Prenanthes purpurea and 3,5-DiCQA Alleviate Cellular Stress in H2O2-Induced Neurotoxicity: An In Vitro Comparative Study
Abstract
1. Introduction
2. Results and Discussion
2.1. In Vitro Evaluation of Neuroprotective Activity
2.1.1. Results of Cell Viability Assays
2.1.2. Results of Compusyn® Analysis
2.1.3. Results of Proteome Profiling
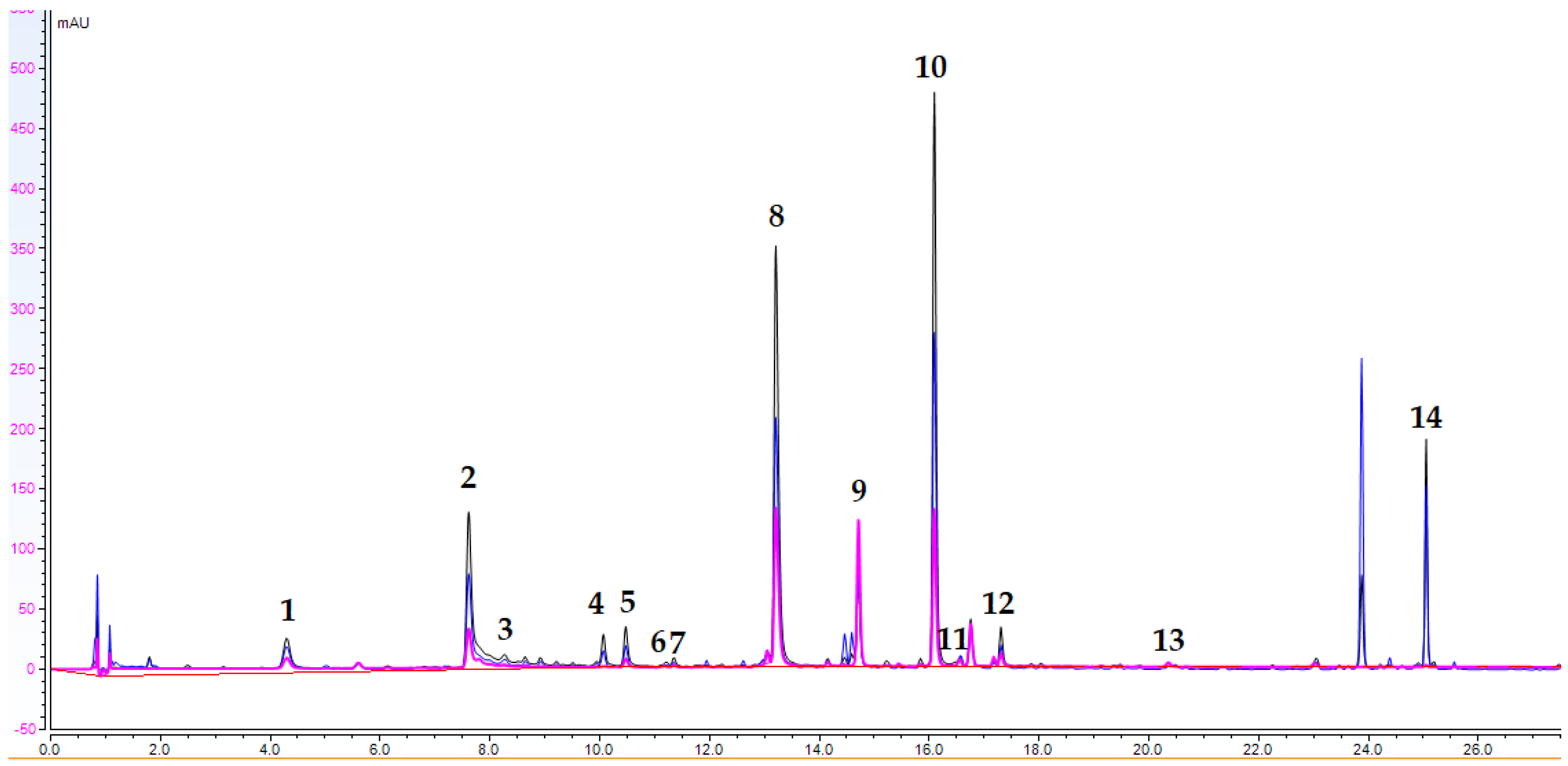
2.2. UHPLC-DAD Analysis
3. Materials and Methods
3.1. Plant Material and Sample Extraction
Chemicals
3.2. In Vitro Cytotoxicity Assays
3.2.1. Cell Lines and Culture Conditions
3.2.2. MTT Cell Viability Assay
3.2.3. Chou–Talalay Method
3.3. Proteomic Analysis
3.4. UHPLC-DAD Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sangwan, N.S. Biotechnology applications in neurodegenerative diseases. In Biotechnology in Healthcare [Internet]; Elsevier: Amsterdam, The Netherlands, 2022; pp. 89–103. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780323900423000050 (accessed on 4 August 2024).
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Allan Butterfield, D. Amyloid β-peptide (1-42)-induced Oxidative Stress and Neurotoxicity: Implications for Neurodegeneration in Alzheimer’s Disease Brain. A Review. Free Radic. Res. 2002, 36, 1307–1313. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative Stress and Neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez De La Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Olaleye Oladele, J.; Oladiji, A.T.; Titilope Oladele, O.; Oyeleke, O.M. Reactive Oxygen Species in Neurodegenerative Diseases: Implications in Pathogenesis and Treatment Strategies. In Biochemistry [Internet]; Ahmad, R., Ed.; IntechOpen: Rijeka, Croatia, 2022; Available online: https://www.intechopen.com/chapters/78522 (accessed on 4 August 2024).
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s Disease: An Update and Insights Into Pathophysiology. Front. Aging Neurosci. 2022, 14, 742408. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.; Zamboni, P.; Mahajan, R. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Hyslop, P.A. Contribution of oxidative stress to neurodegenerative disease. Open Access Gov. 2024, 42, 214–215. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The Interplay of Oxidative Stress and Inflammation: Mechanistic Insights and Therapeutic Potential of Antioxidants. Oxidative Med. Cell. Longev. 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Tesco, G.; Lomoio, S. Pathophysiology of neurodegenerative diseases: An interplay among axonal transport failure, oxidative stress, and inflammation? Semin. Immunol. 2022, 59, 101628. [Google Scholar] [CrossRef]
- He, J.; Zhu, G.; Wang, G.; Zhang, F. Oxidative Stress and Neuroinflammation Potentiate Each Other to Promote Progression of Dopamine Neurodegeneration. Oxidative Med. Cell. Longev. 2020, 2020, 6137521. [Google Scholar] [CrossRef]
- Ekiert, H.M.; Ramawat, K.G.; Arora, J. (Eds.) Plant Antioxidants and Health [Internet]; Springer International Publishing: Cham, Switzerland, 2022; (Reference Series in Phytochemistry); Available online: https://link.springer.com/10.1007/978-3-030-78160-6 (accessed on 4 August 2024).
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Gevrenova, R.; Balabanova, V.; Zheleva-Dimitrova, D.; Momekov, G. The most promising Southeastern European Tanacetum species: A review of chemical composition and biological studies. Pharmacia 2023, 70, 1067–1081. [Google Scholar] [CrossRef]
- Mihaylova, R.; Gevrenova, R.; Stefanova, A.; Zheleva-Dimitrova, D.; Balabanova, V.; Zengin, G.; Simeonova, R.; Momekov, G. The Phytochemical Profiling, In Vitro Antioxidant, and Hepatoprotective Activity of Prenanthes purpurea L. and Caffeoylquinic Acids in Diclofenac-Induced Hepatotoxicity on HEP-G2 Cells. Int. J. Mol. Sci. 2023, 24, 14148. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef] [PubMed]
- Socała, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaź, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef]
- Gul, Z.; Demircan, C.; Bagdas, D.; Buyukuysal, R.L. Protective Effects of Chlorogenic Acid and its Metabolites on Hydrogen Peroxide-Induced Alterations in Rat Brain Slices: A Comparative Study with Resveratrol. Neurochem. Res. 2016, 41, 2075–2085. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Simeonova, R.; Vitcheva, V.; Zheleva-Dimitrova, D.; Balabanova, V.; Savov, I.; Yagi, S.; Dimitrova, B.; Voynikov, Y.; Gevrenova, R. Trans-3,5-dicaffeoylquinic acid from Geigeria alata Benth. & Hook.f. ex Oliv. & Hiern with beneficial effects on experimental diabetes in animal model of essential hypertension. Food Chem. Toxicol. 2019, 132, 110678. [Google Scholar] [CrossRef]
- Mihaylova, R.; Gevrenova, R.; Petrova, A.; Savov, Y.; Zheleva-Dimitrova, D.; Balabanova, V.; Momekov, G.; Simeonova, R. Mitigating Effects of Tanacetum balsamita L. on Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD). Plants 2024, 13, 2086. [Google Scholar] [CrossRef]
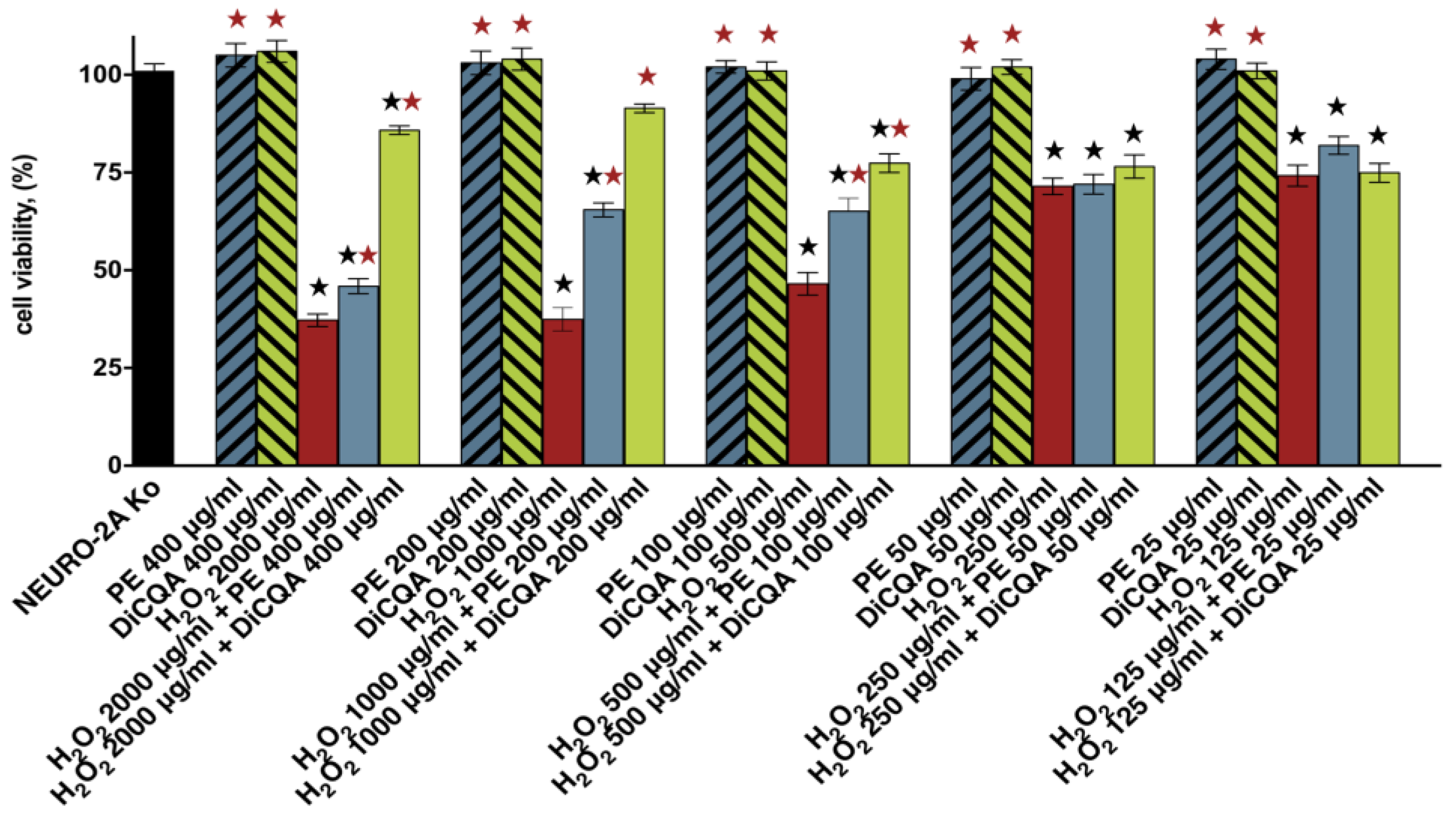
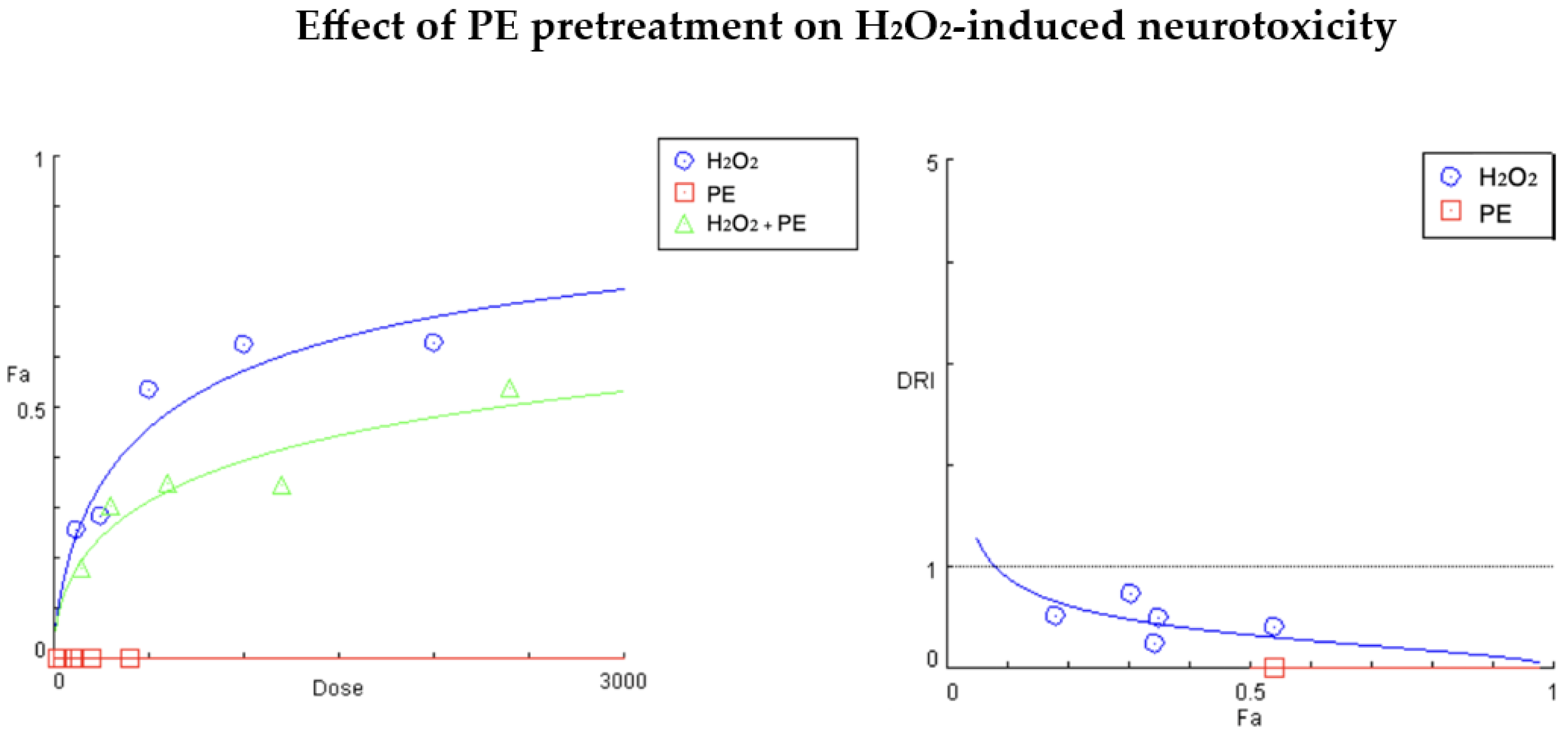
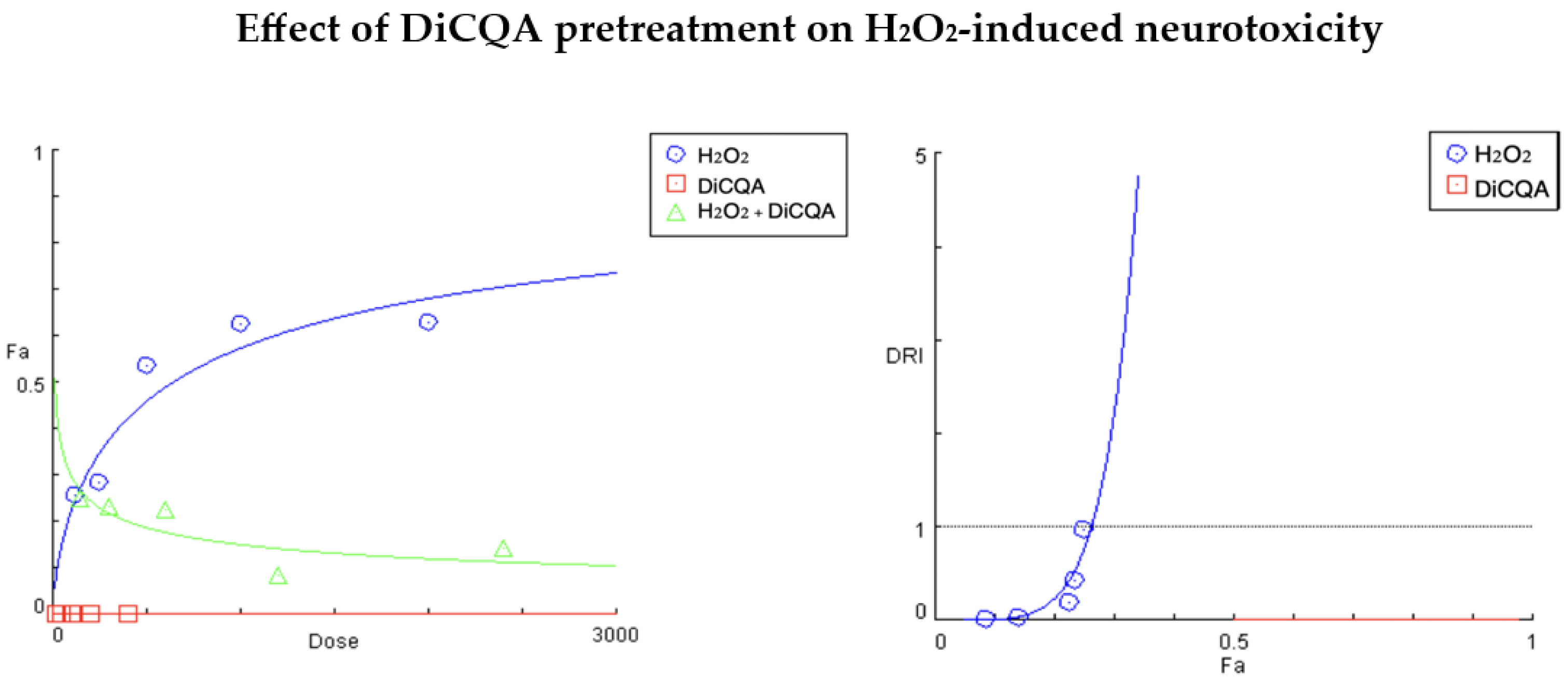
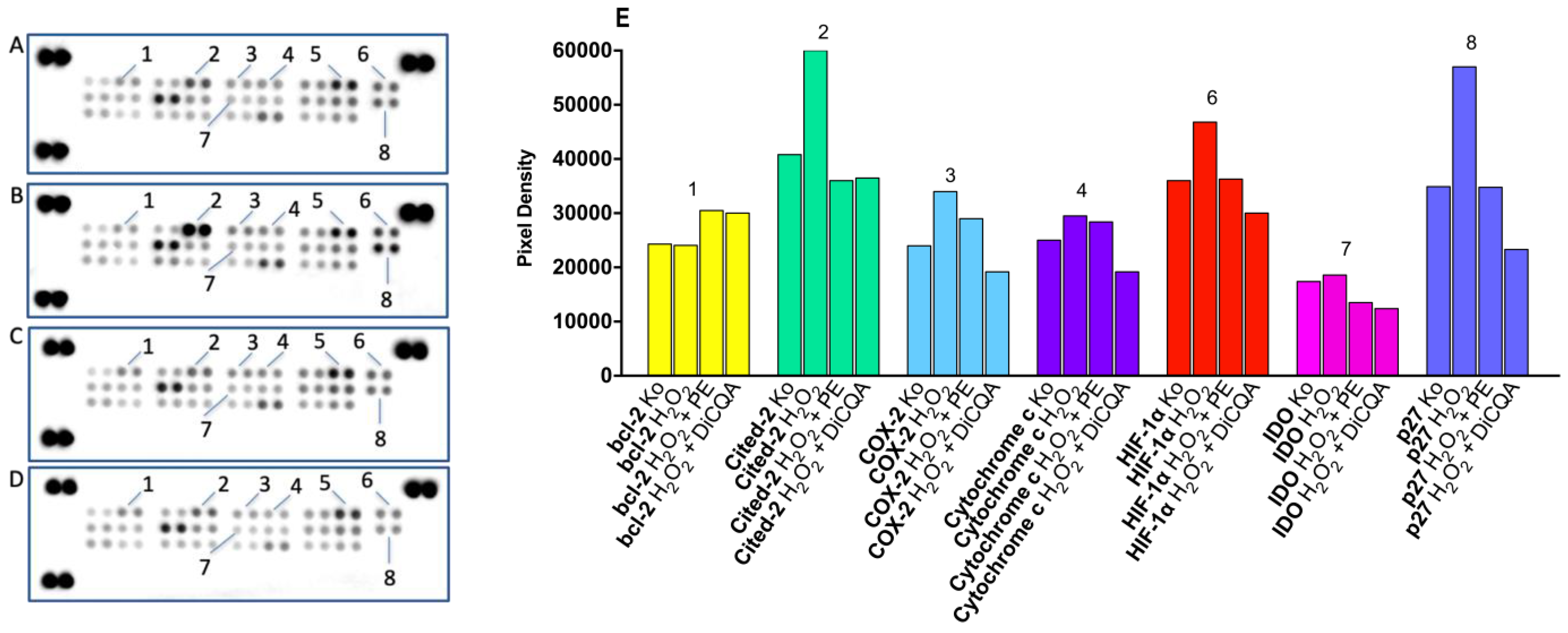
| Cell Line/Treatment Group | H2O2 | H2O2 + PE | H2O2 +DiCQA |
|---|---|---|---|
| SH-SY5Y 1 | 583.5 ± 18.4 | >2000 | >7000 |
| Fa (H2O2 Alone) | Fa’ (Combo) | Dose H2O2 | Dose PE | DRI H2O2 | CI Value |
|---|---|---|---|---|---|
| 0.628 | 0.540 | 2000.0 | 400.0 | 0.40569 | Infinit |
| 0.625 | 0.345 | 1000.0 | 200.0 | 0.24193 | Infinit |
| 0.535 | 0.349 | 500.0 | 100.0 | 0.49694 | Infinit |
| 0.285 | 0.270 | 250.0 | 50.0 | 0.93989 | → 1 |
| 0.258 | 0.18 | 125.0 | 25.0 | 0.51623 | Infinit |
| Fa (H2O2 Alone) | Fa’ (Combo) | Dose H2O2 | Dose DiCQA | DRI H2O2 | CI Value |
|---|---|---|---|---|---|
| 0.628 | 0.142 | 2000.0 | 400.0 | 0.02106 | Infinit |
| 0.625 | 0.085 | 1000.0 | 200.0 | 0.01761 | Infinit |
| 0.535 | 0.225 | 500.0 | 100.0 | 0.19684 | Infinit |
| 0.285 | 0.234 | 250.0 | 50.0 | 0.42513 | Infinit |
| 0.258 | 0.251 | 125.0 | 25.0 | 0.97783 | 1.09452 |
| № | Analyte | tR | Content (μg/mg de) |
|---|---|---|---|
| 1. | caftaric acid | 4.30 | 4.78 ± 0.13 |
| 2. | chlorogenic acid | 7.61 | 11.80 ± 0.03 |
| 3. | caffeic acid | 8.27 | 1.73 ± 0.10 |
| 4. | AQA1 | 10.07 | 1.93 ± 0.04 |
| 5. | AQA2 | 10.47 | 2.49 ± 0.10 |
| 6. | AQA3 | 11.12 | 0.090 ± 0.008 |
| 7. | p-coumaric acid | 11.35 | 0.26 ± 0.01 |
| 8. | cichoric acid | 13.21 | 26.63 ± 1.45 |
| 9. | luteolin 7-O-glucoside | 14.71 | 2.33 ± 0.27 |
| 10. | 3,5-diCQA | 16.09 | 30.30 ± 3.24 |
| 11. | AQA4 | 16.76 | 1.68 ± 0.33 |
| 12. | AQA5 | 17.31 | 1.73 ± 0.10 |
| 13. | luteolin | 20.36 | 0.100 ± 0.002 |
| 14. | AQA6 | 25.05 | 7.60 ± 0.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaylova, R.; Zheleva-Dimitrova, D.; Elincheva, V.; Gevrenova, R.; Momekov, G.; Simeonova, R. Prenanthes purpurea and 3,5-DiCQA Alleviate Cellular Stress in H2O2-Induced Neurotoxicity: An In Vitro Comparative Study. Int. J. Mol. Sci. 2024, 25, 9805. https://doi.org/10.3390/ijms25189805
Mihaylova R, Zheleva-Dimitrova D, Elincheva V, Gevrenova R, Momekov G, Simeonova R. Prenanthes purpurea and 3,5-DiCQA Alleviate Cellular Stress in H2O2-Induced Neurotoxicity: An In Vitro Comparative Study. International Journal of Molecular Sciences. 2024; 25(18):9805. https://doi.org/10.3390/ijms25189805
Chicago/Turabian StyleMihaylova, Rositsa, Dimitrina Zheleva-Dimitrova, Viktoria Elincheva, Reneta Gevrenova, Georgi Momekov, and Rumyana Simeonova. 2024. "Prenanthes purpurea and 3,5-DiCQA Alleviate Cellular Stress in H2O2-Induced Neurotoxicity: An In Vitro Comparative Study" International Journal of Molecular Sciences 25, no. 18: 9805. https://doi.org/10.3390/ijms25189805
APA StyleMihaylova, R., Zheleva-Dimitrova, D., Elincheva, V., Gevrenova, R., Momekov, G., & Simeonova, R. (2024). Prenanthes purpurea and 3,5-DiCQA Alleviate Cellular Stress in H2O2-Induced Neurotoxicity: An In Vitro Comparative Study. International Journal of Molecular Sciences, 25(18), 9805. https://doi.org/10.3390/ijms25189805










