Comparative Analysis of Casparian Strip Membrane Domain Protein Family in Oryza sativa (L.) and Arabidopsis thaliana (L.)
Abstract
1. Introduction
2. Results
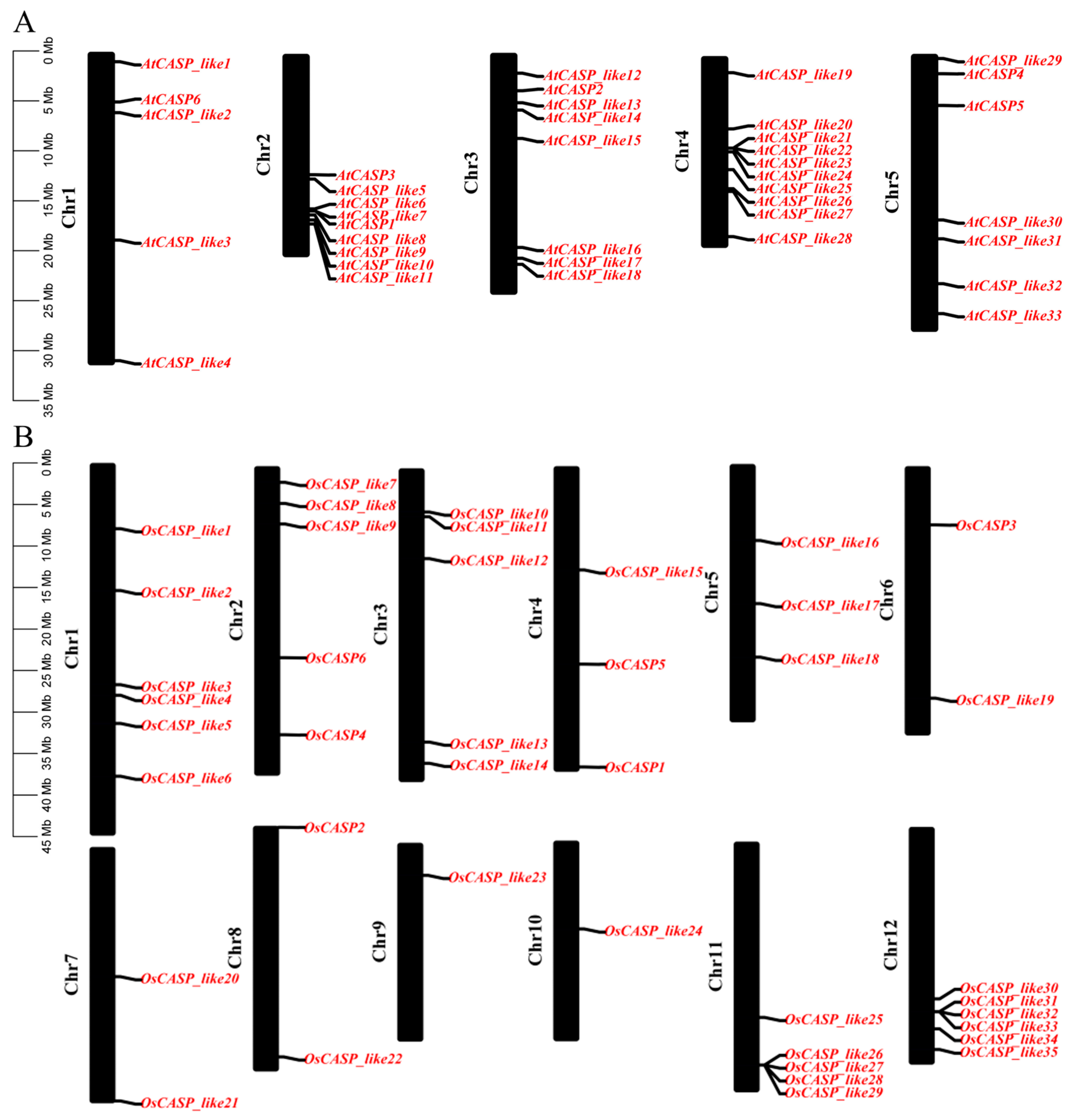
2.1. Identification, Gene Location and Physicochemical Properties of Rice and Arabidopsis CASPs
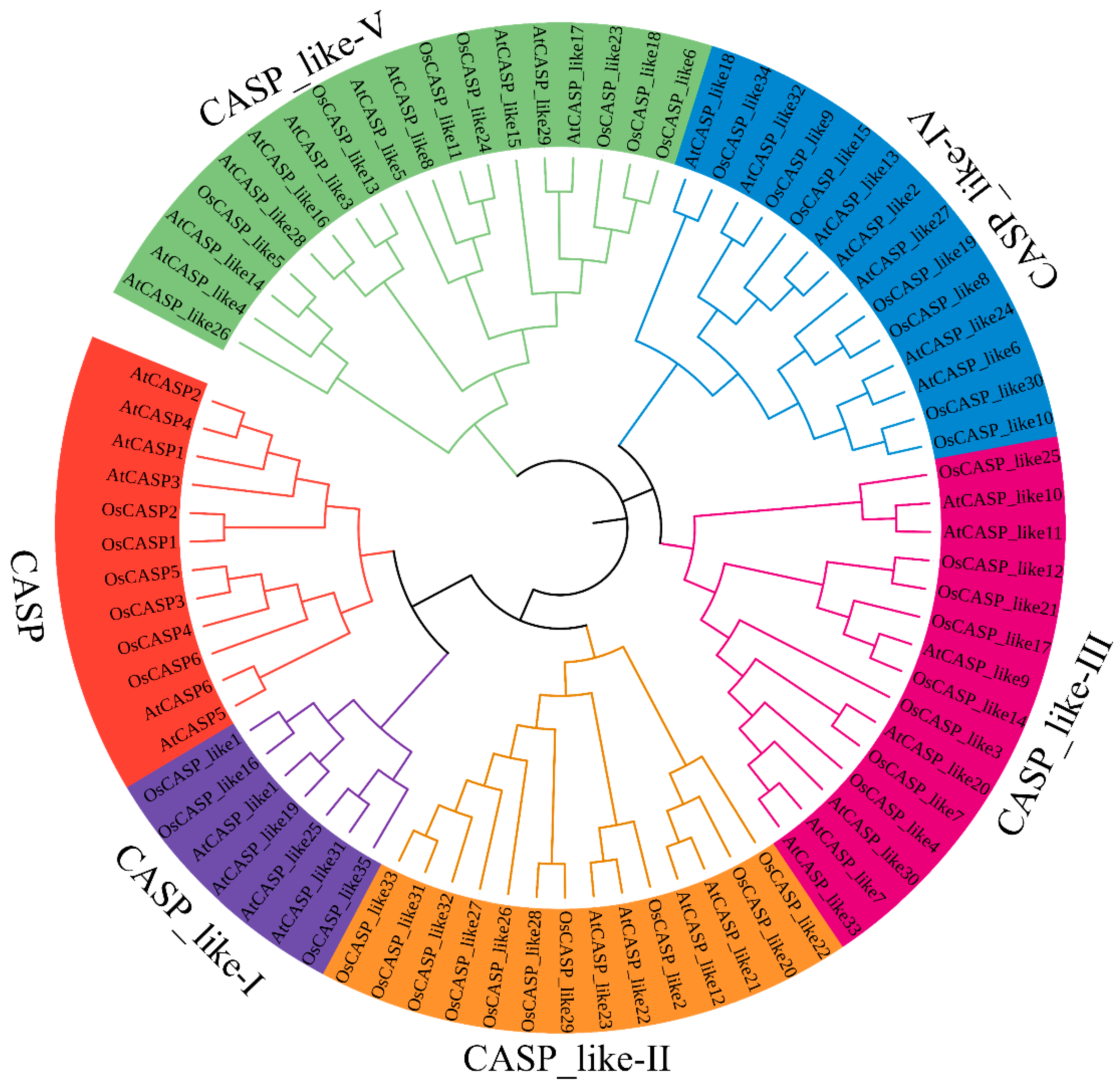
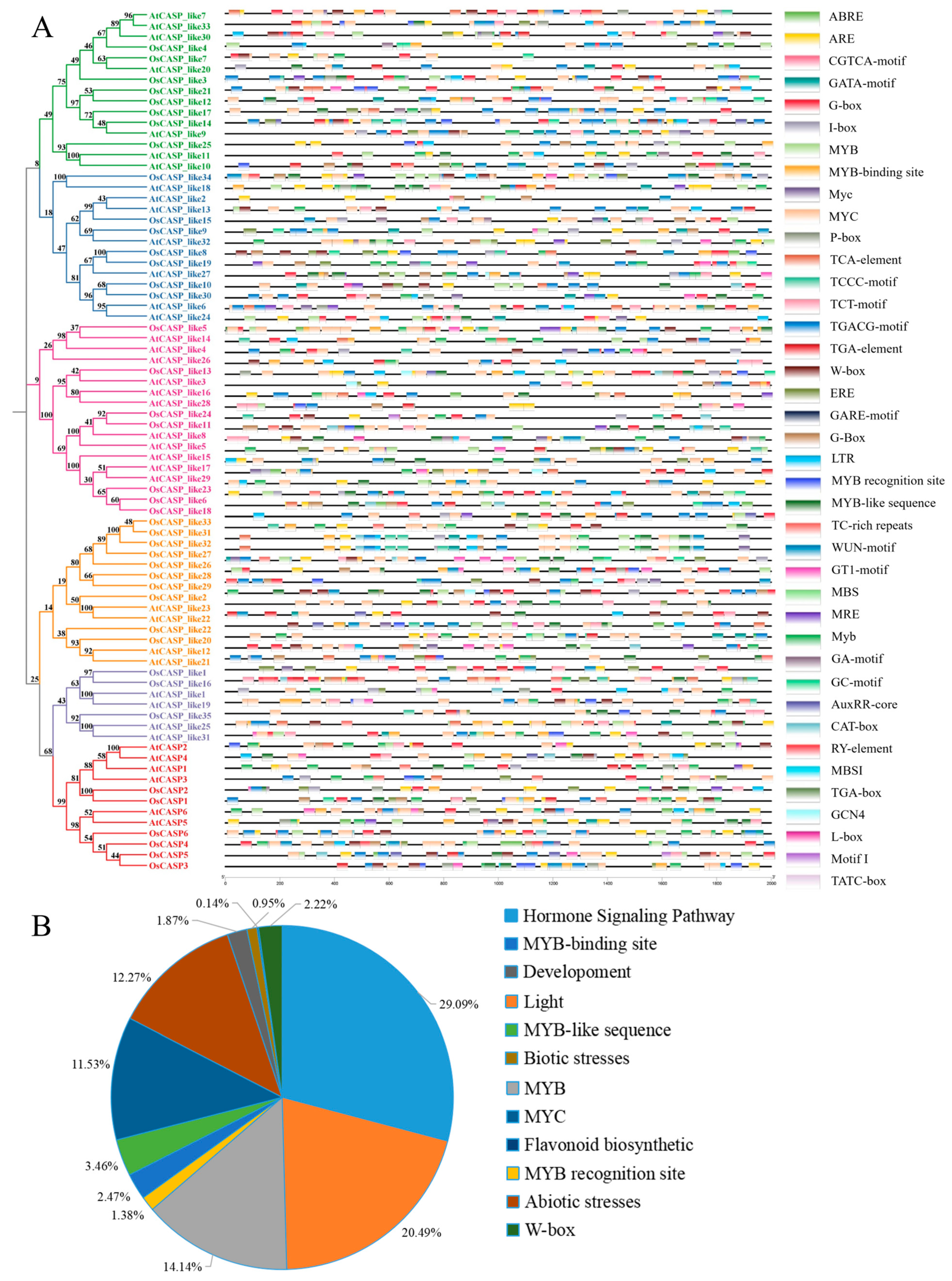
2.2. Phylogenetic Analysis of AtCASPs and OsCASPs
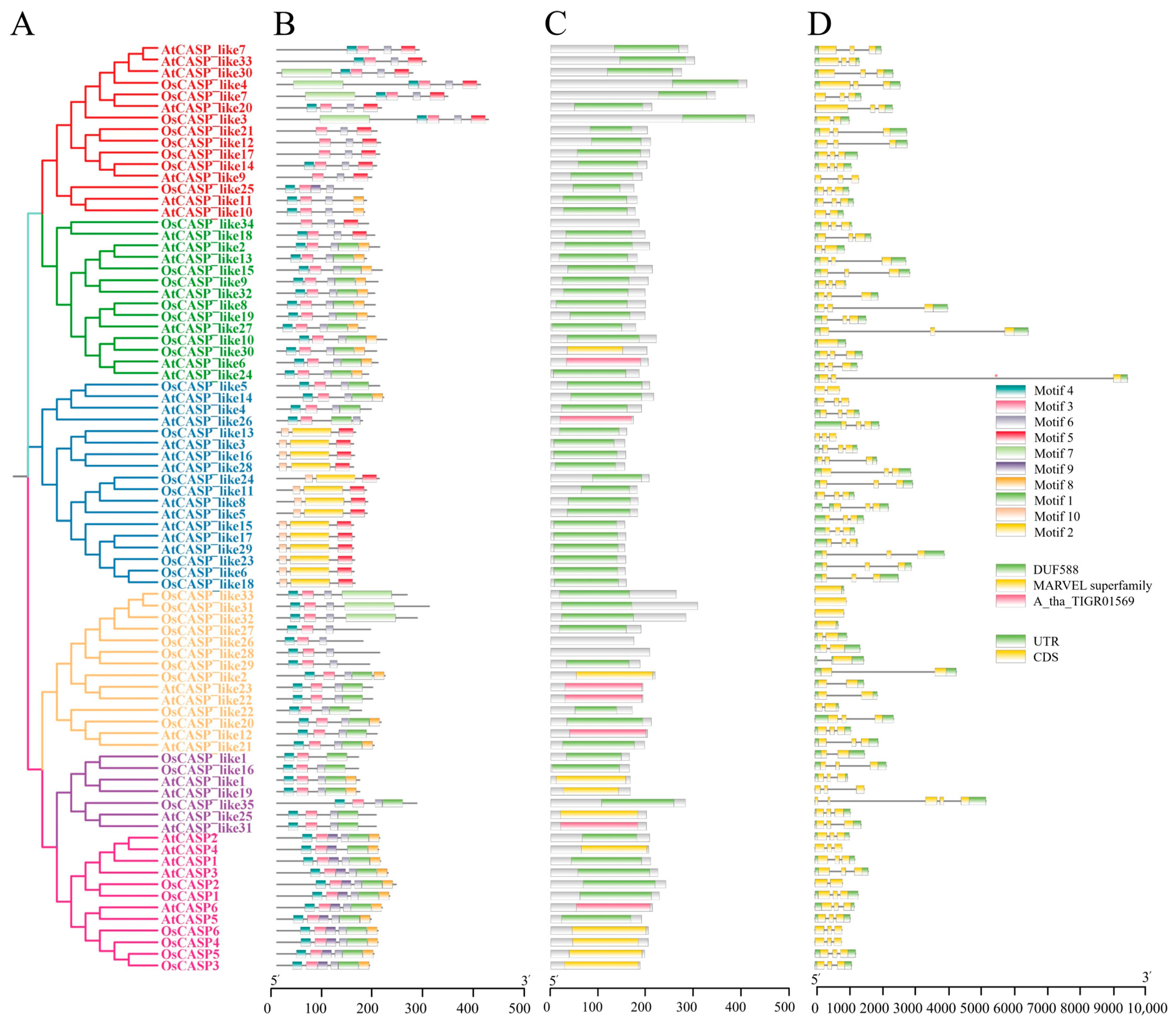
2.3. Gene Structure and Motif of AtCASPs and OsCASPs
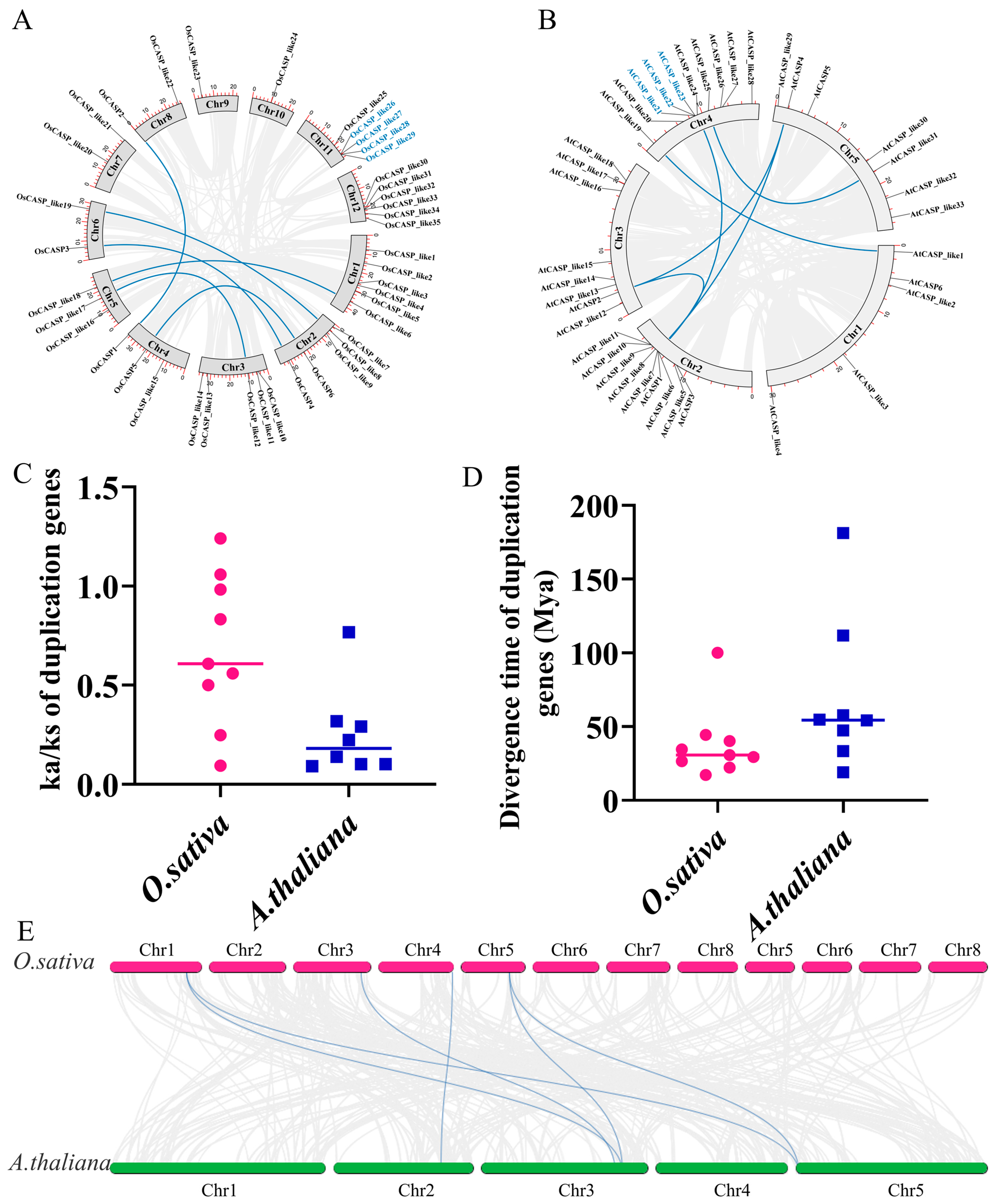
2.4. Duplication Events, Synteny and Ka/Ks Analysis of AtCASP and OsCASP Genes
2.5. Cis-Element Analysis of AtCASPs and OsCASPs
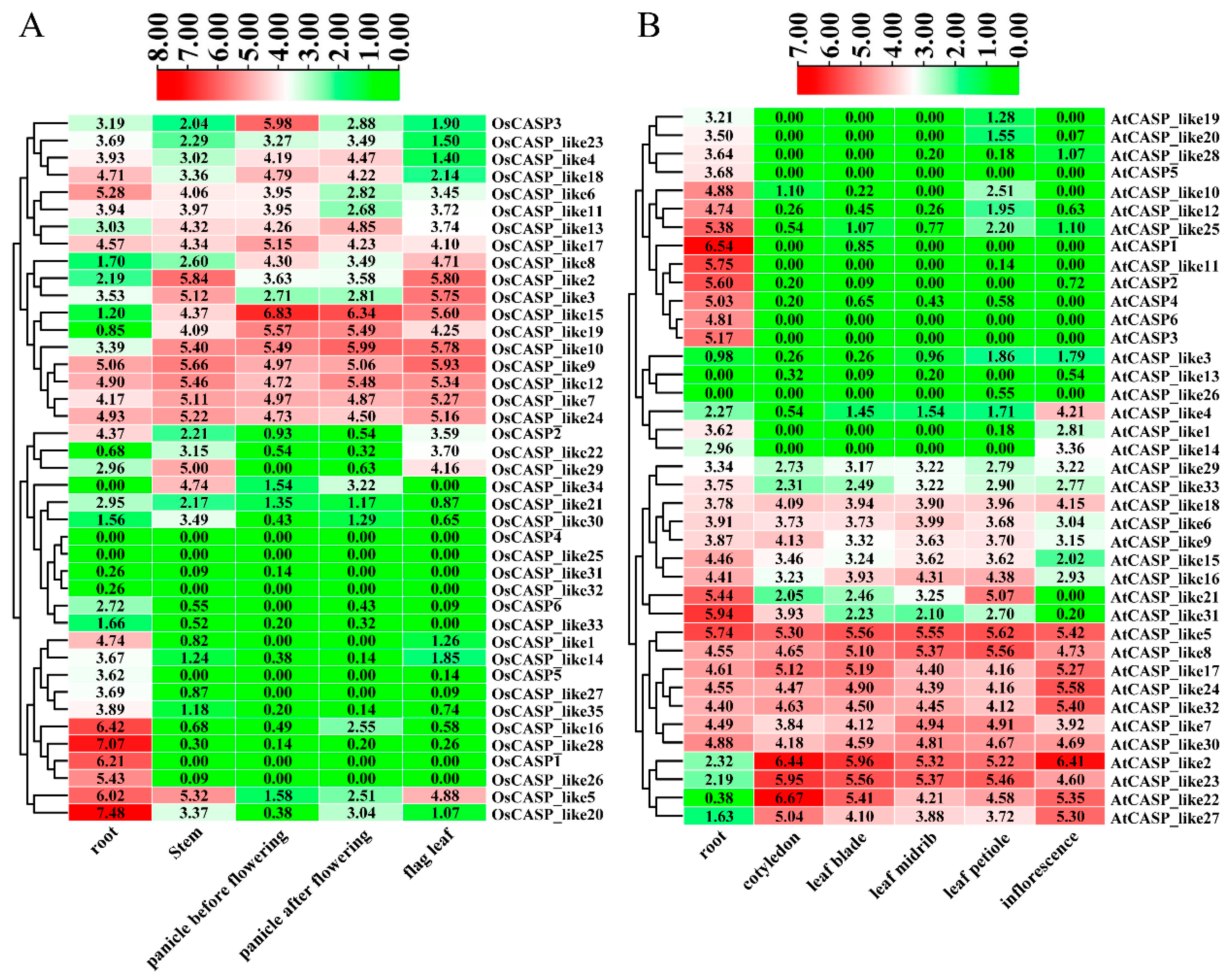
2.6. Tissue-Specific Expression Patterns of AtCASP and OsCASP Genes by RNA-Seq
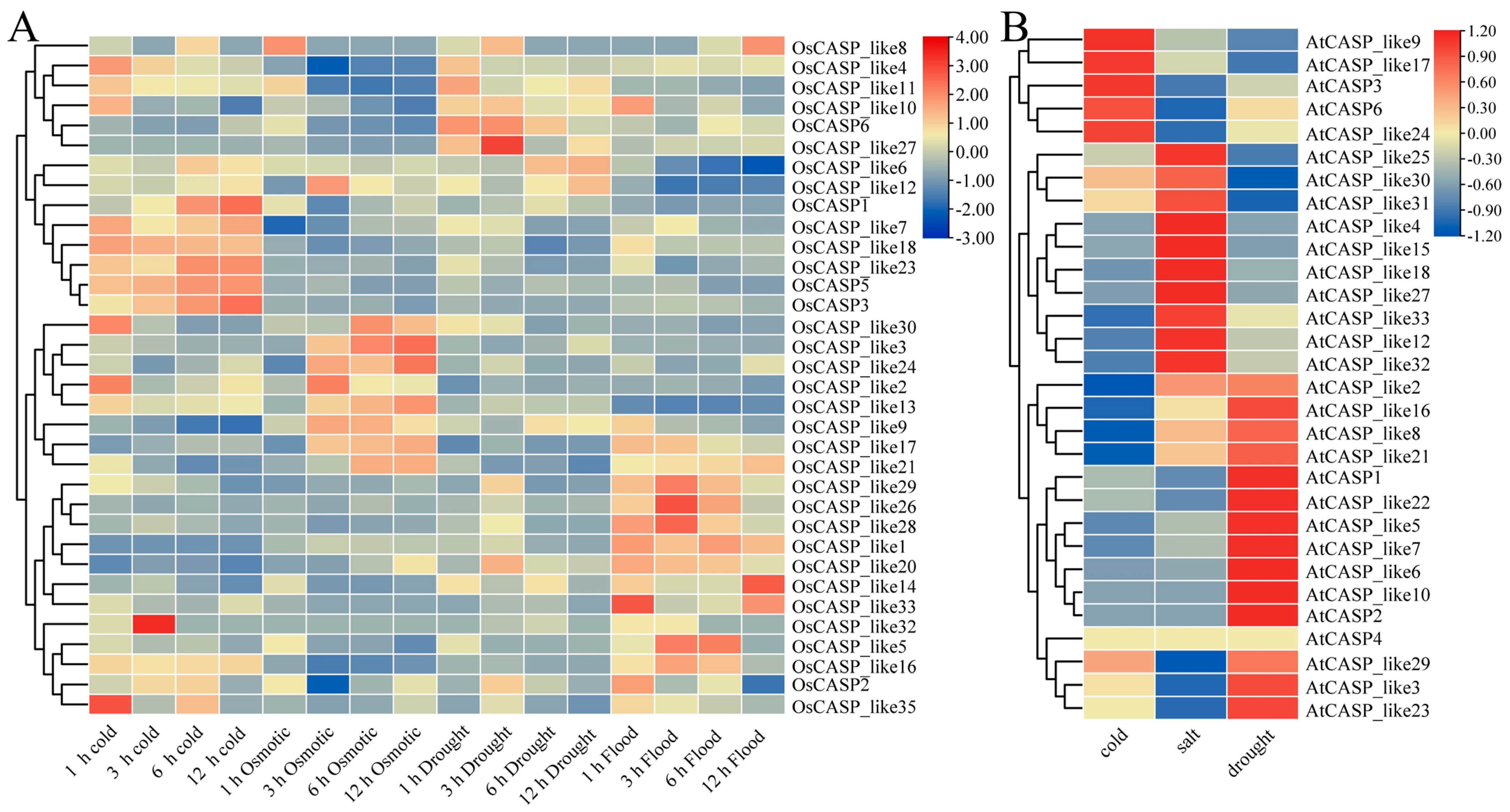
2.7. The Expression Patterns of OsCASP Genes in Different Abiotic Stresses by Transcriptome Data
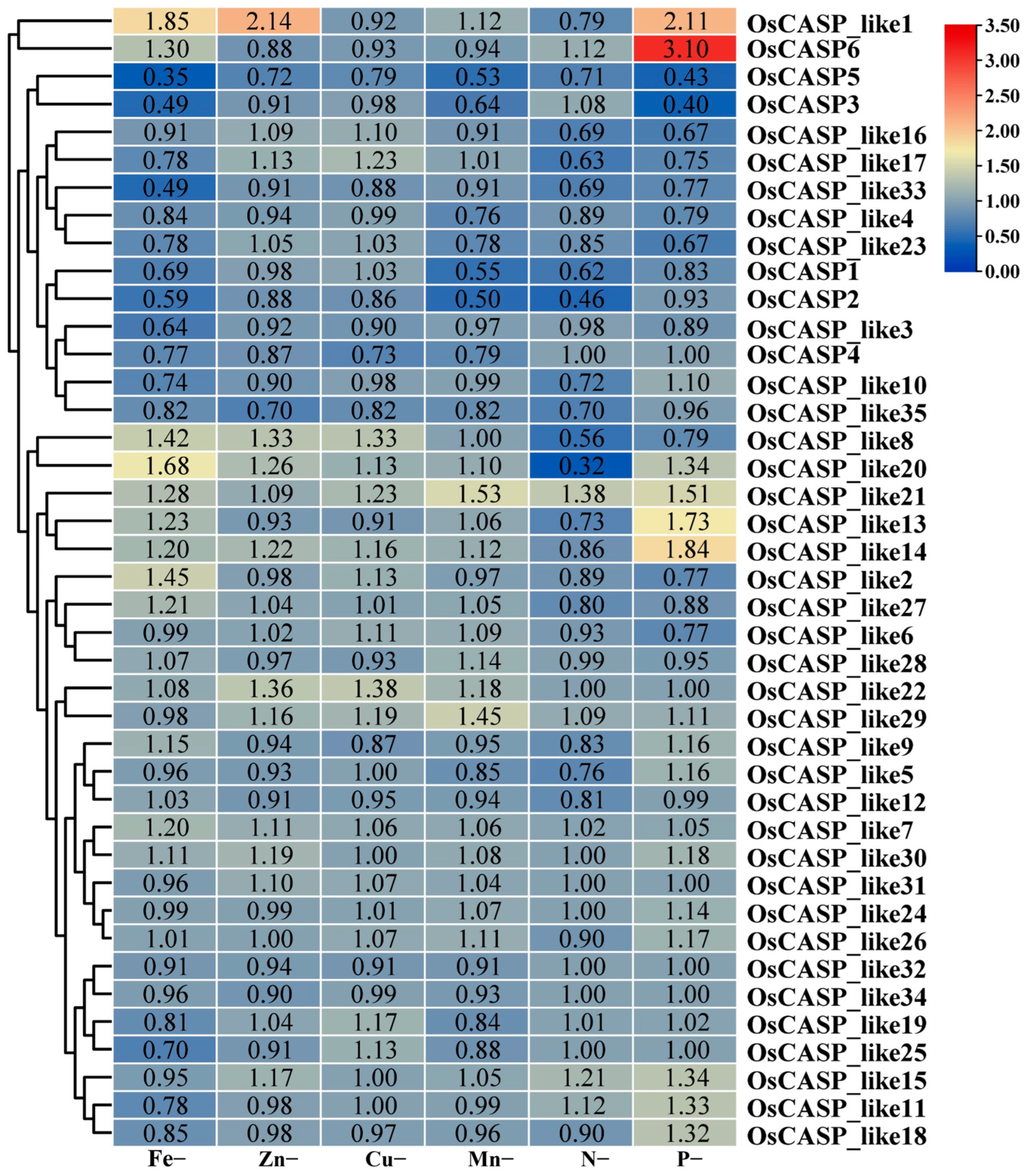
2.8. The Expression Patterns of OsCASP Genes in Ion Defects by RNA-Seq Data
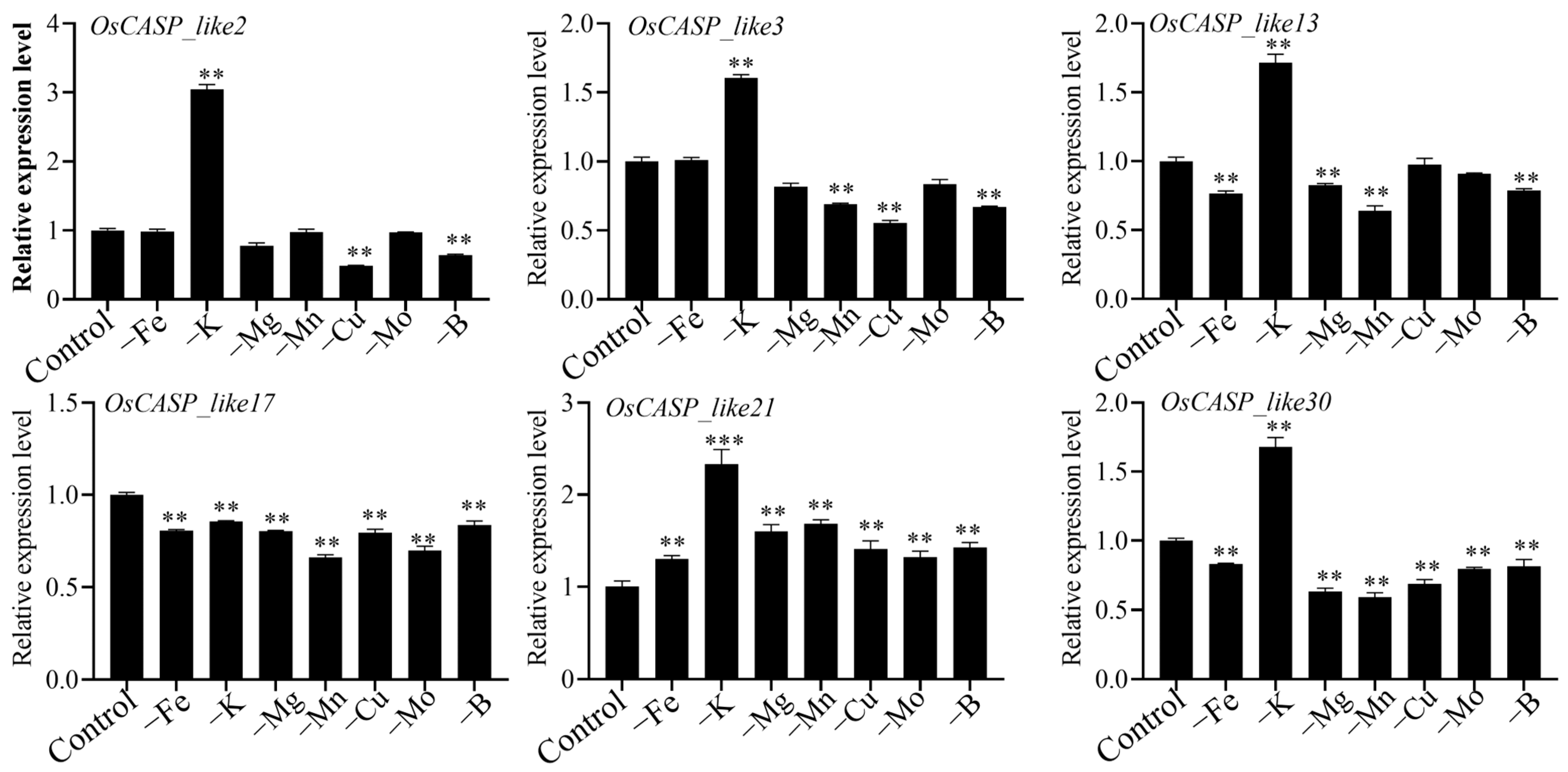
2.9. Expression Patterns of OsCASP Genes in Different Ion Defects by RT-qPCR
3. Discussion
4. Methods
4.1. Identification, Chromosomal Location and Phylogenetic Analysis of AtCASPs and OsCASPs
4.2. Gene Structure and Conserved Motifs Analysis of AtCASPs and OsCASPs
4.3. Duplication Events, Ka/Ks and Synteny Analysis of AtCASPs and OsCASPs
4.4. Cis-Acting Element Analysis of AtCASPs and OsCASPs
4.5. Expression Patterns of AtCASP and OsCASP Genes with RNA-Seq
4.6. Plant Materials, Growth Conditions and Treatments
4.7. RNA Extraction and RT-qPCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barberon, M.; Geldner, N. Radial Transport of Nutrients: The Plant Root as a Polarized Epithelium. Plant Physiol. 2014, 166, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Barberon, M. The endodermis as a checkpoint for nutrients. New Phytol. 2016, 213, 1604–1610. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, P.; Barberon, M. Polarized transport across root epithelia. Curr. Opin. Plant Biol. 2019, 52, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yamaji, N.; Huang, S.; Zhang, X.; Shi, M.; Fu, S.; Yang, G.; Ma, J.F.; Xia, J. OsCASP1 Is Required for Casparian Strip Formation at Endodermal Cells of Rice Roots for Selective Uptake of Mineral Elements. Plant Cell 2019, 31, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, B.; Chen, Z.; Wu, M.; Chao, D.; Wei, Q.; Xin, Y.; Li, L.; Ming, Z.; Xia, J. Three OsMYB36 members redundantly regulate Casparian strip formation at the root endodermis. Plant Cell 2022, 34, 2948–2968. [Google Scholar] [CrossRef]
- Geldner, N. The Endodermis. Annu. Rev. Plant Biol. 2013, 64, 531–558. [Google Scholar] [CrossRef]
- Robbins, N.E., II; Trontin, C.; Duan, L.; Dinneny, J.R. Beyond the Barrier: Communication in the Root through the Endodermis. Plant Physiol. 2014, 166, 551–559. [Google Scholar] [CrossRef]
- Roppolo, D.; De Rybel, B.; Tendon, V.D.; Pfister, A.; Alassimone, J.; Vermeer, J.E.M.; Yamazaki, M.; Stierhof, Y.-D.; Beeckman, T. A novel protein family mediates Casparian strip formation in the endodermis. Nature 2011, 473, 380–383. [Google Scholar] [CrossRef]
- Hosmani, P.S.; Kamiya, T.; Danku, J.; Naseer, S.; Geldner, N.; Guerinot, M.L.; Salt, D.E. Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based Casparian strip in the root. Proc. Natl. Acad. Sci. USA 2013, 110, 14498–14503. [Google Scholar] [CrossRef]
- Kalmbach, L.; Hématy, K.; De Bellis, D.; Barberon, M.; Fujita, S.; Ursache, R.; Daraspe, J. Transient cell-specific EXO70A1 activity in the CASP domain and Casparian strip localization. Nat. Plants 2017, 3, 17058. [Google Scholar] [CrossRef]
- Reyt, G.; Ramakrishna, P.; Salas-González, I.; Fujita, S.; Love, A.; Tiemessen, D.; Lapierre, C.; Morreel, K.; Calvo-Polanco, M.; Flis, P.; et al. Two chemically distinct root lignin barriers control solute and water balance. Nat. Commun. 2021, 12, 2320. [Google Scholar] [CrossRef] [PubMed]
- Pfister, A.; Barberon, M.; Alassimone, J.; Kalmbach, L.; Lee, Y.; Vermeer, J.E.M.; Yamazaki, M.; Li, G.; Maurel, C.; Takano, J.; et al. A receptor-like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. eLife 2014, 3, e03115. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Borghi, M.; Wang, P.; Danku, J.M.C.; Kalmbach, L.; Hosmani, P.S.; Naseer, S.; Fujiwara, T.; Geldner, N.; Salt, D.E.; et al. The MYB36 transcription factor orchestrates Casparian strip formation. Proc. Natl. Acad. Sci. USA 2015, 112, 10533–10538. [Google Scholar] [CrossRef] [PubMed]
- Alassimone, J.; Fujita, S.; Doblas, V.G.; van Dop, M.; Barberon, M.; Kalmbach, L.; Vermeer, J.E.M.; Rojas-Murcia, N.; Santuari, L.; Hardtke, C.S.; et al. Polarly localized kinase SGN1 is required for Casparian strip integrity and positioning. Nat. Plants 2016, 2, 16113. [Google Scholar] [CrossRef]
- Doblas, V.G.; Geldner, N.; Barberon, M. The endodermis, a tightly controlled barrier for nutrients. Curr. Opin. Plant Biol. 2017, 39, 136–143. [Google Scholar] [CrossRef]
- Fujita, S.; De Bellis, D.; Edel, K.H.; Köster, P.; Andersen, T.G.; Schmid-Siegert, E.; Tendon, V.D.; Pfister, A.; Marhavý, P.; Ursache, R.; et al. SCHENGEN receptor module drives localized ROS production and lignification in plant roots. EMBO J. 2020, 39, e103894. [Google Scholar] [CrossRef]
- Okuda, S.; Fujita, S.; Moretti, A.; Hohmann, U.; Doblas, V.G.; Ma, Y.; Pfister, A.; Brandt, B.; Geldner, N.; Hothorn, M. Molecular mechanism for the recognition of sequence-divergent CIF peptides by the plant receptor kinases GSO1/SGN3 and GSO2. Proc. Natl. Acad. Sci. USA 2020, 117, 2693–2703. [Google Scholar] [CrossRef]
- Liberman, L.M.; Sparks, E.E.; Moreno-Risueno, M.A.; Petricka, J.J.; Benfey, P.N. MYB36 regulates the transition from proliferation to differentiation in the Arabidopsis root. Proc. Natl. Acad. Sci. USA 2015, 112, 12099–12104. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, L.; Pan, Z.; He, S.; Gao, Q.; Chen, B.; Gong, W.; Du, X. Casparian strip membrane domain proteins in Gossypium arboreum: Genome-wide identification and negative regulation of lateral root growth. BMC Genom. 2020, 21, 340. [Google Scholar] [CrossRef]
- Su, Y.; Haq, M.Z.U.; Liu, X.; Li, Y.; Yu, J.; Liu, Y. A Genome-Wide Identification and Expression Analysis of the Casparian Strip Membrane Domain Protein-like Gene Family in Pogostemon cablin in Response to p-HBA-Induced Continuous Cropping Obstacles. Plants 2023, 12, 3901. [Google Scholar] [CrossRef]
- Duan, W.; Xue, B.; He, Y.; Liao, S.; Li, X.; Li, X.; Liang, Y.-K. Genome-Wide Identification and Expression Pattern Analysis of Dirigent Members in the Genus Oryza. Int. J. Mol. Sci. 2023, 24, 7189. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. Tbtools v2.121: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Journal, M.A.; Mandlik, R.; Varanavasiappan, S.; Kokiladevi, E.; Sudhakar, D.; Arul, L. Cloning and In silico analysis of Casparian strip membrane domain protein (CASP) from rice. Madras Agric. J. 2019, 106, 155–159. [Google Scholar]
- Liu, L.; Wei, X.; Yang, Z.; Yuan, F.; Han, G.; Guo, J.; Wang, B. SbCASP-LP1C1 improves salt exclusion by enhancing the root apoplastic barrier. Plant Mol. Biol. 2023, 111, 73–88. [Google Scholar] [CrossRef]
- Hu, B.; Jiang, Z.; Wang, W.; Qiu, Y.; Zhang, Z.; Liu, Y.; Li, A.; Gao, X.; Liu, L.; Qian, Y.; et al. Nitrate-NRT1.1B-SPX4 Cascade Integrates Nitrogen and Phosphorus Signalling Networks in Plants. Nat. Plants 2019, 5, 401–413. [Google Scholar] [CrossRef]
- Freeling, M. Bias in Plant Gene Content Following Different Sorts of Duplication: Tandem, Whole-Genome, Segmental, or by Transposition. Annu. Rev. Plant Biol. 2009, 60, 433–453. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Davies, T.J.; Barraclough, T.G.; Chase, M.W.; Soltis, P.S.; Soltis, D.E.; Savolainen, V. Darwin’s abominable mystery: Insights from a supertree of the angiosperms. Proc. Natl. Acad. Sci. USA 2004, 101, 1904–1909. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.L.; Bremer, K.; Friis, E.M. Dating phylogenetically basal eudicots using rbcL sequences and multiple fossil reference points. Am. J. Bot. 2005, 92, 1737–1748. [Google Scholar] [CrossRef]
- Shaul, O. How introns enhance gene expression. Int. J. Biochem. Cell Biol. 2017, 91, 145–155. [Google Scholar] [CrossRef]
- Lersten, N.R. Occurrence of endodermis with a casparian strip in stem and leaf. Bot. Rev. 1997, 63, 265–272. [Google Scholar] [CrossRef]
- Hao, N.; Yao, H.; Suzuki, M.; Li, B.; Wang, C.; Cao, J.; Fujiwara, T.; Wu, T.; Kamiya, T. Novel lignin-based extracellular barrier in glandular trichome. Nat. Plants 2024, 10, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7, Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME Suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, H.; Long, Y.; Shu, Y.; Zhai, J. Plant Public RNA-seq Database: A comprehensive online database for expression analysis of 45,000 plant public RNA-Seq libraries. Plant Biotechnol. J. 2022, 20, 806–808. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Chen, Y.; Liu, Y.; Lin, W.-H.; Wang, J.-W. Single-cell transcriptome atlas and chromatin accessibility landscape reveal differentiation trajectories in the rice root. Nat. Commun. 2021, 2, 2053. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, B.; Liang, Z.; Liu, Y.; Li, D.; Cao, P.; Liu, C. Comparative Analysis of Casparian Strip Membrane Domain Protein Family in Oryza sativa (L.) and Arabidopsis thaliana (L.). Int. J. Mol. Sci. 2024, 25, 9858. https://doi.org/10.3390/ijms25189858
Xue B, Liang Z, Liu Y, Li D, Cao P, Liu C. Comparative Analysis of Casparian Strip Membrane Domain Protein Family in Oryza sativa (L.) and Arabidopsis thaliana (L.). International Journal of Molecular Sciences. 2024; 25(18):9858. https://doi.org/10.3390/ijms25189858
Chicago/Turabian StyleXue, Baoping, Zicong Liang, Yue Liu, Dongyang Li, Peng Cao, and Chang Liu. 2024. "Comparative Analysis of Casparian Strip Membrane Domain Protein Family in Oryza sativa (L.) and Arabidopsis thaliana (L.)" International Journal of Molecular Sciences 25, no. 18: 9858. https://doi.org/10.3390/ijms25189858
APA StyleXue, B., Liang, Z., Liu, Y., Li, D., Cao, P., & Liu, C. (2024). Comparative Analysis of Casparian Strip Membrane Domain Protein Family in Oryza sativa (L.) and Arabidopsis thaliana (L.). International Journal of Molecular Sciences, 25(18), 9858. https://doi.org/10.3390/ijms25189858





