Abstract
Skeletal muscle development is spotlighted in mammals since it closely relates to animal health and economic benefits to the breeding industry. Researchers have successfully unveiled many regulatory factors and mechanisms involving myogenesis. However, the effect of N6-methyladenosine (m6A) modification, especially demethylase and its regulated genes, on muscle development remains to be further explored. Here, we found that the typical demethylase FTO (fat mass- and obesity-associated protein) was highly enriched in goats’ longissimus dorsi (LD) muscles. In addition, the level of m6A modification on transcripts was negatively regulated by FTO during the proliferation of goat skeletal muscle satellite cells (MuSCs). Moreover, a deficiency of FTO in MuSCs significantly retarded their proliferation and promoted the expression of dystrophin-associated protein 1 (DAG1). m6A modifications of DAG1 mRNA were efficiently altered by FTO. Intriguingly, the results of DAG1 levels and its m6A enrichment from FB23-2 (FTO demethylase inhibitor)-treated cells were consistent with those of the FTO knockdown, indicating that the regulation of FTO on DAG1 depended on m6A modification. Further experiments showed that interfering FTO improved m6A modification at site DAG1-122, recognized by Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) and consequently stabilized DAG1 transcripts. Our study suggests that FTO promotes the proliferation of MuSCs by regulating the expression of DAG1 through m6A modification. This will extend our knowledge of the m6A-related mechanism of skeletal muscle development in animals.
1. Introduction
Goat meat is important in agriculture and animal husbandry in many countries [1]. The growth and development process of skeletal muscle includes several steps: progenitor cell/satellite cell generation, myoblast proliferation, directional differentiation, myocyte fusion to form multinuclear muscle tubes, and further formation of mature muscle fibers [2]. Recent studies suggest that in addition to many transcription factors, such as myogenic regulatory factors (MRFs) [3], specific epigenetic mechanisms may play an important role in controlling skeletal muscle transcription patterns [4]. N6-methyladenosine (m6A) modification is the most common mRNA methylation modification in eukaryotes [5]. The relationship between m6A modification and muscle development is complex and may involve multiple genes and regulatory factors. Therefore, it is necessary to explore the role of m6A modification on myogenesis further.
m6A methyltransferase (writer), demethylase (eraser), and reader protein (reader) participate in the RNA m6A methylation modification process. m6A writing requires the participation of a variety of proteins to form a methyltransferase complex with methyltransferase-like 3 (METTL3) and methyltransferase-like 14 (METTL14) as the main components [6]. METTL3 acts as a catalyst, and METTL14 stabilizes the structure of the complex, recruiting proteins such as WTAP to recognize catalytic substrates—specific RNA sequence RRACH (R: A, G; A: m6A; H: A, C, U) [7].
As a demethylase (eraser), FTO (fat mass- and obesity-associated protein) was discovered in 2011 [8]. In 2013, the second RNA demethylase of the same family was discovered—AlkB homology 5 (ALKBH5) [9]. These findings suggest that m6A modification is a dynamic and reversible process. Moreover, reading proteins, including the YTHDF family, YTHDC family, and Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1), recognize the m6A sites and consequently alter mRNA processing, such as by affecting the shearing process [10], regulating stability [11], and promoting translation and degradation [12]. As the first demethylase to be discovered [8], FTO is closely related to the proliferation and differentiation of myoblasts. However, its regulatory mechanism on myocyte proliferation and whether it depends on m6A modification remains to be further explored.
Here, we explored and found the high enrichment of FTO in goat skeletal muscles and its close association with m6A modification on transcripts of goat proliferating muscle satellite cells (MuSCs). Mechanistically, during myocyte proliferation, FTO inhibits dystrophin-associated protein 1 (DAG1) mRNA dependent on m6A modification associated with the m6A reading protein IGF2BP1. Our results provide a reference for further understanding the relationship between m6A modification and skeletal muscle development.
2. Results
2.1. FTO Knockdown Suppresses the Proliferation of Goat MuSCs
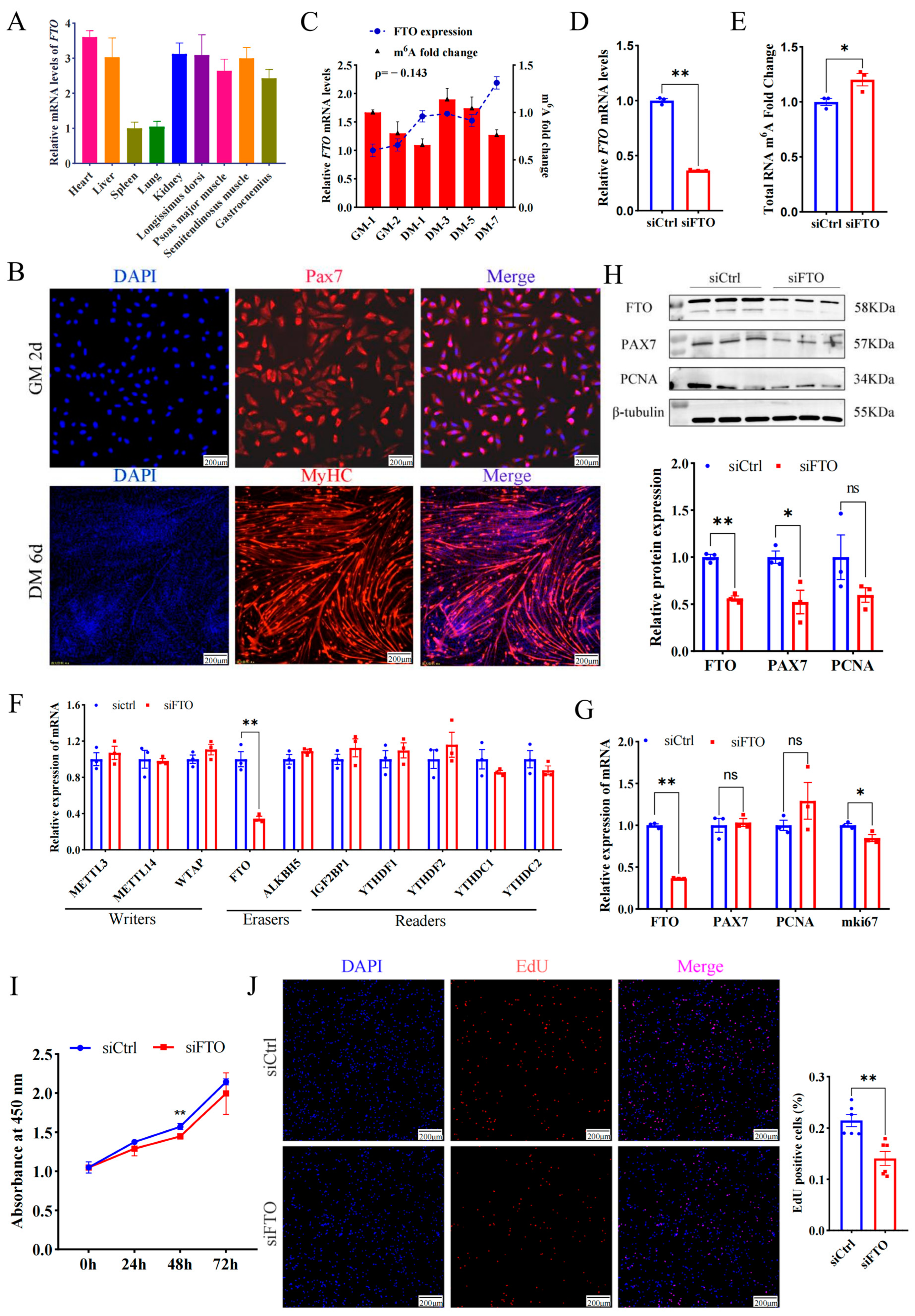
In addition to the spleen and lung, FTO mRNA was highly enriched in the heart, liver, kidney, and four types of skeletal muscles, including the longissimus dorsi muscle (LD), semitendinosus muscle, psoas major muscle, and gastrocnemius muscle (Figure 1A), implying FTO is closely related to muscle development. To profile FTO in the proliferating myoblasts, MuSCs from newborn goat LD muscles were isolated, purified, and identified (Figure 1B). FTO gradually increased during the proliferation of myoblasts. On the contrary, the m6A modification of total RNA gradually decreased (Figure 1C), roughly suggesting the demethylase function FTO plays on mRNA.
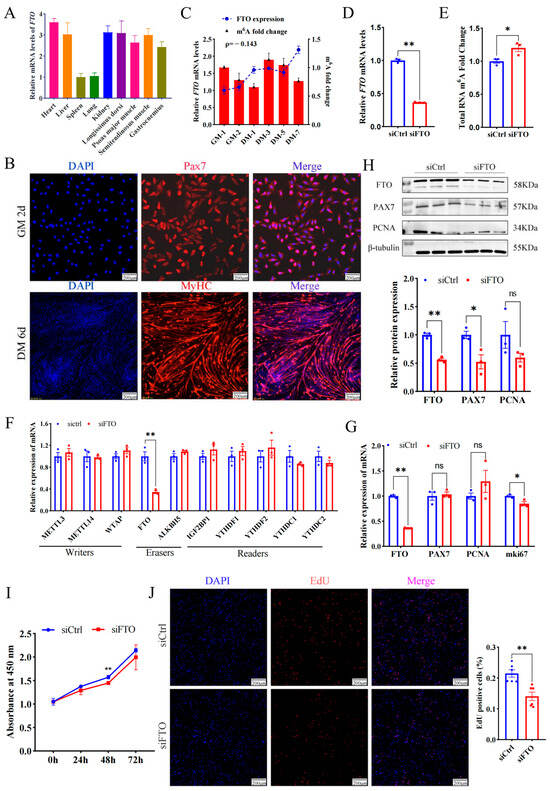
Figure 1.
Deficiency of FTO suppresses the proliferation of goat MuSCs. (A) Expression of FTO in different tissues of goats. (B) Cells immunofluorescent stained with anti-PAX7 (MuSCs cultured in growth medium (GM) for 2 days) and anti-MYHC (MuSCs cultured in differentiation medium (DM) for 6 days). Scale bar: 200 μm. (C) FTO mRNA and m6A changes during MuSCs (cultured in the growth medium for 1 and 2 days and differentiation medium for 1, 3, 5, and 7 days). (D) mRNA level of FTO in cells treated with siFTO. (E) The m6A of total RNA affected by FTO knockdown. (F) Effect of FTO knockdown on gene expression of m6A modified enzymes. (G) mRNA changes in myoblast proliferation marker genes after FTO knockdown. (H) Protein of myoblast proliferation genes affected by deficiency of FTO. (I) CCK8 assay of the viability of MuSCs. (J) The number of new cells stained with EdU. Scale bar: 200 μm. Results are represented as the mean ± SEM, * p < 0.05, ** p < 0.01, and ns indicate insignificance.
To explore the effect of FTO on myoblast proliferation, siRNA targeting FTO mRNA (siFTO) was transfected into MuSCs cells and successfully knocked down FTO transcripts by ~0.6 fold (p < 0.01) (Figure 1D), while significantly up-regulating the m6A modification level of total RNA (p < 0.05) (Figure 1E). Interference with FTO only decreased mRNA levels of itself, but failed to change the expression of most methyltransferases, demethylases, and methyl-reading proteins (Figure 1F). In addition, the deficiency of FTO significantly decreased proliferation marker gene mki67 (p < 0.05), but mRNA levels of PAX7 and PCNA changed insignificantly (p > 0.05, Figure 1G). The WB experiment showed that the knockdown of FTO significantly reduced the protein of FTO and PAX7 by ~0.4 fold (p < 0.01 or 0.05) (Figure 1H). Moreover, the number of newly formed cells measured by the CCK8 (OD value at 450 nm) was lower in the siFTO group than in the control group at 24 h, 48 h, and 72 h and reached a significant level at 48 h (p < 0.01) (Figure 1I). On the contrary, overexpressed FTO resulted in more new cells than the control group (Figure S1). Consistently, compared with the control, FTO knockdown dramatically decreased the number of EdU-positive cells (p < 0.01) (Figure 1J).
These results indicate that FTO promotes muscle cell proliferation.
2.2. DAG1 Is Targeted by FTO and Inhibits Cell Proliferation
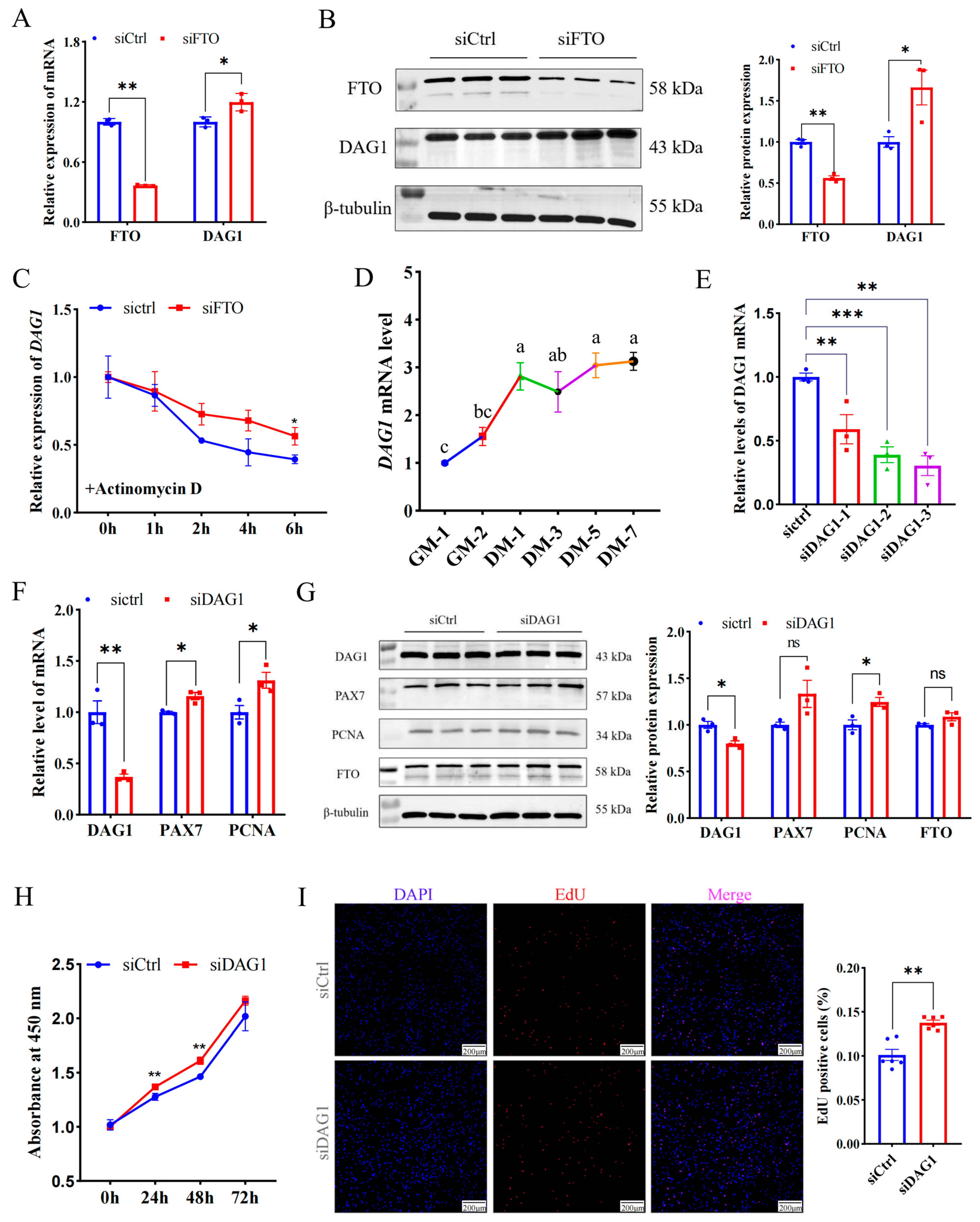
To explore the regulatory mechanism of FTO in promoting muscle cell proliferation, the potential targets of FTO were further screened using previously published data, and it was found that dystroglycan 1 (DAG1) is the gene most affected by it (Figure S2) [13]. In addition, several studies have unveiled that DAG1 regulates cell proliferation [14,15,16,17]. Here, we found interfering FTO significantly elevated DAG1 mRNA (p < 0.05) (Figure 2A) and protein (p < 0.05) (Figure 2B). Since m6A closely regulates mRNA stability [10], we assessed whether FTO stabilizes DAG1 mRNA. siFTO-transfected cells were treated with actinomycin D for 0 h, 1 h, 2 h, 4 h, and 6 h. The results showed that compared with the control, a deficiency of FTO significantly increased the stability of DAG1 mRNA (p < 0.05) (Figure 2C). These results suggest that FTO inhibited the expression of DAG1 mRNA during myoblast proliferation by affecting its stability.
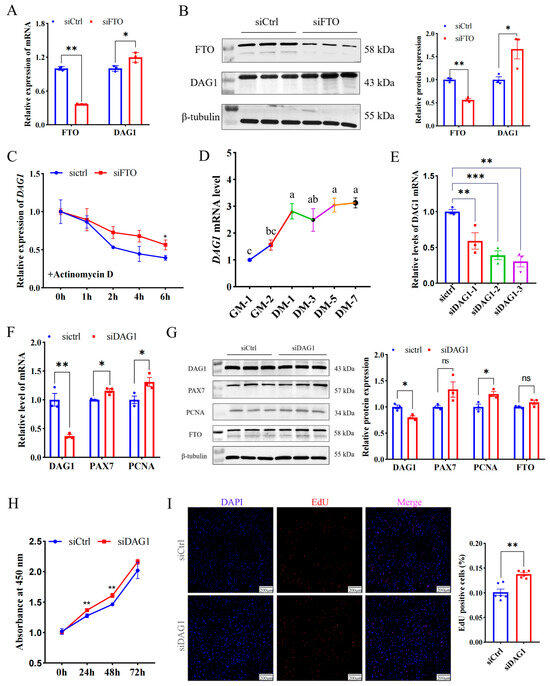
Figure 2.
The FTO-targeted gene DAG1 inhibits cell proliferation. (A) DAG1 mRNA increased by FTO knockdown. (B) DAG1 protein elevated by inhibiting FTO. (C) DAG1 mRNA stability affected by FTO knockdown. (D) The profile of DAG1 in cell proliferation and differentiation. (E) siRNA targeting DAG1 knockdown on mRNA expression. (F) mRNA changes in cell proliferation marker genes in cells deficiency of DAG1. (G) Effect of DAG1 knockdown on protein of cell proliferation marker genes. (H) Viability of cells tested by CCK8. (I) EdU staining cells altered by siDAG1. Scale bar: 200 μm. Results are represented as the mean ± SEM, * p < 0.05, ** p < 0.01, *** p < 0.001, and ns indicates no significance. In the picture marked with lower case letters, means shared at least one letter indicate no significance (p > 0.05), and on the contrary, no common letters indicate a significant difference (p < 0.05).
Furthermore, DAG1 mRNA expression increased significantly from the proliferative stage to the first day of differentiation, and then slowly decreased and then increased. (Figure 2D). Meanwhile, the successful knockdown of DAG1 using three siRNAs (p < 0.01) (Figure 2E) significantly promoted mRNA levels of the proliferation marker genes PAX7 and PCNA (p < 0.05) (Figure 2F). The WB results implied that a deficiency of DAG1 increased the protein of PCNA (p < 0.05) and PAX7 (p = 0.08), without a significant change in FTO (Figure 2G). The number of new cells tested by CCK8 in the siDAG1 group was higher than that in the control group at 24 h, 48 h, and 72 h (Figure 2H). Expectedly, overexpressing DAG1 decreased the cell number at 24 h, 48 h, and 72 h and reached a significant level at 72 h (p < 0.01) (Figure S3). Additionally, the knockdown of DAG1 significantly increased EdU-positive cells (p < 0.01) (Figure 2I). These results suggest that FTO negatively regulates DAG1, a suppressor of myogenic proliferation.
2.3. FTO Regulates DAG1 in an m6A-Dependent Manner
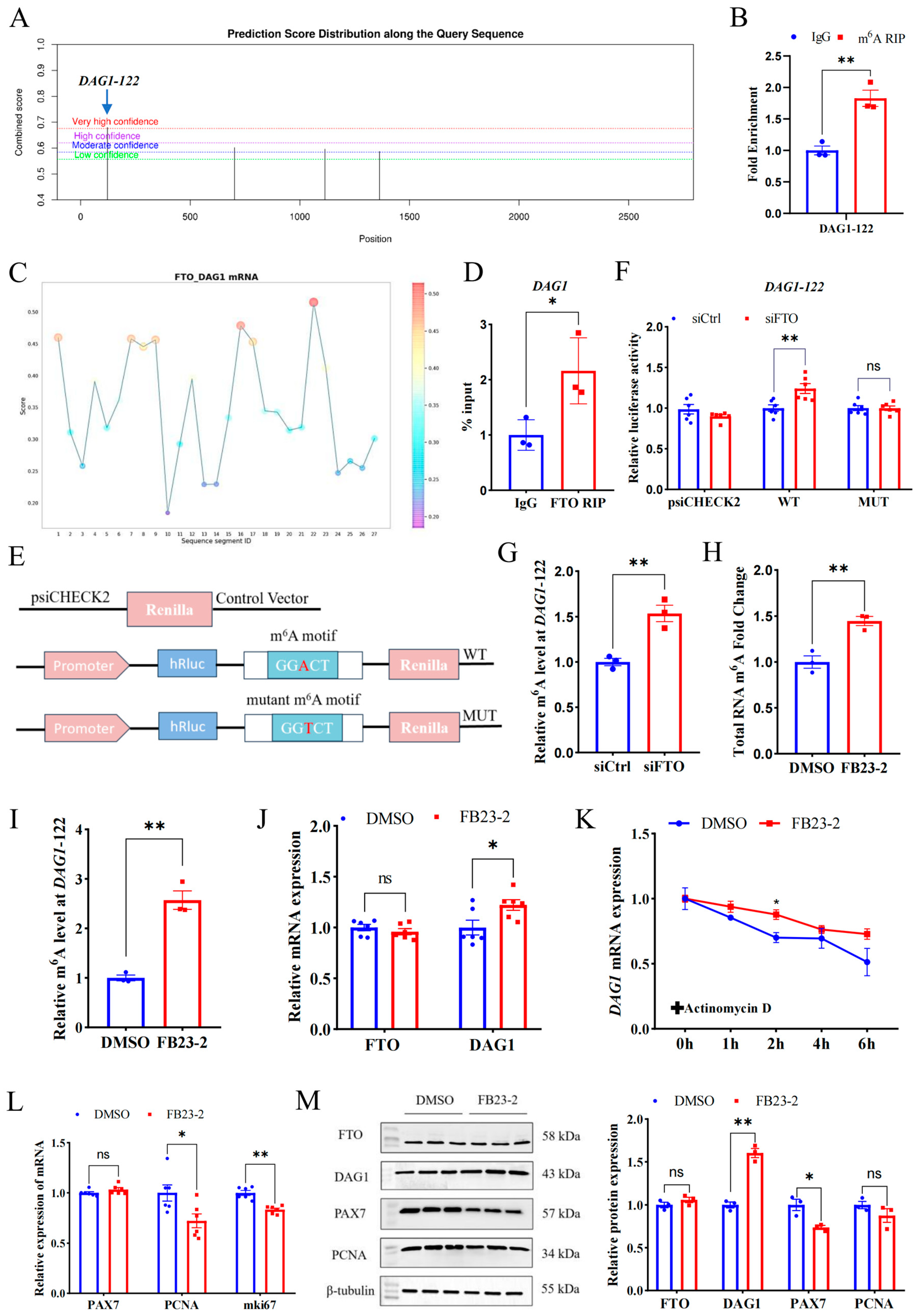
To investigate the mechanism underpinning FTO’s regulation on DAG1, we employed SRAMP, an online mammalian m6A modification website, to screen the m6A sites in the full sequence of the DAG1 gene. The top loci with the highest confidence (DAG1-122, chr22) were selected for verification (Figure 3A). Using the specific primers targeting the site and m6A MeRIP samples performed with goat LD muscles, we verified that anti-m6A was highly enriched at DAG1-122 sites compared with anti-IgG (Figure 3B). Further, we used the online website RBPsuite and found that FTO potentially bound to DAG1 mRNA, with high confidence at DAG1-122 sites (Figure 3C). Moreover, the results from FTO-RIP performed on the LD confirmed the binding of FTO to DAG1 transcripts (Figure 3D).
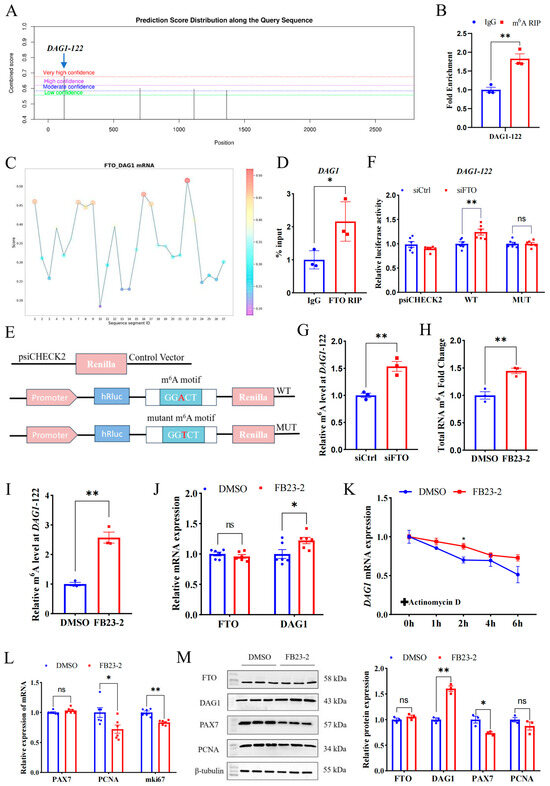
Figure 3.
FTO regulates DAG1 and other proliferation genes in an m6A-dependent manner. (A) The m6A modification sites on DAG1 mRNA predicted using the SRAMP. (B) The m6A modification of DAG1 mRNA verified by MeRIP-qPCR. (C) The FTO binding sites on DAG1 mRNA predicted by RBPsuite. (D) FTO binding on DAG1 mRNA verified by RIP-qPCR. (E) The wild-type (WT) and mutant (MUT) m6A motif dual luciferase reporter vectors. (F) Effect of interfering FTO on luciferase activity in m6A-modified fragments of DAG1 mRNA. (G) DAG1 m6A modification levels affected by interfering FTO. (H) Changes in total RNA m6A modification of cells treated by FB23-2. (I) MeRIP-qPCR of DAG1-122 after FB23-2 treatment. (J) FTO and DAG1 mRNA altered by FB23-2. (K) Effect of FB23-2 on DAG1 stability. (L) mRNA levels of cell proliferation marker genes changed by FB23-2. (M) Protein of proliferation marker genes influenced by FB23-2. Results are represented as the mean ± SEM, * p < 0.05, ** p < 0.01, and ns indicates no significance.
A dual luciferase reporter assay was performed to further determine the active sites of FTO on DAG1 mRNA. Wild and mutant types of DAG1-122 were constructed into the psiCHECK2 vector (Figure 3E) and co-transfected with siCtrl and siFTO. The results showed that siFTO significantly increased the luciferase activity of the DAG1-122 wild-type (p < 0.01), and site mutation abolished this change (Figure 3F). Moreover, the results of the MeRIP-qPCR showed that the m6A modification level of DAG1-122 was significantly increased after siFTO (p < 0.05) (Figure 3G). These results indicate that DAG1-122 plays a critical role in FTO’s regulation on DAG1.
In addition, FB23-2 was used to selectively inhibit FTO demethylase activity. Compared with the control DMSO, FB23-2 treatment failed to significantly change the FTO mRNA and protein levels (Figure 3J,M). Nevertheless, the m6A levels of total RNA and the DAG1-122 site were dramatically upregulated (p < 0.01) in the FB23-2 treatment group (Figure 3H,I). Notably, FB23-2 treatment significantly increased DAG1 at both the mRNA and protein levels (p < 0.05 or p < 0.01) (Figure 3J,M). Furthermore, FB23-2-conducted cells were treated with actinomycin D for 0 h, 1 h, 2 h, 4 h, and 6 h. The results showed that FB23-2 increased the stability of DAG1 mRNA (Figure 3K). Moreover, the addition of FB23-2 dramatically reduced transcripts of PCNA and mki67 (p < 0.05 or p < 0.01) (Figure 3L) and the PAX7 protein (p < 0.05) (Figure 3M).
These results suggest that FTO suppresses m6A’s modification of transcripts including DAG1 mRNA at DAG1-122 dependent on their demethylase activity and further affects the myogenic proliferation of MuSCs.
2.4. IGF2BP1 Stabilizes DAG1 mRNA through Recognizing Its m6A Modification
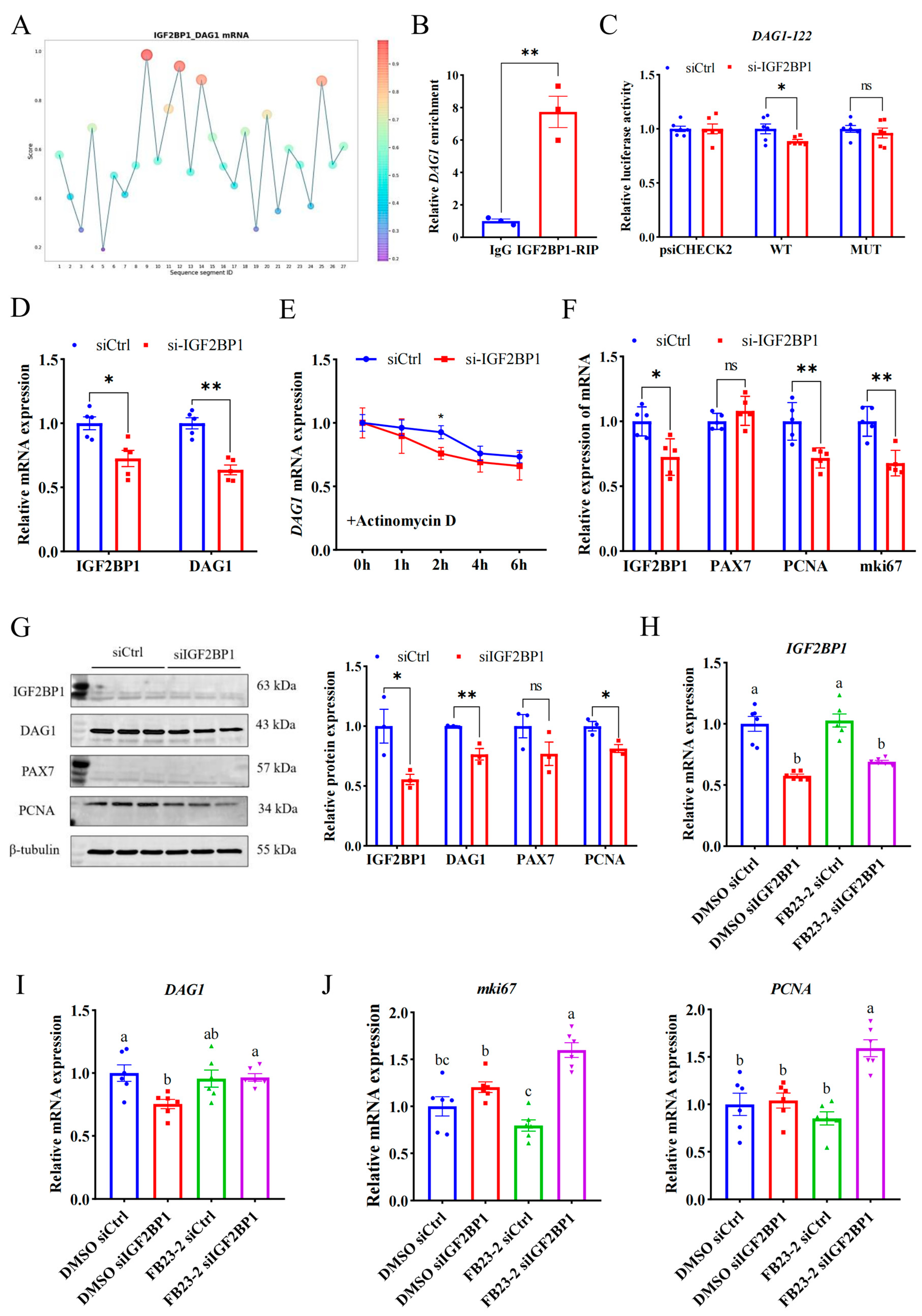
A reading protein is critical for recognizing the m6A modification site and downstream function, so it is important to explore the reading protein that recognizes the m6A modification site of DAG1 mRNA. Since the m6A modification promotes DAG1 mRNA expression, and IGF2BP1 is a typical reading protein that promotes mRNA stability [18,19,20], we used an online website and found that the IGF2BP1 potentially binds to DAG1 mRNA at high confidence sites (DAG1-122) (Figure 4A). And the RIP assay confirmed that DAG1 mRNA was significantly enriched by the IGF2BP1 antibody (Figure 4B). Furthermore, compared with the control, interference with IGF2BP1 significantly reduced the wild-type luciferase activity of DAG1-122 (p < 0.05), without changing after the mutation (Figure 4C). In addition, both mRNA and proteins of DAG1 were dramatically reduced after interfering with IGF2BP1 (p < 0.01) (Figure 4D,G). These results show that IGF2BP1 acts on the DAG1-122 locus and promotes DAG1 expression.
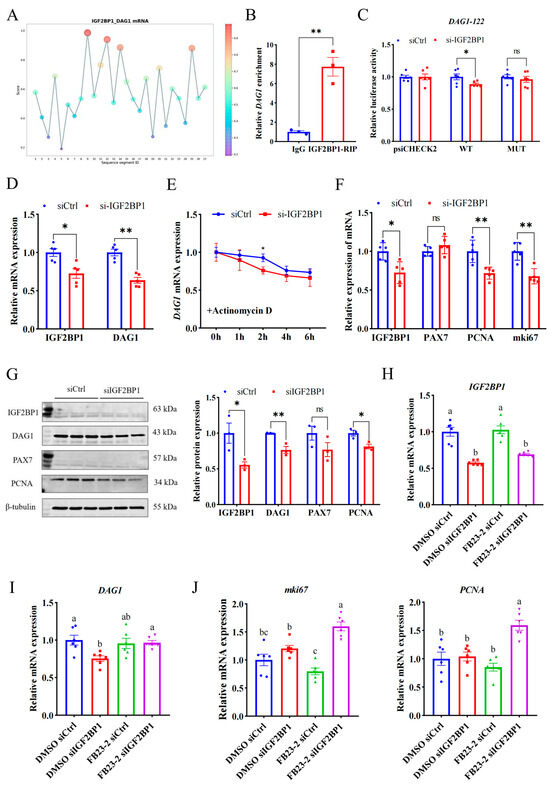
Figure 4.
IGF2BP1 stabilizes DAG1 mRNA through recognizing its m6A modification. (A) The DAG1-IGF2BP1 interaction sites predicted by RBPsuite. (B) DAG1 mRNA enriched by IGF2BP1 protein. (C) Luciferase activity of DAG1-122 altered by interfering IGF2BP1. (D) IGF2BP1 and DAG1 mRNA altered by interfering IGF2BP1. (E) DAG1 mRNA stability caused by knockdown of IGF2BP1. (F) mRNA profiles of cell proliferation marker genes altered by deficiency of IGF2BP1. (G) Protein of cell proliferation marker genes affected by interfering IGF2BP1. (H) Expression of IGF2BP1 transcripts in cells treated with FB23-2 combined with siIGF2BP1. (I) DAG1 mRNA, (J) mki67 mRNA, and PCNA mRNA in cells cotransfected with FB23-2 and siIGF2BP1. Results are represented as the mean ± SEM, * p < 0.05, ** p < 0.01, and ns indicates no significance. Means with totally different lowercase letters indicate p < 0.05.
To detect the effect of IGF2BP1 on DAG1 stability, siIGF2BP1-transfected proliferation cells were treated with actinomycin D for 0 h, 1 h, 2 h, 4 h, and 6 h. The results showed that compared with the control, the stability of DAG1 mRNA was significantly reduced by a deficiency of IGF2BP1 and reached a significant level at 2 h (p < 0.05) (Figure 4E), suggesting that the recognition and binding of IGF2BP1 on DAG1-122 affected the stability of DAG1 mRNA and promoted its expression during myoblast proliferation. In addition, the mRNA levels of PCNA and mki67 were significantly reduced after siIGF2BP1 (p < 0.01) (Figure 4F), and the level of PCNA protein was significantly reduced (p < 0.05) (Figure 4G), indicating that IGF2BP1 promotes the proliferation of muscle cells.
In order to explore whether the regulation of IGF2BP1 on DAG1 depends on the demethylase activity of FTO, we co-transfected cells with siIGF2BP1 and FB23-2 to conduct a salvage test. The results showed that FB23-2 solely failed to affected IGF2BP1 levels, while the levels of IGF2BP1 mRNA were significantly decreased by interfering itself (p < 0.05) (Figure 3H). Moreover, DAG1 mRNA was downregulated by a deficiency of IGF2BP1 when FB23-2 was absent (p < 0.05), and this decrease was successfully restored by adding FB23-2 (p < 0.05) (Figure 3I). Additionally, the expression of mki67 and PCNA was greatly elevated by the cotransfection of siIGF2BP1 and FB23-2 (p < 0.05), though FB23-2 solely tended to downregulate them (Figure 3J). These results suggest that IGF2BP1 positively regulates DAG1 expression through m6A modification, subsequently affecting myoblast proliferation.
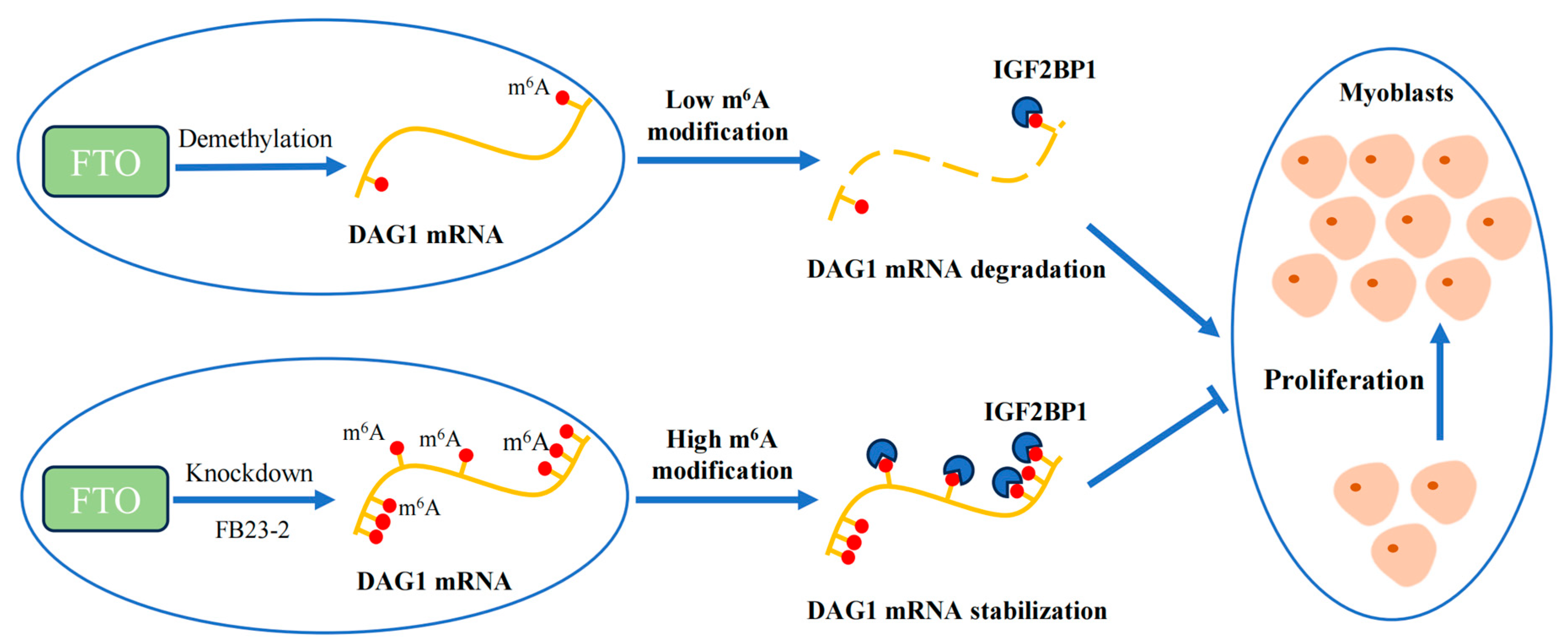
During the proliferation of goat MuSCs, the demethylase FTO reduces the m6A methylation level of DAG1 mRNA, which results in fewer recognition sites of IGF2BP1 and a consequently decreased DAG1 level and eventually promotes the proliferation of muscle cells. On the contrary, the knockdown of FTO or inhibition of its demethylation effect by adding FB23-2 increases the m6A methylation level of DAG1 mRNA and the recognition of IGF2BP1 on the m6A site, promoting its expression, thereby inhibiting the proliferation of muscle cells.
3. Discussion
The regulatory network of muscle development is complex, and currently, most research focuses on various myogenic regulatory factors and non-coding RNA [21]. However, the post-transcriptional regulatory mechanisms affecting skeletal muscle development remain largely unknown. m6A modification is a methylation modification that occurs on the sixth nitrogen atom of RNA adenine and affects mRNA nucleation [18], mRNA splicing [19], stability [20], and other biological processes. Due to the development of m6A high-throughput sequencing technology, many studies have shown a correlation between m6A modification and skeletal muscle [22,23].
MeRIP-seq and RNA-seq combined analysis and comparison of m6A mRNA in yaks at different developmental stages showed that the gene regulating cell differentiation and muscle development was continuously expressed, and the abundance of m6A was negatively correlated with the gene expression level [24]. Whole-transcriptome studies revealed that m6A has a regulatory effect on embryonic muscle development in Wenchang chickens [25], Dingan goose [26], and ducks [27,28]. The m6A-seq analysis of skeletal muscle of wild boar, Landrace, and Rong chang pig (obese breed) showed that the m6A modification pattern had obvious differences among breeds [23]. In this study, FTO transcripts was enriched in goat skeletal muscles. Moreover, m6A modification levels of total RNAs exhibited two waves of decrease during myogenic development of MuSCs from the proliferative stage to the pre-differentiation stage (GM1 to DM1) and during differentiation stage (DM3 to DM7). This indicates that m6A modification is key in regulating myogenic development in goats.
Previous studies have shown that FTO relies on m6A modification to regulate the expression of NFATC1 to maintain the formation of slow muscle fibers in mice [29]. To further regulate the cell cycle process, the cyclin CCND1 is demethylated [30]. Previous studies have shown that the knockdown of both methyltransferase FTO and demethylase METTL3 promotes bovine myoblast proliferation, suggesting that m6A modifiers regulate skeletal muscle development with a complex and contradictory network of regulatory systems [31]. In addition, m6A modification-related enzymes are also regulated by other genes and small-molecule compounds [32,33,34,35,36]. It was reported that FTO knockdown significantly decreased the expression of cyclin CCND1 and inhibited the proliferation of goat myoblasts [13]. In this study, we came to a similar conclusion that FTO knockdown inhibited myocyte proliferation. In addition, the proliferation process of myoblasts is impaired when the expression of anti-muscular dystrophin is lost [37]. We found that decreased FTO expression level can promote the expression of DAG1, and DAG1 knockdown can promote myoblast proliferation.
In 2019, a study reported the discovery of a compound, FB23-2, with high activity and selectivity against FTO [38]. Subsequently, the compound was used to study cancer [39,40], bone development [41,42], and muscle cell proliferation [13]. In this study, the dual luciferin report test of m6A modification site mutation (DAG1-122) found that this site is a key site for FTO function. The agreement between the addition of FB23-2 to MuSCs and the interference with FTO strongly suggests that the regulation of DAG1 by FTO in proliferative MuSCs depends on m6A modification.
m6A modification requires reading proteins to recognize m6A and regulate mRNA processing [43]. m6A modification promotes DAG1 mRNA expression, so the IGF2BPS family that promotes mRNA stability is selected. IGF2BP3 has been reported to promote the human myocardial regeneration process by promoting MMP3 mRNA stability through m6A modification [44]. The IGF2BP1 protein can enhance its stability and facilitate translation by recognizing RNA N6-methyladenosine [11]. IGF2BP1 was also found to target many myogenic genes in skeletal muscle, such as MYH2 and MyoD, suggesting that IGF2BP1 may regulate skeletal muscle development by binding to target genes that undergo m6A modification [45]. Here, we found the binding of IGF2BP1 and DAG1 mRNA through the RIP test, and IGF2BP1 recognized DAG1 m6A modification sites and increased mRNA expression, which affected the proliferation of goat muscle cells.
As part of epigenetic modification, m6A modification regulates a variety of developmental processes. In studies of FTO catalytic substrates, they were initially reported to catalyze the demethylation of 3-methylthymine (3-meT) [46] and 3-methyluracil (3-meU) [47] in single-stranded DNA, subsequently catalyzing N6-methyladenosine (m6A) [8] as the first RNA demethylase. The second base in many mRNAs can be methylated by 2′-O-methylation [48], with a portion of the base forming m6Am from m6A [49]. N6,2′-O-dimethyladenosine (m6Am) is also a reversible modification that affects the fate of cellular mRNA [50]. FTO preferentially demethylates m6Am rather than m6A and reduces the stability of m6Am mRNA. However, under certain conditions, the overexpression efficiency of FTO is high, and the level of m6A in mRNA will be reduced [51]. Our findings are consistent with a slight increase in total m6A levels in cell lines after FTO knockdown [8]. It is worth noting that the overall abundance of m6Am is much lower than that of m6A. Most importantly, m6A modification has a strong correlation with muscle development, and there are currently no studies showing a relationship between m6Am and muscle development, which may also be a direction for future research.
Conclusively, our study shows that demethylase FTO affects the m6A modification of total RNA during muscle development. In addition, FTO regulates DAG1 levels and thus promotes muscle cell proliferation. Mechanistically, FTO demethylates the m6A modification of DAG1 mRNA, which interrupts the m6A-related mRNA stability recognized by IGF2BP1, thereby promoting muscle cell proliferation (Figure 5).
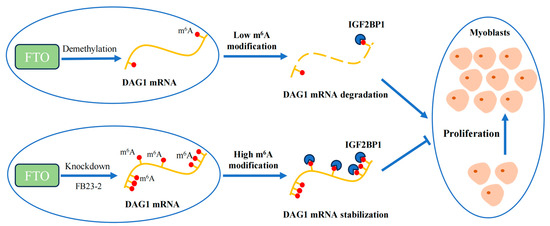
Figure 5.
Proposed mechanism of FTO/IGF2BP1/DAG1 on myocyte proliferation.
4. Materials and Methods
4.1. Animal and Sample Collection
Goats were from the Chengdu Ma Goat breeding farm in Dayi County, Chengdu, China. Three healthy 1-month-old Chengdu Ma goats were humanely sacrificed. Their hearts, livers, spleen, lungs, kidneys, longissimus dorsi muscles, psoas major muscles, gastrocnemius, and semitendinosus muscles were sampled. The samples were quickly placed in liquid nitrogen for RNA extraction.
4.2. Isolation and Identification of MuSCs
The longissimus dorsi muscles of the newborn Chengdu Ma goats were quickly collected and cleaned in PBS buffer. After removing fascia, adipocyte tissue, and blood vessels, the muscle was cut into pieces and digested with trypsin and collagenase I + II. When the tissue digestion was completed, 10% FBS was added to terminate digestion, and then, the tissue was mixed and slowly filtered with a 70 μm cell sieve. Filtrate was collected in a 50 mL centrifuge tube and centrifuged at 2000 rpm for 5 min, and the supernatant was discarded. After full cleaning with PBS, centrifuge it was centrifuged and the supernatant was discarded under the same conditions. Finally, 5 mL of 10% FBS resuspension cells was added, mixed, and cultured in an incubator (37 °C, 5% CO2) [51]. Moreover, the isolated cells were identified by staining with a PAX7 antibody (with a positive rate > 95%) and could successfully differentiate into myocytes and myotubes (MyHC-positive). These isolated cells were considered MuSCs and were used for subsequent experiments.
4.3. Cell Culture and Transfection
MuSCs were cultured in a growth medium containing 10% FBS (Gibco, Grand Island, NY, USA) and 1% penicillin–streptomycin (Invitrogen, Bohemia, NY, USA). When the cell density reached more than 85%, it was replaced with 2% equine serum (Gibco, Grand Island, NY, USA)—cultured in a differentiation medium to promote myogenic differentiation. Lipofectamine 3000 (Life Technologies, Waltham, MA, USA) was used in transfected plasmids.
4.4. Cell Proliferation Assay
An appropriate amount of 50 μM EdU medium was prepared, and on the second day after experimental treatment (transfection of interfering RNA) in MuSCs, 100 μL of 50 uM EdU medium was added to each well and incubated for 2 h, the medium was discarded, and the cells were cleaned with PBS 1–2 times, for 5 min each time. Follow-up cell fixation, apollo staining, and other steps were carried out according to the instructions for the Cell-Light™ EdU Apollo In Vitro Kit (RiboBio, Guangzhou, China).
The cells were inoculated on 96-well plates and treated (transfected with interference RNA) after adhesion. After incubation for 24, 48, and 72 h in the incubator, 10 μL of CCK-8 solution was added to each well and incubated in the incubator for 2 h in the dark to detect the absorbance of 450 nm. The CCK8 experiment used Cell Counting Kit-8 (Beyotime, Shanghai, China).
4.5. Target Gene Screening Methods
Genes related to muscle development were screened according to qval < 0.05 and 0 < |log2.FC| < 2. As shown in the figure below, DAG1 was the gene most affected by FTO in proliferation cells, and the level of m6A was high. Therefore, DAG1 was finally selected as the target of FTO for further exploration.
4.6. Interfering RNA and Plasmid Construction
The siRNA used in this experiment was designed and synthesized by Ruibo Biology (RiboBio, Guangzhou, China), and the siRNA information is shown in Table S1. The complete CDS of the FTO (NC_030825.1) gene was amplified and inserted into the pEGFP-N1 (Promega, Madison, WI, USA) vector using a homologous recombinant cloning kit (Vazyme, Nanjing, China) to construct the overexpressed plasmid. The DAG1 (NC_030829.1) overexpression vector was synthesized by Qingke (Beijing, China).
4.7. Total RNA Extraction and qPCR
RNAiso Plus (Takara, Dalian, China) was used to extract RNA from cells and tissues. cDNA was obtained using a PrimeScript™ RT kit (Takara, Dalian, China). In addition, SYBR premix Ex TaqTM II (Takara, Dalian, China) was employed to quantify gene expression by qPCR. The 2−ΔΔCt methods were used to normalize gene levels with GAPDH or β-actin as the internal control. All primers are shown in Table S2.
4.8. Immunofluorescence
MuSC was washed thrice with PBS (5 min each time) and fixed with 4% paraformaldehyde. The cells were permeated with 0.5% TritonX-100. The cells were then incubated with the primary antibody (1:120, 4 °C, 12 h) and the secondary antibody (1:120, 37 °C, 2 h). Finally, the calculation was performed using ImageJ software (Version 1.54j). Antibodies are as follows: anti-Pax7 (1:200, abs124153, absin, Shanghai, China), anti-MyHC (1:300, sc-378137, Santa Cruz, CA, USA), Cy3 Goat Anti-Mouse IgG (1:200, AS008, ABclonal, Wuhan, China), and FITC Goat Anti-Rabbit IgG (AS011, 1:200, ABclonal, Wuhan, China).
4.9. Western Blot (WB) Analysis
About 20 micrograms of protein was separated using 8%–12% SDS-PAGE gel, transferred to activated (PVDF) membrane (Millipore, MA, USA), cleaned with TBST solution, and sealed with 5% skim milk at 37 °C for 2 h. After TBST cleaning, the membrane was incubated with the required primary antibody at 4 °C overnight. After TBST cleaning, the corresponding secondary antibody was incubated at 37 °C for 1.5 h after dilution. TBST was cleaned in sequence with TBS and strip visualization was performed using Ge1DocTM XR & ChemiDocTM XRS (Bio-Rad, Hercules, CA, USA).
The total protein extraction kit (Beibo Biology, Shanghai, China) was used to extract protein, and the BCA protein quantification kit (Beibo, Shanghai, China) was used for protein quantification. WB antibodies mainly include anti-β-tubulin (1:1000, 250007, ZENBIO, Chengdu, China), anti-MyHC (1:1000, sc-378137, Santa Cruz, Dallas, TX, USA), anti-FTO (1:1000, bs-7056R, Bioss, Woburn, MA, USA), anti-DAG1 (1:1000, A10076, ABclonal, Woburn, MA, USA), anti-PAX7 (1:200, sc-81975, Santa Cruz, CA, USA), anti-Mouse IgG (1:5000, 511103, ZENBIO, Chengdu, China), and anti-Rabbit IgG (AS014, 1:5000, ABclonal, Wuhan, China).
4.10. Luciferase Reporter Assays
We downloaded the mature mRNA sequence of goat DAG1 (NC_030829.1) from the NCBI official website. Wild-type (WT) and m6A motif mutants (modification sites A to T mutation, MUT) from the DAG1-122 regions were inserted into the psiCHECK-2 vector. siCtrl and siFTO were transfected into MuSCs, and subsequently, psi-CHRCK2, WT, and MUT vectors were transfected. Luciferase activity was quantified using the dual luciferase reporter gene assay kit (Transgen, Beijing, China). The MUT vector was synthesized by Cytology (Qingke, Beijing, China). These luciferase vector sequence are shown in Table S3.
4.11. Total m6A Modification Level Analysis
RNAiso Plus was used to isolate total RNA from MuSCs at proliferative stages (GM-1, GM-2) and differentiation stages (DM-1, DM-3, DM-5 and DM-7). The global m6A methylation level of RNA was detected using the m6A RNA methylation quantitative kit (Epigentek, Farmingdale, NY, USA).
4.12. RNA Immunoprecipitation (RIP) Assay
The MuSC was collected and cleaved for RIP assays to evaluate the binding capacity of proteins and DAG1 mRNA. Subsequently, RIP was tested using the Magna RIP™ RNA-binding protein immunoprecipitation kit (Sigma-Aldrich, St. Louis, MO, USA). The antibodies used in RIP assays include FTO (1:1000, 27226-1-AP, Proteintech, Rosemont, IL, USA), IGF2BP1 (1:1000, EPR18797, abcam, Cambridge, UK), and IgG (Sigma-Aldrich, St. Louis, MO, USA).
4.13. RNA Stability Assays
The cells were treated according to the experimental requirements (transfected with siFTO or siIGF2BP1 or added with FB23-2 on the second day of muscle cell proliferation), and actinomycin D was added at the appropriate time (48 h after transfection and 72 h after the addition of FB23-2). The cells were collected 0, 1, 2, 4, and 6 h later, and RNA was extracted for subsequent experiments.
4.14. Methylated RNA Immunoprecipitation (MeRIP)
First, RNA was extracted from LD tissues of Chengdu Ma goats and cells were transfected with siFTO/FB23-2 on the second day of proliferation; then, we enriched the m6A-modified RNA fragments in cells with antibodies that exclusively recognized m6A modification through the Magna MeRIP™ m6A Kit (Sigma-Aldrich, St. Louis, MI, USA). The enriched RNA was purified using the RNeasy mini kit (Qiagen, Hilden, Germany). Finally, the m6A level of DAG1 mRNA was quantified by qPCR.
4.15. Online Prediction of m6A Modification Sites and RBP Binding
To predict m6A modification sites on the DAG1 transcripts, the mature mRNA sequence was put into the prediction box using the online tool SRAMP with mature mRNA mode (http://www.cuilab.cn/sramp, accessed online 5 May 2023).
We employed RBPsuite (http://www.csbio.sjtu.edu.cn/bioinf/RBPsuite/, accessed online 21 June 2023) to explore the potential interaction between proteins and transcripts. We chose the human species, linear RNA type, and general protein model and then filled in the RNA sequence as required. Subsequently, we obtained a map of the protein distribution on RNA.
4.16. Statistical Analysis
At least three independent biological replicates were performed for each experiment or treatment. Data were analyzed using GraphPad Prism version 9.0. An unpaired two-tailed Student’s t-test was employed to compare the means difference between the two groups when the homogeneity of variance met Levene’s test. The one-way or two-way ordinary ANOVA with Tukey’s correction for multiple comparisons was performed to analyze three or more means with equal variances. All results were presented as mean ± SEM. Statistical significance was denoted as * p < 0.05, ** p < 0.01, and *** p < 0.001.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25189804/s1.
Author Contributions
Conceptualization, writing—original draft, and data curation, J.Y.; methodology, L.X.; validation, Z.Z.; software, D.D.; formal analysis, S.Z.; investigation, J.G.; resources, T.Z.; writing—review and editing, L.L.; visualization and resources, L.W. and J.C.; conceptualization, project administration and funding acquisition, L.L. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 32072715) and the Sichuan Natural Science Foundation (Grant No. 2023NSFSC0232).
Institutional Review Board Statement
Experimental protocols, collection of animal tissue, and sampling procedures associated with animals were reviewed and approved by the Sichuan Agricultural University Animal Ethical and Welfare Committee on 15 March 2021 (Sichuan, China, approval No.2021202053).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data from the current study are exhibited in the manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Teixeira, A.; Silva, S.; Guedes, C.; Rodrigues, S. Sheep and Goat Meat Processed Products Quality: A Review. Foods 2020, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, O.; Avila-Aviles, R.D.; Hernandez-Hernandez, J.M. Chromatin Landscape During Skeletal Muscle Differentiation. Front. Genet. 2020, 11, 578712. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Widmann, M.; Nieß, A.M.; Munz, B. Physical Exercise and Epigenetic Modifications in Skeletal Muscle. Sports Med. 2019, 49, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Du, J.; Zhang, J.; Yin, S. MRNA N6-methyladenosine (m6A) modification and developmental regulation. Chin. J. Histochem. Cytochem. 2018, 27, 575–580. (In Chinese) [Google Scholar]
- Liu, J.; Yue, Y.; Han, D.; Wang, X.; Fu, Y.; Zhang, L.; Jia, G.; Yu, M.; Lu, Z.; Deng, X.; et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014, 10, 93–95. [Google Scholar] [CrossRef]
- Cao, G.; Li, H.B.; Yin, Z.; Flavell, R.A. Recent advances in dynamic m6A RNA modification. Open Biol. 2016, 6, 160003. [Google Scholar] [CrossRef]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.-G.; et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.L.; Song, S.H.; et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, Q.; Chen, X.; He, L.; Wang, D.; Su, R.; Xue, Y.; Sun, H.; Wang, H. Nuclear m6A reader YTHDC1 promotes muscle stem cell activation/proliferation by regulating mRNA splicing and nuclear export. Elife 2023, 9, e82703. [Google Scholar] [CrossRef]
- Huang, H.; Weng, H.; Sun, W.; Qin, X.; Shi, H.; Wu, H.; Zhao, B.S.; Mesquita, A.; Liu, C.; Yuan, C.L.; et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018, 20, 1098. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wei, Q.; Jin, J.; Luo, Q.; Liu, Y.; Yang, Y.; Cheng, C.; Li, L.; Pi, J.; Si, Y.; et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020, 48, 3816–3831. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Zhang, Z.; Ren, C.; Liang, Y.; Gao, X.; Fan, Y.; Wang, F. FTO regulates myoblast proliferation by controlling CCND1 expression in an m6A-YTHDF2-dependent manner. Exp Cell Res. 2021, 401, 112524. [Google Scholar] [CrossRef]
- Xu, R.; Ding, P.; Zhao, X.; Li, Z.; Liu, F.; Gu, L.; Zheng, Y.; Sang, M.; Meng, L. Circular RNA circ-TNRC6B inhibits the proliferation and invasion of esophageal squamous cell carcinoma cells by regulating the miR-452-5p/DAG1 axis. Mol Oncol. 2023, 17, 1437–1452. [Google Scholar] [CrossRef]
- Morikawa, Y.; Heallen, T.; Leach, J.; Xiao, Y.; Martin, J.F. Dystrophin-glycoprotein complex sequesters Yap to inhibit cardiomyocyte proliferation. Nature 2017, 547, 227–231. [Google Scholar] [CrossRef]
- Quereda, C.; Pastor, À.; Martín-Nieto, J. Involvement of abnormal dystroglycan expression and matriglycan levels in cancer pathogenesis. Cancer Cell Int. 2022, 22, 395. [Google Scholar] [CrossRef]
- Cohn, R.D.; Henry, M.D.; Michele, D.E.; Barresi, R.; Saito, F.; Moore, S.A.; Flanagan, J.D.; Skwarchuk, M.W.; Robbins, M.E.; Mendell, J.R.; et al. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell 2002, 110, 639–648. [Google Scholar] [CrossRef]
- Xu, W.; He, C.; Kaye, E.G.; Li, J.; Mu, M.; Nelson, G.M.; Dong, L.; Wang, J.; Wu, F.; Shi, Y.G.; et al. Dynamic control of chromatin-associated m6A methylation regulates nascent RNA synthesis. Mol Cell. 2022, 82, 1156–1168.e7. [Google Scholar] [CrossRef]
- Cui, X.; Nilsson, K.; Kajitani, N.; Schwartz, S. Overexpression of m6A-factors METTL3, ALKBH5, and YTHDC1 alters HPV16 mRNA splicing. Virus Genes 2022, 58, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Chen, D.X.; Zhang, Y.; Xu, X.; Cai, Y.; Wei, W.Q.; Hao, J.J.; Wang, M.R. Elevated expression of the RNA-binding protein IGF2BP1 enhances the mRNA stability of INHBA to promote the invasion and migration of esophageal squamous cancer cells. Exp. Hematol. Oncol. 2023, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, Y.; Li, T.; Ma, Z.; Jia, H.; Chen, Q.; Zhao, Y.; Zhai, L.; Zhong, R.; Li, C.; et al. Long non-coding RNA Linc-RAM enhances myogenic differentiation by interacting with MyoD. Nat. Commun. 2017, 8, 14016. [Google Scholar] [CrossRef] [PubMed]
- Gheller, B.J.; Blum, J.E.; Fong, E.; Malysheva, O.V.; Cosgrove, B.D.; Thalacker-Mercer, A.E. A defined N6-methyladenosine (m6A) profile conferred by METTL3 regulates muscle stem cell/myoblast state transitions. Cell Death Discov. 2020, 6, 95. [Google Scholar] [CrossRef]
- Tao, X.; Chen, J.; Jiang, Y.; Wei, Y.; Chen, Y.; Xu, H.; Zhu, L.; Tang, G.; Li, M.; Jiang, A.; et al. Transcriptome-wide N6-methyladenosine methylome profiling of porcine muscle and adipose tissues reveals a potential mechanism for transcriptional regulation and differential methylation pattern. BMC Genom. 2017, 18, 336. [Google Scholar] [CrossRef]
- Ma, X.; La, Y.; Bao, P.; Chu, M.; Guo, X.; Wu, X.; Pei, J.; Ding, X.; Liang, C.; Yan, P. Regulatory Role of N6-Methyladenosine in Longissimus Dorsi Development in Yak. Front. Vet. Sci. 2022, 9, 757115. [Google Scholar] [CrossRef]
- Gu, L.; Jiang, Q.; Chen, Y.; Zheng, X.; Zhou, H.; Xu, T. Transcriptome-wide study revealed m6A and miRNA regulation of embryonic breast muscle development in Wenchang chickens. Front. Vet. Sci. 2022, 9, 934728. [Google Scholar] [CrossRef]
- Xu, T.; Xu, Z.; Lu, L.; Zeng, T.; Gu, L.; Huang, Y.; Zhang, S.; Yang, P.; Wen, Y.; Lin, D.; et al. Transcriptome-wide study revealed m6A regulation of embryonic muscle development in Dingan goose (Anser cygnoides orientalis). BMC Genom. 2021, 22, 270. [Google Scholar] [CrossRef]
- Chen, B.; Liu, S.; Zhang, W.; Xiong, T.; Zhou, M.; Hu, X.; Mao, H.; Liu, S. Profiling Analysis of N6-Methyladenosine mRNA Methylation Reveals Differential m6A Patterns during the Embryonic Skeletal Muscle Development of Ducks. Animals 2022, 12, 2593. [Google Scholar] [CrossRef]
- Gu, L.; Zhang, S.; Li, B.; Jiang, Q.; Xu, T.; Huang, Y.; Lin, D.; Xing, M.; Huang, L.; Zheng, X.; et al. m6A and miRNA jointly regulate the development of breast muscles in duck embryonic stages. Front. Vet. Sci. 2022, 9, 933850. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Du, X.; Luo, M.; Yang, N. FTO-dependent m6A regulates muscle fiber remodeling in an NFATC1-YTHDF2 dependent manner. Clin. Epigenet. 2023, 15, 109. [Google Scholar] [CrossRef]
- Hirayama, M.; Wei, F.Y.; Chujo, T.; Oki, S.; Yakita, M.; Kobayashi, D.; Araki, N.; Takahashi, N.; Yoshida, R.; Nakayama, H.; et al. FTO Demethylates Cyclin D1 mRNA and Controls Cell-Cycle Progression. Cell Rep. 2020, 31, 107464. [Google Scholar] [CrossRef]
- Yang, X.; Mei, C.; Ma, X.; Du, J.; Wang, J.; Zan, L. m6A Methylases Regulate Myoblast Proliferation, Apoptosis and Differentiation. Animals 2022, 12, 773. [Google Scholar] [CrossRef]
- Wang, T.; Kong, S.; Tao, M.; Ju, S. The potential role of RNA N6-methyladenosine in Cancer progression. Mol. Cancer 2020, 19, 88. [Google Scholar] [CrossRef] [PubMed]
- Selberg, S.; Blokhina, D.; Aatonen, M.; Koivisto, P.; Siltanen, A.; Mervaala, E.; Kankuri, E.; Karelson, M. Discovery of Small Molecules that Activate RNA Methylation through Cooperative Binding to the METTL3-14-WTAP Complex Active Site. Cell Rep. 2019, 26, 3762–3771.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Chen, R.; Zhu, X.; Lian, N. EGR1 mediates METTL3/m6A/CHI3L1 to promote osteoclastogenesis in osteoporosis. Genomics 2023, 115, 110696. [Google Scholar] [CrossRef]
- Zhang, J.; Tsoi, H.; Li, X.; Wang, H.; Gao, J.; Wang, K.; Go, M.Y.; Ng, S.C.; Chan, F.K.; Sung, J.J.; et al. Carbonic anhydrase IV inhibits colon cancer development by inhibiting the Wnt signalling pathway through targeting the WTAP-WT1-TBL1 axis. Gut. 2016, 65, 1482–1493. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ye, F.; Yu, L.; Jia, G.; Huang, X.; Zhang, X.; Peng, S.; Chen, K.; Wang, M.; Gong, S.; et al. Development of cell-active N6-methyladenosine RNA demethylase FTO inhibitor. J. Am. Chem. Soc. 2012, 134, 17963–17971. [Google Scholar] [CrossRef]
- Gosselin, M.R.; Mournetas, V.; Borczyk, M.; Verma, S.; Occhipinti, A.; Róg, J.; Bozycki, L.; Korostynski, M.; Robson, S.C.; Angione, C.; et al. Loss of full-length dystrophin expression results in major cell-autonomous abnormalities in proliferating myoblasts. eLife 2022, 11, e75521. [Google Scholar] [CrossRef]
- Huang, Y.; Su, R.; Sheng, Y.; Dong, L.; Dong, Z.; Xu, H.; Ni, T.; Zhang, Z.S.; Zhang, T.; Li, C.; et al. Small-Molecule Targeting of Oncogenic FTO Demethylase in Acute Myeloid Leukemia. Cancer Cell 2019, 35, 677–691.E10. [Google Scholar] [CrossRef]
- Huang, W.M.; Li, Z.X.; Wu, Y.H.; Shi, Z.L.; Mi, J.L.; Hu, K. m6A demethylase FTO renders radioresistance of nasopharyngeal carcinoma via promoting OTUB1-mediated anti-ferroptosis. Transl. Oncol. 2023, 27, 101576. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ye, L.H.; Zhao, A.Q.; Gao, W.R.; Dai, N.; Yin, Y.; Zhang, X. M6A modification regulates tumor suppressor DIRAS1 expression in cervical cancer cells. Cancer Biol Ther. 2024, 25, 2306674. [Google Scholar] [CrossRef]
- He, J.; Zhao, Y.; Zhang, Y.; Zhang, Z.; Li, D.; Xu, Q. FTO regulates osteoclast development by modulating the proliferation and apoptosis of osteoclast precursors in inflammatory conditions. Cell. Signal. 2024, 117, 111098. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Zhang, M.; Chen, P.; Xiong, X.F.; Liu, P.Q.; Wang, H.B.; Wang, J.J.; Shen, J. The m6A demethylase FTO promotes the osteogenesis of mesenchymal stem cells by downregulating PPARG. Acta Pharmacol Sin. 2022, 43, 1311–1323. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, J.; He, C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015, 29, 1343–1355. [Google Scholar] [CrossRef]
- Li, S.; Shen, S.; Xu, H.; Cai, S.; Yuan, X.; Wang, C.; Zhang, X.; Chen, S.; Chen, J.; Shi, D.L.; et al. IGF2BP3 promotes adult myocardial regeneration by stabilizing MMP3 mRNA through interaction with m6A modification. Cell Death Discov. 2023, 9, 164. [Google Scholar] [CrossRef]
- Nordin, A.; Larsson, E.; Holmberg, M. The defective splicing caused by the ISCU intron mutation in patients with myopathy with lactic acidosis is repressed by PTBP1 but can be derepressed by IGF2BP1. Hum Mutat. 2012, 33, 467–470. [Google Scholar] [CrossRef]
- Jia, G.; Yang, C.G.; Yang, S.; Jian, X.; Yi, C.; Zhou, Z.; He, C. Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett. 2008, 582, 3313–3319. [Google Scholar] [CrossRef]
- He, C. Grand challenge commentary: RNA epigenetics? Nat. Chem. Biol. 2010, 6, 863–865. [Google Scholar] [CrossRef]
- Wei, C.; Gershowitz, A.; Moss, B. N6, O2′-dimethyladenosine a novel methylated ribonucleoside next to the 5′ terminal of animal cell and virus mRNAs. Nature 1975, 257, 251–253. [Google Scholar] [CrossRef]
- Mauer, J.; Luo, X.; Blanjoie, A.; Jiao, X.; Grozhik, A.V.; Patil, D.P.; Linder, B.; Pickering, B.F.; Vasseur, J.J.; Chen, Q.; et al. Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature 2017, 541, 371–375. [Google Scholar] [CrossRef]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.B.; Jaffrey, S.R. 5′ UTR m6A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cao, J.; Sun, Y.; Zhou, H.; Zhu, Q.; Dai, D.; Zhan, S.; Guo, J.; Zhong, T.; Wang, L.; et al. METTL3 Promotes the Differentiation of Goat Skeletal Muscle Satellite Cells by Regulating MEF2C mRNA Stability in a m6A-Dependent Manner. Int. J. Mol. Sci. 2023, 24, 14115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).