Wine, Polyphenols, and the Matrix Effect: Is Alcohol Always the Same?
Abstract
:1. Introduction
2. Fundamental Concepts for the Study of Plant-Derived Bioactive Compounds
2.1. The Importance of the Phytocomplex
2.2. Vehicle, Matrix and Food Matrix
3. Polyphenols: General Properties and Lipophilic Phenols in Wine
3.1. Bioavailability
3.2. Polyphenols as Wine Matrix Constituents
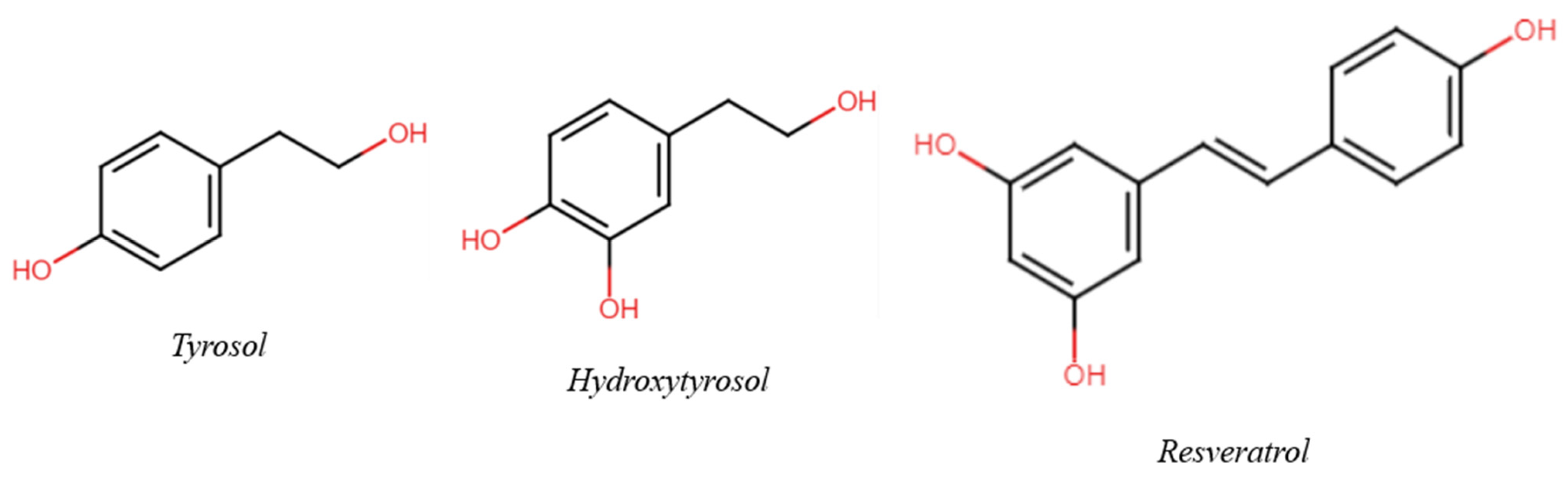
3.3. Tyrosol, Hydroxytyrosol and Resveratrol
3.3.1. When Matrix Effect Is Respected: The Case of Tyrosol and Hydroxytyrosol
3.3.2. When Matrix Effect Is Disregarded: Resveratrol
4. Alcohol and Alcoholic Beverages
4.1. Absorption and Metabolism of Ethanol
4.2. Physiological Effects and Toxicity of Alcohol
The Dark Side of the Matrix Effect: Alcohol and Smoke
4.3. When the Matrix Effect Is Ignored: Alcohol Beverages Specificity
5. In Vivo Studies on Polyphenol Biological Activities
| Reference | N° of Studies | Treatment | Results | Outcomes |
|---|---|---|---|---|
| [143] | 12 | Wine (242–94.308 mg/L/die) | ↓ CICAM-1 ↓ VCAM-1 ↓ TNF-α ↓ CCR2 ↑αMβ2 (Mac-1) | Improvement of the markers associated with atherosclerotic inflammation in healthy patients, but not in those with CVD |
| [144] | 4 | Red wine vs. white wine and/or other alcoholic beverages | / | Red wine decreased the risk of Alzheimer’s Disease |
| [145] | 4 | Grape extract (700–1400 mg/die) | ↓ blood pressure ↑ FMD | Improvement of vascular health, particularly in at risk human populations |
| [146] | 11 | Wine (0–84.9 g/die) | / | There is not a statical significant correlation between wine consumption and prostate cancer |
| [147] | 9 | Wine (118–300 mL/die) | ↓ total cholesterol ↓ diastolic blood pressure | Improvement of some cardiovascular parameters in type 2 diabetes mellitus patients |
| [148] | 99 | Red wine/grape extract (100–2000 mg/die) Red wine (250–400 mL/die) | ↓ total cholesterol ↓ blood pressure ↑ HDL-C ↑ FMD ↓TAGs | Improvement of cardiovascular health Support in the treatment of metabolic disorders |
| [149] | 6 | Red wine (moderate consumption, max one glass/die) | / | Decrease in the risk of developing Non-Hodgkin Lymphoma, epithelial ovarian cancer, prostate cancer, lung cancer, breast cancer, esophageal adenocarcinoma (Barret’s esophagus) |
6. When Natural Vehicle in Wine Is Targeted: Non-Alcoholic Alternatives to Wine
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADH | Alcohol dehydrogenase |
| ALD | Alcohol-related liver disease |
| ALDH | Acetaldehyde dehydrogenase |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| BBB | Blood–brain barrier |
| BMD | Bone Mineral Density |
| CO2 | Carbon dioxide |
| CYP2E1 | Cytochrome P450 isoform 2E1 |
| EFSA | European Food Safety Agency |
| EMA | European Medicines Agency |
| EtG | Ethyl glucuronide |
| EtOH | Ethanol |
| EtS | Ethyl sulfate |
| FAEE | Fatty acid ethyl ester |
| FMD | Flow-Mediated Dilatation |
| GABA | Gamma-aminobutyric acid |
| H2O | Water |
| HDL-C | High-Density Lipoprotein-Cholesterol |
| HNC | Head and Neck Cancer |
| HTyr | Hydroxytyrosol |
| IL | Interleukin |
| LDL | Low-Density Lipoprotein |
| MCP | Monocyte Chemoattractant Protein |
| MeOH | Methanol |
| MEOS | Microsomal ethanol-oxidizing system |
| MUFAs | Mono-Unsaturated Fatty Acids |
| PEth | Phosphatidylethanol |
| PUFAs | Poly-Unsaturated Fatty Acids |
| ROS | Reactive Oxygen Species |
| Rsv | Resveratrol |
| RWE | Red Wine Extract |
| TAG | Triacylglycerol |
| TNF | Tumor Necrosis Factor |
| Tyr | Tyrosol |
| USDA | United States Department of Agriculture |
| UV | Ultraviolet |
| WHO | World Health Organization |
References
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Katan, M.B. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1999, 37, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Biagi, M.; Pecorari, R.; Appendino, G.; Miraldi, E.; Magnano, A.R.; Governa, P.; Cettolin, G.; Giachetti, D. Herbal Products in Italy: The Thin Line between Phytotherapy, Nutrition and Parapharmaceuticals; A Normative Overview of the Fastest Growing Market in Europe. Pharmaceuticals 2016, 9, 65. [Google Scholar] [CrossRef]
- Bertuccioli, A.; Cardinali, M.; Di Pierro, F.; Zonzini, G.B.; Matera, M.R. Ketogenic and Low FODMAP Diet in Therapeutic Management of a Young Autistic Patient with Epilepsy and Dysmetabolism Poorly Responsive to Therapies: Clinical Response and Effects of Intestinal Microbiota. Int. J. Mol. Sci. 2022, 23, 8829. [Google Scholar] [CrossRef]
- Governa, P.; Manetti, F.; Miraldi, E.; Biagi, M. Effects of in vitro simulated digestion on the antioxidant activity of different Camellia sinensis (L.) Kuntze leaves extracts. Eur. Food Res. Technol. 2021, 248, 119–128. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consultation on Selected Medicinal Plants. Available online: https://iris.who.int/handle/10665/42052 (accessed on 2 August 2024).
- European Medicines Agency (EMA). Herbal Medicinal Products. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/herbal-medicinal-products (accessed on 2 August 2024).
- Ahlemeyer, B.; Krieglstein, J. Pharmacological studies supporting the therapeutic use of Ginkgo biloba extract for Alzheimer’s disease. Pharmacopsychiatry 2003, 36 (Suppl. S1), S8–S14. [Google Scholar] [CrossRef]
- Butterweck, V. Mechanism of action of St John’s wort in depression: What is known? CNS Drugs 2003, 17, 539–562. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex-02015R2283-20210327-EN. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02015R2283-20210327 (accessed on 2 August 2024).
- NAL. Agricultural Thesaurus: NALT: Food Matrix. Available online: https://agclass.nal.usda.gov/vocabularies/nalt/concept?uri=https://lod.nal.usda.gov/nalt/17238 (accessed on 2 August 2024).
- Capuano, E.; Oliviero, T.; van Boekel, M.A.J.S. Modeling food Matrix effects on chemical reactivity: Challenges and perspectives. Crit. Rev. Food Sci. Nutr. 2018, 58, 2814–2828. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Stiller, A.; Garrison, K.; Gurdyumov, K.; Kenner, J.; Yasmin, F.; Yates, P.; Song, B.H. From Fighting Critters to Saving Lives: Polyphenols in Plant Defense and Human Health. Int. J. Mol. Sci. 2021, 22, 8995. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef]
- Bernardi, S.; Del Bo’, C.; Marino, M.; Gargari, G.; Cherubini, A.; Andrés-Lacueva, C.; Hidalgo-Liberona, N.; Peron, G.; González-Dominguez, R.; Kroon, P.; et al. Polyphenols and Intestinal Permeability: Rationale and Future Perspectives. J. Agric. Food Chem. 2020, 68, 1816–1829. [Google Scholar] [CrossRef]
- Reis, A.; Perez-Gregorio, R.; Mateus, N.; de Freitas, V. Interactions of dietary polyphenols with epithelial lipids: Advances from membrane and cell models in the study of polyphenol absorption, transport and delivery to the epithelium. Crit. Rev. Food Sci. Nutr. 2021, 61, 3007–3030. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Terracone, C.; Gambacorta, G.; La Notte, E. Phenolic content and antioxidant activity of Primitivo wine: Comparison among winemaking technologies. J. Food Sci. 2009, 74, C258–C267. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. S6), 1402S–1406S. [Google Scholar] [CrossRef]
- Ditano-Vázquez, P.; Torres-Peña, J.D.; Galeano-Valle, F.; Pérez-Caballero, A.I.; Demelo-Rodríguez, P.; Lopez-Miranda, J.; Katsiki, N.; Delgado-Lista, J.; Alvarez-Sala-Walther, L.A. The Fluid Aspect of the Mediterranean Diet in the Prevention and Management of Cardiovascular Disease and Diabetes: The Role of Polyphenol Content in Moderate Consumption of Wine and Olive Oil. Nutrients 2019, 11, 2833. [Google Scholar] [CrossRef]
- Visioli, F.; Panaite, S.A.; Tomé-Carneiro, J. Wine’s Phenolic Compounds and Health: A Pythagorean View. Molecules 2020, 25, 4105. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; De Sanctis, P.; Giovannini, C.; D’Archivio, M.; Masella, R. Anti-inflammatory Activity of Extra Virgin Olive Oil Polyphenols: Which Role in the Prevention and Treatment of Immune-Mediated Inflammatory Diseases? Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 36–50. [Google Scholar] [CrossRef]
- Boronat, A.; Mateus, J.; Soldevila-Domenech, N.; Guerra, M.; Rodríguez-Morató, J.; Varon, C.; Muñoz, D.; Barbosa, F.; Morales, J.C.; Gaedigk, A.; et al. Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free Radic. Biol. Med. 2019, 143, 471–481. [Google Scholar] [CrossRef]
- D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Mañá, C.; Farré, M.; Rodríguez-Morató, J.; Papaseit, E.; Pujadas, M.; Fitó, M.; Robledo, P.; Covas, M.I.; Cheynier, V.; Meudec, E.; et al. Moderate consumption of wine, through both its phenolic compounds and alcohol content, promotes hydroxytyrosol endogenous generation in humans. A randomized controlled trial. Mol. Nutr. Food Res. 2015, 59, 1213–1216. [Google Scholar] [CrossRef]
- Warleta, F.; Quesada, C.S.; Campos, M.; Allouche, Y.; Beltrán, G.; Gaforio, J.J. Hydroxytyrosol protects against oxidative DNA damage in human breast cells. Nutrients 2011, 3, 839–857. [Google Scholar] [CrossRef] [PubMed]
- Soldevila-Domenech, N.; Boronat, A.; Mateus, J.; Diaz-Pellicer, P.; Matilla, I.; Pérez-Otero, M.; Aldea-Perona, A.; de la Torre, R. Generation of the Antioxidant Hydroxytyrosol from Tyrosol Present in Beer and Red Wine in a Randomized Clinical Trial. Nutrients 2019, 11, 2241. [Google Scholar] [CrossRef]
- Miró-Casas, E.; Covas, M.I.; Fitó, M.; Farré-Albadalejo, M.; Marrugat, J.; de la Torre, R. Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur. J. Clin. Nutr. 2003, 57, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, Á. Pharmacokinetics and bioavailability of hydroxytyrosol are dependent on the food Matrix in humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Sakavitsi, M.E.; Breynaert, A.; Nikou, T.; Lauwers, S.; Pieters, L.; Hermans, N.; Halabalaki, M. Availability and Metabolic Fate of Olive Phenolic Alcohols Hydroxytyrosol and Tyrosol in the Human GI Tract Simulated by the In Vitro GIDM-Colon Model. Metabolites 2022, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of hydroxytyrosol as a novel food pursuant to Regulation (EC) No 258/97. EFSA Journal. Eur. Food Saf. Auth. 2017, 15, e04728. [Google Scholar] [CrossRef]
- Implementing Decision—2017/2373—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32017D2373 (accessed on 2 August 2024).
- Decision of the Intergovernmental Committee: 5.COM 6.41—Intangible Heritage—Culture Sector—UNESCO. Available online: https://ich.unesco.org/en/decisions/5.COM/6.41 (accessed on 20 August 2024).
- Santos-Buelga, C.; González-Manzano, S.; González-Paramás, A.M. Wine, Polyphenols, and Mediterranean Diets. What Else Is There to Say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef]
- Gallardo-Fernández, M.; Gonzalez-Ramirez, M.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Hydroxytyrosol in Foods: Analysis, Food Sources, EU Dietary Intake, and Potential Uses. Foods 2022, 11, 2355. [Google Scholar] [CrossRef]
- Pezzuto, J.M. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol. Ther. 2019, 27, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef] [PubMed]
- Bandiwadekar, A.; Jose, J.; Gopan, G.; Augustin, V.; Ashtekar, H.; Khot, K.B. Transdermal delivery of resveratrol loaded solid lipid nanoparticle as a microneedle patch: A novel approach for the treatment of Parkinson’s disease. Drug Deliv. Transl. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ferraz da Costa, D.C.; Pereira Rangel, L.; Quarti, J.; Santos, R.A.; Silva, J.L.; Fialho, E. Bioactive Compounds and Metabolites from Grapes and Red Wine in Breast Cancer Chemoprevention and Therapy. Molecules 2020, 25, 3531. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Sessa, M.; Balestrieri, M.L.; Ferrari, G.; Servillo, L.; Castaldo, D.; D’Onofrio, N.; Donsì, F.; Tsao, R. Bioavailability of encapsulated resveratrol into nanoemulsion-based delivery systems. Food Chem. 2014, 147, 42–50. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Giovannini, L.; Bernini, W.; Migliori, M.; Fregoni, M.; Bavaresco, L.; Bertelli, A. Antiplatelet activity of cis-resveratrol. Drugs Exp. Clin. Res. 1996, 22, 61–63. [Google Scholar]
- Nair, M.S. Spectroscopic study on the interaction of resveratrol and pterostilbene with human serum albumin. J. Photochem. Photobiol. B Biol. 2015, 149, 58–67. [Google Scholar] [CrossRef]
- Geng, T.; Zhao, X.; Ma, M.; Zhu, G.; Yin, L. Resveratrol-Loaded Albumin Nanoparticles with Prolonged Blood Circulation and Improved Biocompatibility for Highly Effective Targeted Pancreatic Tumor Therapy. Nanoscale Res. Lett. 2017, 12, 437. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Y.; Liu, H.; Yuan, J.; Zheng, Z.; Zou, G. Transport of a Cancer Chemopreventive Polyphenol, Resveratrol: Interaction with Serum Albumin and Hemoglobin. J. Fluoresc. 2007, 17, 580–587. [Google Scholar] [CrossRef]
- Bertelli, A.A.E. Wine, research and cardiovascular disease: Instructions for use. Atherosclerosis 2007, 195, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.A.; Giovannini, L.; Stradi, R.; Urien, S.; Tillement, J.P.; Bertelli, A. Kinetics of trans- and cis-resveratrol (3,4′,5-trihydroxystilbene) after red wine oral administration in rats. Int. J. Clin. Pharmacol. Res. 1996, 16, 77–81. [Google Scholar] [PubMed]
- Urpi-Sarda, M.; Zamora-Ros, R.; Lamuela-Raventos, R.; Cherubini, A.; Jauregui, O.; de la Torre, R.; Covas, M.I.; Estruch, R.; Jaeger, W.; Andres-Lacueva, C. HPLC-tandem mass spectrometric method to characterize resveratrol metabolism in humans. Clin. Chem. 2007, 53, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol. Res. 2012, 66, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation with Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Bertelli, A.A.; Giovannini, L.; Giannessi, D.; Migliori, M.; Bernini, W.; Fregoni, M.; Bertelli, A. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int. J. Tissue React. 1995, 17, 1–3. [Google Scholar]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Sánchez-Ortiz, A.; Mateo-Sanz, J.M.; Mateos-Fernández, M.A.; Figueras, M.L. New Labeling Rules for Wine: Wine Alcohol-Derived Calories and Polyphenol Consumption on Health. Foods 2024, 13, 295. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Addolorato, G.; Capristo, E.; Greco, A.V.; Stefanini, G.F.; Gasbarrini, G. Energy expenditure, substrate oxidation, and body composition in subjects with chronic alcoholism: New findings from metabolic assessment. Alcohol. Clin. Exp. Res. 1997, 21, 962–967. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Alcohol metabolism. Clin. Liver Dis. 2012, 16, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Kwo, P.Y.; Ramchandani, V.A.; O’Connor, S.; Amann, D.; Carr, L.G.; Sandrasegaran, K.; Kopecky, K.K.; Li, T.K. Gender differences in alcohol metabolism: Relationship to liver volume and effect of adjusting for body mass. Gastroenterology 1998, 115, 1552–1557. [Google Scholar] [CrossRef] [PubMed]
- Lands, W.E. Alcohol and energy intake. Am. J. Clin. Nutr. 1995, 62 (Suppl. S5), 1101S–1106S. [Google Scholar] [CrossRef]
- Pikaar, N.A.; Wedel, M.; Hermus, R.J. Influence of several factors on blood alcohol concentrations after drinking alcohol. Alcohol Alcohol. 1988, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, P.K.; Sedman, A.J.; Sakmar, E.; Kay, D.R.; Wagner, J.G. Pharmacokinetics of ethanol after oral administration in the fasting state. J. Pharmacokinet. Biopharm. 1977, 5, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Comporti, M.; Signorini, C.; Leoncini, S.; Gardi, C.; Ciccoli, L.; Giardini, A.; Vecchio, D.; Arezzini, B. Ethanol-inducedoxidative stress: Basic knowledge. Genes Nutr. 2010, 5, 101–109. [Google Scholar] [CrossRef]
- Heier, C.; Xie, H.; Zimmermann, R. Nonoxidative ethanol metabolism in humans-from biomarkers to bioactive lipids. IUBMB Life 2016, 68, 916–923. [Google Scholar] [CrossRef]
- Holford, N.H. Clinical pharmacokinetics of ethanol. Clin. Pharmacokinet. 1987, 13, 273–292. [Google Scholar] [CrossRef]
- Laposata, E.A.; Lange, L.G. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science 1986, 231, 497–499. [Google Scholar] [CrossRef]
- Lieber, C.S. Ethanol metabolism, cirrhosis and alcoholism. Clin. Acta Int. J. Clin. Chem. 1997, 257, 59–84. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health 2006, 29, 245–254. [Google Scholar] [PubMed]
- Hendriks, H.F.J. Alcohol and Human Health: What Is the Evidence? Annu. Rev. Food Sci. Technol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Holstege, A.; Bedossa, P.; Poynard, T.; Kollinger, M.; Chaput, J.C.; Houglum, K.; Chojkier, M. Acetaldehyde-modified epitopes in liver biopsy specimens of alcoholic and nonalcoholic patients: Localization and association with progression of liver fibrosis. Hepatology 1994, 19, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Han, J.; Lee, C.; Yoon, M.; Jung, Y. Pathophysiological Aspects of Alcohol Metabolism in the Liver. Int. J. Mol. Sci. 2021, 22, 5717. [Google Scholar] [CrossRef]
- Lieber, C.S. Hepatic and metabolic effects of ethanol: Pathogenesis and prevention. Ann. Med. 1994, 26, 325–330. [Google Scholar] [CrossRef]
- Setshedi, M.; Wands, J.R.; Monte, S.M. Acetaldehyde adducts in alcoholic liver disease. Oxidative Med. Cell. Longev. 2010, 3, 178–185. [Google Scholar] [CrossRef]
- Tuma, D.J.; Casey, C.A. Dangerous byproducts of alcohol breakdown—Focus on adducts. Alcohol Res. Health 2003, 27, 285–290. [Google Scholar]
- Wu, D.; Cederbaum, A.I. Alcohol, oxidative stress, and free radical damage. Alcohol Res. Health 2003, 27, 277–284. [Google Scholar]
- Seth, D.; Haber, P.S.; Syn, W.K.; Diehl, A.M.; Day, C.P. Pathogenesis of alcohol-induced liver disease: Classical concepts and recent advances. J. Gastroenterol. Hepatol. 2011, 26, 1089–1105. [Google Scholar] [CrossRef]
- Goldstein, B.Y.; Chang, S.C.; Hashibe, M.; La Vecchia, C.; Zhang, Z.F. Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: An update. Eur. J. Cancer Prev. 2010, 19, 431–465. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Gallus, S.; Garavello, W.; Bosetti, C.; La Vecchia, C. Alcohol and tobacco use, and cancer risk for upper aerodigestive tract and liver. Eur. J. Cancer Prev. 2008, 17, 340–344. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Personal Habits and Indoor Combustions; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100 E, pp. 1–538. [Google Scholar]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Schatzkin, A.; Leitzmann, M.F.; Hollenbeck, A.R.; Abnet, C.C. Alcohol and head and neck cancer risk in a prospective study. Br. J. Cancer 2007, 96, 1469–1474. [Google Scholar] [CrossRef]
- Purdue, M.P.; Hashibe, M.; Berthiller, J.; La Vecchia, C.; Dal Maso, L.; Herrero, R.; Franceschi, S.; Castellsague, X.; Wei, Q.; Sturgis, E.M.; et al. Type of alcoholic beverage and risk of head and neck cancer--a pooled analysis within the INHANCE Consortium. Am. J. Epidemiol. 2009, 169, 132–142. [Google Scholar] [CrossRef]
- Freedman, N.D.; Abnet, C.C.; Leitzmann, M.F.; Hollenbeck, A.R.; Schatzkin, A. Prospective investigation of the cigarette smoking-head and neck cancer association by sex. Cancer 2007, 110, 1593–1601. [Google Scholar] [CrossRef]
- Gandini, S.; Botteri, E.; Iodice, S.; Boniol, M.; Lowenfels, A.B.; Maisonneuve, P.; Boyle, P. Tobacco smoking and cancer: A meta-analysis. Int. J. Cancer 2008, 122, 155–164. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; Fernandez, L.; et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007, 99, 777–789. [Google Scholar] [CrossRef]
- Lubin, J.H.; Purdue, M.; Kelsey, K.; Zhang, Z.F.; Winn, D.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; Sturgis, E.M.; Smith, E.; et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: A pooled analysis of case-control studies. Am. J. Epidemiol. 2009, 170, 937–947. [Google Scholar] [CrossRef]
- Marron, M.; Boffetta, P.; Zhang, Z.F.; Zaridze, D.; Wünsch-Filho, V.; Winn, D.M.; Wei, Q.; Talamini, R.; Szeszenia-Dabrowska, N.; Sturgis, E.M.; et al. Cessation of alcohol drinking, tobacco smoking and the reversal of head and neck cancer risk. Int. J. Epidemiol. 2010, 39, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Zeka, A.; Gore, R.; Kriebel, D. Effects of alcohol and tobacco on aerodigestive cancer risks: A meta-regression analysis. Cancer Causes Control CCC 2003, 14, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Maasland, D.H.; van den Brandt, P.A.; Kremer, B.; Goldbohm, R.A.; Schouten, L.J. Alcohol consumption, cigarette smoking and the risk of subtypes of head-neck cancer: Results from the Netherlands Cohort Study. BMC Cancer 2014, 14, 187. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lodi, G.; Iriti, M. Ethanol versus Phytochemicals in Wine: Oral Cancer Risk in a Light Drinking Perspective. Int. J. Mol. Sci. 2015, 16, 17029–17047. [Google Scholar] [CrossRef]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; Ghissassi, F.E.; Bouvard, V.; Altieri, A.; Cogliano, V. Carcinogenicity of alcoholic beverages. Lancet Oncol. Lancet Oncol. 2007, 8, 292–293. [Google Scholar] [CrossRef]
- Kubo, A.; Levin, T.R.; Block, G.; Rumore, G.J.; Quesenberry, C.P., Jr.; Buffler, P.; Corley, D.A. Alcohol types and sociodemographic characteristics as risk factors for Barrett’s esophagus. Gastroenterology 2009, 136, 806–815. [Google Scholar] [CrossRef]
- Vioque, J.; Barber, X.; Bolumar, F.; Porta, M.; Santibáñez, M.; de la Hera, M.G.; Moreno-Osset, E.; PANESOES Study Group. Esophageal cancer risk by type of alcohol drinking and smoking: A case-control study in Spain. BMC Cancer 2008, 8, 221. [Google Scholar] [CrossRef]
- Anderson, L.A.; Cantwell, M.M.; Watson, R.G.; Johnston, B.T.; Murphy, S.J.; Ferguson, H.R.; McGuigan, J.; Comber, H.; Reynolds, J.V.; Murray, L.J. The association betweenalcohol and reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Gastroenterology 2009, 136, 799–805. [Google Scholar] [CrossRef]
- Pandeya, N.; Williams, G.; Green, A.C.; Webb, P.M.; Whiteman, D.C. Australian Cancer Study Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology 2009, 136, 1215-e2. [Google Scholar] [CrossRef]
- Gammon, M.D.; Schoenberg, J.B.; Ahsan, H.; Risch, H.A.; Vaughan, T.L.; Chow, W.H.; Rotterdam, H.; West, A.B.; Dubrow, R.; Stanford, J.L.; et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J. Natl. Cancer Inst. 1997, 89, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Grønbaek, M. Epidemiologic evidence for the cardioprotective effects associated with consumption of alcoholic beverages. Pathophysiology 2004, 10, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, J.S. Long-Term Health Outcomes of Regular, Moderate Red Wine Consumption. Cureus 2023, 15, e46786. [Google Scholar] [CrossRef] [PubMed]
- Cucciniello, R.; Tomasini, M.; Russo, A.; Falivene, L.; Gambuti, A.; Forino, M. Experimental and theoretical studies on the acetaldehyde reaction with (+)-catechin. Food Chem. 2023, 426, 136556. [Google Scholar] [CrossRef]
- Maciel, M.E.; Castro, J.A.; Castro, G.D. Inhibition of rat mammary microsomal oxidation of ethanol to acetaldehyde by plant polyphenols. Hum. Exp. Toxicol. 2011, 30, 656–664. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Kumatoriya, K.; Tando, M.; Kometani, T.; Shinohara, M. Polyphenols from persimmon fruit attenuate acetaldehyde-induced DNA double-strand breaks by scavenging acetaldehyde. Sci. Rep. 2022, 12, 10300. [Google Scholar] [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, L.; Xu, J.; Tang, J. The antioxidant (-)-epigallocatechin-3-gallate inhibits activated hepatic stellate cell growth and suppresses acetaldehyde-induced gene expression. Biochem. J. 2002, 368 Pt 3, 695–704. [Google Scholar] [CrossRef]
- Silva, P.; Latruffe, N.; de Gaetano, G. Wine Consumption and Oral Cavity Cancer: Friend or Foe, Two Faces of Janus. Molecules 2020, 25, 2569. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.Y.; Mou, Y.H.; Wang, L.H.; Zhou, Y.N.; Wu, C.F. Differences in the activities of resveratrol and ascorbic acid in protection of ethanol-induced oxidative DNA damage in human peripheral lymphocytes. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2012, 50, 168–174. [Google Scholar] [CrossRef]
- Chen, C.H.; Budas, G.R.; Churchill, E.N.; Disatnik, M.H.; Hurley, T.D.; Mochly-Rosen, D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science 2008, 321, 1493–1495. [Google Scholar] [CrossRef]
- Gál, R.; Halmosi, R.; Gallyas, F., Jr.; Tschida, M.; Mutirangura, P.; Tóth, K.; Alexy, T.; Czopf, L. Resveratrol and beyond: The Effect of Natural Polyphenols on the Cardiovascular System: A Narrative Review. Biomedicines 2023, 11, 2888. [Google Scholar] [CrossRef]
- Corder, R.; Mullen, W.; Khan, N.Q.; Marks, S.C.; Wood, E.G.; Carrier, M.J.; Crozier, A. Oenology: Red wine procyanidins and vascular health. Nature 2006, 444, 566. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- van Bussel, B.C.T.; Henry, R.M.A.; Schalkwijk, C.G.; Dekker, J.M.; Nijpels, G.; Feskens, E.J.M.; Stehouwer, C.D.A. Alcohol and red wine consumption, but not fruit, vegetables, fish or dairy products, are associated with less endothelial dysfunction and less low-grade inflammation: The Hoorn Study. Eur. J. Nutr. 2018, 57, 1409–1419. [Google Scholar] [CrossRef]
- Larsen, B.A.; Klinedinst, B.S.; Le, S.T.; Pappas, C.; Wolf, T.; Meier, N.F.; Lim, Y.L.; Willette, A.A. Beer, wine, and spirits differentially influence body composition in older white adults-a United Kingdom Biobank study. Obes. Sci. Pract. 2022, 8, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Whincup, P.H. Alcohol and adiposity: Effects of quantity and type of drink and time relation with meals. Int. J. Obes. 2005, 29, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Isath, A.; Rosenson, R.S.; Khawaja, M.; Wang, Z.; Fogg, S.E.; Virani, S.S.; Qi, L.; Cao, Y.; Long, M.T.; et al. Alcohol Consumption and Cardiovascular Health. Am. J. Med. 2022, 135, 1213–1230.e3. [Google Scholar] [CrossRef] [PubMed]
- Schutte, R.; Papageorgiou, M.; Najlah, M.; Huisman, H.W.; Ricci, C.; Zhang, J.; Milner, N.; Schutte, A.E. Drink types unmask the health risks associated with alcohol intake—Prospective evidence from the general population. Clin. Nutr. 2020, 39, 3168–3174. [Google Scholar] [CrossRef]
- GBD 2020 Alcohol Collaborators. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: A systematic analysis for the Global Burden of Disease Study 2020. Lancet 2022, 400, 185–235. [Google Scholar] [CrossRef]
- WHO. WHO Scientific Group on Cardiovascular Disease Risk Factors—New Areas for Research Cardiovascular Disease Risk Factors: New Areas for Research: Report of a WHO Scientific Group. World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 1994; Volume 841, pp. 1–53. [Google Scholar]
- Santos-Buelga, C.; González-Paramás, A.M.; Oludemi, T.; Ayuda-Durán, B.; González-Manzano, S. Plant phenolics as functional food ingredients. Adv. Food Nutr. Res. 2019, 90, 183–257. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Herranz-López, M.; Joven, J.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A.; Micol, V. Molecular promiscuity of plant polyphenols in the management of age-related diseases: Far beyond their antioxidant properties. Adv. Exp. Med. Biol. 2014, 824, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Joven, J.; Micol, V.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A. Bioactive Food Components Platform Polyphenols and the modulation of gene expression pathways: Can we eat our way out of the danger of chronic disease? Crit. Rev. Food Sci. Nutr. 2014, 54, 985–1001. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; Jáuregui, O.; Mena, P.; Hanhineva, K.; Tinahones, F.J.; Angelino, D.; Andrés-Lacueva, C. Quantifying the human diet in the crosstalk between nutrition and health by multi-targeted metabolomics of food and microbiota-derived metabolites. Int. J. Obes. 2020, 44, 2372–2381. [Google Scholar] [CrossRef]
- Bladé, C.; Arola, L.; Salvadó, M.J. Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59. [Google Scholar] [CrossRef]
- Faria, A.; Meireles, M.; Fernandes, I.; Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; de Freitas, V.; Mateus, N.; Calhau, C. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014, 149, 190–196. [Google Scholar] [CrossRef]
- Talavéra, S.; Felgines, C.; Texier, O.; Besson, C.; Gil-Izquierdo, A.; Lamaison, J.L.; Rémésy, C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005, 53, 3902–3908. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, L.; Hu, G.; Chen, X.; Niu, F.; Yuan, L.; Liu, H.; Xiong, H.; Arikkath, J.; Buch, S. Regulation of morphine-induced synaptic alterations: Role of oxidative stress, ER stress, and autophagy. J. Cell Biol. 2016, 215, 245–258. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R.; et al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remón, A.; Lamuela-Raventos, R.M.; et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharmacol. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J. Bone Miner. Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Kutleša, Z.; Budimir Mršić, D. Wine and bone health: A review. J. Bone Miner. Metab. 2016, 34, 11–22. [Google Scholar] [CrossRef]
- Gorelik, S.; Ligumsky, M.; Kohen, R.; Kanner, J. The stomach as a “bioreactor”: When red meat meets red wine. J. Agric. Food Chem. 2008, 56, 5002–5007. [Google Scholar] [CrossRef]
- Timmers, P.R.H.J.; Wilson, J.F.; Joshi, P.K.; Deelen, J. Multivariate genomic scan implicates novel loci and haem metabolism in human ageing. Nat. Commun. 2020, 11, 3570. [Google Scholar] [CrossRef]
- Brizuela, L.; Dayon, A.; Doumerc, N.; Ader, I.; Golzio, M.; Izard, J.C.; Hara, Y.; Malavaud, B.; Cuvillier, O. The sphingosine kinase-1 survival pathway is a molecular target for the tumor-suppressive tea and wine polyphenols in prostate cancer. FASEB J. 2010, 24, 3882–3894. [Google Scholar] [CrossRef]
- He, X.; Andersson, G.; Lindgren, U.; Li, Y. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem. Biophys. Res. Commun. 2010, 401, 356–362. [Google Scholar] [CrossRef]
- Habold, C.; Momken, I.; Ouadi, A.; Bekaert, V.; Brasse, D. Effect of prior treatment with resveratrol on density and structure of rat long bones under tail-suspension. J. Bone Miner. Metab. 2011, 29, 15–22. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, G.; Yang, J.; Pan, J.; Sha, B.; Luo, M.; Yang, W.; Liu, J.; Zeng, L. Effects of resveratrol in an animal model of osteoporosis: A meta-analysis of preclinical evidence. Front. Nutr. 2023, 10, 1234756. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Valls-Fonayet, J.; Richard, T.; Cantos-Villar, E. A rapid quantification of stilbene content in wine by ultra-high pressure liquid chromatography–Mass spectrometry. Food Control 2020, 108, 106821. [Google Scholar] [CrossRef]
- Beaumont, P.; Courtois, A.; Atgié, C.; Richard, T.; Krisa, S. In the shadow of resveratrol: Biological activities of epsilon-viniferin. J. Physiol. Biochem. 2022, 78, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate Wine Consumption and Health: A Narrative Review. Nutrients 2022, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Meng, G.; Li, G.; Wang, J. Red wine alleviates atherosclerosis-related inflammatory markers in healthy subjects rather than in high cardiovascular risk subjects: A systematic review and meta-analysis. Medicine 2024, 103, e38229. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Feng, Y. Alcohol consumption and risk of Alzheimer’s disease: A dose-response meta-analysis. Geriatr. Gerontol. Int. 2022, 22, 278–285. [Google Scholar] [CrossRef]
- Weaver, S.R.; Rendeiro, C.; McGettrick, H.M.; Philp, A.; Lucas, S.J.E. Fine wine or sour grapes? A systematic review and meta-analysis of the impact of red wine polyphenols on vascular health. Eur. J. Nutr. 2021, 60, 1–28. [Google Scholar] [CrossRef]
- Hong, S.; Khil, H.; Lee, D.H.; Keum, N.; Giovannucci, E.L. Alcohol Consumption and the Risk of Prostate Cancer: A Dose-Response Meta-Analysis. Nutrients 2020, 12, 2188. [Google Scholar] [CrossRef]
- Ye, J.; Chen, X.; Bao, L. Effects of wine on blood pressure, glucose parameters, and lipid profile in type 2 diabetes mellitus: A meta-analysis of randomized interventional trials (PRISMA Compliant). Medicine 2019, 98, e15771. [Google Scholar] [CrossRef]
- García-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andrés-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Konic Ristic, A.; Hollands, W.J.; et al. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef]
- Sancho, M.; Mach, N. Efecto de lospolifenoles del vino sobre la prevención del cáncer [Effects of wine polyphenols on cancer prevention]. Nutr. Hosp. 2014, 31, 535–551. [Google Scholar] [CrossRef]
- Meng, X.; Maliakal, P.; Lu, H.; Lee, M.J.; Yang, C.S. Urinary and plasma levels of resveratrol and quercetin in humans, mice, and rats after ingestion of pure compounds and grape juice. J. Agric. Food Chem. 2004, 52, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, L.; Pitozzi, V.; Luceri, C.; Giannini, L.; Toti, S.; Salvini, S.; Sera, F.; Souquet, J.M.; Cheynier, V.; Sofi, F.; et al. Effects of de-alcoholised wines with different polyphenol content on DNA oxidative damage, gene expression of peripheral lymphocytes, and haemorheology: An intervention study in post-menopausal women. Eur. J. Nutr. 2011, 50, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Naissides, M.; Mamo, J. Polyphenolics and fat absorption. Int. J. Obes. 2004, 28, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Boban, M.; Modun, D.; Music, I.; Vukovic, J.; Brizic, I.; Salamunic, I.; Obad, A.; Palada, I.; Dujic, Z. Red wine induced modulation of vascular function: Separating the role of polyphenols, ethanol, and urates. J. Cardiovasc. Pharmacol. 2006, 47, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Lekakis, J.; Rallidis, L.S.; Andreadou, I.; Vamvakou, G.; Kazantzoglou, G.; Magiatis, P.; Skaltsounis, A.L.; Kremastinos, D.T. Polyphenolic compounds from red grapes acutely improve endothelial function in patients with coronary heart disease. Eur. J. Prev. Cardiol. 2005, 12, 596–600. [Google Scholar] [CrossRef]
- van Mierlo, L.A.; Zock, P.L.; van der Knaap, H.C.; Draijer, R. Grape polyphenols do not affect vascular function in healthy men. J. Nutr. 2010, 140, 1769–1773. [Google Scholar] [CrossRef]
- Wallerath, T.; Li, H.; Gödtel-Ambrust, U.; Schwarz, P.M.; Förstermann, U. A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase. Nitric Oxide Biol. Chem. 2005, 12, 97–104. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.; Zhao, A.; Wang, K.; Fan, Z.; Yang, H.; Liao, W.; Bao, S.; Zhao, L.; Zhang, Y.; et al. Transcriptomic and metabonomic profiling reveal synergistic effects of quercetin and resveratrol supplementation in high fat diet fed mice. J. Proteome Res. 2012, 11, 4961–4971. [Google Scholar] [CrossRef]
- Chan, M.M.; Mattiacci, J.A.; Hwang, H.S.; Shah, A.; Fong, D. Synergy between ethanol and grape polyphenols, quercetin, and resveratrol, in the inhibition of the inducible nitric oxide synthase pathway. Biochem. Pharmacol. 2000, 60, 1539–1548. [Google Scholar] [CrossRef]
- Spaak, J.; Tomlinson, G.; McGowan, C.L.; Soleas, G.J.; Morris, B.L.; Picton, P.; Notarius, C.F.; Floras, J.S. Dose-related effects of red wine and alcohol on heart rate variability. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H2226–H2231. [Google Scholar] [CrossRef]
- Stranieri, C.; Guzzo, F.; Gambini, S.; Cominacini, L.; Fratta Pasini, A.M. Intracellular Polyphenol Wine Metabolites Oppose Oxidative Stress and Upregulate Nrf2/ARE Pathway. Antioxidants 2022, 11, 2055. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Supplementation with Red Wine Extract Increases Insulin Sensitivity and Peripheral Blood Mononuclear Sirt1 Expression in Nondiabetic Humans. Nutrients 2020, 12, 3108. [Google Scholar] [CrossRef] [PubMed]
- Mannari, C.; Bertelli, A.A.E.; Stiaccini, G.; Giovannini, L. Wine, sirtuins and nephroprotection: Not only resveratrol. Med. Hypotheses 2010, 75, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Resolution OENO 8/2003—Specificity of Wine and Scientific Research. Available online: https://www.oiv.int/public/medias/8284/oeno-8-2003-en.pdf (accessed on 2 August 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miraldi, E.; Baini, G.; Biagi, M.; Cappellucci, G.; Giordano, A.; Vaccaro, F.; Bertelli, A.A.E. Wine, Polyphenols, and the Matrix Effect: Is Alcohol Always the Same? Int. J. Mol. Sci. 2024, 25, 9796. https://doi.org/10.3390/ijms25189796
Miraldi E, Baini G, Biagi M, Cappellucci G, Giordano A, Vaccaro F, Bertelli AAE. Wine, Polyphenols, and the Matrix Effect: Is Alcohol Always the Same? International Journal of Molecular Sciences. 2024; 25(18):9796. https://doi.org/10.3390/ijms25189796
Chicago/Turabian StyleMiraldi, Elisabetta, Giulia Baini, Marco Biagi, Giorgio Cappellucci, Alessandro Giordano, Federica Vaccaro, and Alberto A. E. Bertelli. 2024. "Wine, Polyphenols, and the Matrix Effect: Is Alcohol Always the Same?" International Journal of Molecular Sciences 25, no. 18: 9796. https://doi.org/10.3390/ijms25189796







