Lacticaseibacillus paracsei HY7207 Alleviates Hepatic Steatosis, Inflammation, and Liver Fibrosis in Mice with Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Results
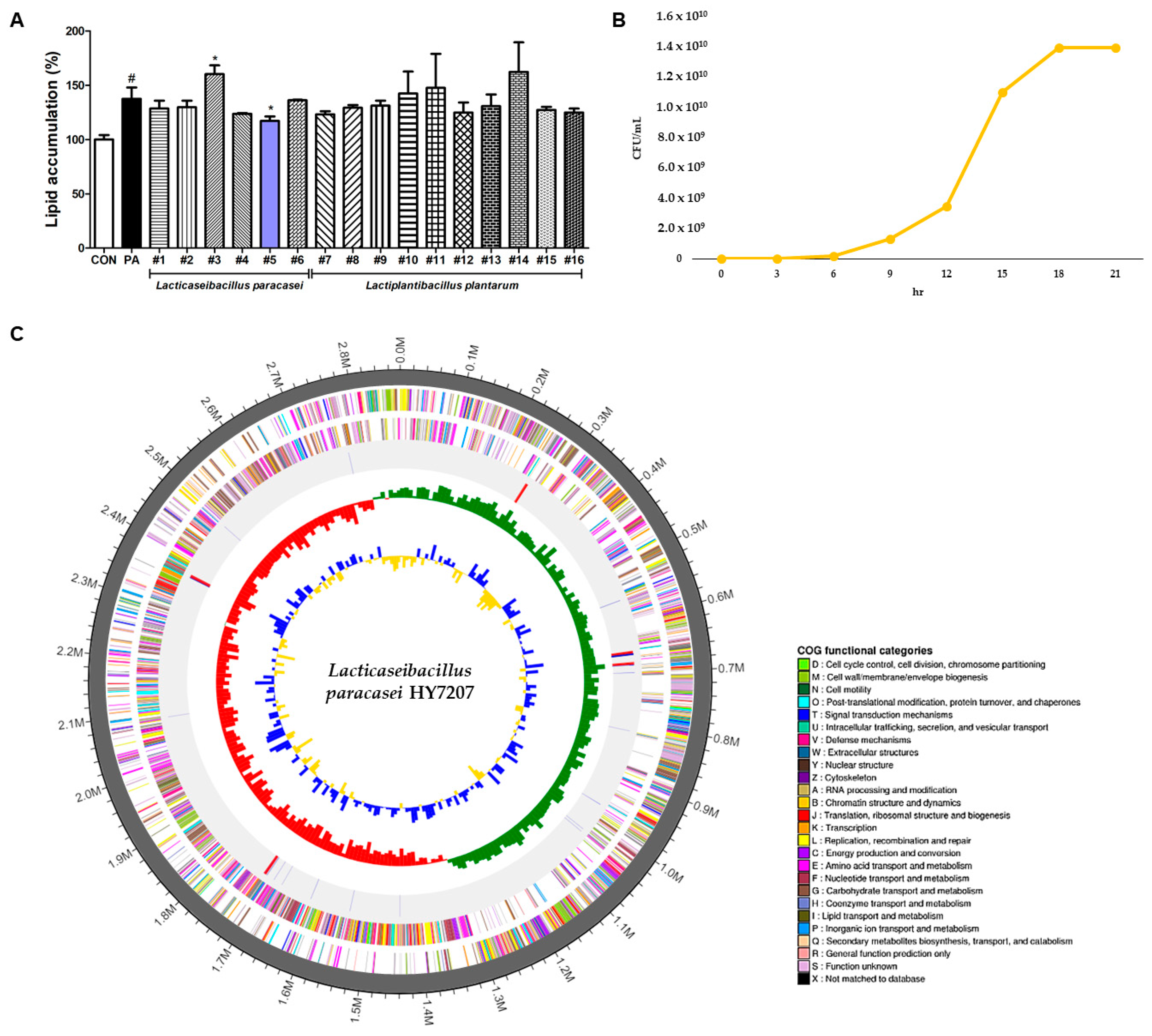
2.1. Screening of LAB Strains Isolated from Traditional Fermented Beverages
2.2. Growth Curve of HY7207 in Industrial Culture
2.3. Whole-Genome Sequencing of HY7207
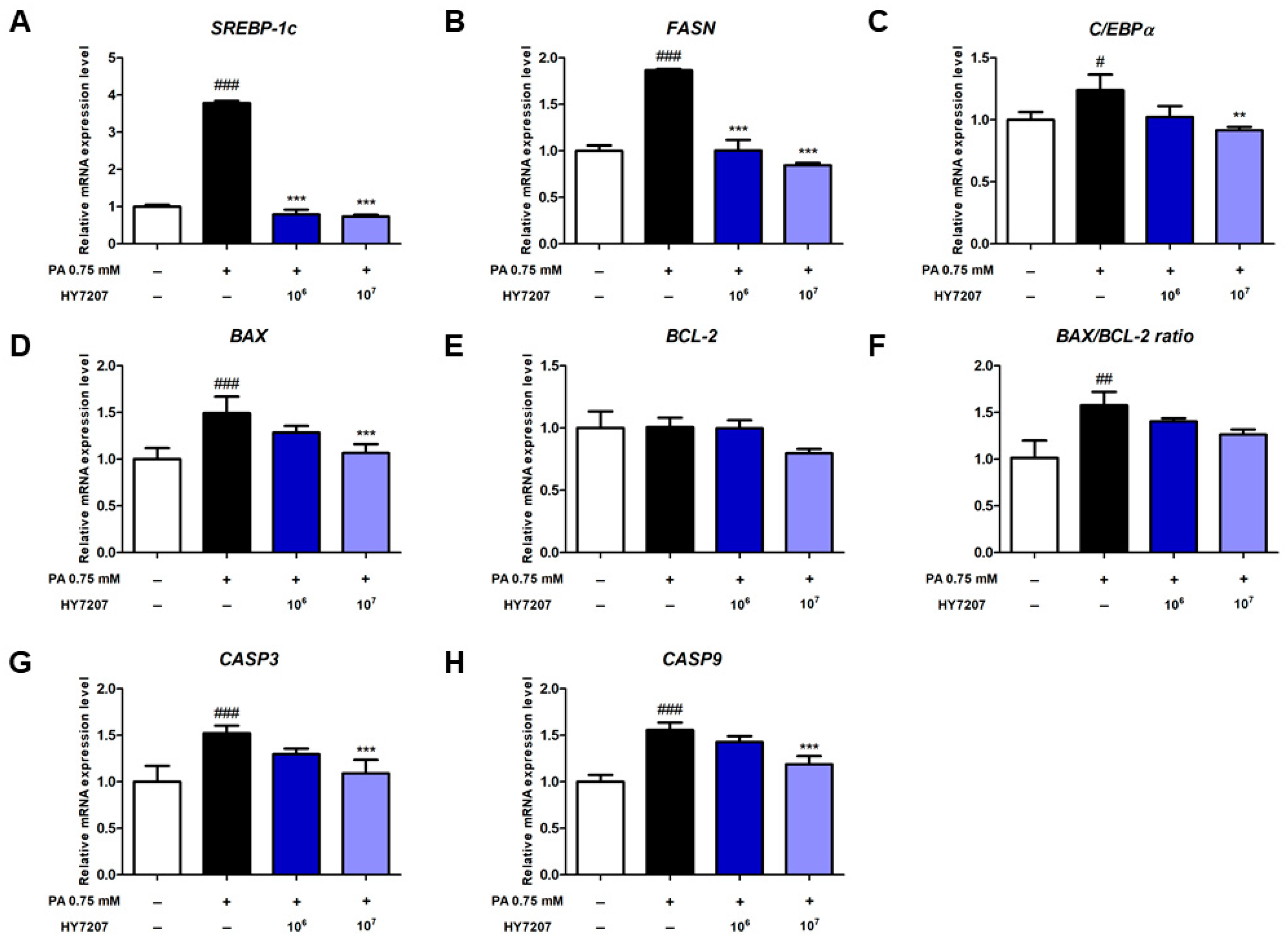
2.4. Effects of HY7207 on the Expression of Genes Involved in Lipogenesis and Apoptosis by PA-Treated HepG2 Cells
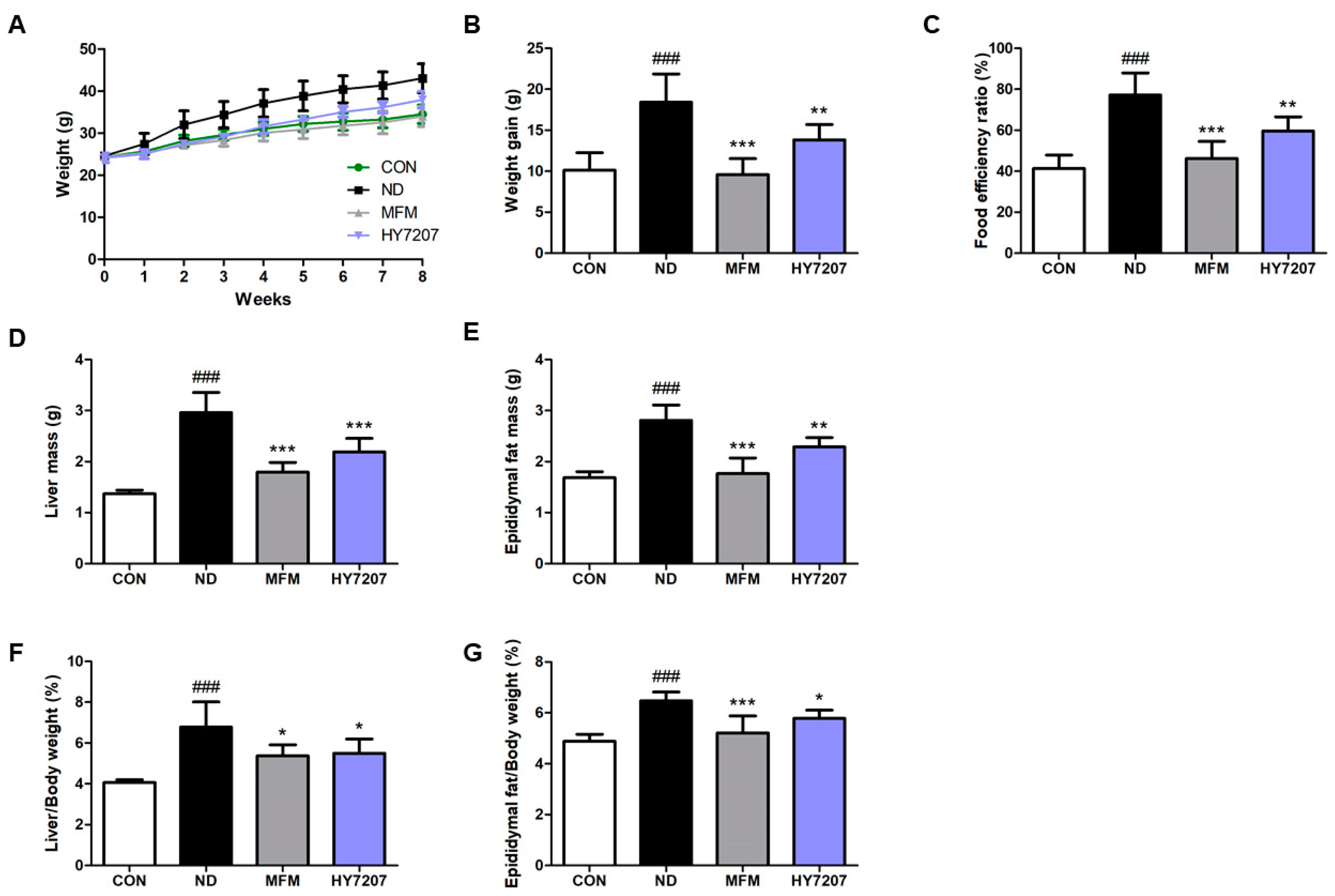
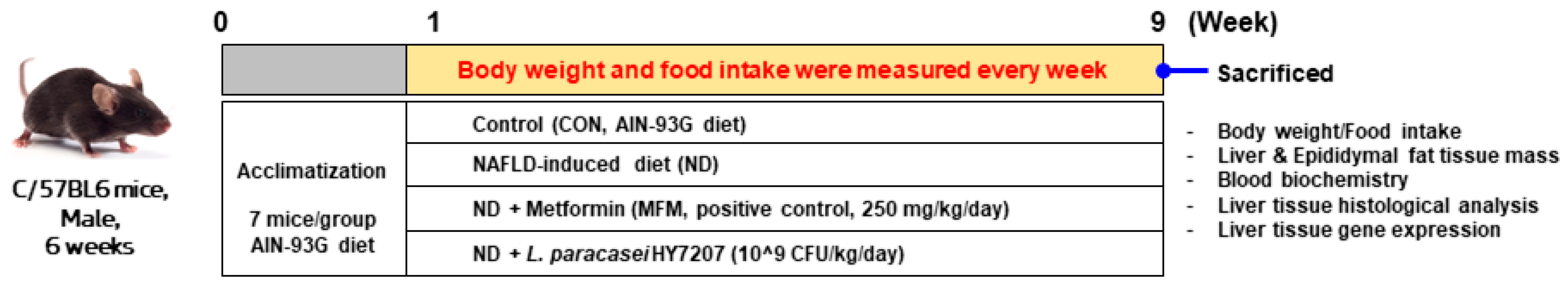
2.5. Effects of HY7207 on Physiologic Parameters in Mice Fed an NAFLD-Inducing Diet
2.6. Effects of HY7207 on Blood Biochemistry
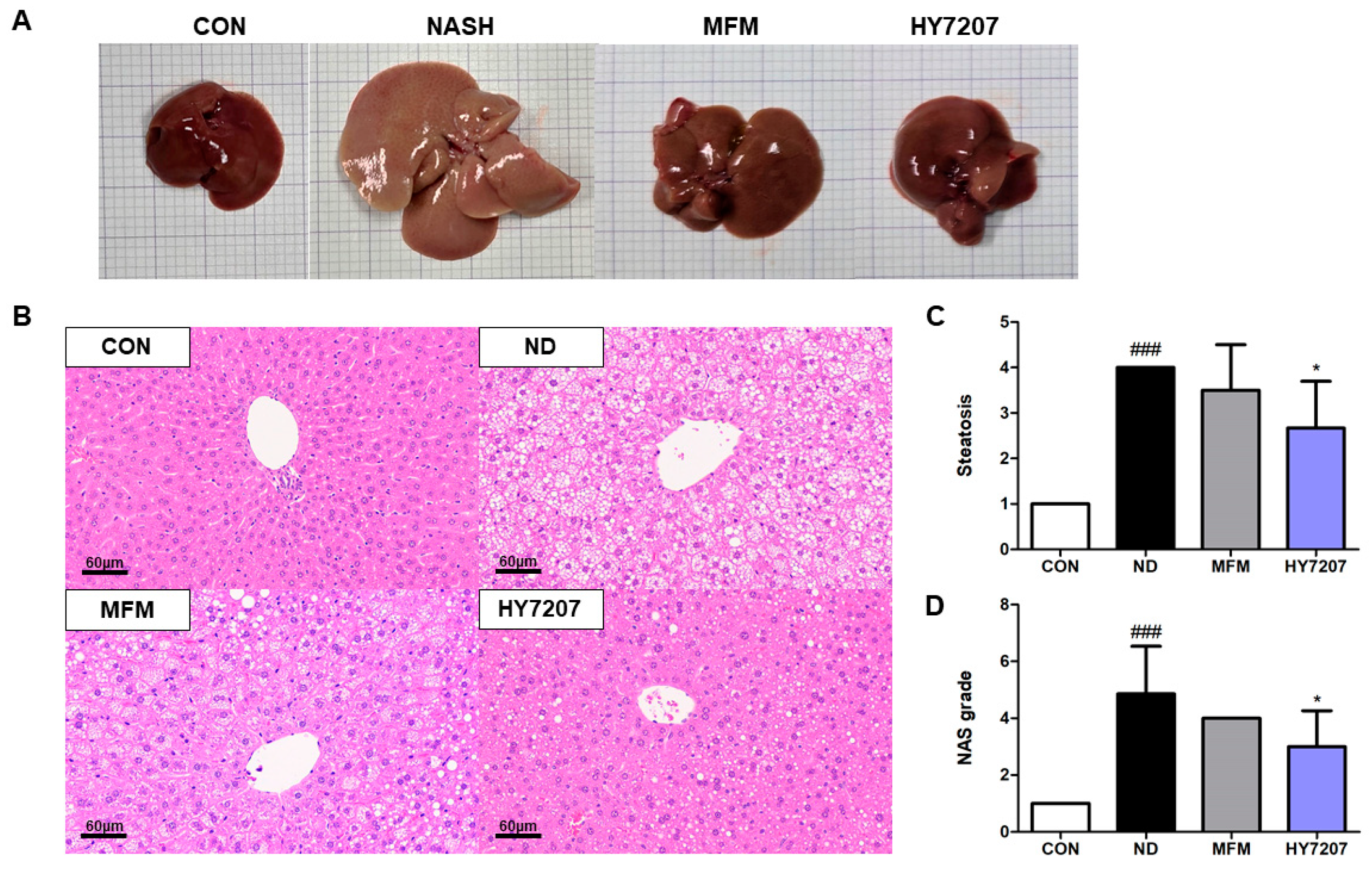
2.7. Effects of HY7207 on the Histology of the Liver
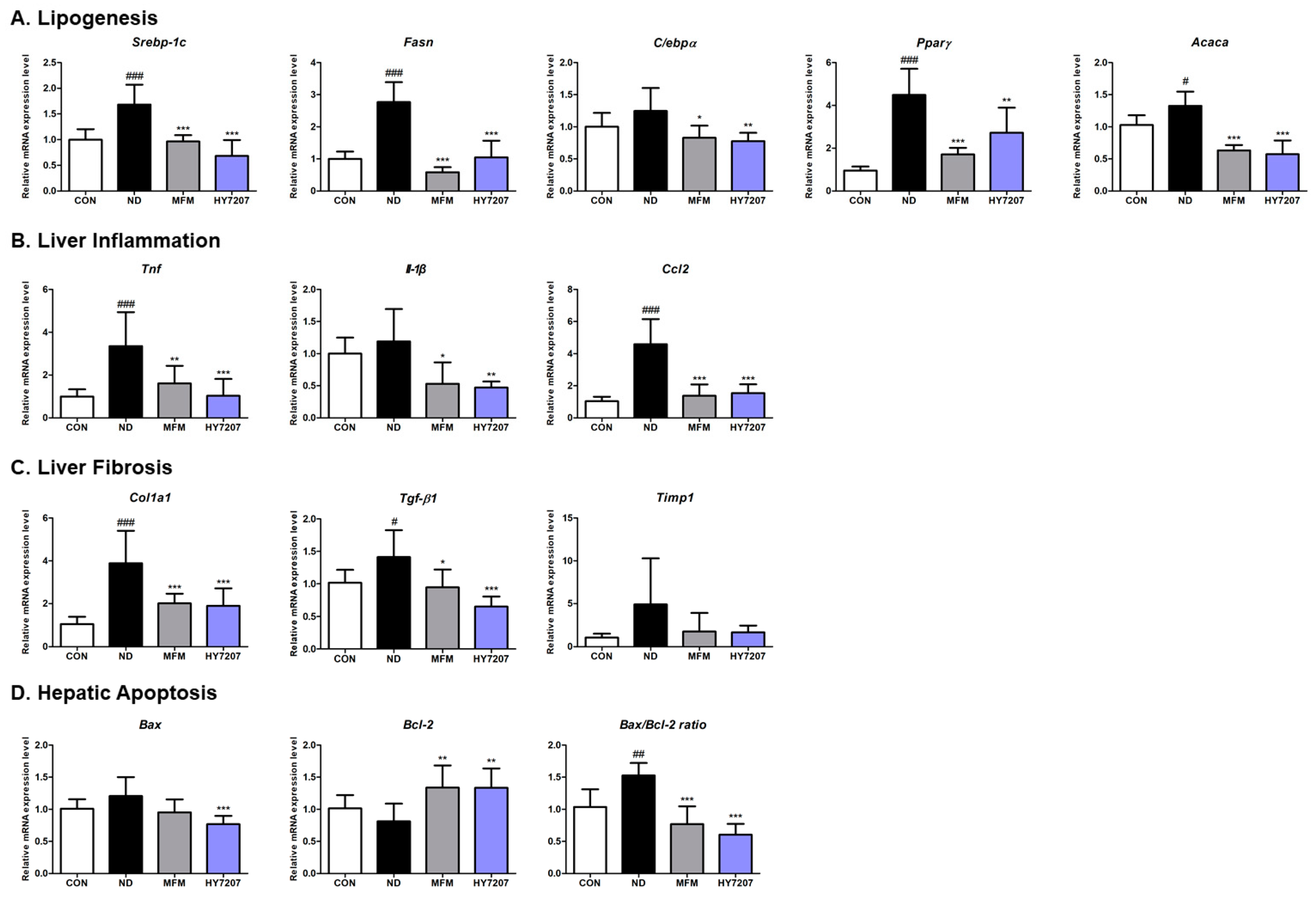
2.8. Effects of HY7207 on the Hepatic Gene Expression of Mice with NAFLD
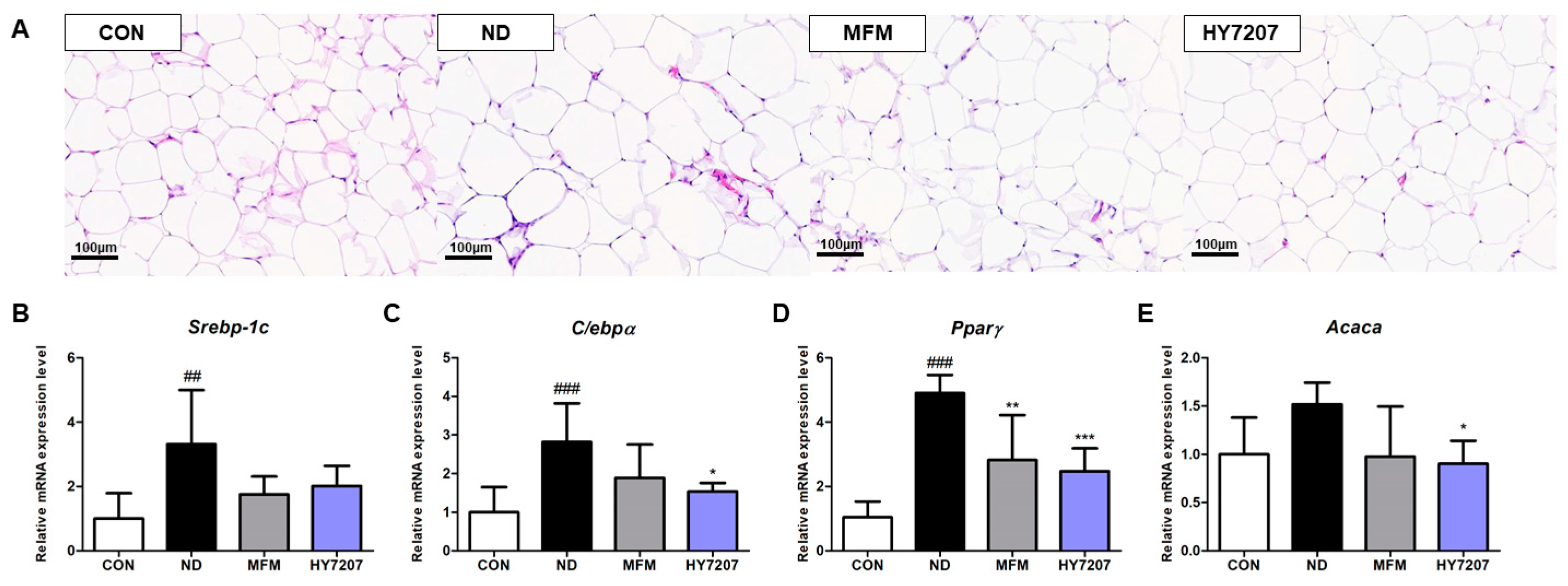
2.9. Effects of HY7207 on the Histology of Epididymal Fat
2.10. Effects of HY7207 on the Expression of Lipogenesis-Related Genes in the Epididymal Fat of Mice with NAFLD
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Isolation of LAB Strains
4.2. Culture of HepG2 Cells
4.3. Assessment of the Inhibition of Lipid Accumulation in HepG2 Cells
4.4. Industrial Culture and Assessment of the Growth Curve of HY7207 Cells
4.5. Whole Genome Sequencing of HY7207
4.6. Animal Experiments
4.7. Blood Biochemical Analysis
4.8. Histological Analysis
4.9. Isolation of RNA, cDNA Synthesis, and Real-Time PCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abd El-Kader, S.M.; El-Den Ashmawy, E.M.S. Non-alcoholic fatty liver disease: The diagnosis and management. World J. Hepatol. 2015, 7, 846. [Google Scholar] [CrossRef] [PubMed]
- Buss, C.; Valle-Tovo, C.; Miozzo, S.; de Mattos, A.A. Probiotics and synbiotics may improve liver aminotransferases levels in non-alcoholic fatty liver disease patients. Ann. Hepatol. 2014, 13, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Paolella, G.; Mandato, C.; Pierri, L.; Poeta, M.; Di Stasi, M.; Vajro, P. Gut-liver axis and probiotics: Their role in non-alcoholic fatty liver disease. World J. Gastroenterol. WJG 2014, 20, 15518. [Google Scholar] [CrossRef] [PubMed]
- Bellentani, S.; Scaglioni, F.; Marino, M.; Bedogni, G. Epidemiology of non-alcoholic fatty liver disease. Dig. Dis. 2010, 28, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.; Baranova, A.; Younossi, Z. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Yin, X.; Guo, X.; Liu, Z.; Wang, J. Advances in the diagnosis and treatment of non-alcoholic fatty liver disease. Int. J. Mol. Sci. 2023, 24, 2844. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Li, N.; Li, X.; Ding, Y.; Liu, X.; Diggle, K.; Kisseleva, T.; Brenner, D.A. SREBP regulation of lipid metabolism in liver disease, and therapeutic strategies. Biomedicines 2023, 11, 3280. [Google Scholar] [CrossRef]
- Souza-Mello, V. Peroxisome proliferator-activated receptors as targets to treat non-alcoholic fatty liver disease. World J. Hepatol. 2015, 7, 1012. [Google Scholar] [CrossRef]
- Ruiz, R.; Jideonwo, V.; Ahn, M.; Surendran, S.; Tagliabracci, V.S.; Hou, Y.; Gamble, A.; Kerner, J.; Irimia-Dominguez, J.M.; Puchowicz, M.A. Sterol regulatory element-binding protein-1 (SREBP-1) is required to regulate glycogen synthesis and gluconeogenic gene expression in mouse liver. J. Biol. Chem. 2014, 289, 5510–5517. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, J.; Calvisi, D.F.; Chen, X. Role of lipogenesis rewiring in hepatocellular carcinoma. In Seminars in Liver Disease; Thieme Medical Publishers, Inc.: New York, NY, USA, 2022; Volume 42, pp. 77–86. [Google Scholar]
- Chatrath, H.; Vuppalanchi, R.; Chalasani, N. Dyslipidemia in patients with nonalcoholic fatty liver disease. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2012; Volume 32, pp. 22–29. [Google Scholar]
- Martin, A.; Lang, S.; Goeser, T.; Demir, M.; Steffen, H.-M.; Kasper, P. Management of dyslipidemia in patients with non-alcoholic fatty liver disease. Curr. Atheroscler. Rep. 2022, 24, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Alkhouri, N.; Carter-Kent, C.; Feldstein, A.E. Apoptosis in nonalcoholic fatty liver disease: Diagnostic and therapeutic implications. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 201–212. [Google Scholar] [CrossRef]
- Lei, Z.; Yu, J.; Wu, Y.; Shen, J.; Lin, S.; Xue, W.; Mao, C.; Tang, R.; Sun, H.; Qi, X. CD1d protects against hepatocyte apoptosis in non-alcoholic steatohepatitis. J. Hepatol. 2024, 80, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.W.; Lee, C.D. Apoptosis and diagnosis of nonalcoholic steatohepatitis. Korean J. Hepatol. 2011, 17, 247. [Google Scholar] [CrossRef]
- Kanda, T.; Matsuoka, S.; Yamazaki, M.; Shibata, T.; Nirei, K.; Takahashi, H.; Kaneko, T.; Fujisawa, M.; Higuchi, T.; Nakamura, H. Apoptosis and non-alcoholic fatty liver diseases. World J. Gastroenterol. 2018, 24, 2661. [Google Scholar] [CrossRef]
- Braunersreuther, V.; Viviani, G.L.; Mach, F.; Montecucco, F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J. Gastroenterol. WJG 2012, 18, 727. [Google Scholar] [CrossRef]
- Nagata, N.; Chen, G.; Xu, L.; Ando, H. An update on the chemokine system in the development of NAFLD. Medicina 2022, 58, 761. [Google Scholar] [CrossRef]
- Dooley, S.; Ten Dijke, P. TGF-β in progression of liver disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Hemarajata, P.; Versalovic, J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013, 6, 39–51. [Google Scholar] [CrossRef]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of probiotics in human gut microbiome-associated diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Bengoa, A.A.; Dardis, C.; Garrote, G.L.; Abraham, A.G. Health-promoting properties of Lacticaseibacillus paracasei: A focus on kefir isolates and exopolysaccharide-producing strains. Foods 2021, 10, 2239. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fan, X.; Li, D.; Zhao, T.; Wu, D.; Liu, Z.; Long, D.; Li, B.; Huang, X. High Antioxidant Capacity of Lacticaseibacillus paracasei TDM-2 and Pediococcus pentosaceus TCM-3 from Qinghai Tibetan Plateau and Their Function towards Gut Modulation. Foods 2023, 12, 1814. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.-K.; Chang, W.-W.; Jhong, J.-H.; Tsai, W.-H.; Chou, C.-H.; Wang, I.-J. Lacticaseibacillus paracasei GM-080 ameliorates allergic airway inflammation in children with allergic rhinitis: From an animal model to a double-blind, randomized, placebo-controlled trial. Cells 2023, 12, 768. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Tang, X.; Zhang, Q.; Xiong, F.; Yan, Y.; Zhao, J.; Mao, B.; Zhang, H.; Cui, S. Lacticaseibacillus paracasei CCFM1222 Ameliorated the Intestinal Barrier and Regulated Gut Microbiota in Mice with Dextran Sulfate Sodium-Induced Colitis. Probiotics Antimicrob. Proteins 2024, 1–13. [Google Scholar] [CrossRef]
- Ji, Y.; Xie, Q.; Meng, X.; Wang, W.; Li, S.; Lang, X.; Zhao, C.; Yuan, Y.; Ye, H. Lactobacillus paracasei improves dietary fatty liver by reducing insulin resistance and inflammation in obese mice model. J. Funct. Foods 2022, 95, 105150. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Chai, W.; Sun, C.; Zhang, T.; Zhao, C.; Yuan, Y.; Wang, X.; Liu, H.; Ye, H. Lactobacillus paracasei Jlus66 extenuate oxidative stress and inflammation via regulation of intestinal flora in rats with non alcoholic fatty liver disease. Food Sci. Nutr. 2019, 7, 2636–2646. [Google Scholar] [CrossRef]
- Fulgencio, J.-P.; Kohl, C.; Girard, J.; Pégorier, J.-P. Effect of metformin on fatty acid and glucose metabolism in freshly isolated hepatocytes and on specific gene expression in cultured hepatocytes. Biochem. Pharmacol. 2001, 62, 439–446. [Google Scholar] [CrossRef]
- Zheng, J.; Woo, S.-L.; Hu, X.; Botchlett, R.; Chen, L.; Huo, Y.; Wu, C. Metformin and metabolic diseases: A focus on hepatic aspects. Front. Med. 2015, 9, 173–186. [Google Scholar] [CrossRef]
- Kumar, R.; Priyadarshi, R.N.; Anand, U. Non-alcoholic fatty liver disease: Growing burden, adverse outcomes and associations. J. Clin. Transl. Hepatol. 2020, 8, 76. [Google Scholar] [CrossRef]
- Negi, C.K.; Babica, P.; Bajard, L.; Bienertova-Vasku, J.; Tarantino, G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism 2022, 126, 154925. [Google Scholar] [CrossRef] [PubMed]
- Meroni, M.; Longo, M.; Dongiovanni, P. The role of probiotics in nonalcoholic fatty liver disease: A new insight into therapeutic strategies. Nutrients 2019, 11, 2642. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, K.; Kim, J.-Y.; Shim, J.-J.; Lim, J.; Kim, J.-Y.; Lee, J.-L. Lactobacillus helveticus Isolated from Raw Milk Improves Liver Function, Hepatic Steatosis, and Lipid Metabolism in Non-Alcoholic Fatty Liver Disease Mouse Model. Microorganisms 2023, 11, 2466. [Google Scholar] [CrossRef] [PubMed]
- Garrison, A.T.; Huigens, R.W., III. Eradicating bacterial biofilms with natural products and their inspired analogues that operate through unique mechanisms. Curr. Top. Med. Chem. 2017, 17, 1954–1964. [Google Scholar] [CrossRef]
- Dewulf, J.P.; Gerin, I.; Rider, M.H.; Veiga-da-Cunha, M.; Van Schaftingen, E.; Bommer, G.T. The synthesis of branched-chain fatty acids is limited by enzymatic decarboxylation of ethyl-and methylmalonyl-CoA. Biochem. J. 2019, 476, 2427–2447. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyoshi, H.; Yasui, K.; Harano, Y.; Endo, M.; Tsuji, K.; Minami, M.; Itoh, Y.; Okanoue, T.; Yoshikawa, T. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatol. Res. 2009, 39, 366–373. [Google Scholar] [CrossRef]
- Dorn, C.; Riener, M.-O.; Kirovski, G.; Saugspier, M.; Steib, K.; Weiss, T.S.; Gäbele, E.; Kristiansen, G.; Hartmann, A.; Hellerbrand, C. Expression of fatty acid synthase in nonalcoholic fatty liver disease. Int. J. Clin. Exp. Pathol. 2010, 3, 505. [Google Scholar]
- Matsusue, K.; Gavrilova, O.; Lambert, G.; Brewer, H.B., Jr.; Ward, J.M.; Inoue, Y.; LeRoith, D.; Gonzalez, F.J. Hepatic CCAAT/enhancer binding protein α mediates induction of lipogenesis and regulation of glucose homeostasis in leptin-deficient mice. Mol. Endocrinol. 2004, 18, 2751–2764. [Google Scholar] [CrossRef]
- Lakhani, H.V.; Sharma, D.; Dodrill, M.W.; Nawab, A.; Sharma, N.; Cottrill, C.L.; Shapiro, J.I.; Sodhi, K. Phenotypic alteration of hepatocytes in non-alcoholic fatty liver disease. Int. J. Med. Sci. 2018, 15, 1591. [Google Scholar] [CrossRef]
- Pang, L.; Liu, K.; Liu, D.; Lv, F.; Zang, Y.; Xie, F.; Yin, J.; Shi, Y.; Wang, Y.; Chen, D. Differential effects of reticulophagy and mitophagy on nonalcoholic fatty liver disease. Cell Death Dis. 2018, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Pinyopornpanish, K.; Leerapun, A.; Pinyopornpanish, K.; Chattipakorn, N. Effects of metformin on hepatic steatosis in adults with nonalcoholic fatty liver disease and diabetes: Insights from the cellular to patient levels. Gut Liver 2021, 15, 827. [Google Scholar] [CrossRef]
- Khosravi, S.; Alavian, S.M.; Zare, A.; Daryani, N.E.; Fereshtehnejad, S.-M.; Daryani, N.E.; Keramati, M.R.; Abdollahzade, S.; Taba Vakili, S.T. Non-alcoholic fatty liver disease and correlation of serum alanin aminotransferase level with histopathologic findings. Hepat. Mon. 2011, 11, 452. [Google Scholar] [PubMed]
- Ali, A.H.; Petroski, G.F.; Diaz-Arias, A.A.; Al Juboori, A.; Wheeler, A.A.; Ganga, R.R.; Pitt, J.B.; Spencer, N.M.; Hammoud, G.M.; Rector, R.S. A model incorporating serum alkaline phosphatase for prediction of liver fibrosis in adults with obesity and nonalcoholic fatty liver disease. J. Clin. Med. 2021, 10, 3311. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Huang, M.S.; Shyu, Y.C.; Chien, R.N. Gamma-glutamyl transpeptidase elevation is associated with metabolic syndrome, hepatic steatosis, and fibrosis in patients with nonalcoholic fatty liver disease: A community-based cross-sectional study. Kaohsiung J. Med. Sci. 2021, 37, 819–827. [Google Scholar] [CrossRef]
- Tu, L.N.; Showalter, M.R.; Cajka, T.; Fan, S.; Pillai, V.V.; Fiehn, O.; Selvaraj, V. Metabolomic characteristics of cholesterol-induced non-obese nonalcoholic fatty liver disease in mice. Sci. Rep. 2017, 7, 6120. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, H.S.; Cho, A.-R.; Lee, Y.-J.; Kwon, Y.-J. Non-alcoholic fatty liver disease is an independent risk factor for LDL cholesterol target level. Int. J. Environ. Res. Public Health 2021, 18, 3442. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Simental-Mendía, E.; Rodríguez-Hernández, H.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of triglycerides and glucose as biomarker for screening simple steatosis and NASH in asymptomatic women. Ann. Hepatol. 2017, 15, 715–720. [Google Scholar]
- Lee, N.Y.; Yoon, S.J.; Han, D.H.; Gupta, H.; Youn, G.S.; Shin, M.J.; Ham, Y.L.; Kwak, M.J.; Kim, B.Y.; Yu, J.S. Lactobacillus and Pediococcus ameliorate progression of non-alcoholic fatty liver disease through modulation of the gut microbiome. Gut Microbes 2020, 11, 882–899. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Singla, T.; Muneshwar, K.N.; Pathade, A.G.; Yelne, S. Hepatocytic Ballooning in Non-alcoholic Steatohepatitis: Bridging the Knowledge Gap and Charting Future Avenues. Cureus 2023, 15, e45884. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Cortez-Pinto, H. Cell death and nonalcoholic steatohepatitis: Where is ballooning relevant? Expert Rev. Gastroenterol. Hepatol. 2011, 5, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, M.; Yamasandhi, P.G. Nonalcoholic fatty liver disease and type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2018, 22, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Vancells Lujan, P.; Vinas Esmel, E.; Sacanella Meseguer, E. Overview of non-alcoholic fatty liver disease (NAFLD) and the role of sugary food consumption and other dietary components in its development. Nutrients 2021, 13, 1442. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Basaranoglu, G.; Bugianesi, E. Carbohydrate intake and nonalcoholic fatty liver disease: Fructose as a weapon of mass destruction. Hepatobiliary Surg. Nutr. 2015, 4, 109. [Google Scholar]
- Gowda, D.; Shekhar, C.; Gowda, S.G.B.; Chen, Y.; Hui, S.P. Crosstalk between Lipids and Non-Alcoholic Fatty Liver Disease. Livers 2023, 3, 687–708. [Google Scholar] [CrossRef]

| Features | Terms |
|---|---|
| Sequencing platforms | Illumina MiSeq PacBio RSII |
| Libraries used | TruSeq DNA Library LT Kit SMRTbell® Prep Kit |
| Genome size (bp) | 2,877,365 |
| G+C contents (%) | 46.43 |
| rRNA genes | 15 |
| tRNA genes | 58 |
| Gene | Gene Name | Catalog Number |
|---|---|---|
| In vitro | ||
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Hs99999905_m1 |
| SREBP-1c | Sterol regulatory element-binding protein 1 | Hs01088691_m1 |
| FASN | Fatty acid synthase | Hs00188012_m1 |
| C/EBPα | CCAAT/enhancer-binding protein alpha | Hs00269972_s1 |
| BAX | BCL2 associated X, apoptosis regulator | Hs00180269_m1 |
| Bcl-2 | BCL2, apoptosis regulator | Hs04986394_s1 |
| CASP3 | Caspase 3 | Hs00234387_m1 |
| CASP9 | Caspase 9 | Hs00962278_m1 |
| In vivo | ||
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | Mm99999915_g1 |
| Srebp-1c | Sterol regulatory element-binding protein 1 | Mm00550338_m1 |
| Fasn | Fatty acid synthase | Mm00433237_m1 |
| C/ebpα | CCAAT/enhancer-binding protein alpha | Mm00514283_m1 |
| Pparγ | Peroxisome proliferator-activated receptor gamma | Mm00440945_m1 |
| Acaca | Acetyl-CoA carboxylase alpha | Mm01304257_m1 |
| Tnf | Tumor necrosis factor | Mm00443258_m1 |
| Il-1β | Interleukin 1 beta | Mm00434228_m1 |
| Ccl2 | C-C motif chemokine ligand 2 | Mm00441242_m1 |
| Col1a1 | Collagen type I alpha 1 | Mm00801666_g1 |
| Tgf- β1 | Transforming growth factor beta 1 | Mm01178820_m1 |
| Timp1 | tissue inhibitor of metalloproteinase 1 | Mm01341361_m1 |
| Bax | BCL2 associated X, apoptosis regulator | Mm00432051_m1 |
| Bcl-2 | BCL2, apoptosis regulator | Mm00477631_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Jeon, H.-J.; Kim, D.-G.; Kim, J.-Y.; Shim, J.-J.; Lee, J.-H. Lacticaseibacillus paracsei HY7207 Alleviates Hepatic Steatosis, Inflammation, and Liver Fibrosis in Mice with Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2024, 25, 9870. https://doi.org/10.3390/ijms25189870
Kim H-J, Jeon H-J, Kim D-G, Kim J-Y, Shim J-J, Lee J-H. Lacticaseibacillus paracsei HY7207 Alleviates Hepatic Steatosis, Inflammation, and Liver Fibrosis in Mice with Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2024; 25(18):9870. https://doi.org/10.3390/ijms25189870
Chicago/Turabian StyleKim, Hyeon-Ji, Hye-Jin Jeon, Dong-Gun Kim, Joo-Yun Kim, Jae-Jung Shim, and Jae-Hwan Lee. 2024. "Lacticaseibacillus paracsei HY7207 Alleviates Hepatic Steatosis, Inflammation, and Liver Fibrosis in Mice with Non-Alcoholic Fatty Liver Disease" International Journal of Molecular Sciences 25, no. 18: 9870. https://doi.org/10.3390/ijms25189870
APA StyleKim, H.-J., Jeon, H.-J., Kim, D.-G., Kim, J.-Y., Shim, J.-J., & Lee, J.-H. (2024). Lacticaseibacillus paracsei HY7207 Alleviates Hepatic Steatosis, Inflammation, and Liver Fibrosis in Mice with Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences, 25(18), 9870. https://doi.org/10.3390/ijms25189870






