] [CrossRef]
- Délèze, J. Calcium ions and the healing over of heart fibers. In Electrophysiology of the Heart; Taccardi, B., Marchetti, C., Eds.; Pergamon Press: Oxford, UK, 1965; pp. 147–148. [Google Scholar]
- Oliveira-Castro, G.M.; Loewenstein, W.R. Junctional membrane permeability: Effects of divalent cations. J. Membr. Biol. 1971, 5, 51–77. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.; Loewenstein, W.R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature 1975, 254, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.; Loewenstein, W.R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: A study with aequorin. J. Membr. Biol. 1976, 28, 87–119. [Google Scholar] [CrossRef] [PubMed]
- Délèze, J.; Loewenstein, W.R. Permeability of a cell junction during intracellular injection of divalent cations. J. Membr. Biol. 1976, 28, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Gap Junction Stucture and Chemical Regulation. Direct Calmodulin Role in Cell-to-Cell Channel Gating; Academic Press: London, UK, 2019. [Google Scholar]
- Spray, D.C.; Stern, J.H.; Harris, A.L.; Bennett, M.V. Gap junctional conductance: Comparison of sensitivities to H and Ca ions. Proc. Natl. Acad. Sci. USA 1982, 79, 441–445. [Google Scholar] [CrossRef]
- Firek, L.; Weingart, R. Modification of gap junction conductance by divalent cations and protons in neonatal rat heart cells. J. Mol. Cell. Cardiol. 1995, 27, 1633–1643. [Google Scholar] [CrossRef]
- Rose, B.; Simpson, I.; Loewenstein, W.R. Calcium ion produces graded changes in permeability of membrane channels in cell junction. Nature 1977, 267, 625–627. [Google Scholar] [CrossRef]
- Weingart, R. The actions of ouabain on intercellular coupling and conduction velocity in mammalian ventricular muscle. J. Physiol. 1977, 264, 341–365. [Google Scholar] [CrossRef]
- Dahl, G.; Isenberg, G. Decoupling of heart muscle cells: Correlation with increased cytoplasmic calcium activity and with changes of nexus ultrastructure. J. Membr. Biol. 1980, 53, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Noma, A.; Tsuboi, N. Direct measurement of the gap junctional conductance under the influence of Ca2+ in dissociated paired myocytes of guinea-pig. Jpn. Heart J. 1986, 27 (Suppl. S1), 161–166. [Google Scholar] [PubMed]
- Noma, A.; Tsuboi, N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J. Physiol. 1987, 382, 193–211. [Google Scholar] [CrossRef]
- Dekker, L.R.; Fiolet, J.W.; VanBavel, E.; Coronel, R.; Opthof, T.; Spaan, J.A.; Janse, M.J. Intracellular Ca2+, intercellular electrical coupling, and mechanical activity in ischemic rabbit papillary muscle. Effects of preconditioning and metabolic blockade. Circ. Res. 1996, 79, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. Increase in gap junction resistance with acidification in crayfish septate axons is closely related to changes in intracellular calcium but not hydrogen ion concentration. J. Membr. Biol. 1990, 113, 75–92. [Google Scholar] [CrossRef]
- Neyton, J.; Trautmann, A. Single-channel currents of an intercellular junction. Nature 1985, 317, 331–335. [Google Scholar] [CrossRef]
- Lazrak, A.; Peracchia, C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys. J. 1993, 65, 2002–2012. [Google Scholar] [CrossRef]
- Lazrak, A.; Peres, A.; Giovannardi, S.; Peracchia, C. Ca-mediated and independent effects of arachidonic acid on gap junctions and Ca-independent effects of oleic acid and halothane. Biophys. J. 1994, 67, 1052–1059. [Google Scholar] [CrossRef]
- Enkvist, M.O.; McCarthy, K.D. Astroglial gap junction communication is increased by treatment with either glutamate or high K+ concentration. J. Neurochem. 1994, 62, 489–495. [Google Scholar] [CrossRef]
- Cotrina, M.L.; Kang, J.; Lin, J.H.; Bueno, E.; Hansen, T.W.; He, L.; Liu, Y.; Nedergaard, M. Astrocytic gap junctions remain open during ischemic conditions. J. Neurosci. 1998, 18, 2520–2537. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Venance, L. Characterization and regulation of gap junction channels in cultured astrocytes. In Gap Junctions in the Nervous System; Spray, D.C., Dermietzel, R., Eds.; R.G Landes Medical Pub.: Austin, TX, USA, 1996; pp. 135–157. [Google Scholar]
- Crow, J.M.; Atkinson, M.M.; Johnson, R.G. Micromolar levels of intracellular calcium reduce gap junctional permeability in lens cultures. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3332–3341. [Google Scholar]
- Mears, D.; Sheppard, N.F., Jr.; Atwater, I.; Rojas, E. Magnitude and modulation of pancreatic beta-cell gap junction electrical conductance in situ. J. Membr. Biol. 1995, 146, 163–176. [Google Scholar] [CrossRef]
- Dakin, K.; Zhao, Y.; Li, W.H. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat. Methods 2005, 2, 55–62. [Google Scholar] [CrossRef]
- Xu, Q.; Kopp, R.F.; Chen, Y.; Yang, J.J.; Roe, M.W.; Veenstra, R.D. Gating of connexin 43 gap junctions by a cytoplasmic loop calmodulin binding domain. Am. J. Physiol. Cell Physiol. 2012, 302, C1548–C1556. [Google Scholar] [CrossRef]
- Iwatsuki, N.; Petersen, O.H. Membrane potential, resistance, and intercellular communication in the lacrimal gland: Effects of acetylcholine and adrenaline. J. Physiol. 1978, 275, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, N.; Petersen, O.H. Pancreatic acinar cells: Acetylcholine-evoked electrical uncoupling and its ionic dependency. J. Physiol. 1978, 274, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, N.; Petersen, O.H. Electrical coupling and uncoupling of exocrine acinar cells. J. Cell Biol. 1978, 79 Pt 1, 533–545. [Google Scholar] [CrossRef]
- Matthews, E.K.; Petersen, O.H. Pancreatic acinar cells: Ionic dependence of the membrane potential and acetycholine-induced depolarization. J. Physiol. 1973, 231, 283–295. [Google Scholar] [CrossRef]
- Scheele, G.A.; Palade, G.E. Studies on the guinea pig pancreas. Parallel discharge of exocrine enzyme activities. J. Biol. Chem. 1975, 250, 2660–2670. [Google Scholar] [CrossRef]
- Ochs, D.L.; Korenbrot, J.I.; Williams, J.A. Intracellular free calcium concentrations in isolated pancreatic acini; effects of secretagogues. Biochem. Biophys. Res. Commun. 1983, 117, 122–128. [Google Scholar] [CrossRef]
- Lurtz, M.M.; Louis, C.F. Calmodulin and protein kinase C regulate gap junctional coupling in lens epithelial cells. Am. J. Physiol. Cell Physiol. 2003, 285, C1475–C1482. [Google Scholar] [CrossRef][Green Version]
- Parker, J.; Daniel, L.W.; Waite, M. Evidence of protein kinase C involvement in phorbol diester-stimulated arachidonic acid release and prostaglandin synthesis. J. Biol. Chem. 1987, 262, 5385–5393. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J.; Burch, R.M.; Jelsema, C.L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: Arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988, 11, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L. The eicosanoids and their biochemical mechanisms of action. Biochem. J. 1989, 259, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Wolfe, L.S. Arachidonic acid cascade and signal transduction. J. Neurochem. 1990, 55, 1–15. [Google Scholar] [CrossRef]
- Bayraktar, E.; Lopez-Pigozzi, D.; Bortolozzi, M. Calcium Regulation of Connexin Hemichannels. Int. J. Mol. Sci. 2024, 25, 6594. [Google Scholar] [CrossRef]
- Persechini, A.; Moncrief, N.D.; Kretsinger, R.H. The EF-hand family of calcium-modulated proteins. Trends Neurosci. 1989, 12, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Kretsinger, R.H. Hipothesis: Calcium modulated proteins contain EF-hands. In Calcium Transport in Contraction and Secretion; Carafoli, E., Clementi, F., Drabinowski, W., Margreth, A., Eds.; Elsevier: Amsterdam, The Netherlands, 1975; pp. 469–478. [Google Scholar]
- Kretsinger, R.H.; Barry, C.D. The predicted structure of the calcium-binding component of troponin. Biochim. Biophys. Acta 1975, 405, 40–52. [Google Scholar] [CrossRef]
- Furia, E.T. Sequestrants in food. In Handbook of Food Additives; Furia, T.E., Ed.; CRC Press: Boca Raton, FL, USA, 1973; pp. 271–294. [Google Scholar]
- Flagg-Newton, J.; Loewenstein, W.R. Experimental depression of junctional membrane permeability in mammalian cell culture. A study with tracer molecules in the 300 to 800 Dalton range. J. Membr. Biol. 1979, 50, 65–100. [Google Scholar] [CrossRef]
- Lurtz, M.M.; Louis, C.F. Intracellular calcium regulation of connexin43. Am. J. Physiol. Cell Physiol. 2007, 293, C1806–C1813. [Google Scholar] [CrossRef] [PubMed]
- Délèze, J. The recovery of resting potential and input resistance in sheep heart injured by knife or laser. J. Physiol. 1970, 208, 547–562. [Google Scholar] [CrossRef] [PubMed]
- De Mello, W.C. Influence of temperature on myocardial healing-over. Experientia 1972, 28, 832–833. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, W.R.; Penn, R.D. Intercellular communication and tissue growth. II. Tissue regeneration. J. Cell Biol. 1967, 33, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Heyman, N.S.; Kurjiaka, D.T.; Ek Vitorin, J.F.; Burt, J.M. Regulation of gap junctional charge selectivity in cells coexpressing connexin 40 and connexin 43. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H450–H459. [Google Scholar] [CrossRef]
- Bukauskas, F.F.; Peracchia, C. Two distinct gating mechanisms in gap junction channels: CO2-sensitive and voltage-sensitive. Biophys. J. 1997, 72, 2137–2142. [Google Scholar] [CrossRef]
- Johnston, M.F.; Ramon, F. Electrotonic coupling in internally perfused crayfish segmented axons. J. Physiol. 1981, 317, 509–518. [Google Scholar] [CrossRef]
- Peracchia, C.; Bernardini, G.; Peracchia, L.L. Is calmodulin involved in the regulation of gap junction permeability? Pflüg. Arch. 1983, 399, 152–154. [Google Scholar] [CrossRef]
- Van Eldik, L.J.; Hertzberg, E.L.; Berdan, R.C.; Gilula, N.B. Interaction of calmodulin and other calcium-modulated proteins with mammalian and arthropod junctional membrane proteins. Biochem. Biophys. Res. Commun. 1985, 126, 825–832. [Google Scholar] [CrossRef]
- Hertzberg, E.L.; Gilula, N.B. Liver gap junctions and lens fiber junctions: Comparative analysis and calmodulin interaction. Cold Spring Harb. Symp. Quant. Biol. 1981, 46, 639–645. [Google Scholar] [CrossRef]
- Arellano, R.O.; Ramon, F.; Rivera, A.; Zampighi, G.A. Calmodulin acts as an intermediary for the effects of calcium on gap junctions from crayfish lateral axons. J. Membr. Biol. 1988, 101, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.M. Identification of Innexins Contributing to the Giant-Fiber Escape Response in Marbled Crayfish. Master’s Thesis, Illinois State University, Normal, IL, USA, 2020. [Google Scholar]
- Nicholson, B.J.; Zhou, L.; Cao, F.; Zhou, H.; Chen, Y. Diverse molecular mechanisms of gap junction channel gating. In Gap Junctions; Werner, R., Ed.; IOS Press: Amsterdam, The Netherlands, 1998; pp. 3–8. [Google Scholar]
- Bevans, C.G.; Harris, A.L. Regulation of connexin channels by pH. Direct action of the protonated form of taurine and other aminosulfonates. J. Biol. Chem. 1999, 274, 3711–3719. [Google Scholar] [CrossRef]
- Peracchia, C. Communicating junctions and calmodulin: Inhibition of electrical uncoupling in Xenopus embryo by calmidazolium. J. Membr. Biol. 1984, 81, 49–58. [Google Scholar] [CrossRef]
- Peracchia, C. Calmodulin-like proteins and communicating junctions. Electrical uncoupling of crayfish septate axons is inhibited by the calmodulin inhibitor W7 and is not affected by cyclic nucleotides. Pflüg. Arch. 1987, 408, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, J.A. Electrical uncoupling induced by general anesthetics: A calcium-independent process? In Gap Junctions; Bennett, M.V.L., Spray, D.C., Eds.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1985; pp. 167–175. [Google Scholar]
- Tuganowski, W.; Korczynska, I.; Wasik, K.; Piatek, G. Effects of calmidazolium and dibutyryl cyclic AMP on the longitudinal internal resistance in sinus node strips. Pflüg. Arch. 1989, 414, 351–353. [Google Scholar] [CrossRef]
- Gandolfi, S.A.; Duncan, G.; Tomlinson, J.; Maraini, G. Mammalian lens inter-fiber resistance is modulated by calcium and calmodulin. Curr. Eye Res. 1990, 9, 533–541. [Google Scholar] [CrossRef]
- Peracchia, C.; Wang, X.; Li, L.; Peracchia, L.L. Inhibition of calmodulin expression prevents low-pH-induced gap junction uncoupling in Xenopus oocytes. Pflü. Arch. 1996, 431, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Wang, X.G.; Peracchia, L.L. Slow gating of gap junction channels and calmodulin. J. Membr. Biol. 2000, 178, 55–70. [Google Scholar] [CrossRef]
- Peracchia, C.; Young, K.C.; Wang, X.G.; Peracchia, L.L. Is the voltage gate of connexins CO2-sensitive? Cx45 channels and inhibition of calmodulin expression. J. Membr. Biol. 2003, 195, 53–62. [Google Scholar] [CrossRef]
- Peracchia, C.; Sotkis, A.; Wang, X.G.; Peracchia, L.L.; Persechini, A. Calmodulin directly gates gap junction channels. J. Biol. Chem. 2000, 275, 26220–26224. [Google Scholar] [CrossRef]
- Sotkis, A.; Wang, X.G.; Yasumura, T.; Peracchia, L.L.; Persechini, A.; Rash, J.E.; Peracchia, C. Calmodulin colocalizes with connexins and plays a direct role in gap junction channel gating. Cell Commun. Adhes. 2001, 8, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Salarian, M.; Chen, Y.; Zhuo, Y.; Brown, N.E.; Hepler, J.R.; Yang, J. Direct Visualization of Interaction between Calmodulin and Connexin45. Biochem. J. 2017, 474, 4035–4051. [Google Scholar] [CrossRef]
- Dodd, R.; Peracchia, C.; Stolady, D.; Török, K. Calmodulin association with connexin32-derived peptides suggests trans-domain interaction in chemical gating of gap junction channels. J. Biol. Chem. 2008, 283, 26911–26920. [Google Scholar] [CrossRef]
- Török, K.; Stauffer, K.; Evans, W.H. Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem. J. 1997, 326 Pt 2, 479–483. [Google Scholar] [CrossRef]
- Tran, O.; Kerruth, S.; Coates, C.; Kaur, H.; Peracchia, C.; Carter, T.; Török, K. Ca2+-Dependent and -Independent Calmodulin Binding to the Cytoplasmic Loop of Gap Junction Connexins. Int. J. Mol. Sci. 2023, 24, 4153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, W.; Lurtz, M.M.; Chen, Y.; Jiang, J.; Huang, Y.; Louis, C.F.; Yang, J.J. Calmodulin mediates the Ca2+-dependent regulation of Cx44 gap junctions. Biophys. J. 2009, 96, 2832–2848. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, W.; Lurtz, M.M.; Ye, Y.; Huang, Y.; Lee, H.W.; Chen, Y.; Louis, C.F.; Yang, J.J. Identification of the calmodulin binding domain of connexin 43. J. Biol. Chem. 2007, 282, 35005–35017. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Lin, X.; Wong, H.C.; Xu, Q.; Jiang, J.; Wang, S.; Lurtz, M.M.; Louis, C.F.; Veenstra, R.D.; et al. Molecular interaction and functional regulation of connexin50 gap junctions by calmodulin. Biochem. J. 2011, 435, 711–722. [Google Scholar] [CrossRef]
- Babu, Y.S.; Bugg, C.E.; Cook, W.J. Structure of calmodulin refined at 2.2 Å resolution. J. Mol. Biol. 1988, 204, 191–204. [Google Scholar] [CrossRef]
- Elvira, M.; Villalobo, A. Calmodulin prevents the proteolysis of connexin32 by calpain. Bioelectrochem. Bioenerg. 1997, 42, 207–211. [Google Scholar] [CrossRef]
- Diez, J.A.; Elvira, M.; Villalobo, A. The epidermal growth factor receptor tyrosine kinase phosphorylates connexin32. Mo. Cell. Biochem. 1998, 187, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Martin, P.E.; Evans, W.H. Assembly of gap junction channels: Mechanism, effects of calmodulin antagonists and identification of connexin oligomerization determinants. Eur. J. Biochem. 2001, 268, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C. The calmodulin hypoyhesis for gap junction regulation six years later. In Gap Junctions; Hertzberg, E., Johnson, R.G., Eds.; Alan R. Liss: New York, NY, USA, 1988; Volume 7, pp. 267–282. [Google Scholar]
- Török, K.; Trentham, D.R. Mechanism of 2-chloro-(epsilon-amino-Lys75)-[6-[4-(N,N- diethylamino)phenyl]-1,3,5-triazin-4-yl]calmodulin interactions with smooth muscle myosin light chain kinase and derived peptides. Biochemistry 1994, 33, 12807–12820. [Google Scholar] [CrossRef]
- Stauch, K.; Kieken, F.; Sorgen, P. Characterization of the structure and intermolecular interactions between the connexin 32 carboxyl-terminal domain and the protein partners synapse-associated protein 97 and calmodulin. J. Biol. Chem. 2012, 287, 27771–27788. [Google Scholar] [CrossRef]
- Sorgen, P.L.; Trease, A.J.; Spagnol, G.; Delmar, M.; Nielsen, M.S. Protein-Protein Interactions with Connexin 43: Regulation and Function. Int. J. Mol. Sci. 2018, 19, 1428–1449. [Google Scholar] [CrossRef]
- Aseervatham, J.L.X.; Mitchell, C.K.; Lin, Y.-P.; Heidelberg, R.; O’Brian, J. Calmodulin binding to connexin 35: Specializations to function as an electrical synapse. Int. J. Mol. Sci. 2020, 21, 6346. [Google Scholar] [CrossRef]
- Wei, S.; Cassara, C.; Lin, X.; Veenstra, R.D. Calcium-calmodulin gating of a pH-insensitive isoform of connexin43 gap junctions. Biochem. J. 2019, 476, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Peracchia, C. Positive charges of the initial C-terminus domain of Cx32 inhibit gap junction gating sensitivity to CO2. Biophys. J. 1997, 73, 798–806. [Google Scholar] [CrossRef]
- Carrer, A.; Leparulo, A.; Crispino, G.; Ciubotaru, C.D.; Marin, O.; Zonta, F.; Bortolozzi, M. Cx32 hemichannel opening by cytosolic Ca2+ is inhibited by the R220X mutation that causes Charcot-Marie-Tooth disease. Hum. Mol. Genet. 2018, 27, 80–94. [Google Scholar] [CrossRef]
- Burr, G.S.; Mitchell, C.K.; Keflemariam, Y.J.; Heidelberger, R.; O’Brien, J. Calcium-dependent binding of calmodulin to neuronal gap junction proteins. Biochem. Biophys. Res. Commun. 2005, 335, 1191–1198. [Google Scholar] [CrossRef][Green Version]
- Siu, R.C.; Smirnova, E.; Brown, C.A.; Zoidl, C.; Spray, D.C.; Donaldson, L.W.; Zoidl, G. Structural and Functional Consequences of Connexin 36 (Cx36) Interaction with Calmodulin. Front. Mol. Neurosci. 2016, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Peracchia, C. Connexin 32/38 chimeras suggest a role for the second half of inner loop in gap junction gating by low pH. Am. J. Physiol. 1996, 271 Pt 1, C1743–C1749. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Peracchia, L.L.; Peracchia, C. Chimeric evidence for a role of the connexin cytoplasmic loop in gap junction channel gating. Pflüg. Arch. 1996, 431, 844–852. [Google Scholar] [CrossRef]
- Kerruth, S.; Coates, C.; Rezavi, S.A.; Peracchia, C.; Torok, K. Calmodulin interaction with gap junction intracellular loop peptides. Biophys. J. 2018, 114, 468a. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Myllykoski, M.; Kuczera, K.; Kursula, P. Complex formation between calmodulin and a peptide from the intracellular loop of the gap junction protein connexin43: Molecular conformation and energetics of binding. Biophys. Chem. 2009, 144, 130–135. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.; Qi, Y. Role of intramolecular interaction in connexin50: Mediating the Ca2+-dependent binding of calmodulin to gap junction. Arch. Biochem. Biophys. 2005, 440, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, T.; Liu, Y.; Qi, Y. The gating effect of calmodulin and calcium on the connexin50 hemichannel. Biol. Chem. 2006, 387, 595–601. [Google Scholar] [CrossRef]
- Peracchia, C. Chemical gating of gap junction channels; roles of calcium, pH and calmodulin. Biochim. Biophys. Acta 2004, 1662, 61–80. [Google Scholar] [CrossRef]
- Peracchia, C. Gap Junctions—Molecular Basis of Cell Communication in Health and Disease; Academic Press: San Diego, CA, USA, 2000; Volume 49. [Google Scholar]
- Peracchia, C. Calmodulin-Cork Model of Gap Junction Channel Gating-One Molecule, Two Mechanisms. Int. J. Mol. Sci. 2020, 21, 4938. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Wang, X.C.; Peracchia, L.L. Behavior of chemical and slow voltage-gates of connexin channels. The cork gating hypothesis. In Gap Junctions—Molecular Basis of Cell Comunication in Health and Disease; Peracchia, C., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 271–295. [Google Scholar]
- Peracchia, C.; Wang, X.G.; Peracchia, L.L. Is the chemical gate of connexins voltage sensitive? Behavior of Cx32 wild-type and mutant channels. Am. J. Physiol. 1999, 276 Pt 1, C1361–C1373. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Salim, M.; Peracchia, L.L. Unusual slow gating of gap junction channels in oocytes expressing connexin32 or its COOH-terminus truncated mutant. J. Membr. Biol. 2007, 215, 161–168. [Google Scholar] [CrossRef]
- Fleishman, S.J.; Unger, V.M.; Yeager, M.; Ben-Tal, N. A Calpha model for the transmembrane alpha helices of gap junction intercellular channels. Mol. Cell 2004, 15, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.; Goodenough, D.; Sosinsky, G. Three-dimensional structure of the gap junction connexon. Biophys. J. 1997, 72 Pt 1, 533–544. [Google Scholar] [CrossRef]
- Maeda, S.; Nakagawa, S.; Suga, M.; Yamashita, E.; Oshima, A.; Fujiyoshi, Y.; Tsukihara, T. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature 2009, 458, 597–602. [Google Scholar] [CrossRef]
- Persechini, A.; Gansz, K.J.; Paresi, R.J. Activation of myosin light chain kinase and nitric oxide synthase activities by engineered calmodulins with duplicated or exchanged EF hand pairs. Biochemistry 1996, 35, 224–228. [Google Scholar] [CrossRef]
- Astegno, A.; La, V.V.; Marino, V.; Dell’Orco, D.; Dominici, P. Biochemical and biophysical characterization of a plant calmodulin: Role of the N- and C-lobes in calcium binding, conformational change, and target interaction. Biochim. Biophys. Acta 2016, 1864, 297–307. [Google Scholar] [CrossRef]
- Bukauskas, F.F.; Jordan, K.; Bukauskiene, A.; Bennett, M.V.; Lampe, P.D.; Laird, D.W.; Verselis, V.K. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc. Natl. Acad. Sci. USA 2000, 97, 2556–2561. [Google Scholar] [CrossRef]
- Peracchia, C.; Leverone Peracchia, L.M. Calmodulin-Connexin Partnership in Gap Junction Channel Regulation-Calmodulin-Cork Gating Model. Int. J. Mol. Sci. 2021, 22, 13055. [Google Scholar] [CrossRef]
- Nyegaard, M.; Overgaard, M.T.; Sondergaard, M.T.; Vranas, M.; Behr, E.R.; Hildebrandt, L.L.; Lund, J.; Hedley, P.L.; Camm, A.J.; Wettrell, G.; et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am. J. Hum. Genet. 2012, 91, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.H.; Brohus, M.; Nyegaard, M.; Overgaard, M.T. Human Calmodulin Mutations. Front. Mol. Neurosci. 2018, 11, 396. [Google Scholar] [CrossRef] [PubMed]
- Badone, B.; Ronchi, C.; Kotta, M.C.; Sala, L.; Ghidoni, A.; Crotti, L.; Zaza, A. Calmodulinopathy: Functional Effects of CALM Mutations and Their Relationship with Clinical Phenotypes. Front. Cardiovasc. Med. 2018, 5, 176. [Google Scholar] [CrossRef] [PubMed]
- Kotta, M.C.; Sala, L.; Ghidoni, A.; Badone, B.; Ronchi, C.; Parati, G.; Zaza, A.; Crotti, L. Calmodulinopathy: A Novel, Life-Threatening Clinical Entity Affecting the Young. Front. Cardiovasc. Med. 2018, 5, 175. [Google Scholar] [CrossRef]
- Chazin, W.J.; Johnson, C.N. Calmodulin Mutations Associated with Heart Arrhythmia: A Status Report. Int. J. Mol. Sci. 2020, 21, 1418. [Google Scholar] [CrossRef]
- Crotti, L.; Spazzolini, C.; Tester, D.J.; Ghidoni, A.; Baruteau, A.E.; Beckmann, B.M.; Behr, E.R.; Bennett, J.S.; Bezzina, C.R.; Bhuiyan, Z.A.; et al. Calmodulin mutations and life-threatening cardiac arrhythmias: Insights from the International Calmodulinopathy Registry. Eur. Heart J. 2019, 40, 2964–2975. [Google Scholar] [CrossRef]
- Prakash, O.; Gupta, N.; Milburn, A.; McCormick, L.; Deugi, V.; Fisch, P.; Wyles, J.; Thomas, N.L.; Antonyuk, S.; Dart, C.; et al. Calmodulin variant E140G associated with long QT syndrome impairs CaMKIIdelta autophosphorylation and L-type calcium channel inactivation. J. Biol. Chem. 2023, 299, 102777. [Google Scholar] [CrossRef]
- Prakash, O.; Held, M.; McCormick, L.F.; Gupta, N.; Lian, L.Y.; Antonyuk, S.; Haynes, L.P.; Thomas, N.L.; Helassa, N. CPVT-associated calmodulin variants N53I and A102V dysregulate Ca2+ signalling via different mechanisms. J. Cell Sci. 2022, 135, jcs258796. [Google Scholar] [CrossRef]
- Peracchia, C. Connexin/Innexin Channels in Cytoplasmic Organelles. Are There Intracellular Gap Junctions? A Hypothesis! Int. J. Mol. Sci. 2020, 21, 2163. [Google Scholar] [CrossRef]
- Peracchia, C. Gap Junction Channelopathies and Calmodulinopathies. Do Disease-Causing Calmodulin Mutants Affect Direct Cell-Cell Communication? Int. J. Mol. Sci. 2021, 22, 9169. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peracchia, C. Calcium Role in Gap Junction Channel Gating: Direct Electrostatic or Calmodulin-Mediated? Int. J. Mol. Sci. 2024, 25, 9789. https://doi.org/10.3390/ijms25189789
Peracchia C. Calcium Role in Gap Junction Channel Gating: Direct Electrostatic or Calmodulin-Mediated? International Journal of Molecular Sciences. 2024; 25(18):9789. https://doi.org/10.3390/ijms25189789
Chicago/Turabian StylePeracchia, Camillo. 2024. "Calcium Role in Gap Junction Channel Gating: Direct Electrostatic or Calmodulin-Mediated?" International Journal of Molecular Sciences 25, no. 18: 9789. https://doi.org/10.3390/ijms25189789
APA StylePeracchia, C. (2024). Calcium Role in Gap Junction Channel Gating: Direct Electrostatic or Calmodulin-Mediated? International Journal of Molecular Sciences, 25(18), 9789. https://doi.org/10.3390/ijms25189789





