Composite Polycaprolactone/Gelatin Nanofiber Membrane Scaffolds for Mesothelial Cell Culture and Delivery in Mesothelium Repair
Abstract
:1. Introduction
2. Results and Discussion
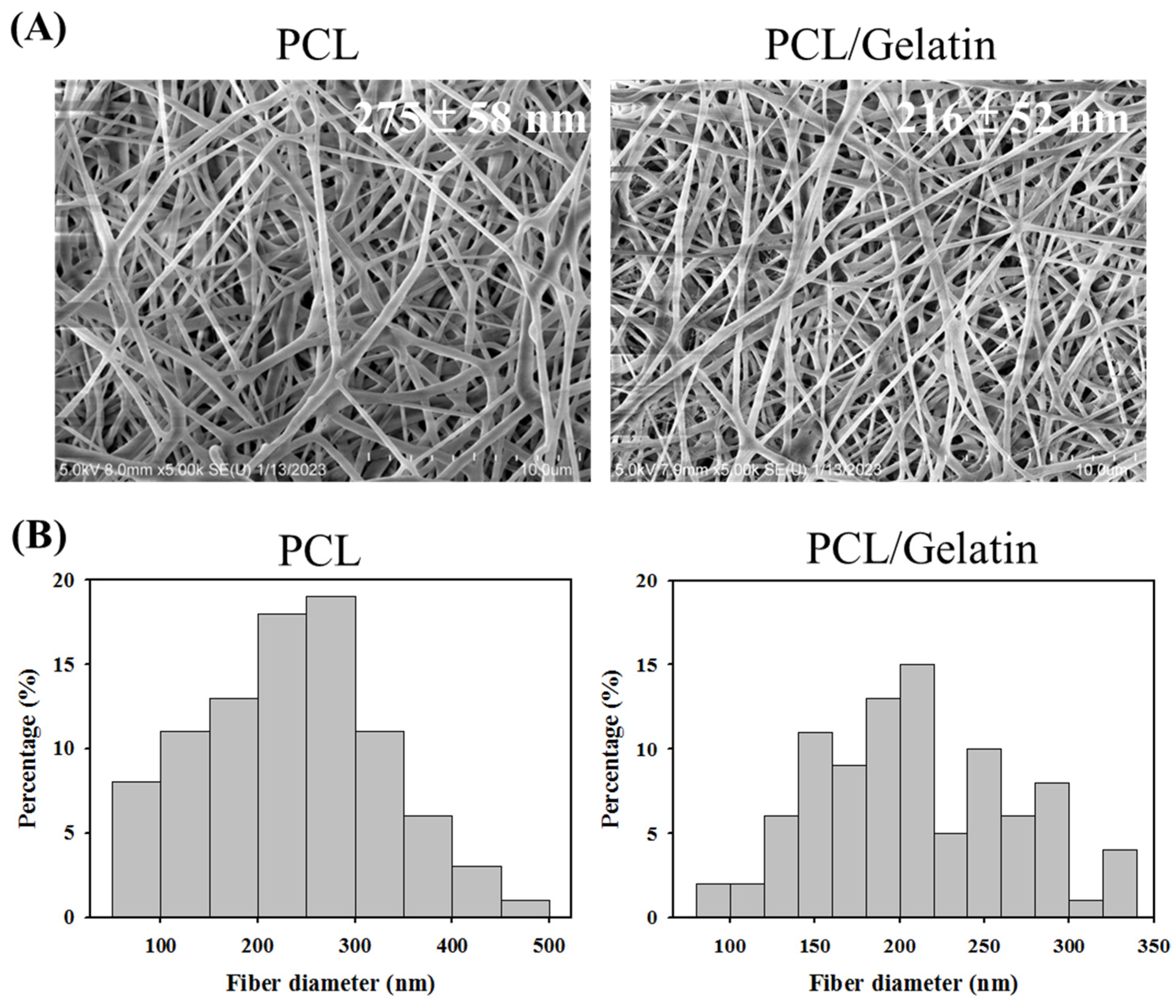
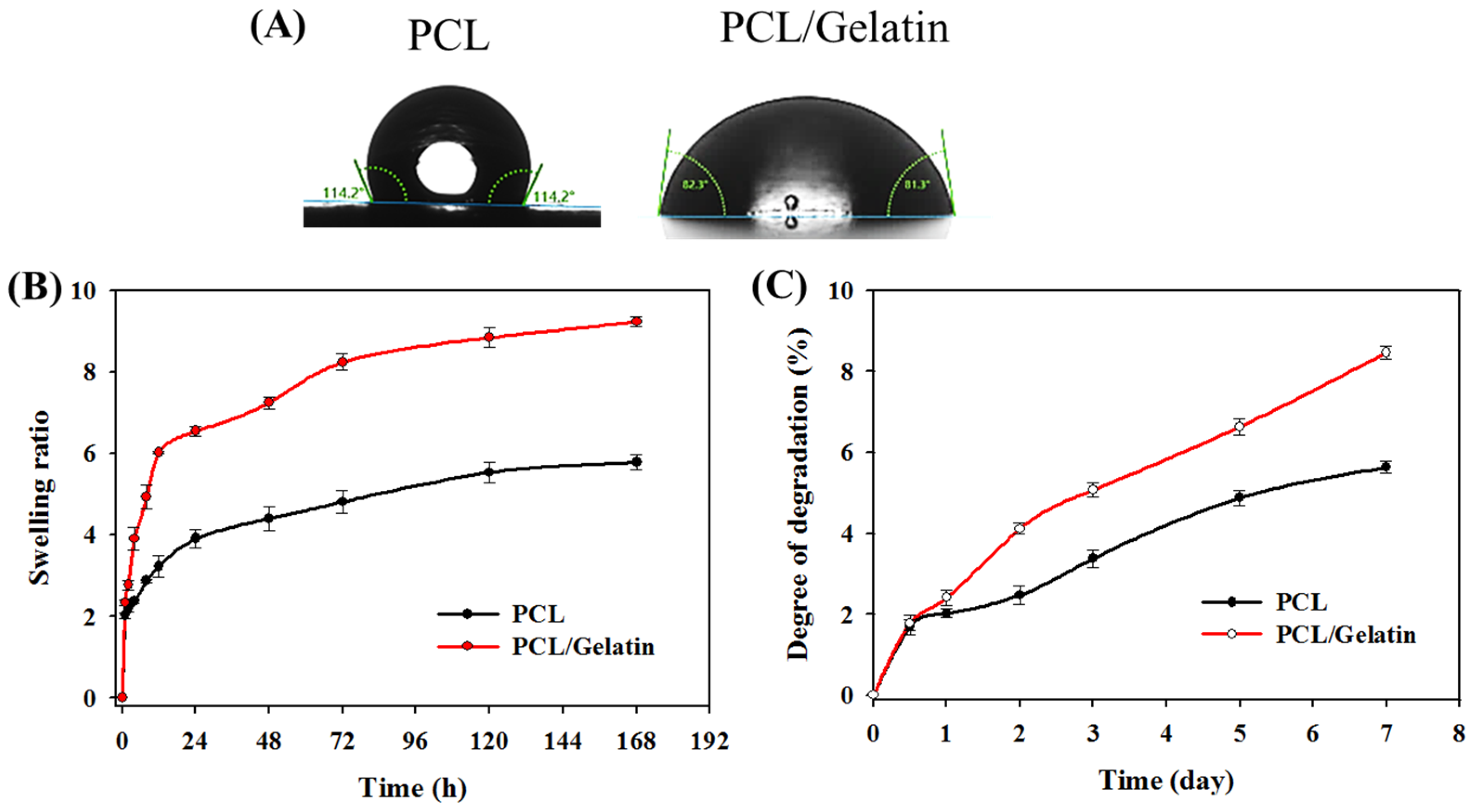
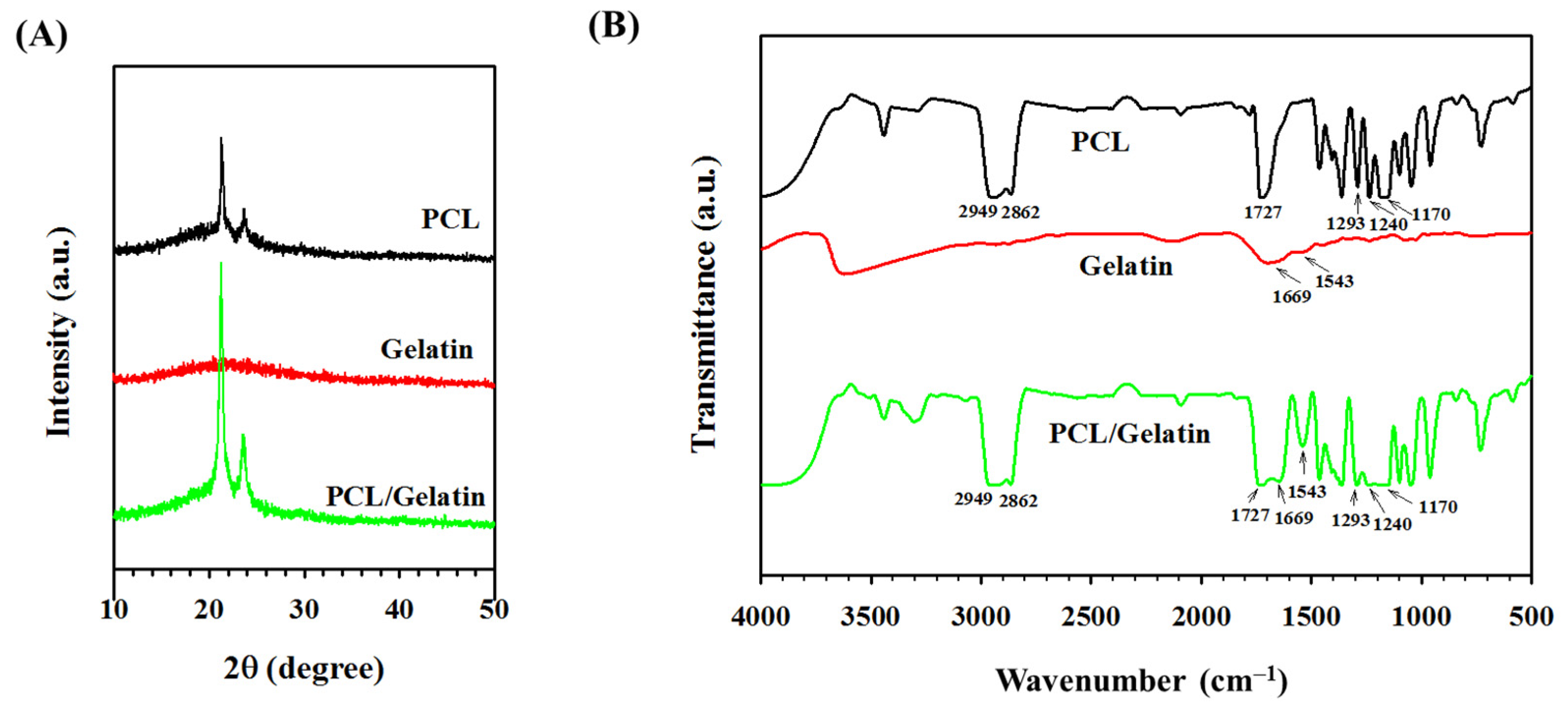
2.1. Characterization of PCL and PCL/Gelatin Nanofiber Membrane Scaffolds (NMSs)
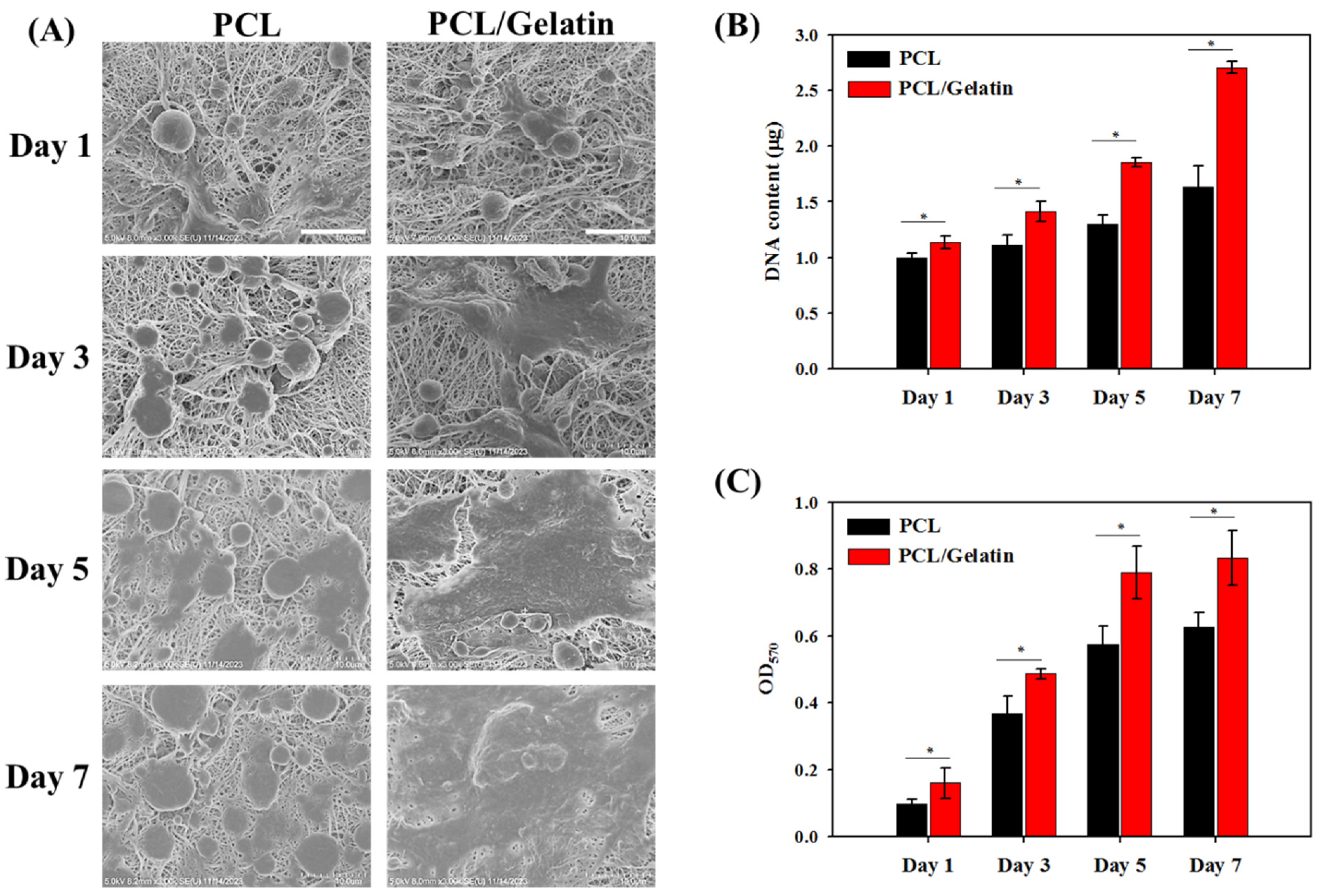
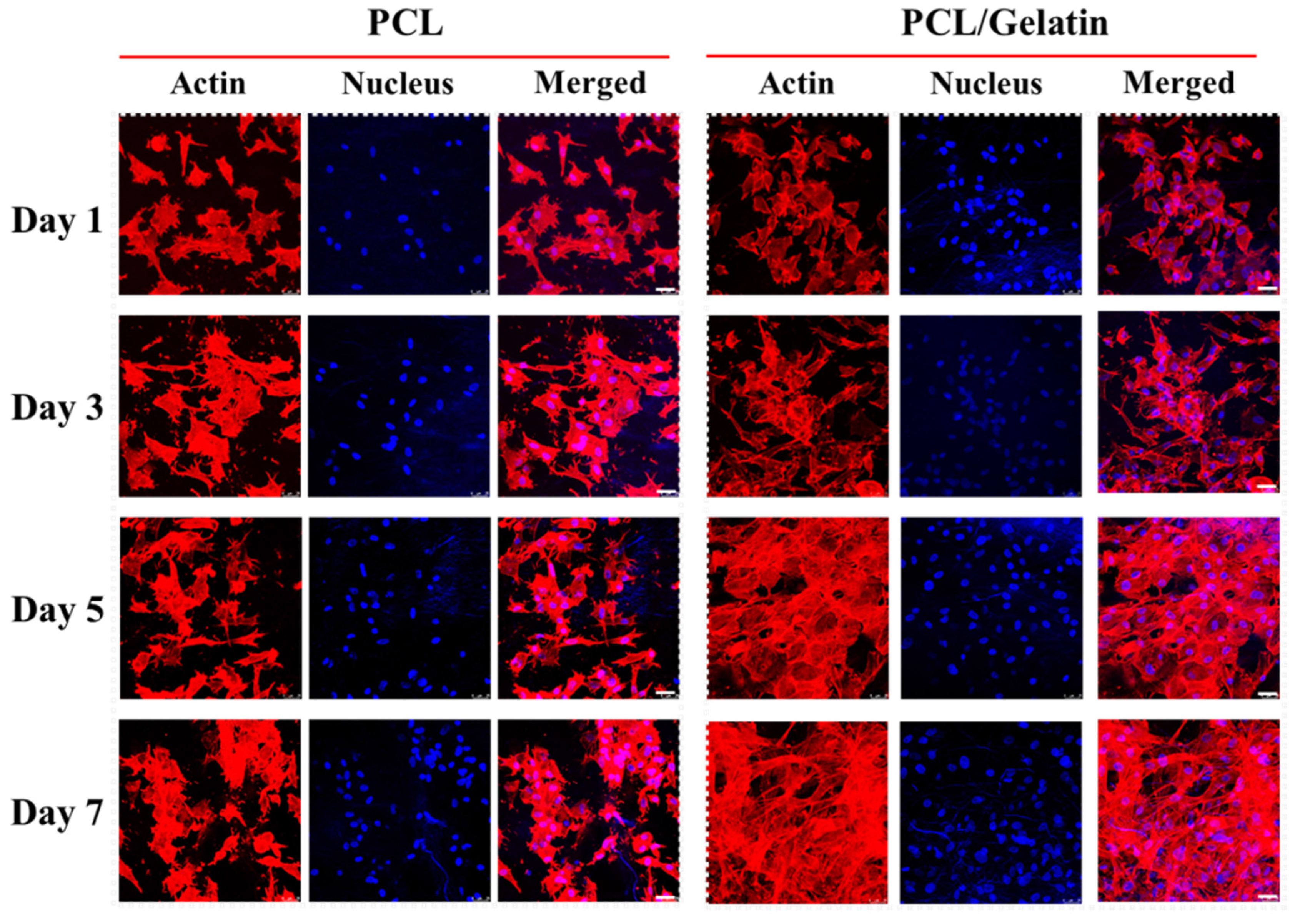
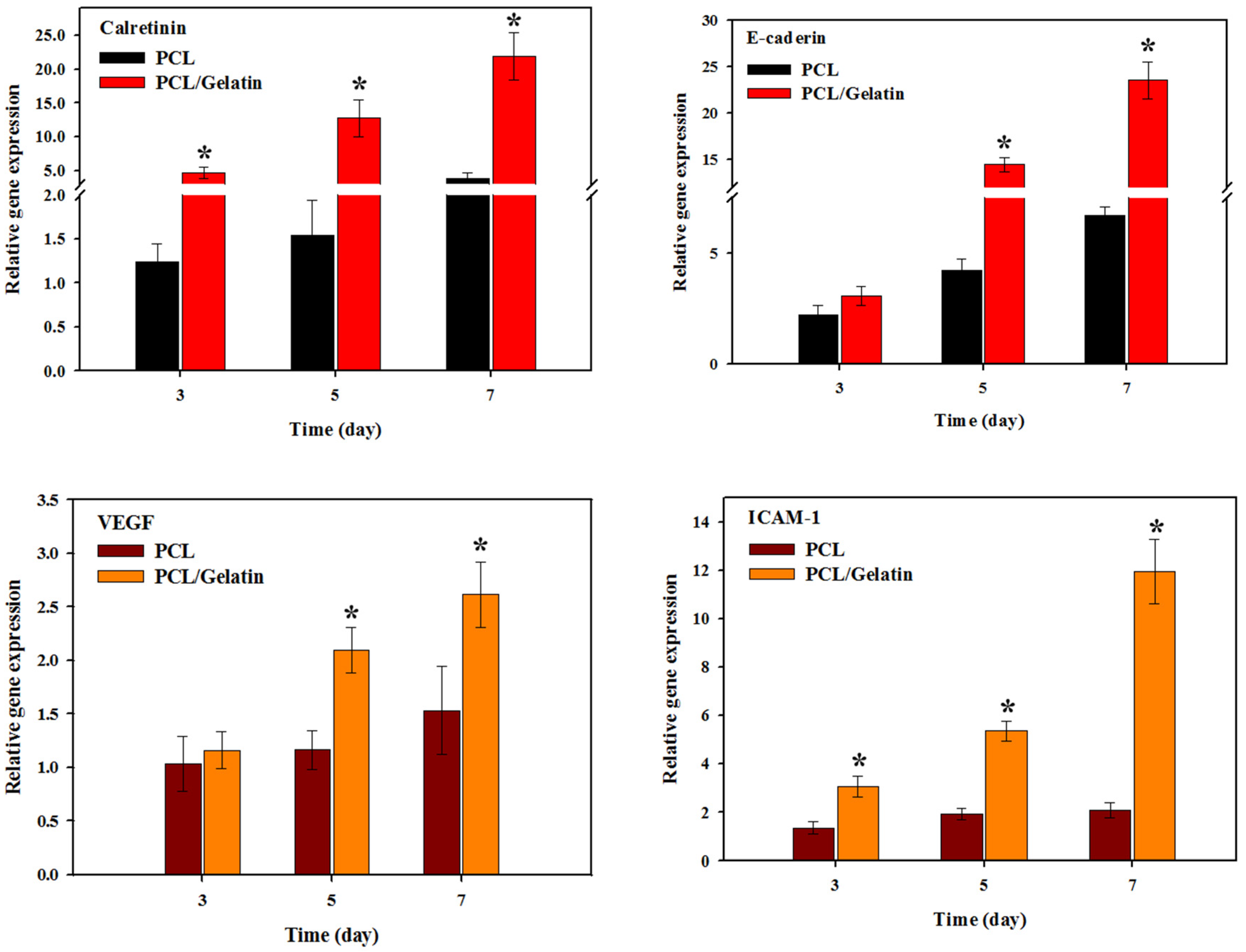
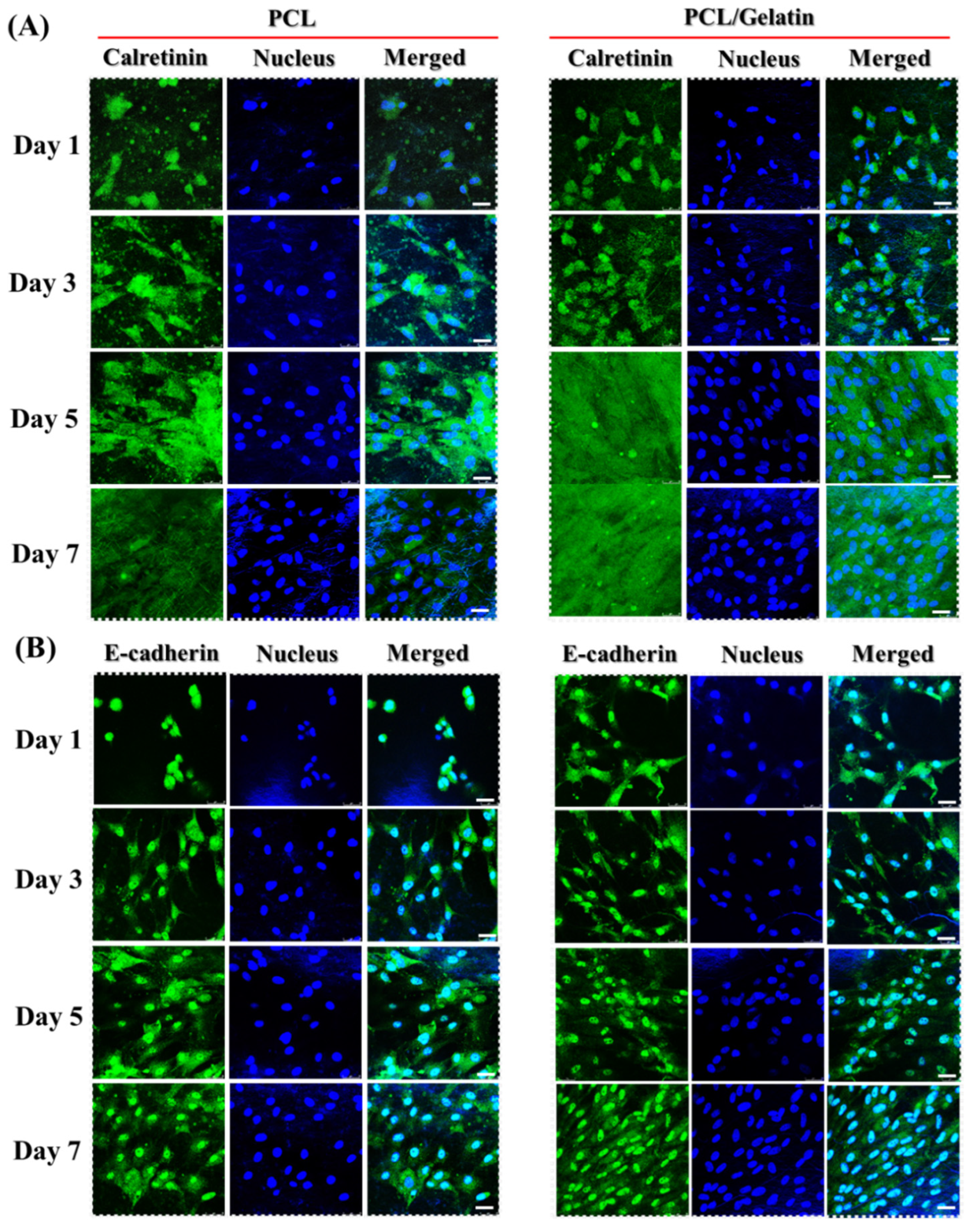
2.2. In Vitro Study
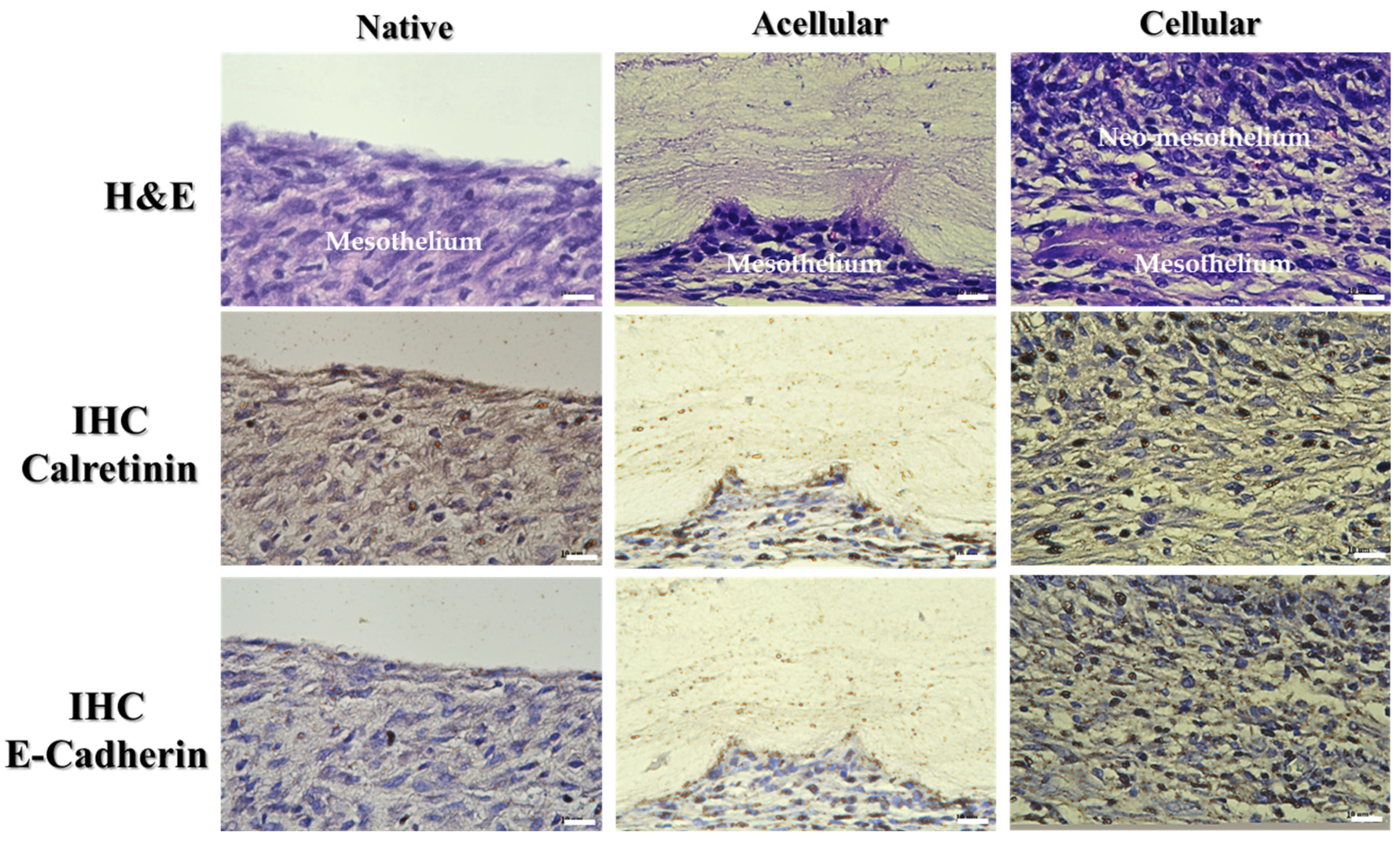
2.3. In Vivo Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of Electrospun Nanofiber Membrane Scaffolds (NMSs)
3.3. Characterization of Electrospun Nanofiber Membrane Scaffolds (NMSs)
3.4. In Vitro Cell Culture
3.5. Cell Morphology by Scanning Electron Microscope (SEM) Observation
3.6. Cell Proliferation by DNA Contents and MTT Assays
3.7. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
3.8. Cell Viability by Live/Dead Staining
3.9. Cytoskeleton Staining
3.10. Immunofluorescence (IF) Staining
3.11. In Vivo Studies
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mutsaers, S.E.; Prêle, C.M.; Lansley, S.M.; Herrick, S.E. The origin of regenerating mesothelium: A historical perspective. Int. J. Artif. Organs 2007, 30, 484–494. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Wilkosz, S. Structure and function of mesothelial cells. Cancer Treat. Res. 2007, 134, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E. Mesothelial cells: Their structure, function and role in serosal repair. Respirology 2002, 7, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Herrick, S.E.; Mutsaers, S.E. Mesothelial progenitor cells and their potential in tissue engineering. Int. J. Biochem. Cell Biol. 2004, 36, 621–642. [Google Scholar] [CrossRef] [PubMed]
- Uiterwijk, H.; Franssen, C.F.M.; Kuipers, J.; Westerhuis, R.; Nauta, F.L. Glucose Exposure in Peritoneal Dialysis Is a Significant Factor Predicting Peritonitis. Am. J. Nephrol. 2020, 51, 237–243. [Google Scholar] [CrossRef]
- Mutsaers, S.E.; Prele, C.M.; Pengelly, S.; Herrick, S.E. Mesothelial cells and peritoneal homeostasis. Fertil. Steril. 2016, 106, 1018–1024. [Google Scholar] [CrossRef]
- Osawa, H.; Nishimura, J.; Hiraki, M.; Takahashi, H.; Haraguchi, N.; Hata, T.; Ikenaga, M.; Murata, K.; Yamamoto, H.; Mizushima, T.; et al. Regeneration of peritoneal mesothelial cells after placement of hyaluronate carboxymethyl-cellulose (Seprafilm®). Surg. Today 2017, 47, 130–136. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Sharma, C.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of natural origin chitosan-gelatin-alginate composite scaffold by foaming method without using surfactant. J. Appl. Polym. Sci. 2013, 127, 3228–3241. [Google Scholar] [CrossRef]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Thomas, K.; Engler, A.J.; Meyer, G.A. Extracellular matrix regulation in the muscle satellite cell niche. Connect. Tissue Res. 2015, 56, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhao, W.; Zhu, J.M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 2013, 34, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Campiglio, C.E.; Marcolin, C.; Draghi, L. Electrospun ECM macromolecules as biomimetic scaffold for regenerative medicine: Challenges for preserving conformation and bioactivity. AIMS Mater. Sci. 2017, 4, 638–669. [Google Scholar] [CrossRef]
- Krithica, N.; Natarajan, V.; Madhan, B.; Sehgal, P.K.; Mandal, A.B. Type I Collagen Immobilized Poly(caprolactone) Nanofibers: Characterization of Surface Modification and Growth of Fibroblasts. Adv. Eng. Mater. 2012, 14, B149–B154. [Google Scholar] [CrossRef]
- Mitragotri, S.; Anderson, D.G.; Chen, X.; Chow, E.K.; Ho, D.; Kabanov, A.V.; Karp, J.M.; Kataoka, K.; Mirkin, C.A.; Petrosko, S.H.; et al. Accelerating the Translation of Nanomaterials in Biomedicine. ACS Nano 2015, 9, 6644–6654. [Google Scholar] [CrossRef]
- Bazmandeh, A.Z.; Mirzaei, E.; Fadaie, M.; Shirian, S.; Ghasemi, Y. Dual spinneret electrospun nanofibrous/gel structure of chitosan-gelatin/chitosan-hyaluronic acid as a wound dressing: In-vitro and in-vivo studies. Int. J. Biol. Macromol. 2020, 162, 359–373. [Google Scholar] [CrossRef]
- Sharifi-Aghdam, M.; Faridi-Majidi, R.; Derakhshan, M.A.; Chegeni, A.; Azami, M. Preparation of collagen/polyurethane/knitted silk as a composite scaffold for tendon tissue engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 652–662. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, R.; Zhou, F.; Streubel, P.N.; Chen, S.; Duan, B. Electrospun thymosin Beta-4 loaded PLGA/PLA nanofiber/microfiber hybrid yarns for tendon tissue engineering application. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110268. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, S.H.; Shalumon, K.T.; Chen, J.P. Dual functional core-sheath electrospun hyaluronic acid/polycaprolactone nanofibrous membranes embedded with silver nanoparticles for prevention of peritendinous adhesion. Acta Biomater. 2015, 26, 225–235. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Sheu, C.; Chen, C.H.; Chen, S.H.; Jose, G.; Kuo, C.Y.; Chen, J.P. Multi-functional electrospun antibacterial core-shell nanofibrous membranes for prolonged prevention of post-surgical tendon adhesion and inflammation. Acta Biomater. 2018, 72, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhang, Z.; Zheng, L.; Song, R.; Zhao, Y. Fabrication and Characterization of Electrospun Polycaprolactone Blended with Chitosan-Gelatin Complex Nanofibrous Mats. J. Nanomater. 2014, 2014, 964621. [Google Scholar] [CrossRef]
- Hsieh, C.F.; Chen, C.H.; Kao, H.H.; Govindaraju, D.T.; Dash, B.S.; Chen, J.P. PLGA/Gelatin/Hyaluronic Acid Fibrous Membrane Scaffold for Therapeutic Delivery of Adipose-Derived Stem Cells to Promote Wound Healing. Biomedicines 2022, 10, 2902. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Liao, H.T.; Li, W.H.; Darshan, T.G.; Mini, P.A.; Chen, J.P. Braided suture-reinforced fibrous yarn bundles as a scaffold for tendon tissue engineering in extensor digitorum tendon repair. Chem. Eng. J. 2023, 454, 140366. [Google Scholar] [CrossRef]
- Jarquin Yanez, K.; Arenas Alatorre, J. Structural Effect of Different EDC Crosslinker Concentration in Gelatin-Hyaluronic Acid Scaffolds. J. Bioeng. Biomed. Sci. 2016, 6, 182. [Google Scholar] [CrossRef]
- Gautam, S.; Chou, C.-F.; Dinda, A.K.; Potdar, P.D.; Mishra, N.C. Fabrication and characterization of PCL/gelatin/chitosan ternary nanofibrous composite scaffold for tissue engineering applications. J. Mater. Sci. 2013, 49, 1076–1089. [Google Scholar] [CrossRef]
- Govindaraju, D.T.; Chen, C.H.; Shalumon, K.T.; Kao, H.H.; Chen, J.P. Bioactive Nanostructured Scaffold-Based Approach for Tendon and Ligament Tissue Engineering. Nanomaterials 2023, 13, 1847. [Google Scholar] [CrossRef]
- Ye, K.; Kuang, H.; You, Z.; Morsi, Y.; Mo, X. Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release. Pharmaceutics 2019, 11, 182. [Google Scholar] [CrossRef]
- Li, D.; Chen, W.; Sun, B.; Li, H.; Wu, T.; Ke, Q.; Huang, C.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X. A comparison of nanoscale and multiscale PCL/gelatin scaffolds prepared by disc-electrospinning. Colloids Surf. B Biointerfaces 2016, 146, 632–641. [Google Scholar] [CrossRef]
- Casper, C.L.; Yamaguchi, N.; Kiick, K.L.; Rabolt, J.F. Functionalizing Electrospun Fibers with Biologically Relevant Macromolecules. Biomacromolecules 2005, 6, 1998–2007. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Yoo, J.J.; Atala, A.; Lee, S.J. Controlled heparin conjugation on electrospun poly(epsilon-caprolactone)/gelatin fibers for morphology-dependent protein delivery and enhanced cellular affinity. Acta Biomater. 2012, 8, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1626. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, S.H.; Kuo, C.Y.; Li, M.L.; Chen, J.P. Response of Dermal Fibroblasts to Biochemical and Physical Cues in Aligned Polycaprolactone/Silk Fibroin Nanofiber Scaffolds for Application in Tendon Tissue Engineering. Nanomaterials 2017, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, B.; Park, J.; Kaliannagounder, V.K.; Awasthi, G.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Regenerated cellulose nanofiber reinforced chitosan hydrogel scaffolds for bone tissue engineering. Carbohydr. Polym. 2021, 251, 117023. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Janas, D.; Vahedi, P. Nanofiber scaffolds based on extracellular matrix for articular cartilage engineering: A perspective. Nanotheranostics 2023, 7, 61–69. [Google Scholar] [CrossRef]
- Kao, H.H.; Kuo, C.Y.; Tagadur Govindaraju, D.; Chen, K.S.; Chen, J.P. Polycaprolactone/Chitosan Composite Nanofiber Membrane as a Preferred Scaffold for the Culture of Mesothelial Cells and the Repair of Damaged Mesothelium. Int. J. Mol. Sci. 2022, 23, 9517. [Google Scholar] [CrossRef]
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 324–332. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Vasilescu, S.; Gu, Z.; Sun, T. Bio-inspired enhancement of friction and adhesion at the polydimethylsiloxane-intestine interface and biocompatibility characterization. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 246–252. [Google Scholar] [CrossRef]
- Kwak, H.W.; Lee, H.; Park, S.; Lee, M.E.; Jin, H.-J. Chemical and physical reinforcement of hydrophilic gelatin film with di-aldehyde nanocellulose. Int. J. Biol. Macromol. 2020, 146, 332–342. [Google Scholar] [CrossRef]
- Doostmohammadi, M.; Forootanfar, H.; Shakibaie, M.; Torkzadeh-Mahani, M.; Rahimi, H.R.; Jafari, E.; Ameri, A.; Ameri, A. Polycaprolactone/gelatin electrospun nanofibres containing biologically produced tellurium nanoparticles as a potential wound dressing scaffold: Physicochemical, mechanical, and biological characterisation. IET Nanobiotechnol. 2021, 15, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Darshan, T.G.; Chen, C.H.; Kuo, C.Y.; Shalumon, K.T.; Chien, Y.M.; Kao, H.H.; Chen, J.P. Development of high resilience spiral wound suture-embedded gelatin/PCL/heparin nanofiber membrane scaffolds for tendon tissue engineering. Int. J. Biol. Macromol. 2022, 221, 314–333. [Google Scholar] [CrossRef]
- Loyo, C.; Cordoba, A.; Palza, H.; Canales, D.; Melo, F.; Vivanco, J.F.; Baier, R.V.; Millán, C.; Corrales, T.; Zapata, P.A. Effect of Gelatin Coating and GO Incorporation on the Properties and Degradability of Electrospun PCL Scaffolds for Bone Tissue Regeneration. Polymers 2024, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Bio-functionalized PCL nanofibrous scaffolds for nerve tissue engineering. Mater. Sci. Eng. C 2010, 30, 1129–1136. [Google Scholar] [CrossRef]
- Longo, R.; Catauro, M.; Sorrentino, A.; Guadagno, L. Thermal and mechanical characterization of complex electrospun systems based on polycaprolactone and gelatin. J. Therm. Anal. Calorim. 2022, 147, 5391–5399. [Google Scholar] [CrossRef]
- Rodríguez-Martín, M.; Aguilar, J.M.; Castro-Criado, D.; Romero, A. Characterization of Gelatin-Polycaprolactone Membranes by Electrospinning. Biomimetics 2024, 9, 70. [Google Scholar] [CrossRef]
- Zuo, K.J.; Shafa, G.; Chan, K.; Zhang, J.; Hawkins, C.; Tajdaran, K.; Gordon, T.; Borschel, G.H. Local FK506 drug delivery enhances nerve regeneration through fresh, unprocessed peripheral nerve allografts. Exp. Neurol. 2021, 341, 113680. [Google Scholar] [CrossRef]
- Abdul Kafi, M.; El-Said, W.A.; Kim, T.H.; Choi, J.W. Cell adhesion, spreading, and proliferation on surface functionalized with RGD nanopillar arrays. Biomaterials 2012, 33, 731–739. [Google Scholar] [CrossRef]
- Wang, F.; Cai, X.; Shen, Y.; Meng, L. Cell-scaffold interactions in tissue engineering for oral and craniofacial reconstruction. Bioact. Mater. 2023, 23, 16–44. [Google Scholar] [CrossRef]
- Doglioni, C.; Dei Tos, A.P.; Laurino, L.; Iuzzolino, P.; Chiarelli, C.; Celio, M.R.; Viale, G. Calretinin: A novel immunocytochemical marker for mesothelioma. Am. J. Surg. Pathol. 1996, 20, 1037–1046. [Google Scholar] [CrossRef]
- Gotzos, V.; Vogt, P.; Celio, M.R. The calcium binding protein calretinin is a selective marker for malignant pleural mesotheliomas of the epithelial type. Pathol. Res. Pract. 1996, 192, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Nagel, H.; Hemmerlein, B.; Ruschenburg, I.; Hüppe, K.; Droese, M. The value of anti-calretinin antibody in the differential diagnosis of normal and reactive mesothelia versus metastatic tumors in effusion cytology. Pathol. Res. Pract. 1998, 194, 759–764. [Google Scholar] [CrossRef]
- Perl, A.K.; Wilgenbus, P.; Dahl, U.; Semb, H.; Christofori, G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature 1998, 392, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 1995, 7, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Suassuna, J.H.; Das Neves, F.C.; Hartley, R.B.; Ogg, C.S.; Cameron, J.S. Immunohistochemical studies of the peritoneal membrane and infiltrating cells in normal subjects and in patients on CAPD. Kidney Int. 1994, 46, 443–454. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Lara-Pezzi, E.; Selgas, R.; Ramírez-Huesca, M.; Domínguez-Jiménez, C.; Jiménez-Heffernan, J.A.; Aguilera, A.; Sánchez-Tomero, J.A.; Bajo, M.A.; Álvarez, V.; et al. Peritoneal Dialysis and Epithelial-to-Mesenchymal Transition of Mesothelial Cells. N. Engl. J. Med. 2003, 348, 403–413. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef]
- Aguilera, A.; Yáñez-Mo, M.; Selgas, R.; Sánchez-Madrid, F.; López-Cabrera, M. Epithelial to mesenchymal transition as a triggering factor of peritoneal membrane fibrosis and angiogenesis in peritoneal dialysis patients. Curr. Opin. Investig. Drugs 2005, 6, 262–268. [Google Scholar]
- Romano, M.; Catalano, A.; Nutini, M.; D’Urbano, E.; Crescenzi, C.; Claria, J.; Libner, R.; Davi, G.; Procopio, A. 5-lipoxygenase regulates malignant mesothelial cell survival: Involvement of vascular endothelial growth factor. FASEB J. 2001, 15, 2326–2336. [Google Scholar] [CrossRef]

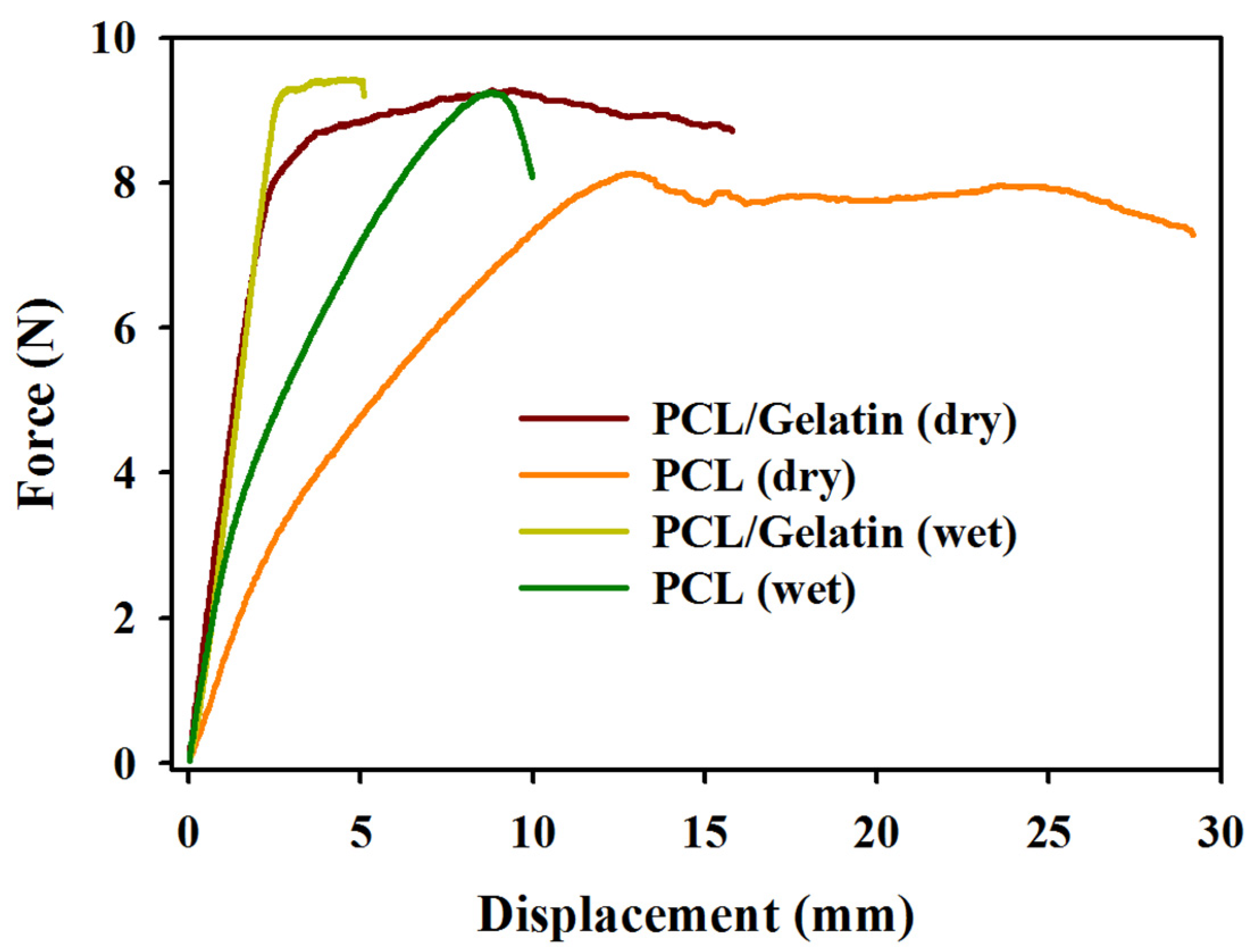

| Scaffolds | Maximum Load (N) | Maximum Displacement (mm) | Stiffness (N/mm) |
|---|---|---|---|
| PCL (dry) | 8.56 ± 0.95 | 25.00 ± 3.63 | 1.26 ± 0.06 |
| PCL/gelatin (dry) | 9.19 ± 0.41 | 14.94 ± 1.18 α | 3.72 ± 0.46 α |
| PCL (wet) | 9.44 ± 0.31 | 11.46 ± 1.86 | 1.96 ± 0.33 |
| PCL/gelatin (wet) | 10.07 ± 0.39 | 5.31 ± 0.89 β | 3.10 ± 0.32 β |
| Culture Time (Days) | Calretinin (%) | E-Cadherin (%) | ||
|---|---|---|---|---|
| PCL | PCL/Gelatin | PCL | PCL/Gelatin | |
| 1 | 10.68 ± 0.57 | 23.13 ± 1.00 * | 10.26 ± 0.35 | 28.10 ± 0.16 * |
| 3 | 20.10 ± 0.30 | 49.88 ± 0.43 * | 21.20 ± 0.36 | 36.82 ± 0.35 * |
| 5 | 38.82 ± 0.33 | 80.20 ± 0.54 * | 38.10 ± 0.20 | 66.20 ± 1.84 * |
| 7 | 41.33 ± 1.00 | 88.10 ± 0.20 * | 48.30 ± 1.45 | 84.70 ± 0.21 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindaraju, D.T.; Kao, H.-H.; Chien, Y.-M.; Chen, J.-P. Composite Polycaprolactone/Gelatin Nanofiber Membrane Scaffolds for Mesothelial Cell Culture and Delivery in Mesothelium Repair. Int. J. Mol. Sci. 2024, 25, 9803. https://doi.org/10.3390/ijms25189803
Govindaraju DT, Kao H-H, Chien Y-M, Chen J-P. Composite Polycaprolactone/Gelatin Nanofiber Membrane Scaffolds for Mesothelial Cell Culture and Delivery in Mesothelium Repair. International Journal of Molecular Sciences. 2024; 25(18):9803. https://doi.org/10.3390/ijms25189803
Chicago/Turabian StyleGovindaraju, Darshan Tagadur, Hao-Hsi Kao, Yen-Miao Chien, and Jyh-Ping Chen. 2024. "Composite Polycaprolactone/Gelatin Nanofiber Membrane Scaffolds for Mesothelial Cell Culture and Delivery in Mesothelium Repair" International Journal of Molecular Sciences 25, no. 18: 9803. https://doi.org/10.3390/ijms25189803







