Deoxynivalenol Induces Local Inflammation and Lesions in Tissues at Doses Recommended by the EU
Abstract
1. Introduction
2. Results
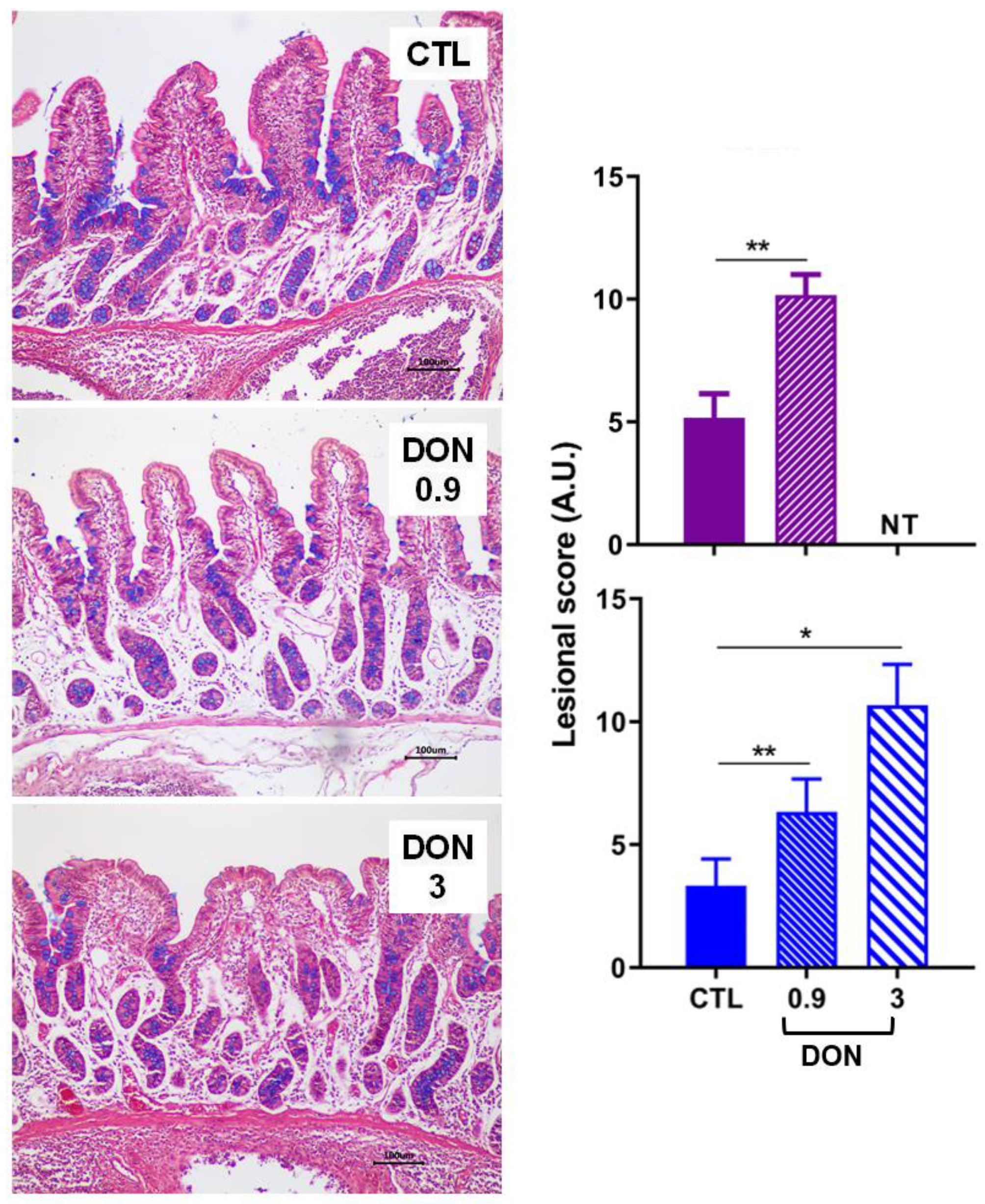
2.1. Low Levels of DON Have an Impact on the Intestine
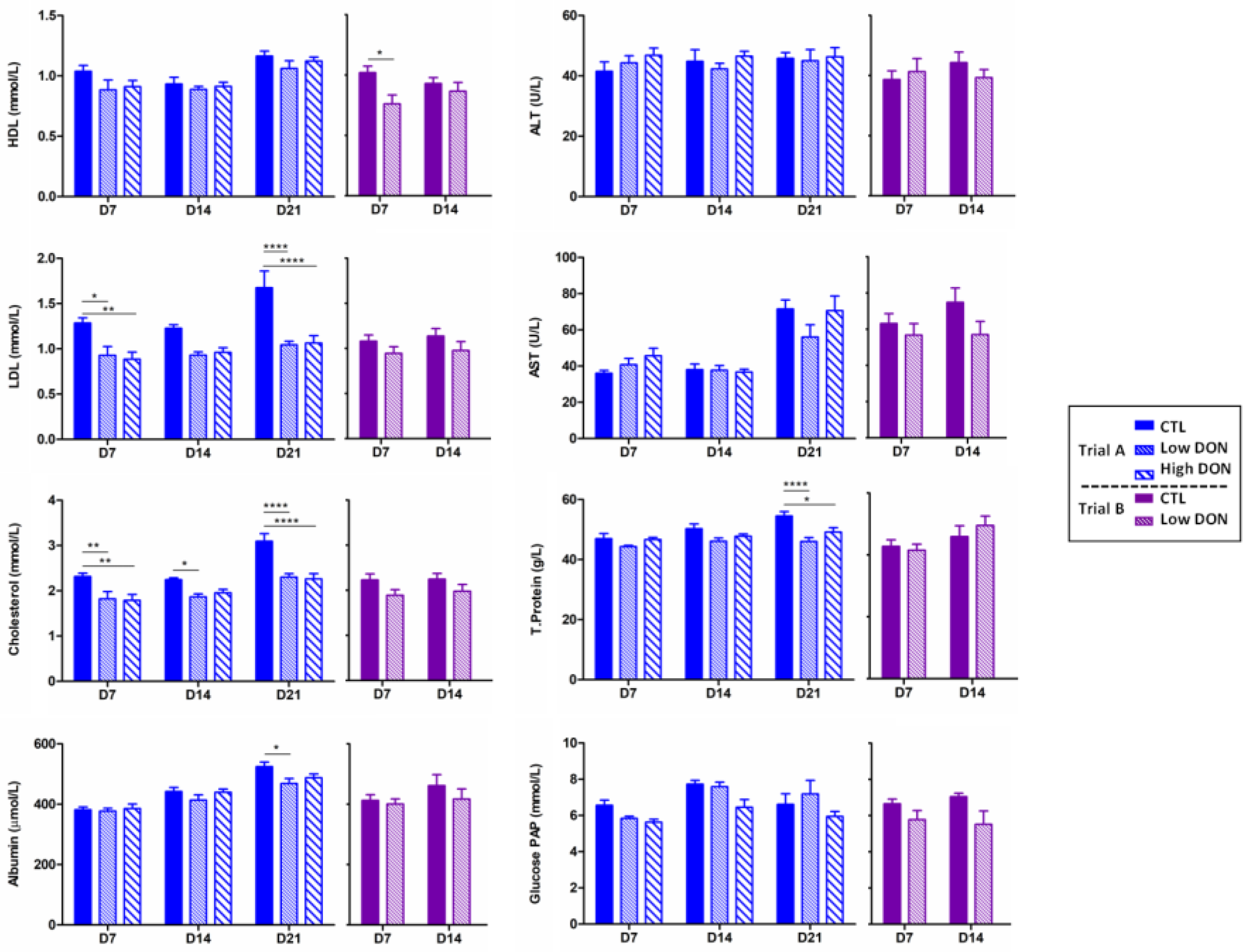
2.2. Low Levels of DON Have an Impact on the Liver
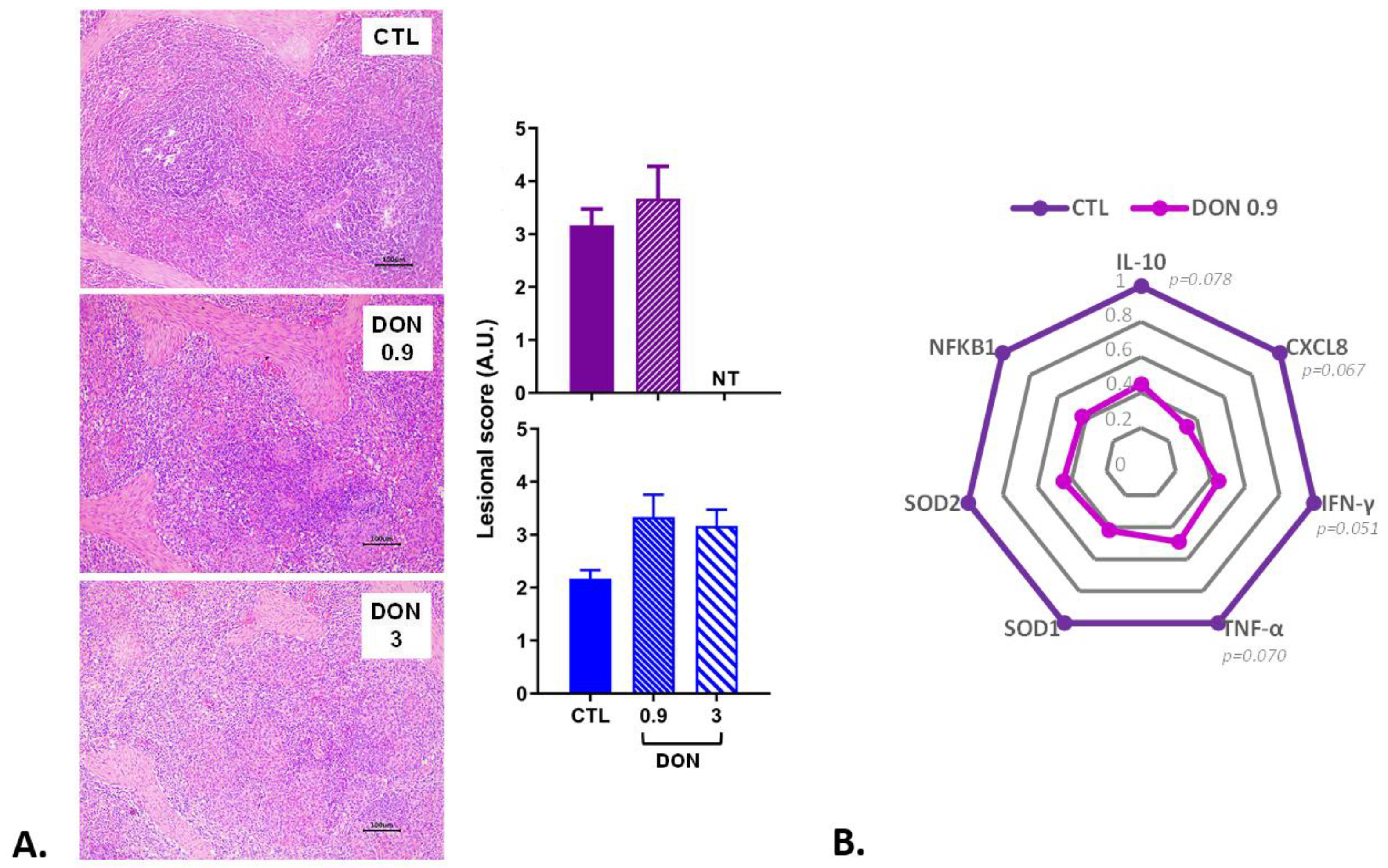
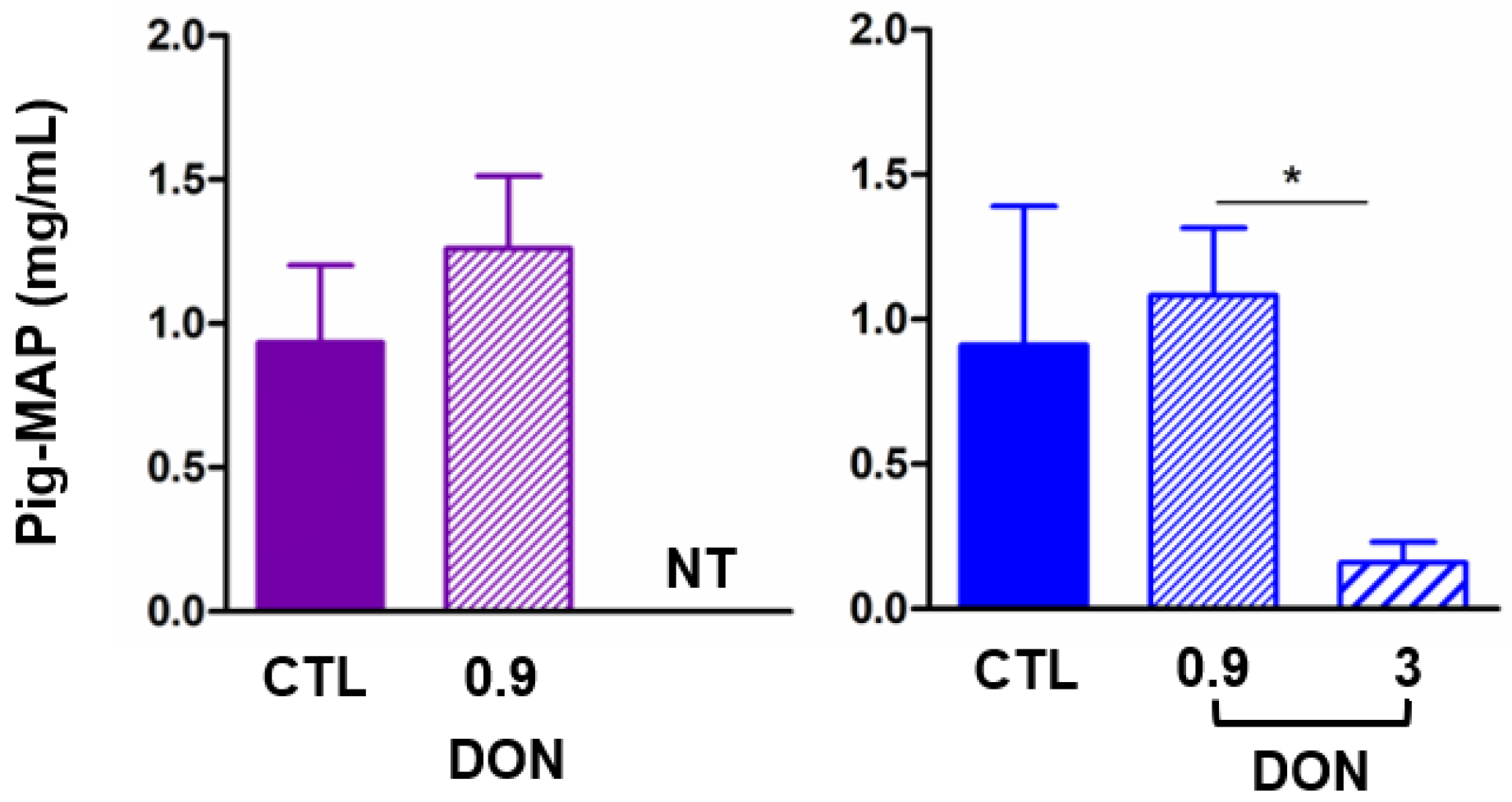
2.3. Low Levels of DON Have an Impact on the Spleen and Inflammatory Proteins
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Tissue and Blood Sampling
4.2. Biochemical Analysis and Acute-Phase Protein Measurement
4.3. Histopathological Analysis
4.4. Expression of mRNA Encoding Cytokines by Real-Time PCR
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Payros, D.; Garofalo, M.; Pierron, A.; Soler-Vasco, L.; Al-Ayoubi, C.; Maruo, V.M.; Alassane-Kpembi, I.; Pinton, P.; Oswald, I.P. Mycotoxins in human food: A challenge for research. Cah. Nutr. Diet. 2021, 56, 170–183. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. Eur. Food Saf. Auth. 2017, 15, e04718. [Google Scholar]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Terciolo, C.; Maresca, M.; Pinton, P.; Oswald, I.P. Review article: Role of satiety hormones in anorexia induction by Trichothecene mycotoxins. Food Chem. Toxicol. 2018, 121, 701–714. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. 2016, 2, 63–68. [Google Scholar] [CrossRef]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other Type B trichothecenes on the intestine: A review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol-Induced Proinflammatory Gene Expression: Mechanisms and Pathological Sequelae. Toxins 2010, 2, 1300–1317. [Google Scholar] [CrossRef]
- Payros, D.; Ménard, S.; Laffitte, J.; Neves, M.; Tremblay-Franco, M.; Luo, S. The food contaminant, deoxynivalenol, modulates the Thelper/Treg balance and increases inflammatory bowel diseases. Arch. Toxicol. 2020, 94, 3173–3184. [Google Scholar] [CrossRef] [PubMed]
- Garreau de Loubresse, N.; Prokhorova, I.; Holtkamp, W.; Rodnina, M.V.; Yusupova, G.; Yusupov, M. Structural basis for the inhibition of the eukaryotic ribosome. Nature 2014, 513, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Mimoun, S.; Murate, L.S.; Loiseau, N.; Lippi, Y.; Bracarense, A.P.; Liaubet, L.; Schatzmayr, G.; Berthiller, F.; Moll, W.D.; et al. Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-D-glucoside. Arch Toxicol. 2016, 90, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Alassane-Kpembi, I.; Laffitte, J.; Lencina, C.; Neves, M.; Bracarense, A.P.; Pinton, P.; Ménard, S.; Oswald, I.P. Dietary Exposure to the Food Contaminant Deoxynivalenol Triggers Colonic Breakdown by Activating the Mitochondrial and the Death Receptor Pathways. Arch. Toxicol. 2021, 65, e2100191. [Google Scholar] [CrossRef]
- Tardivel, C.; Airault, C.; Djelloul, M.; Guillebaud, F.; Barbouche, R.; Troadec, J.D.; Gaigé, S.; Dallaporta, M. The food born mycotoxin deoxynivalenol induces low-grade inflammation in mice in the absence of observed-adverse effects. Toxicol. Lett. 2015, 232, 601–611. [Google Scholar] [CrossRef]
- Pierron, A.; Vatzia, E.; Stadler, M.; Mair, K.H.; Schmidt, S.; Stas, M.R.; Dürlinger, S.; Kreutzmann, H.; Knecht, C.; Balka, G.; et al. Influence of deoxynivalenol-contaminated feed on the immune response of pigs after PRRSV vaccination and infection. Arch. Toxicol. 2023, 97, 1079–1089. [Google Scholar] [CrossRef]
- Vignal, C.; Djouina, M.; Pichavant, M.; Caboche, S.; Waxin, C.; Beury, D.; Hot, D.; Gower-Rousseau, C.; Body-Malapel, M. Chronic ingestion of deoxynivalenol at human dietary levels impairs intestinal homeostasis and gut microbiota in mice. Arch. Toxicol. 2018, 92, 2327–2338. [Google Scholar] [CrossRef]
- Hasuda, A.L.; Person, E.; Khoshal, A.K.; Bruel, S.; Puel, S.; Oswald, I.P.; Bracarense, A.P.F.R.L.; Pinton, P. Deoxynivalenol induces apoptosis and inflammation in the liver: Analysis using precision-cut liver slices. Food Chem. Toxicol. 2022, 163, 112930. [Google Scholar] [CrossRef]
- Karlovsky, P. Biological detoxification of the mycotoxin deoxynivalenol and its use in genetically engineered crops and feed additives. Appl. Microbiol. Biotechnol. 2011, 91, 491–504. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2024/1022 as Regards Maximum Levels of Deoxynivalenol in Food. Available online: http://data.europa.eu/eli/reg/2024/1022/oj (accessed on 4 September 2024).
- EC Recommendations 2006/576/EC on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding. Available online: http://data.europa.eu/eli/reco/2006/576/oj (accessed on 4 September 2024).
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu Rev Food Sci Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef]
- Holanda, D.M.; Kim, S.W. Mycotoxin occurrence, toxicity, and detoxifying agents in pig production with an emphasis on deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Gerdes, R.G.; Underhill, K.L.; Rotter, B.A.; Jui, P.Y.; Trenholm, H.L. Effects of low-level dietary deoxynivalenol on haematological and clinical parameters of the pig. Nat. Toxins 1994, 2, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.W.; Trenholm, H.L.; Elliot, J.I.; Hartin, K.E.; Thompson, B.K. Effet of feeding vomitoxin-contaminated wheat to pigs. Can. J. Anim. Sci. 1982, 62, 1211–1222. [Google Scholar] [CrossRef]
- Alizadeh, A.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol Impairs Weight Gain and Affects Markers of Gut Health after Low-Dose, Short-Term Exposure of Growing Pigs. Toxins 2015, 7, 2071–2095. [Google Scholar] [CrossRef]
- Bracarense, A.P.F.L.; Pierron, A.; Pinton, P.; Gerez, J.R.; Schatzmayr, G.; Moll, W.D.; Zhou, T.; Oswald, I.P. Reduced toxicity of 3-epi-deoxynivalenol and de-epoxy-deoxynivalenol through deoxynivalenol bacterial biotransformation: In vivo analysis in piglets. Food Chem. Toxicol. 2020, 140, 111241. [Google Scholar] [CrossRef]
- Pierron, A.; Bracarense, A.P.F.L.; Cossalter, A.M.; Laffitte, J.; Schwartz-Zimmermann, H.E.; Schatzmayr, G.; Pinton, P.; Moll, W.D.; Oswald, I.P. Deepoxy-deoxynivalenol retains some immune-modulatory properties of the parent molecule deoxynivalenol in piglets. Arch. Toxicol. 2018, 92, 3381–3389. [Google Scholar] [CrossRef]
- Skiepko, N.; Przybylska-Gornowicz, B.; Gajęcka, M.; Gajęcki, M.; Lewczuk, B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins 2020, 12, 463. [Google Scholar] [CrossRef]
- Grenier, B.; Loureiro-Bracarense, A.P.; Lucioli, J.; Pacheco, G.D.; Cossalter, A.M.; Moll, W.D.; Schatzmayr, G.; Oswald, I.P. Individual and combined effects of subclinical doses of deoxynivalenol and fumonisins in piglets. Mol. Nutr. Food Res. 2011, 55, 761–771. [Google Scholar] [CrossRef]
- Schrenk, D.; Bignami, M.; Bodin, L.; Del Mazo, J.K.C.J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.C.; Nielsen, E.; Ntzani, E.; Petersen, A.; et al. Assessment of information as regards the toxicity of deoxynivalenol for horses and poultry. EFSA J. 2023, 21, e07806. [Google Scholar]
- Diack, A.B.; Gladney, C.D.; Mellencamp, M.A.; Stear, M.J.; Eckersall, P.D. Characterisation of plasma acute phase protein concentrations in a high health boar herd. Vet. Immunol. Immunopathol. 2011, 139, 107–112. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, X.; Sun, Y.; Zhang, S. Activation of NF-κB contributes to production of pig-major acute protein and serum amyloid A in pigs experimentally infected with porcine circovirus type 2. Res. Vet. Sci. 2013, 95, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Pomorska-Mól, M.; Markowska-Daniel, I.; Kwit, K.; Stępniewska, K.; Pejsak, Z. C-reactive protein, haptoglobin, serum amyloid A and pig major acute phase protein response in pigs simultaneously infected with H1N1 swine influenza virus and Pasteurella multocida. BMC Vet. Res. 2013, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Bannert, E.; Tesch, T.; Kersten, S.; Frahm, J.; Bühler, S.; Sauerwein, H.; Görs, S.; Kahlert, S.; Rothkötter, H.J.; et al. Oral exposure of pigs to the mycotoxin deoxynivalenol does not modulate the hepatic albumin synthesis during a LPS-induced acute-phase reaction. Innate Immun. 2020, 26, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Muroi, M.; Kinoshita, M.; Hamada, O.; Minai, Y.; Sugita-Konishi, Y.; Kamata, Y.; Tanamoto, K. NF-κB activation via MyD88-dependent Toll-like receptor signaling is inhibited by trichothecene mycotoxin deoxynivalenol. J. Toxicol. Sci. 2016, 41, 273–279. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhao, J.; Cao, L.; Zhu, L.; Huang, Y.; Chen, X.; Rahman, S.U.; Feng, S.; Li, Y.; et al. Deoxynivalenol Induces Inflammatory Injury in IPEC-J2 Cells via NF-kappaB Signaling Pathway. Toxins 2019, 11, 12. [Google Scholar] [CrossRef]
- Li, J.; Bai, Y.; Ma, K.; Ren, Z.; Li, J.; Zhang, J.; Shan, A. Dihydroartemisinin alleviates deoxynivalenol induced liver apoptosis and inflammation in piglets. Ecotoxicol. Environ. Saf. 2022, 241, 113811. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Puel, O.; Pinton, P.; Cossalter, A.M.; Chou, T.C.; Oswald, I.P. Co-exposure to low doses of the food contaminants deoxynivalenol and nivalenol has a synergistic inflammatory effect on intestinal explants. Arch. Toxicol. 2017, 91, 2677–2687. [Google Scholar] [CrossRef]
- Ge, L.; Liu, D.; Mao, X.; Liu, S.; Guo, J.; Hou, L.; Chen, X.; Huang, K. Low Dose of Deoxynivalenol Aggravates Intestinal Inflammation and Barrier Dysfunction Induced by Enterotoxigenic Escherichia coli Infection through Activating Macroautophagy/NLRP3 Inflammasomes. J. Agric. Food Chem. 2022, 70, 3009–3022. [Google Scholar] [CrossRef]
- Vandenbroucke, V.; Croubels, S.; Martel, A.; Verbrugghe, E.; Goossens, J.; Van Deun, K.; Boyen, F.; Thompson, A.; Shearer, N.; De Backer, P.; et al. The mycotoxin deoxynivalenol potentiates intestinal inflammation by Salmonella typhimurium in porcine ileal loops. PLoS ONE 2011, 6, 23871. [Google Scholar] [CrossRef]
- Pierron, A.; Neves, M.; Puel, S.; Lippi, Y.; Soler, L.; Miller, J.D.; Oswald, I.P. Intestinal toxicity of the new type A trichothecenes, NX and 3ANX. Chemosphere 2022, 288, 132415. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Braicu, C.; Nougayrede, J.P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, S.G.; Hwang, J.H.; Park, D.H.; Kim, T.W.; Kim, C.W. Selection of appropriate reference genes for RT-qPCR analysis in Berkshire, Duroc, Landrace, and Yorkshire pigs. Gene 2015, 558, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Cano, P.M.; Seeboth, J.; Meurens, F.; Cognie, J.; Abrami, R.; Oswald, I.P.; Guzylack-Piriou, L. Deoxynivalenol as a new factor in the persistence of intestinal inflammatory diseases: An emerging hypothesis through possible modulation of Th17-mediated response. PLoS ONE 2013, 8, e53647. [Google Scholar] [CrossRef]
- Pinton, P.; Graziani, F.; Pujol, A.; Nicoletti, C.; Paris, O.; Ernouf, P.; Di Pasquale, E.; Perrier, J.; Oswald, I.P.; Maresca, M. Deoxynivalenol inhibits the expression by goblet cells of intestinal mucins through a PKR and MAP kinase dependent repression of the resistin-like molecule β. Mol. Nutr. Food Res. 2015, 59, 1076–1087. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierron, A.; Balbo, L.C.; Soler, L.; Pinton, P.; Puel, S.; Laffitte, J.; Albin, M.; Bracarense, A.-P.F.R.L.; Rodriguez, M.A.; Oswald, I.P. Deoxynivalenol Induces Local Inflammation and Lesions in Tissues at Doses Recommended by the EU. Int. J. Mol. Sci. 2024, 25, 9790. https://doi.org/10.3390/ijms25189790
Pierron A, Balbo LC, Soler L, Pinton P, Puel S, Laffitte J, Albin M, Bracarense A-PFRL, Rodriguez MA, Oswald IP. Deoxynivalenol Induces Local Inflammation and Lesions in Tissues at Doses Recommended by the EU. International Journal of Molecular Sciences. 2024; 25(18):9790. https://doi.org/10.3390/ijms25189790
Chicago/Turabian StylePierron, Alix, Luciana C. Balbo, Laura Soler, Philippe Pinton, Sylvie Puel, Joëlle Laffitte, Mickaël Albin, Ana-Paula F. R. Loureiro Bracarense, Maria A. Rodriguez, and Isabelle P. Oswald. 2024. "Deoxynivalenol Induces Local Inflammation and Lesions in Tissues at Doses Recommended by the EU" International Journal of Molecular Sciences 25, no. 18: 9790. https://doi.org/10.3390/ijms25189790
APA StylePierron, A., Balbo, L. C., Soler, L., Pinton, P., Puel, S., Laffitte, J., Albin, M., Bracarense, A.-P. F. R. L., Rodriguez, M. A., & Oswald, I. P. (2024). Deoxynivalenol Induces Local Inflammation and Lesions in Tissues at Doses Recommended by the EU. International Journal of Molecular Sciences, 25(18), 9790. https://doi.org/10.3390/ijms25189790






