The Role of the CX3CR1-CX3CL1 Axis in Respiratory Syncytial Virus Infection and the Triggered Immune Response
Abstract
1. Generalities
2. Infection
3. Biological Aspects of CX3CL1-CX3CR1
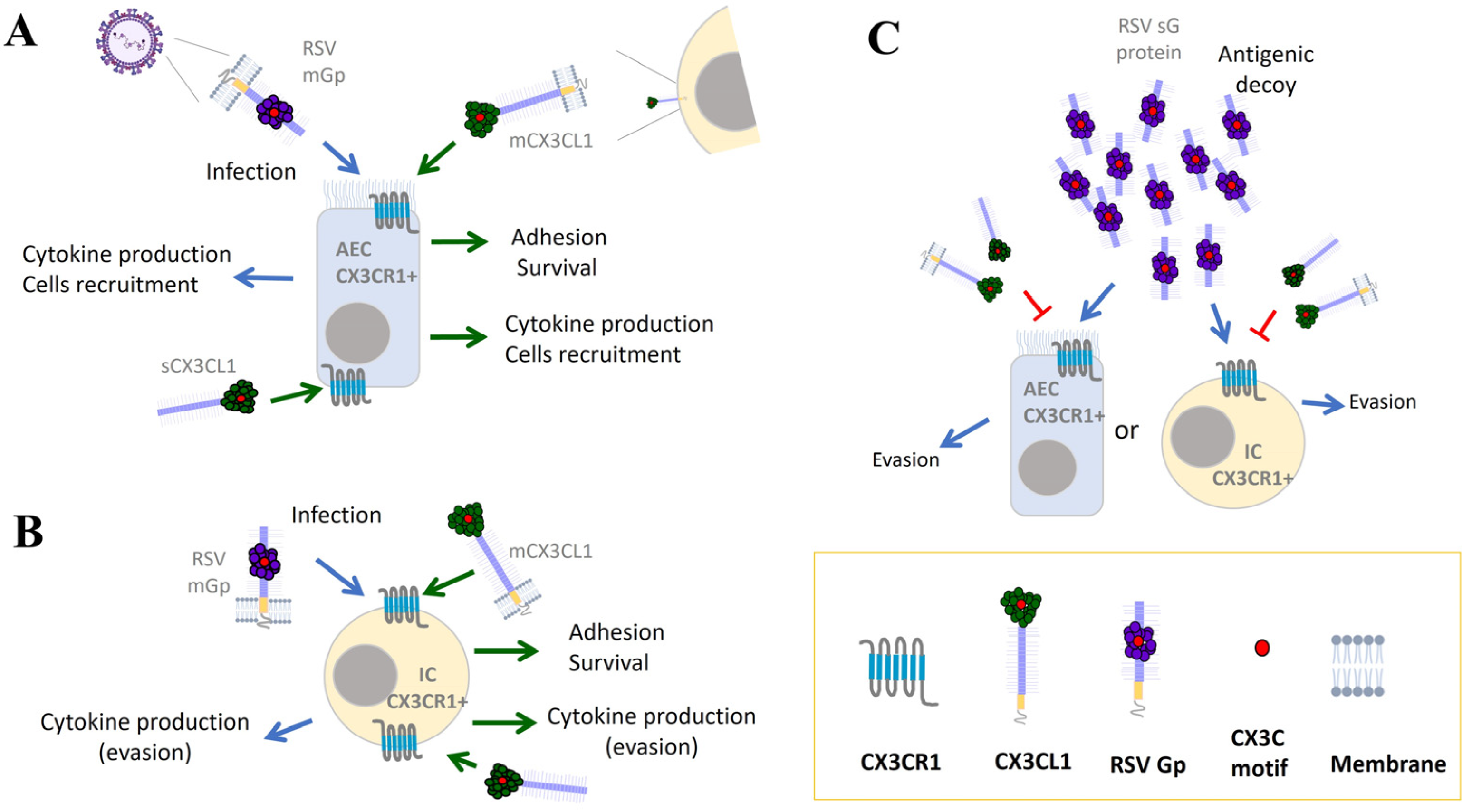
4. CX3CR1 and RSV Infection
5. Immune Response against RSV
6. CX3CR1-CX3CL1 Contribution to the Immune Response against RSV
7. Future Directions
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lehners, N.; Tabatabai, J.; Prifert, C.; Wedde, M.; Puthenparambil, J.; Weissbrich, B.; Biere, B.; Schweiger, B.; Egerer, G.; Schnitzler, P. Long-Term Shedding of Influenza Virus, Parainfluenza Virus, Respiratory Syncytial Virus and Nosocomial Epidemiology in Patients with Hematological Disorders. PLoS ONE 2016, 11, e0148258. [Google Scholar] [CrossRef]
- Malinczak, C.-A.; Lukacs, N.W.; Fonseca, W. Early-Life Respiratory Syncytial Virus Infection, Trained Immunity and Subsequent Pulmonary Diseases. Viruses 2020, 12, 505. [Google Scholar] [CrossRef]
- Eisenhut, M. Extrapulmonary Manifestations of Severe Respiratory Syncytial Virus Infection—A Systematic Review. Crit. Care 2006, 10, R107. [Google Scholar] [CrossRef]
- Bottino, P.; Miglino, R.; Pastrone, L.; Barbui, A.M.; Botta, G.; Zanotto, E.; Sidoti, F.; Costa, C.; Cavallo, R. Clinical Features of Respiratory Syncytial Virus Bronchiolitis in an Infant: Rapid and Fatal Brain Involvement. BMC Pediatr. 2021, 21, 556. [Google Scholar] [CrossRef]
- Soni, A.; Kabra, S.K.; Lodha, R. Respiratory Syncytial Virus Infection: An Update. Indian J. Pediatr. 2023, 90, 1245–1253. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Children Younger than 5 Years in 2019: A Systematic Analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- Arola, M.; Ruuskanen, O.; Ziegler, T.; Mertsola, J.; Näntö-Salonen, K.; Putto-Laurila, A.; Viljanen, M.K.; Halonen, P. Clinical Role of Respiratory Virus Infection in Acute Otitis Media. Pediatrics 1990, 86, 848–855. [Google Scholar] [CrossRef]
- Belongia, E.A.; King, J.P.; Kieke, B.A.; Pluta, J.; Al-Hilli, A.; Meece, J.K.; Shinde, V. Clinical Features, Severity, and Incidence of RSV Illness During 12 Consecutive Seasons in a Community Cohort of Adults ≥ 60 Years Old. Open Forum Infect. Dis. 2018, 5, ofy316. [Google Scholar] [CrossRef]
- Moyes, J.; Walaza, S.; Pretorius, M.; Groome, M.; von Gottberg, A.; Wolter, N.; Haffejee, S.; Variava, E.; Cohen, A.L.; Tempia, S.; et al. Respiratory Syncytial Virus in Adults with Severe Acute Respiratory Illness in a High HIV Prevalence Setting. J. Infect. 2017, 75, 346–355. [Google Scholar] [CrossRef]
- Anderson, C.S.; Chu, C.-Y.; Wang, Q.; Mereness, J.A.; Ren, Y.; Donlon, K.; Bhattacharya, S.; Misra, R.S.; Walsh, E.E.; Pryhuber, G.S.; et al. CX3CR1 as a Respiratory Syncytial Virus Receptor in Pediatric Human Lung. Pediatr. Res. 2020, 87, 862–867. [Google Scholar] [CrossRef]
- Agac, A.; Kolbe, S.M.; Ludlow, M.; Osterhaus, A.D.M.E.; Meineke, R.; Rimmelzwaan, G.F. Host Responses to Respiratory Syncytial Virus Infection. Viruses 2023, 15, 1999. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.L.; Kim, J.S.; Malloy, A.M.W. Development of Innate and Adaptive Immunity to RSV in Young Children. Cell. Immunol. 2024, 399–400, 104824. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Oliver, C.; Prince, G.A.; Hemming, V.G.; Pfarr, D.S.; Wang, S.C.; Dormitzer, M.; O’Grady, J.; Koenig, S.; Tamura, J.K.; et al. Development of a Humanized Monoclonal Antibody (MEDI-493) with Potent in Vitro and in Vivo Activity against Respiratory Syncytial Virus. J. Infect. Dis. 1997, 176, 1215–1224. [Google Scholar] [CrossRef]
- O’Hagan, S.; Galway, N.; Shields, M.D.; Mallett, P.; Groves, H.E. Review of the Safety, Efficacy and Tolerability of Palivizumab in the Prevention of Severe Respiratory Syncytial Virus (RSV) Disease. Drug Healthc. Patient Saf. 2023, 15, 103–112. [Google Scholar] [CrossRef]
- Drysdale, S.B.; Cathie, K.; Flamein, F.; Knuf, M.; Collins, A.M.; Hill, H.C.; Kaiser, F.; Cohen, R.; Pinquier, D.; Felter, C.T.; et al. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N. Engl. J. Med. 2023, 389, 2425–2435. [Google Scholar] [CrossRef]
- Attaianese, F.; Guiducci, S.; Trapani, S.; Barbati, F.; Lodi, L.; Indolfi, G.; Azzari, C.; Ricci, S. Reshaping Our Knowledge: Advancements in Understanding the Immune Response to Human Respiratory Syncytial Virus. Pathogens 2023, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Verwey, C.; Dangor, Z.; Madhi, S.A. Approaches to the Prevention and Treatment of Respiratory Syncytial Virus Infection in Children: Rationale and Progress to Date. Paediatr. Drugs 2024, 26, 101–112. [Google Scholar] [CrossRef]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef]
- Simões, E.A.F.; Center, K.J.; Tita, A.T.N.; Swanson, K.A.; Radley, D.; Houghton, J.; McGrory, S.B.; Gomme, E.; Anderson, M.; Roberts, J.P.; et al. Prefusion F Protein-Based Respiratory Syncytial Virus Immunization in Pregnancy. N. Engl. J. Med. 2022, 386, 1615–1626. [Google Scholar] [CrossRef]
- Carvalho, T. mRNA Vaccine Effective against RSV Respiratory Disease. Nat. Med. 2023, 29, 755–756. [Google Scholar] [CrossRef]
- Ke, Z.; Dillard, R.S.; Chirkova, T.; Leon, F.; Stobart, C.C.; Hampton, C.M.; Strauss, J.D.; Rajan, D.; Rostad, C.A.; Taylor, J.V.; et al. The Morphology and Assembly of Respiratory Syncytial Virus Revealed by Cryo-Electron Tomography. Viruses 2018, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, H.C.; Tripp, R.A. Immunopathology of RSV: An Updated Review. Viruses 2021, 13, 2478. [Google Scholar] [CrossRef]
- Mufson, M.A.; Orvell, C.; Rafnar, B.; Norrby, E. Two Distinct Subtypes of Human Respiratory Syncytial Virus. J. Gen. Virol. 1985, 66 Pt 10, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Escalante, J.C.; Comas-García, A.; Bernal-Silva, S.; Robles-Espinoza, C.D.; Gómez-Leal, G.; Noyola, D.E. Respiratory Syncytial Virus A Genotype Classification Based on Systematic Intergenotypic and Intragenotypic Sequence Analysis. Sci. Rep. 2019, 9, 20097. [Google Scholar] [CrossRef]
- Goya, S.; Galiano, M.; Nauwelaers, I.; Trento, A.; Openshaw, P.J.; Mistchenko, A.S.; Zambon, M.; Viegas, M. Toward Unified Molecular Surveillance of RSV: A Proposal for Genotype Definition. Influenza Respir. Viruses 2020, 14, 274–285. [Google Scholar] [CrossRef]
- Muñoz-Escalante, J.C.; Comas-García, A.; Bernal-Silva, S.; Noyola, D.E. Respiratory Syncytial Virus B Sequence Analysis Reveals a Novel Early Genotype. Sci. Rep. 2021, 11, 3452. [Google Scholar] [CrossRef] [PubMed]
- Goya, S.; Ruis, C.; Neher, R.A.; Meijer, A.; Aziz, A.; Hinrichs, A.S.; von Gottberg, A.; Roemer, C.; Amoako, D.G.; Acuña, D.; et al. Standardized Phylogenetic Classification of Human Respiratory Syncytial Virus below the Subgroup Level. Emerg. Infect. Dis. 2024, 30, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Kaler, J.; Hussain, A.; Patel, K.; Hernandez, T.; Ray, S. Respiratory Syncytial Virus: A Comprehensive Review of Transmission, Pathophysiology, and Manifestation. Cureus 2023, 15, e36342. [Google Scholar] [CrossRef]
- Van der Gucht, W.; Stobbelaar, K.; Govaerts, M.; Mangodt, T.; Barbezange, C.; Leemans, A.; De Winter, B.; Van Gucht, S.; Caljon, G.; Maes, L.; et al. Isolation and Characterization of Clinical RSV Isolates in Belgium during the Winters of 2016-2018. Viruses 2019, 11, 1031. [Google Scholar] [CrossRef]
- DeFord, D.M.; Nosek, J.M.; Castiglia, K.R.; Hasik, E.F.; Franke, M.E.; Nick, B.C.; Abdelnour, A.M.; Haas, C.E.; Junod, N.A.; Latsko, K.N.; et al. Evaluation of the Role of Respiratory Syncytial Virus Surface Glycoproteins F and G on Viral Stability and Replication: Implications for Future Vaccine Design. J. Gen. Virol. 2019, 100, 1112–1122. [Google Scholar] [CrossRef]
- Levine, S.; Klaiber-Franco, R.; Paradiso, P.R. Demonstration That Glycoprotein G Is the Attachment Protein of Respiratory Syncytial Virus. J. Gen. Virol. 1987, 68Pt 9, 2521–2524. [Google Scholar] [CrossRef]
- Johnson, S.M.; McNally, B.A.; Ioannidis, I.; Flano, E.; Teng, M.N.; Oomens, A.G.; Walsh, E.E.; Peeples, M.E. Respiratory Syncytial Virus Uses CX3CR1 as a Receptor on Primary Human Airway Epithelial Cultures. PLoS Pathog. 2015, 11, e1005318. [Google Scholar] [CrossRef]
- Feng, Z.; Xu, L.; Xie, Z. Receptors for Respiratory Syncytial Virus Infection and Host Factors Regulating the Life Cycle of Respiratory Syncytial Virus. Front. Cell. Infect. Microbiol. 2022, 12, 858629. [Google Scholar] [CrossRef]
- Tonello, F.; Massimino, M.L.; Peggion, C. Nucleolin: A Cell Portal for Viruses, Bacteria, and Toxins. Cell. Mol. Life Sci. CMLS 2022, 79, 271. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Cruz, C.; Villarreal Camacho, J.L.; De Ávila-Arias, M.; Hurtado-Gomez, L.; Rodriguez, A.; San-Juan-Vergara, H. Respiratory Syncytial Virus Entry Mechanism in Host Cells: A General Overview. Mol. Microbiol. 2023, 120, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Tran, K.C.; Luthra, P.; Teng, M.N.; He, B. Function of the Respiratory Syncytial Virus Small Hydrophobic Protein. J. Virol. 2007, 81, 8361–8366. [Google Scholar] [CrossRef]
- Galloux, M.; Risso-Ballester, J.; Richard, C.-A.; Fix, J.; Rameix-Welti, M.-A.; Eléouët, J.-F. Minimal Elements Required for the Formation of Respiratory Syncytial Virus Cytoplasmic Inclusion Bodies In Vivo and In Vitro. mBio 2020, 11, e01202-20. [Google Scholar] [CrossRef]
- Henderson, G.; Murray, J.; Yeo, R.P. Sorting of the Respiratory Syncytial Virus Matrix Protein into Detergent-Resistant Structures Is Dependent on Cell-Surface Expression of the Glycoproteins. Virology 2002, 300, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.N.; Collins, P.L. Identification of the Respiratory Syncytial Virus Proteins Required for Formation and Passage of Helper-Dependent Infectious Particles. J. Virol. 1998, 72, 5707–5716. [Google Scholar] [CrossRef]
- Shahriari, S.; Gordon, J.; Ghildyal, R. Host Cytoskeleton in Respiratory Syncytial Virus Assembly and Budding. Virol. J. 2016, 13, 161. [Google Scholar] [CrossRef]
- Tripp, R.A.; Jones, L.P.; Haynes, L.M.; Zheng, H.; Murphy, P.M.; Anderson, L.J. CX3C Chemokine Mimicry by Respiratory Syncytial Virus G Glycoprotein. Nat. Immunol. 2001, 2, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Zhuang, R.; Yang, K.; Chen, L.; Jin, B.; Ma, Y.; Zhang, Y.; Tang, K. Alterations in CX3CL1 Levels and Its Role in Viral Pathogenesis. Int. J. Mol. Sci. 2024, 25, 4451. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A New Classification System and Their Role in Immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.D.; Chadwick, N.; Warren, B.F.; Jewell, D.P.; Gordon, S.; Powrie, F.; Greaves, D.R. The Transmembrane Form of the CX3CL1 Chemokine Fractalkine Is Expressed Predominantly by Epithelial Cells in Vivo. Am. J. Pathol. 2001, 158, 855–866. [Google Scholar] [CrossRef]
- Matsumiya, T.; Ota, K.; Imaizumi, T.; Yoshida, H.; Kimura, H.; Satoh, K. Characterization of Synergistic Induction of CX3CL1/Fractalkine by TNF-Alpha and IFN-Gamma in Vascular Endothelial Cells: An Essential Role for TNF-Alpha in Post-Transcriptional Regulation of CX3CL1. J. Immunol. 2010, 184, 4205–4214. [Google Scholar] [CrossRef]
- Murphy, P.M. International Union of Pharmacology. XXX. Update on Chemokine Receptor Nomenclature. Pharmacol. Rev. 2002, 54, 227–229. [Google Scholar] [CrossRef]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A New Class of Membrane-Bound Chemokine with a CX3C Motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, M.A.; Hermand, P.; Saindoy, E.; Guillou, N.; Guellec, J.; Coens, A.; Hattab, C.; Desuzinges-Mandon, E.; Jawhari, A.; Iatmanen-Harbi, S.; et al. CX3CL1 Homo-Oligomerization Drives Cell-to-Cell Adherence. Sci. Rep. 2020, 10, 9069. [Google Scholar] [CrossRef]
- Rivas-Fuentes, S.; Salgado-Aguayo, A.; Arratia-Quijada, J.; Gorocica-Rosete, P. Regulation and Biological Functions of the CX3CL1-CX3CR1 Axis and Its Relevance in Solid Cancer: A Mini-Review. J. Cancer 2021, 12, 571–583. [Google Scholar] [CrossRef]
- McCulloch, D.R.; Akl, P.; Samaratunga, H.; Herington, A.C.; Odorico, D.M. Expression of the Disintegrin Metalloprotease, ADAM-10, in Prostate Cancer and Its Regulation by Dihydrotestosterone, Insulin-like Growth Factor I, and Epidermal Growth Factor in the Prostate Cancer Cell Model LNCaP. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004, 10, 314–323. [Google Scholar] [CrossRef]
- Arora, P.; Cuevas, B.D.; Russo, A.; Johnson, G.L.; Trejo, J. Persistent Transactivation of EGFR and ErbB2/HER2 by Protease-Activated Receptor-1 Promotes Breast Carcinoma Cell Invasion. Oncogene 2008, 27, 4434–4445. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, K.S.; Crue, T.; Nall, J.M.; Foster, D.; Sajuthi, S.; Correll, K.A.; Nakamura, M.; Everman, J.L.; Downey, G.P.; Seibold, M.A.; et al. Influenza Virus Infection Increases ACE2 Expression and Shedding in Human Small Airway Epithelial Cells. Eur. Respir. J. 2021, 58, 2003988. [Google Scholar] [CrossRef]
- Lan, Y.-Y.; Yeh, T.-H.; Lin, W.-H.; Wu, S.-Y.; Lai, H.-C.; Chang, F.-H.; Takada, K.; Chang, Y. Epstein-Barr Virus Zta Upregulates Matrix Metalloproteinases 3 and 9 That Synergistically Promote Cell Invasion in Vitro. PLoS ONE 2013, 8, e56121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Foronjy, R.F.; Dabo, A.J.; Taggart, C.C.; Weldon, S.; Geraghty, P. Respiratory Syncytial Virus Infections Enhance Cigarette Smoke Induced COPD in Mice. PLoS ONE 2014, 9, e90567. [Google Scholar] [CrossRef] [PubMed]
- Kehrl, J.H. Chemoattractant Receptor Signaling and the Control of Lymphocyte Migration. Immunol. Res. 2006, 34, 211–227. [Google Scholar] [CrossRef]
- Garton, K.J.; Gough, P.J.; Blobel, C.P.; Murphy, G.; Greaves, D.R.; Dempsey, P.J.; Raines, E.W. Tumor Necrosis Factor-Alpha-Converting Enzyme (ADAM17) Mediates the Cleavage and Shedding of Fractalkine (CX3CL1). J. Biol. Chem. 2001, 276, 37993–38001. [Google Scholar] [CrossRef]
- Hundhausen, C.; Misztela, D.; Berkhout, T.A.; Broadway, N.; Saftig, P.; Reiss, K.; Hartmann, D.; Fahrenholz, F.; Postina, R.; Matthews, V.; et al. The Disintegrin-like Metalloproteinase ADAM10 Is Involved in Constitutive Cleavage of CX3CL1 (Fractalkine) and Regulates CX3CL1-Mediated Cell-Cell Adhesion. Blood 2003, 102, 1186–1195. [Google Scholar] [CrossRef]
- Imai, T.; Hieshima, K.; Haskell, C.; Baba, M.; Nagira, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Nomiyama, H.; Schall, T.J.; et al. Identification and Molecular Characterization of Fractalkine Receptor CX3CR1, Which Mediates Both Leukocyte Migration and Adhesion. Cell 1997, 91, 521–530. [Google Scholar] [CrossRef]
- Combadiere, C.; Salzwedel, K.; Smith, E.D.; Tiffany, H.L.; Berger, E.A.; Murphy, P.M. Identification of CX3CR1. A Chemotactic Receptor for the Human CX3C Chemokine Fractalkine and a Fusion Coreceptor for HIV-1. J. Biol. Chem. 1998, 273, 23799–23804. [Google Scholar] [CrossRef]
- Chandrasekar, B.; Mummidi, S.; Perla, R.P.; Bysani, S.; Dulin, N.O.; Liu, F.; Melby, P.C. Fractalkine (CX3CL1) Stimulated by Nuclear Factor kappaB (NF-kappaB)-Dependent Inflammatory Signals Induces Aortic Smooth Muscle Cell Proliferation through an Autocrine Pathway. Biochem. J. 2003, 373, 547–558. [Google Scholar] [CrossRef]
- Das, S.; Raundhal, M.; Chen, J.; Oriss, T.B.; Huff, R.; Williams, J.V.; Ray, A.; Ray, P. Respiratory Syncytial Virus Infection of Newborn CX3CR1-Deficient Mice Induces a Pathogenic Pulmonary Innate Immune Response. JCI Insight 2017, 2, e94605. [Google Scholar] [CrossRef]
- Gkentzi, D.; Dimitriou, G.; Karatza, A. Non-Pulmonary Manifestations of Respiratory Syncytial Virus Infection. J. Thorac. Dis. 2018, 10, S3815–S3818. [Google Scholar] [CrossRef]
- Sjöstedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An Atlas of the Protein-Coding Genes in the Human, Pig, and Mouse Brain. Science 2020, 367, eaay5947. Available online: www.proteinatlas.org/ENSG00000168329-CX3CR1/tissue (accessed on 1 June 2024). [CrossRef] [PubMed]
- Openshaw, P.J.M.; Chiu, C.; Culley, F.J.; Johansson, C. Protective and Harmful Immunity to RSV Infection. Annu. Rev. Immunol. 2017, 35, 501–532. [Google Scholar] [CrossRef]
- García-Sastre, A.; Biron, C.A. Type 1 Interferons and the Virus-Host Relationship: A Lesson in Détente. Science 2006, 312, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, H.C.; Hansen, M.R.; Tripp, R.A. Interferons-Implications in the Immune Response to Respiratory Viruses. Microorganisms 2023, 11, 2179. [Google Scholar] [CrossRef] [PubMed]
- Chirkova, T.; Lin, S.; Oomens, A.G.P.; Gaston, K.A.; Boyoglu-Barnum, S.; Meng, J.; Stobart, C.C.; Cotton, C.U.; Hartert, T.V.; Moore, M.L.; et al. CX3CR1 Is an Important Surface Molecule for Respiratory Syncytial Virus Infection in Human Airway Epithelial Cells. J. Gen. Virol. 2015, 96, 2543–2556. [Google Scholar] [CrossRef]
- Jung, H.E.; Kim, T.H.; Lee, H.K. Contribution of Dendritic Cells in Protective Immunity against Respiratory Syncytial Virus Infection. Viruses 2020, 12, 102. [Google Scholar] [CrossRef]
- Geerdink, R.J.; Pillay, J.; Meyaard, L.; Bont, L. Neutrophils in Respiratory Syncytial Virus Infection: A Target for Asthma Prevention. J. Allergy Clin. Immunol. 2015, 136, 838–847. [Google Scholar] [CrossRef]
- Currie, S.M.; Gwyer Findlay, E.; McFarlane, A.J.; Fitch, P.M.; Böttcher, B.; Colegrave, N.; Paras, A.; Jozwik, A.; Chiu, C.; Schwarze, J.; et al. Cathelicidins Have Direct Antiviral Activity against Respiratory Syncytial Virus In Vitro and Protective Function In Vivo in Mice and Humans. J. Immunol. 2016, 196, 2699–2710. [Google Scholar] [CrossRef]
- Latsko, K.N.; Jacob, A.T.; Junod, N.A.; Haas, C.E.; Castiglia, K.R.; Kastelitz, S.R.; Huffman, E.R.; Trombley, M.P.; Stobart, C.C. Role of Differences in Respiratory Syncytial Virus F and G Glycoproteins on Susceptibility to Inactivation by Antimicrobial Peptides LL-37 and Human Beta-Defensins. Viral Immunol. 2022, 35, 559–565. [Google Scholar] [CrossRef] [PubMed]
- van Erp, E.A.; Feyaerts, D.; Duijst, M.; Mulder, H.L.; Wicht, O.; Luytjes, W.; Ferwerda, G.; van Kasteren, P.B. Respiratory Syncytial Virus Infects Primary Neonatal and Adult Natural Killer Cells and Affects Their Antiviral Effector Function. J. Infect. Dis. 2019, 219, 723–733. [Google Scholar] [CrossRef]
- Schlender, J.; Hornung, V.; Finke, S.; Günthner-Biller, M.; Marozin, S.; Brzózka, K.; Moghim, S.; Endres, S.; Hartmann, G.; Conzelmann, K.-K. Inhibition of Toll-like Receptor 7- and 9-Mediated Alpha/Beta Interferon Production in Human Plasmacytoid Dendritic Cells by Respiratory Syncytial Virus and Measles Virus. J. Virol. 2005, 79, 5507–5515. [Google Scholar] [CrossRef] [PubMed]
- de Graaff, P.M.A.; de Jong, E.C.; van Capel, T.M.; van Dijk, M.E.A.; Roholl, P.J.M.; Boes, J.; Luytjes, W.; Kimpen, J.L.L.; van Bleek, G.M. Respiratory Syncytial Virus Infection of Monocyte-Derived Dendritic Cells Decreases Their Capacity to Activate CD4 T Cells. J. Immunol. 2005, 175, 5904–5911. [Google Scholar] [CrossRef] [PubMed]
- Welliver, T.P.; Garofalo, R.P.; Hosakote, Y.; Hintz, K.H.; Avendano, L.; Sanchez, K.; Velozo, L.; Jafri, H.; Chavez-Bueno, S.; Ogra, P.L.; et al. Severe Human Lower Respiratory Tract Illness Caused by Respiratory Syncytial Virus and Influenza Virus Is Characterized by the Absence of Pulmonary Cytotoxic Lymphocyte Responses. J. Infect. Dis. 2007, 195, 1126–1136. [Google Scholar] [CrossRef]
- Jans, J.; Pettengill, M.; Kim, D.; van der Made, C.; de Groot, R.; Henriet, S.; de Jonge, M.I.; Ferwerda, G.; Levy, O. Human Newborn B Cells Mount an Interferon-α/β Receptor-Dependent Humoral Response to Respiratory Syncytial Virus. J. Allergy Clin. Immunol. 2017, 139, 1997–2000.e4. [Google Scholar] [CrossRef][Green Version]
- Lee, D.C.P.; Harker, J.A.E.; Tregoning, J.S.; Atabani, S.F.; Johansson, C.; Schwarze, J.; Openshaw, P.J.M. CD25+ Natural Regulatory T Cells Are Critical in Limiting Innate and Adaptive Immunity and Resolving Disease Following Respiratory Syncytial Virus Infection. J. Virol. 2010, 84, 8790–8798. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.N.; Whitehead, S.S.; Bermingham, A.; St Claire, M.; Elkins, W.R.; Murphy, B.R.; Collins, P.L. Recombinant Respiratory Syncytial Virus That Does Not Express the NS1 or M2-2 Protein Is Highly Attenuated and Immunogenic in Chimpanzees. J. Virol. 2000, 74, 9317–9321. [Google Scholar] [CrossRef]
- Han, B.; Wang, Y.; Zheng, M. Inhibition of Autophagy Promotes Human RSV NS1-Induced Inflammation and Apoptosis in Vitro. Exp. Ther. Med. 2021, 22, 1054. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Zeng, R.; Yang, J.; Liu, J.; Zhang, Z.; Song, X.; Yao, Z.; Ma, C.; Li, W.; et al. Respiratory Syncytial Virus Replication Is Promoted by Autophagy-Mediated Inhibition of Apoptosis. J. Virol. 2018, 92, e02193-17. [Google Scholar] [CrossRef]
- Pei, J.; Beri, N.R.; Zou, A.J.; Hubel, P.; Dorando, H.K.; Bergant, V.; Andrews, R.D.; Pan, J.; Andrews, J.M.; Sheehan, K.C.F.; et al. Nuclear-Localized Human Respiratory Syncytial Virus NS1 Protein Modulates Host Gene Transcription. Cell Rep. 2021, 37, 109803. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, T.; Pang, L.; Li, K.; Garofalo, R.P.; Casola, A.; Bao, X. A Novel Mechanism for the Inhibition of Interferon Regulatory Factor-3-Dependent Gene Expression by Human Respiratory Syncytial Virus NS1 Protein. J. Gen. Virol. 2011, 92, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, Y.; Song, J.; Lee, J.; Chang, S.-Y. Tissue-Specific Role of CX3CR1 Expressing Immune Cells and Their Relationships with Human Disease. Immune Netw. 2018, 18, e5. [Google Scholar] [CrossRef]
- Park, M.H.; Lee, J.S.; Yoon, J.H. High Expression of CX3CL1 by Tumor Cells Correlates with a Good Prognosis and Increased Tumor-Infiltrating CD8+ T Cells, Natural Killer Cells, and Dendritic Cells in Breast Carcinoma. J. Surg. Oncol. 2012, 106, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.-Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim Monocytes Patrol and Sense Nucleic Acids and Viruses via TLR7 and TLR8 Receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Tacke, R.; Hedrick, C.C.; Hanna, R.N. Nonclassical Patrolling Monocyte Function in the Vasculature. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1306–1316. [Google Scholar] [CrossRef]
- Becker, S.; Soukup, J.M. Airway Epithelial Cell-Induced Activation of Monocytes and Eosinophils in Respiratory Syncytial Viral Infection. Immunobiology 1999, 201, 88–106. [Google Scholar] [CrossRef]
- Soukup, J.M.; Becker, S. Role of Monocytes and Eosinophils in Human Respiratory Syncytial Virus Infection in Vitro. Clin. Immunol. 2003, 107, 178–185. [Google Scholar] [CrossRef]
- Amanatidou, V.; Sourvinos, G.; Apostolakis, S.; Tsilimigaki, A.; Spandidos, D.A. T280M Variation of the CX3C Receptor Gene Is Associated with Increased Risk for Severe Respiratory Syncytial Virus Bronchiolitis. Pediatr. Infect. Dis. J. 2006, 25, 410–414. [Google Scholar] [CrossRef]
- McDermott, D.H.; Fong, A.M.; Yang, Q.; Sechler, J.M.; Cupples, L.A.; Merrell, M.N.; Wilson, P.W.F.; D’Agostino, R.B.; O’Donnell, C.J.; Patel, D.D.; et al. Chemokine Receptor Mutant CX3CR1-M280 Has Impaired Adhesive Function and Correlates with Protection from Cardiovascular Disease in Humans. J. Clin. Investig. 2003, 111, 1241–1250. [Google Scholar] [CrossRef]
- Collar, A.L.; Swamydas, M.; O’Hayre, M.; Sajib, M.S.; Hoffman, K.W.; Singh, S.P.; Mourad, A.; Johnson, M.D.; Ferre, E.M.; Farber, J.M.; et al. The Homozygous CX3CR1-M280 Mutation Impairs Human Monocyte Survival. JCI Insight 2018, 3, e95417. [Google Scholar] [CrossRef] [PubMed]
- Bukreyev, A.; Yang, L.; Fricke, J.; Cheng, L.; Ward, J.M.; Murphy, B.R.; Collins, P.L. The Secreted Form of Respiratory Syncytial Virus G Glycoprotein Helps the Virus Evade Antibody-Mediated Restriction of Replication by Acting as an Antigen Decoy and through Effects on Fc Receptor-Bearing Leukocytes. J. Virol. 2008, 82, 12191–12204. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, J.; Alvarez, R.; Jones, L.P.; Henderson, C.; Anderson, L.J.; Tripp, R.A. Respiratory Syncytial Virus G Protein and G Protein CX3C Motif Adversely Affect CX3CR1+ T Cell Responses. J. Immunol. 2006, 176, 1600–1608. [Google Scholar] [CrossRef]
- Chirkova, T.; Boyoglu-Barnum, S.; Gaston, K.A.; Malik, F.M.; Trau, S.P.; Oomens, A.G.P.; Anderson, L.J. Respiratory Syncytial Virus G Protein CX3C Motif Impairs Human Airway Epithelial and Immune Cell Responses. J. Virol. 2013, 87, 13466–13479. [Google Scholar] [CrossRef]
- Midulla, F.; Huang, Y.T.; Gilbert, I.A.; Cirino, N.M.; McFadden, E.R.; Panuska, J.R. Respiratory Syncytial Virus Infection of Human Cord and Adult Blood Monocytes and Alveolar Macrophages. Am. Rev. Respir. Dis. 1989, 140, 771–777. [Google Scholar] [CrossRef]
- Openshaw, P.J.M. RSV Takes Control of Neonatal Breg Cells: Two Hands on the Wheel. Immunity 2017, 46, 171–173. [Google Scholar] [CrossRef]
- Menon, M.; Hussell, T.; Ali Shuwa, H. Regulatory B Cells in Respiratory Health and Diseases. Immunol. Rev. 2021, 299, 61–73. [Google Scholar] [CrossRef]
- Zhivaki, D.; Lemoine, S.; Lim, A.; Morva, A.; Vidalain, P.-O.; Schandene, L.; Casartelli, N.; Rameix-Welti, M.-A.; Hervé, P.-L.; Dériaud, E.; et al. Respiratory Syncytial Virus Infects Regulatory B Cells in Human Neonates via Chemokine Receptor CX3CR1 and Promotes Lung Disease Severity. Immunity 2017, 46, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Langedijk, A.C.; Bont, L.J. Respiratory Syncytial Virus Infection and Novel Interventions. Nat. Rev. Microbiol. 2023, 21, 734–749. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Murray, J.; Nuñez Castrejon, A.M.; DuBois, R.M.; Tripp, R.A. Respiratory Syncytial Virus (RSV) G Protein Vaccines With Central Conserved Domain Mutations Induce CX3C-CX3CR1 Blocking Antibodies. Viruses 2021, 13, 352. [Google Scholar] [CrossRef]
- Lee, Y.; Klenow, L.; Coyle, E.M.; Grubbs, G.; Golding, H.; Khurana, S. Monoclonal Antibodies Targeting Sites in Respiratory Syncytial Virus Attachment G Protein Provide Protection against RSV-A and RSV-B in Mice. Nat. Commun. 2024, 15, 2900. [Google Scholar] [CrossRef] [PubMed]
- Piloto, J.V.; Dias, R.V.R.; Mazucato, W.S.A.; Fossey, M.A.; de Melo, F.A.; Almeida, F.C.L.; de Souza, F.P.; Caruso, I.P. Computational Insights into the Interaction of the Conserved Cysteine-Noose Domain of the Human Respiratory Syncytial Virus G Protein with the Canonical Fractalkine Binding Site of Transmembrane Receptor CX3CR1 Isoforms. Membranes 2024, 14, 84. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivas-Fuentes, S.; Salgado-Aguayo, A.; Santos-Mendoza, T.; Sevilla-Reyes, E. The Role of the CX3CR1-CX3CL1 Axis in Respiratory Syncytial Virus Infection and the Triggered Immune Response. Int. J. Mol. Sci. 2024, 25, 9800. https://doi.org/10.3390/ijms25189800
Rivas-Fuentes S, Salgado-Aguayo A, Santos-Mendoza T, Sevilla-Reyes E. The Role of the CX3CR1-CX3CL1 Axis in Respiratory Syncytial Virus Infection and the Triggered Immune Response. International Journal of Molecular Sciences. 2024; 25(18):9800. https://doi.org/10.3390/ijms25189800
Chicago/Turabian StyleRivas-Fuentes, Selma, Alfonso Salgado-Aguayo, Teresa Santos-Mendoza, and Edgar Sevilla-Reyes. 2024. "The Role of the CX3CR1-CX3CL1 Axis in Respiratory Syncytial Virus Infection and the Triggered Immune Response" International Journal of Molecular Sciences 25, no. 18: 9800. https://doi.org/10.3390/ijms25189800
APA StyleRivas-Fuentes, S., Salgado-Aguayo, A., Santos-Mendoza, T., & Sevilla-Reyes, E. (2024). The Role of the CX3CR1-CX3CL1 Axis in Respiratory Syncytial Virus Infection and the Triggered Immune Response. International Journal of Molecular Sciences, 25(18), 9800. https://doi.org/10.3390/ijms25189800





