Genome-Wide Identification, Characterization, and Expression Analysis of the MYB-R2R3 Gene Family in Black Pepper (Piper nigrum L.)
Abstract
:1. Introduction
2. Results
2.1. Identification and Physicochemical Properties Analysis of Black Pepper MYB
2.2. Multiple Sequence Alignment and Phylogenetic Analysis
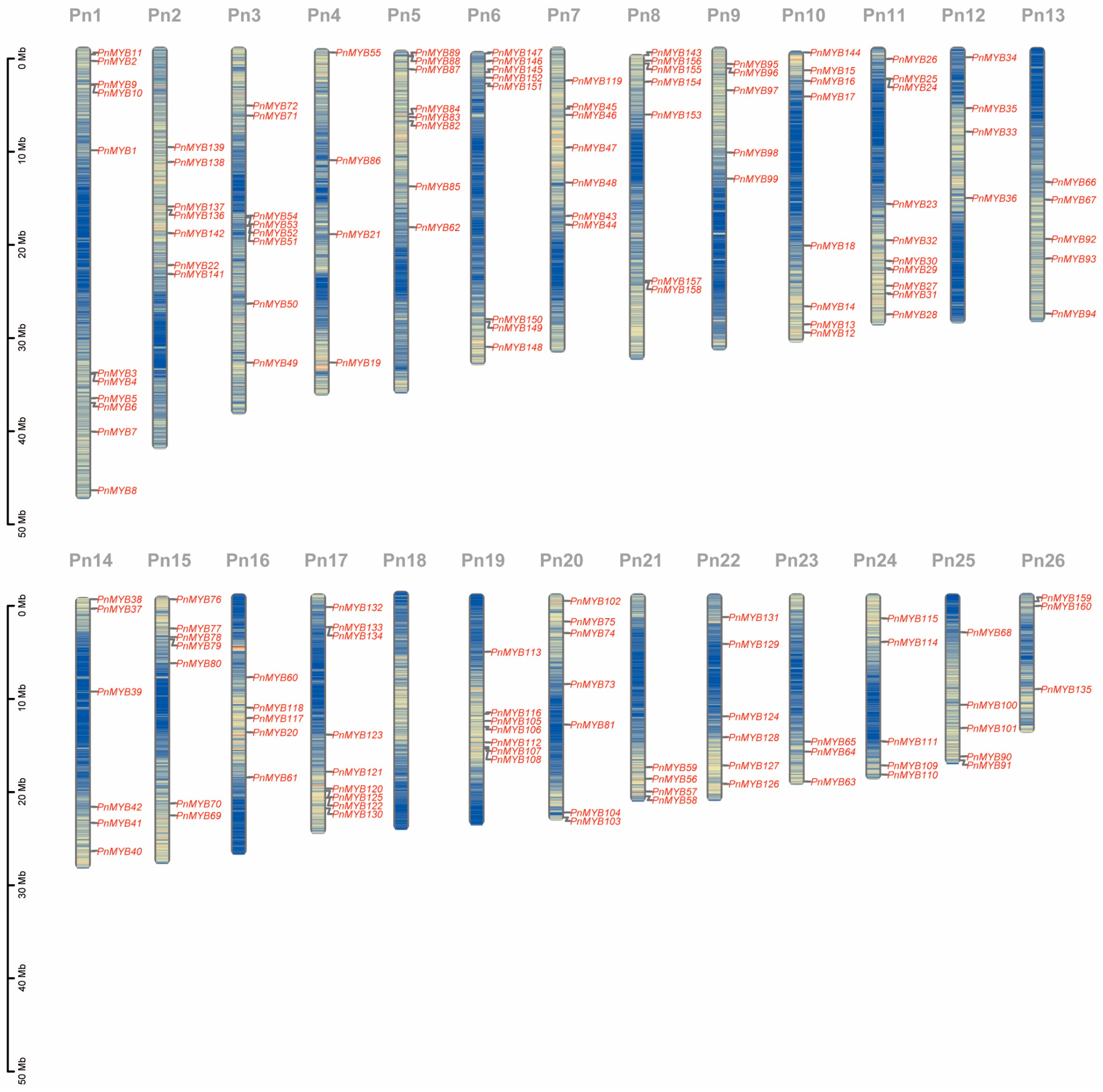
2.3. Gene Structure, Motif Patterns, and Chromosome Location of MYBs
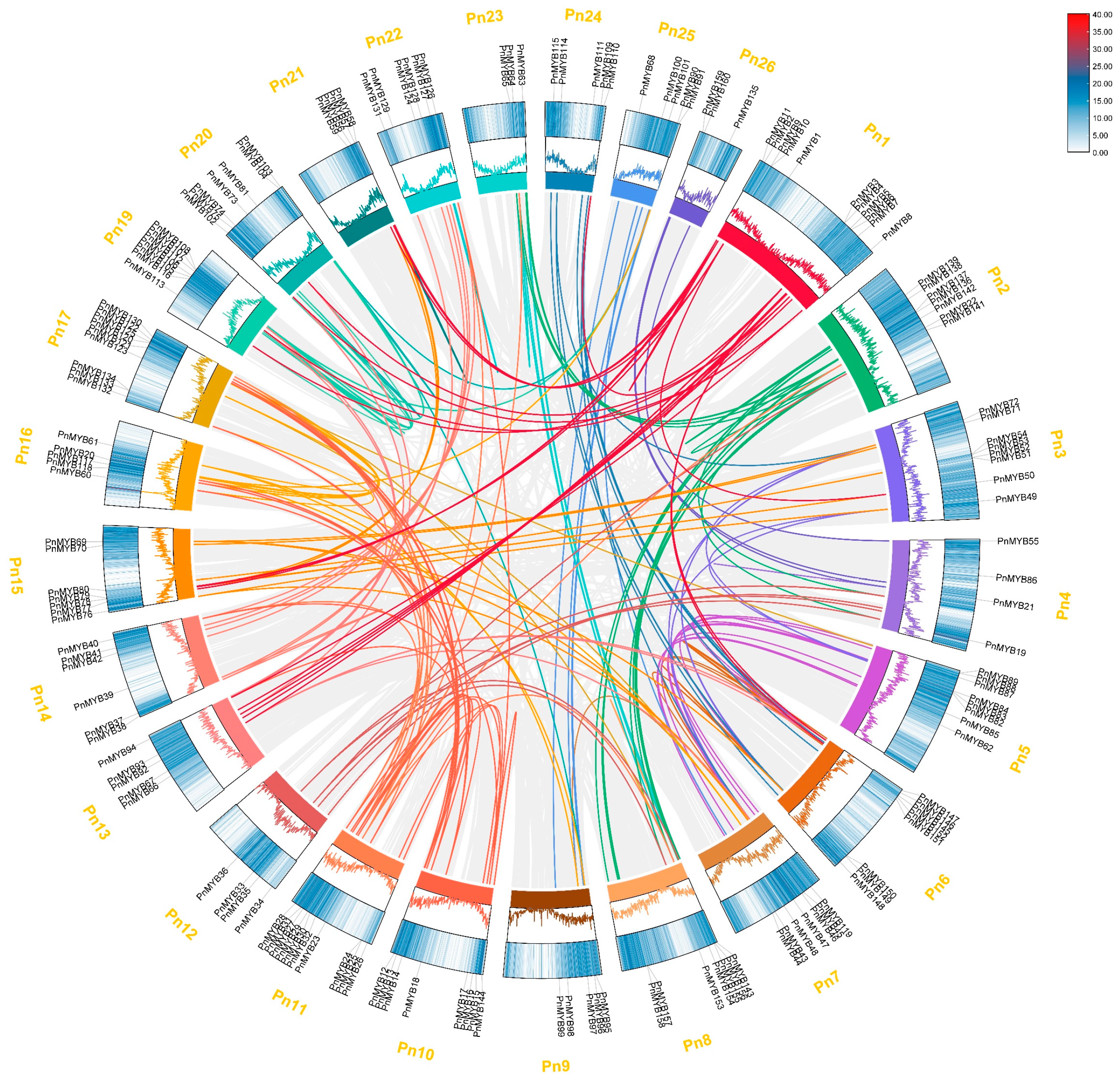
2.4. Collinearity Analysis of the MYB Family in Black Pepper
2.5. Subcellular Localization of PnMYB Family
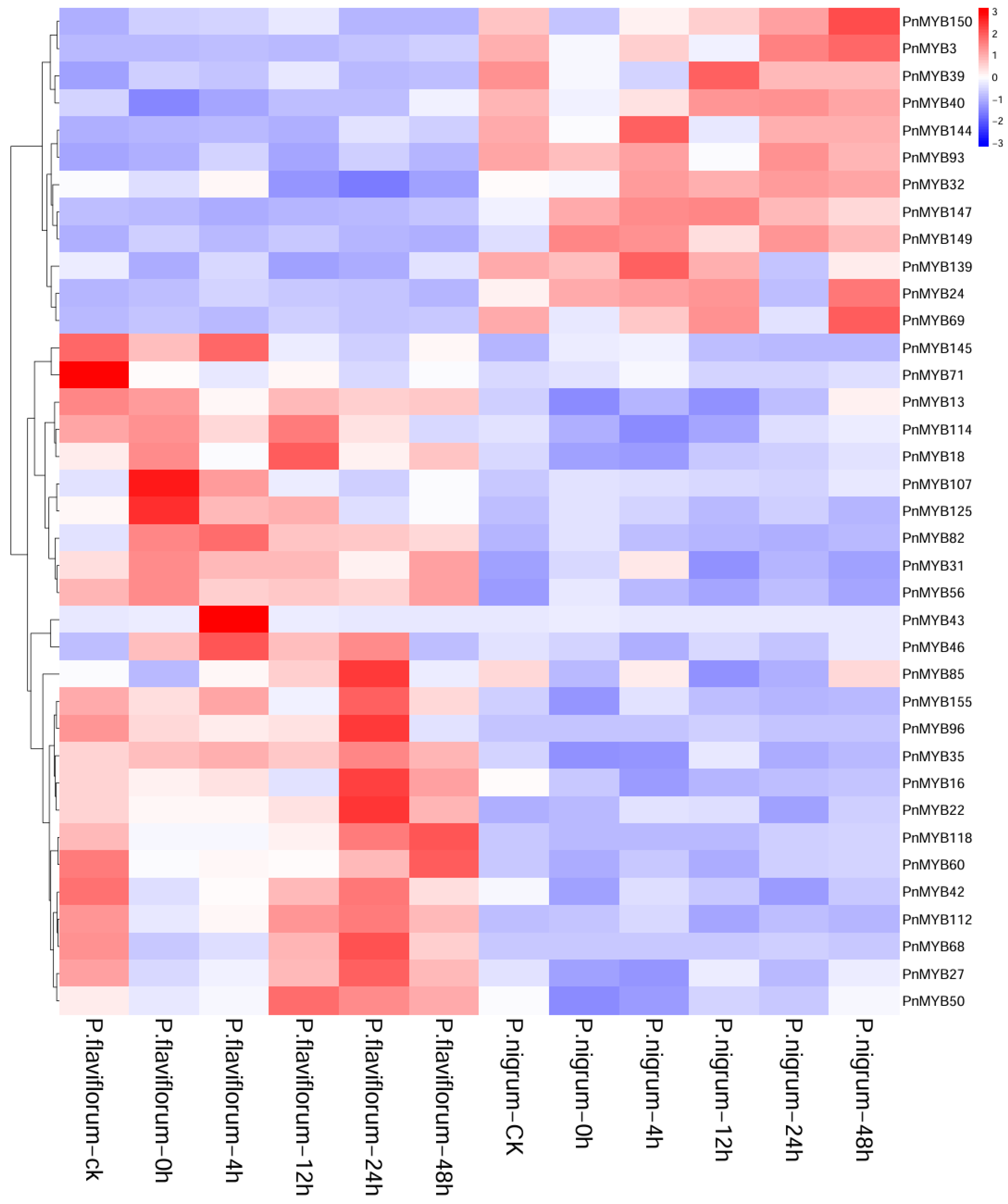
2.6. Expression Pattern Analysis of PnMYB Gene Family
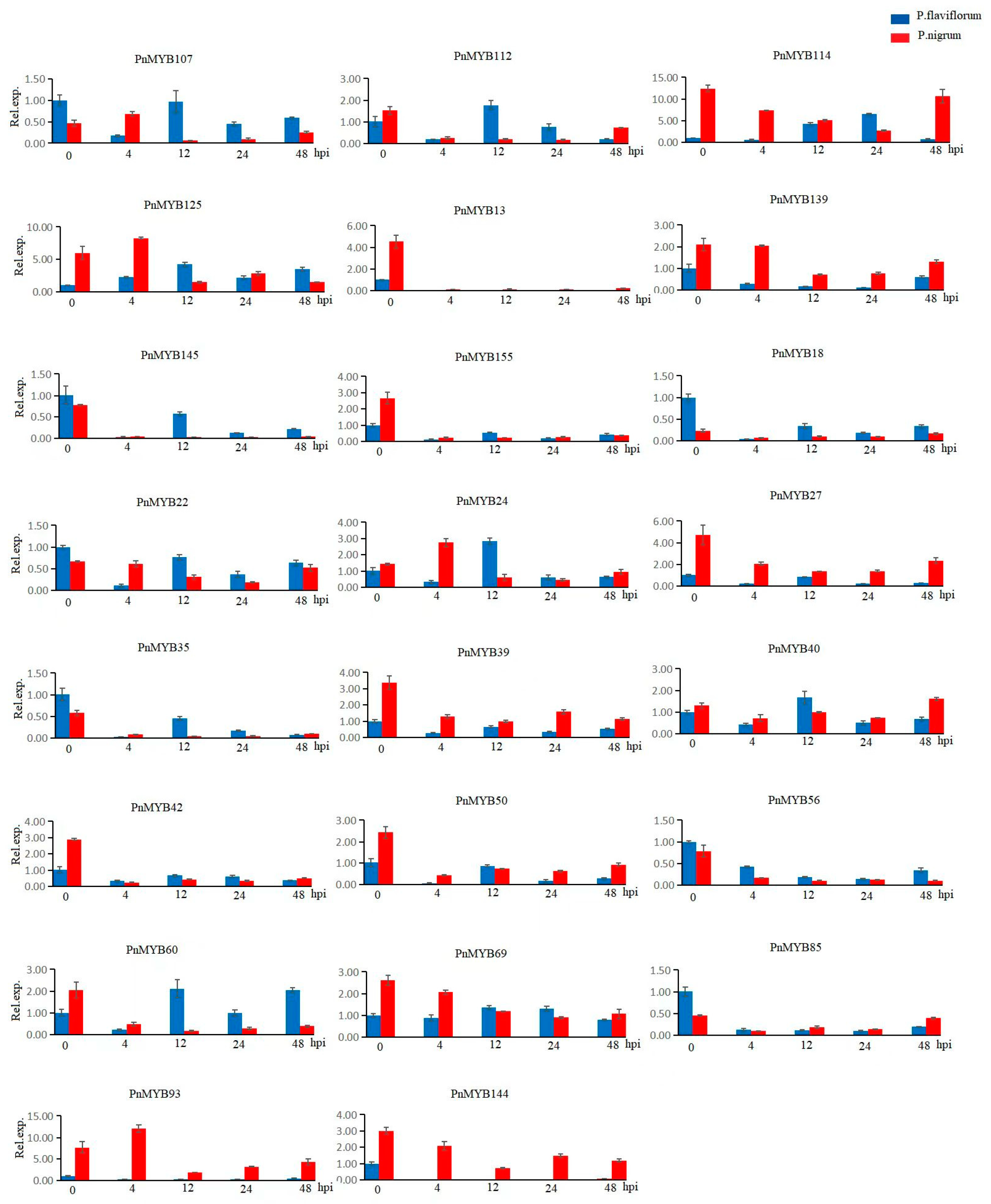
2.7. Expression Differences in MYB Genes in Two Piper Species
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Identification of MYB Genes in Pepper
4.3. Phylogenetic and Gene Structure Analysis
4.4. Chromosomal Localization and Synteny Analysis
4.5. Expression Profile Analysis of the MYB Gene Family in Black Pepper
4.6. Quantitative Analysis of Candidate MYB Genes in Black Pepper
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Umadevi, P.; Anandaraj, M. Genotype Specific Host Resistance for Phytophthora in Black Pepper (Piper nigrum L.). Physiol. Mol. Plant Pathol. 2017, 100, 237–241. [Google Scholar] [CrossRef]
- Ding, Y.; Mao, Y.; Cen, Y.; Hu, L.; Su, Y.; Ma, X.; Long, L.; Hu, H.; Hao, C.; Luo, J. Small RNA Sequencing Reveals Various microRNAs Involved in Piperine Biosynthesis in Black Pepper (Piper nigrum L.). BMC Genom. 2021, 22, 838. [Google Scholar] [CrossRef] [PubMed]
- Quy, P.T.; Hai, N.T.T.; My, T.T.A.; Bui, T.Q.; Hoa, T.T.; Phu, N.V.; Loan, H.T.P.; Nhung, N.T.A. A Theoretical Study on Inhibitability of Silver(I) N-heterocyclic Carbene and Dimer Silver(I) N-heterocyclic Carbene Complexes against Phytophthora capsici and Fusarium Sporotrichioides in Piper nigrum L. Vietnam J. Chem. 2021, 59, 405–415. [Google Scholar] [CrossRef]
- Suraby, E.J.; Prasath, D.; Babu, K.N.; Anandaraj, M. Identification of Resistance Gene Analogs Involved in Phytophthora capsici Recognition in Black Pepper (Piper nigrum L.). J. Plant Pathol. 2020, 102, 1121–1131. [Google Scholar] [CrossRef]
- Nysanth, N.S.; Divya, S.; Nair, C.B.; Anju, A.B.; Praveena, R.; Anith, K.N. Biological Control of Foot Rot (Phytophthora capsici Leonian) Disease in Black Pepper (Piper nigrum L.) with Rhizospheric Microorganisms. Rhizosphere 2022, 23, 100578. [Google Scholar] [CrossRef]
- Malik, N.; George, J.K. Resistance Genes in Piper colubrinum: In Silico Survey from Leaf Transcriptome and Expression Studies Upon Challenge Inoculation with Phytophthora capsici. Appl. Biochem. Biotechnol. 2018, 184, 987–1008. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Ali, M.; Feng, X.-H.; Jin, J.-H.; Huang, L.-J.; Khan, A.; Lv, J.-G.; Gao, S.-Y.; Luo, D.-X.; Gong, Z.-H. A Novel Transcription Factor CaSBP12 Gene Negatively Regulates the Defense Response against Phytophthora capsici in Pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2018, 20, 48. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Feng, X.-H.; Jin, J.-H.; Khan, A.; Guo, W.-L.; Du, X.-H.; Gong, Z.-H. CaSBP11 Participates in the Defense Response of Pepper to Phytophthora Capsici through Regulating the Expression of Defense-Related Genes. Int. J. Mol. Sci. 2020, 21, 9065. [Google Scholar] [CrossRef]
- Xiang, Q.; Judelson, H.S. Myb Transcription Factors and Light Regulate Sporulation in the Oomycete Phytophthora infestans. PLoS ONE 2014, 9, e92086. [Google Scholar] [CrossRef]
- Vandana, V.V.; Suseela Bhai, R. Differential Expression of PR Genes in Response to Phytophthora capsici Inoculation in Resistant and Susceptible Black Pepper (Piper nigrum L.) Lines. Eur. J. Plant Pathol. 2018, 150, 713–724. [Google Scholar] [CrossRef]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB Gene Family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB Transcription Factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Kundan, M.; Gani, U.; Fayaz, M.; Angmo, T.; Kesari, R.; Rahul, V.P.; Gairola, S.; Misra, P. Two R2R3-MYB Transcription Factors, CsMYB33 and CsMYB78 Are Involved in the Regulation of Anthocyanin Biosynthesis in Cannabis sativa L. Ind. Crops Prod. 2022, 188, 115546. [Google Scholar] [CrossRef]
- Baldoni, E.; Genga, A.; Cominelli, E. Plant MYB Transcription Factors: Their Role in Drought Response Mechanisms. Int. J. Mol. Sci. 2015, 16, 15811–15851. [Google Scholar] [CrossRef]
- Wang, X.; Wu, R.; Shen, T.; Li, Z.; Li, C.; Wu, B.; Jiang, H.; Zhao, G. An R2R3-MYB Transcription Factor OsMYBAS1 Promotes Seed Germination under Different Sowing Depths in Transgenic Rice. Plants 2022, 11, 139. [Google Scholar] [CrossRef]
- Yang, R.; Wang, S.; Zou, H.; Li, L.; Li, Y.; Wang, D.; Xu, H.; Cao, X. R2R3-MYB Transcription Factor SmMYB52 Positively Regulates Biosynthesis of Salvianolic Acid B and Inhibits Root Growth in Salvia miltiorrhiza. Int. J. Mol. Sci. 2021, 22, 9538. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Liu, H.; Dong, K.; Wang, Y.; Zhang, Y. Over-Expression of an R2R3 MYB Gene, MdMYB108L, Enhances Tolerance to Salt Stress in Transgenic Plants. Int. J. Mol. Sci. 2022, 23, 9428. [Google Scholar] [CrossRef]
- Duan, A.-Q.; Tan, S.-S.; Deng, Y.-J.; Xu, Z.-S.; Xiong, A.-S. Genome-Wide Identification and Evolution Analysis of R2R3-MYB Gene Family Reveals S6 Subfamily R2R3-MYB Transcription Factors Involved in Anthocyanin Biosynthesis in Carrot. Int. J. Mol. Sci. 2022, 23, 11859. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Xu, J.; Lu, X.; Liu, Y. Anthocyanin Biosynthesis Induced by MYB Transcription Factors in Plants. Int. J. Mol. Sci. 2022, 23, 11701. [Google Scholar] [CrossRef]
- Li, W.; Zhong, J.; Zhang, L.; Wang, Y.; Song, P.; Liu, W.; Li, X.; Han, D. Overexpression of a Fragaria Vesca MYB Transcription Factor Gene (FvMYB82) Increases Salt and Cold Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 10538. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Liu, Y.; Shang, X.; Fang, S. Identification and Expression Analysis of R2R3-MYB Family Genes Associated with Salt Tolerance in Cyclocarya paliurus. Int. J. Mol. Sci. 2022, 23, 3429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhao, H.; Hu, Y.; Zhang, S.; He, S.; Zhang, H.; Zhao, N.; Liu, Q.; Gao, S.; Zhai, H. Expression of the Sweet Potato MYB Transcription Factor IbMYB48 Confers Salt and Drought Tolerance in Arabidopsis. Genes 2022, 13, 1883. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, S.; Yu, Y.; Cui, N.; Yu, G.; Zhao, H.; Meng, X.; Fan, H. The Pivotal Role of MYB Transcription Factors in Plant Disease Resistance. Planta 2023, 258, 16. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.-L.; Chu, R.-Z.; Zhang, Y.; Luo, J.; Su, Y.-Y.; Xie, L.-J.; Zhang, H.-S.; Wang, J.-F.; Bao, Y.-M. OsJAMyb, a R2R3-Type MYB Transcription Factor, Enhanced Blast Resistance in Transgenic Rice. Physiol. Mol. Plant Pathol. 2015, 92, 154–160. [Google Scholar] [CrossRef]
- Qiu, Z.; Yan, S.; Xia, B.; Jiang, J.; Yu, B.; Lei, J.; Chen, C.; Chen, L.; Yang, Y.; Wang, Y.; et al. The Eggplant Transcription Factor MYB44 Enhances Resistance to Bacterial Wilt by Activating the Expression of Spermidine Synthase. J. Exp. Bot. 2019, 70, 5343–5354. [Google Scholar] [CrossRef]
- Wei, X.; Shan, T.; Hong, Y.; Xu, H.; Liu, X.; Zhang, Z. TaPIMP2, a Pathogen-Induced MYB Protein in Wheat, Contributes to Host Resistance to Common Root Rot Caused by Bipolaris sorokiniana. Sci. Rep. 2017, 7, 1754. [Google Scholar] [CrossRef]
- Liu, Z.; Luan, Y.; Li, J.; Yin, Y. Expression of a Tomato MYB Gene in Transgenic Tobacco Increases Resistance to Fusarium Oxysporum and Botrytis Cinerea. Eur. J. Plant Pathol. 2016, 144, 607–617. [Google Scholar] [CrossRef]
- Hawku, M.D.; He, F.; Bai, X.; Islam, M.A.; Huang, X.; Kang, Z.; Guo, J. A R2R3 MYB Transcription Factor, TaMYB391, Is Positively Involved in Wheat Resistance to Puccinia striiformis f. sp. tritici. Int. J. Mol. Sci. 2022, 23, 14070. [Google Scholar] [CrossRef]
- Wang, H.; Hou, J.; Ye, P.; Hu, L.; Huang, J.; Dai, Z.; Zhang, B.; Dai, S.; Que, J.; Min, H.; et al. A Teosinte-Derived Allele of a MYB Transcription Repressor Confers Multiple Disease Resistance in Maize. Mol. Plant 2021, 14, 1846–1863. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, C.; Khan, N.; Chen, M.; Fu, W.; Guan, L.; Leng, X. Genome-Wide Identification of the Class III POD Gene Family and Their Expression Profiling in Grapevine (Vitis vinifera L.). BMC Genom. 2020, 21, 444. [Google Scholar] [CrossRef]
- Zhu, M.; Yan, B.; Hu, Y.; Cui, Z.; Wang, X. Genome-Wide Identification and Phylogenetic Analysis of Rice FTIP Gene Family. Genomics 2020, 112, 3803–3814. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Xu, W.; Zhang, J.; Shen, T.; Chen, C.; Xi, M.; Zhong, Y.; Xu, M. Genome-Scale Identification, Classification, and Expression Profiling of MYB Transcription Factor Genes in Cinnamomum camphora. Int. J. Mol. Sci. 2022, 23, 14279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Luo, R.; Sun, Y.; Yang, C.; Li, X.; Gao, A.; Pu, J. Genome-Wide Characterization, Identification and Expression Profile of MYB Transcription Factor Gene Family during Abiotic and Biotic Stresses in Mango (Mangifera indica). Plants 2022, 11, 3141. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, X.; Wang, F.; Zhang, L.; Xin, M.; Hu, Z.; Yao, Y.; Ni, Z.; Sun, Q.; Peng, H. Characterization of Wheat MYB Genes Responsive to High Temperatures. BMC Plant Biol. 2017, 17, 208. [Google Scholar] [CrossRef]
- Fan, R.; Tao, X.; Xia, Z.; Sim, S.; Hu, L.; Wu, B.; Wang, Q.; Hao, C. Comparative Transcriptome and Metabolome Analysis of Resistant and Susceptible piper Species Upon Infection by the Oomycete Phytophthora capsici. Front. Plant Sci. 2022, 13, 864927. [Google Scholar] [CrossRef]
- Paz-Ares, J.; Ghosal, D.; Wienand, U.; Peterson, P.A.; Saedler, H. The Regulatory C1 Locus of Zea Mays Encodes a Protein with Homology to Myb Proto-Oncogene Products and with Structural Similarities to Transcriptional Activators. EMBO J. 1987, 6, 3553–3558. [Google Scholar] [CrossRef]
- Katiyar, A.; Smita, S.; Lenka, S.K.; Rajwanshi, R.; Chinnusamy, V.; Bansal, K.C. Genome-Wide Classification and Expression Analysis of MYB Transcription Factor Families in Rice and Arabidopsis. BMC Genom. 2012, 13, 544. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Tang, B.; Dai, X.; Xie, L.; Liu, F.; Zou, X. Genome-Wide Identification and Capsaicinoid Biosynthesis-Related Expression Analysis of the R2R3-MYB Gene Family in Capsicum annuum L. Front. Genet. 2020, 11, 598183. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, J.; Zhou, X.; Luan, Y.; Luan, F. Genome-Wide Identification, Characterization and Expression Analysis of the TLP Gene Family in Melon (Cucumis melo L.). Genomics 2020, 112, 2499–2509. [Google Scholar] [CrossRef]
- Liu, J.-J.; Sturrock, R.; Ekramoddoullah, A.K.M. The Superfamily of Thaumatin-like Proteins: Its Origin, Evolution, and Expression towards Biological Function. Plant Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The Roles of Segmental and Tandem Gene Duplication in the Evolution of Large Gene Families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, B.; Gu, G.; Yuan, J.; Shen, S.; Jin, L.; Lin, Z.; Lin, J.; Xie, X. Genome-Wide Identification and Expression Analysis of the R2R3-MYB Gene Family in Tobacco (Nicotiana tabacum L.). BMC Genom. 2022, 23, 432. [Google Scholar] [CrossRef]
- Tarrío, R.; Ayala, F.J.; Rodríguez-Trelles, F. Alternative Splicing: A Missing Piece in the Puzzle of Intron Gain. Proc. Natl. Acad. Sci. USA 2008, 105, 7223–7228. [Google Scholar] [CrossRef]
- Du, H.; Feng, B.-R.; Yang, S.-S.; Huang, Y.-B.; Tang, Y.-X. The R2R3-MYB Transcription Factor Gene Family in Maize. PLoS ONE 2012, 7, e37463. [Google Scholar] [CrossRef]
- Li, Y.; Liang, J.; Zeng, X.; Guo, H.; Luo, Y.; Kear, P.; Zhang, S.; Zhu, G. Genome-Wide Analysis of MYB Gene Family in Potato Provides Insights into Tissue-Specific Regulation of Anthocyanin Biosynthesis. Hortic. Plant J. 2021, 7, 129–141. [Google Scholar] [CrossRef]
- Zhu, N.; Cheng, S.; Liu, X.; Du, H.; Dai, M.; Zhou, D.-X.; Yang, W.; Zhao, Y. The R2R3-Type MYB Gene OsMYB91 Has a Function in Coordinating Plant Growth and Salt Stress Tolerance in Rice. Plant Sci. 2015, 236, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Xia, Z.; Fan, R.; Tan, L.; Hu, L.; Wu, B.; Wu, H. De Novo Transcriptome Sequencing of Black Pepper (Piper nigrum L.) and an Analysis of Genes Involved in Phenylpropanoid Metabolism in Response to Phytophthora capsici. BMC Genom. 2016, 17, 822. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liao, X.; Jin, X.; Tan, L.; Lu, Q.; Yuan, C.; Xue, Y.; Yin, N.; Lin, N.; Chai, Y. MYB43 in Oilseed Rape (Brassica napus) Positively Regulates Vascular Lignification, Plant Morphology and Yield Potential but Negatively Affects Resistance to Sclerotinia sclerotiorum. Genes 2020, 11, 581. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Jiang, W.; Fang, L.; Guan, X.; Chen, J.; Zhang, J.; Saski, C.A.; Scheffler, B.E.; Stelly, D.M.; et al. Sequencing of Allotetraploid Cotton (Gossypium hirsutum L. Acc. TM-1) Provides a Resource for Fiber Improvement. Nat. Biotechnol. 2015, 33, 531–537. [Google Scholar] [CrossRef]
- Tombuloglu, H. Genome-Wide Identification and Expression Analysis of R2R3, 3R- and 4R-MYB Transcription Factors during Lignin Biosynthesis in Flax (Linum usitatissimum). Genomics 2020, 112, 782–795. [Google Scholar] [CrossRef]
- Bhatia, N.; Tiwari, J.K.; Kumari, C.; Zinta, R.; Sharma, S.; Thakur, A.K.; Buckseth, T.; Dalamu, D.; Singh, R.K.; Kumar, V. Screening of Wild Species and Transcriptome Profiling to Identify Differentially Regulated Genes in Response to Late Blight Resistance in Potato. Front. Plant Sci. 2023, 14, 1212135. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Wang, M.; Jiang, J.; Zhou, Q.; Yin, J.; Li, J.; Lian, J.; Xue, Y.; Chai, Y. Downregulation of Brassica napus MYB69 (BnMYB69) Increases Biomass Growth and Disease Susceptibility via Remodeling Phytohormone, Chlorophyll, Shikimate and Lignin Levels. Front. Plant Sci. 2023, 14, 1157836. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Xu, Z.; Wang, M.; Fan, R.; Yuan, D.; Wu, B.; Wu, H.; Qin, X.; Yan, L.; Tan, L.; et al. The Chromosome-Scale Reference Genome of Black Pepper Provides Insight into Piperine Biosynthesis. Nat. Commun. 2019, 10, 4702. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, R.; Huang, K.; Zhao, Z.; Hao, Y.; Guan, X.; Luo, H.; Hao, C. Genome-Wide Identification, Characterization, and Expression Analysis of the MYB-R2R3 Gene Family in Black Pepper (Piper nigrum L.). Int. J. Mol. Sci. 2024, 25, 9851. https://doi.org/10.3390/ijms25189851
Fan R, Huang K, Zhao Z, Hao Y, Guan X, Luo H, Hao C. Genome-Wide Identification, Characterization, and Expression Analysis of the MYB-R2R3 Gene Family in Black Pepper (Piper nigrum L.). International Journal of Molecular Sciences. 2024; 25(18):9851. https://doi.org/10.3390/ijms25189851
Chicago/Turabian StyleFan, Rui, Kai Huang, Zhican Zhao, Yupeng Hao, Xueying Guan, Haiyan Luo, and Chaoyun Hao. 2024. "Genome-Wide Identification, Characterization, and Expression Analysis of the MYB-R2R3 Gene Family in Black Pepper (Piper nigrum L.)" International Journal of Molecular Sciences 25, no. 18: 9851. https://doi.org/10.3390/ijms25189851
APA StyleFan, R., Huang, K., Zhao, Z., Hao, Y., Guan, X., Luo, H., & Hao, C. (2024). Genome-Wide Identification, Characterization, and Expression Analysis of the MYB-R2R3 Gene Family in Black Pepper (Piper nigrum L.). International Journal of Molecular Sciences, 25(18), 9851. https://doi.org/10.3390/ijms25189851




