Exposure of Colon-Derived Epithelial Monolayers to Fecal Luminal Factors from Patients with Colon Cancer and Ulcerative Colitis Results in Distinct Gene Expression Patterns
Abstract
1. Introduction
2. Results
2.1. Study Subjects, Study Samples, and Assessment of Transepithelial Electrical Resistance (TEER) in the Colon-Derived Two-Dimensional Epithelial Monolayers
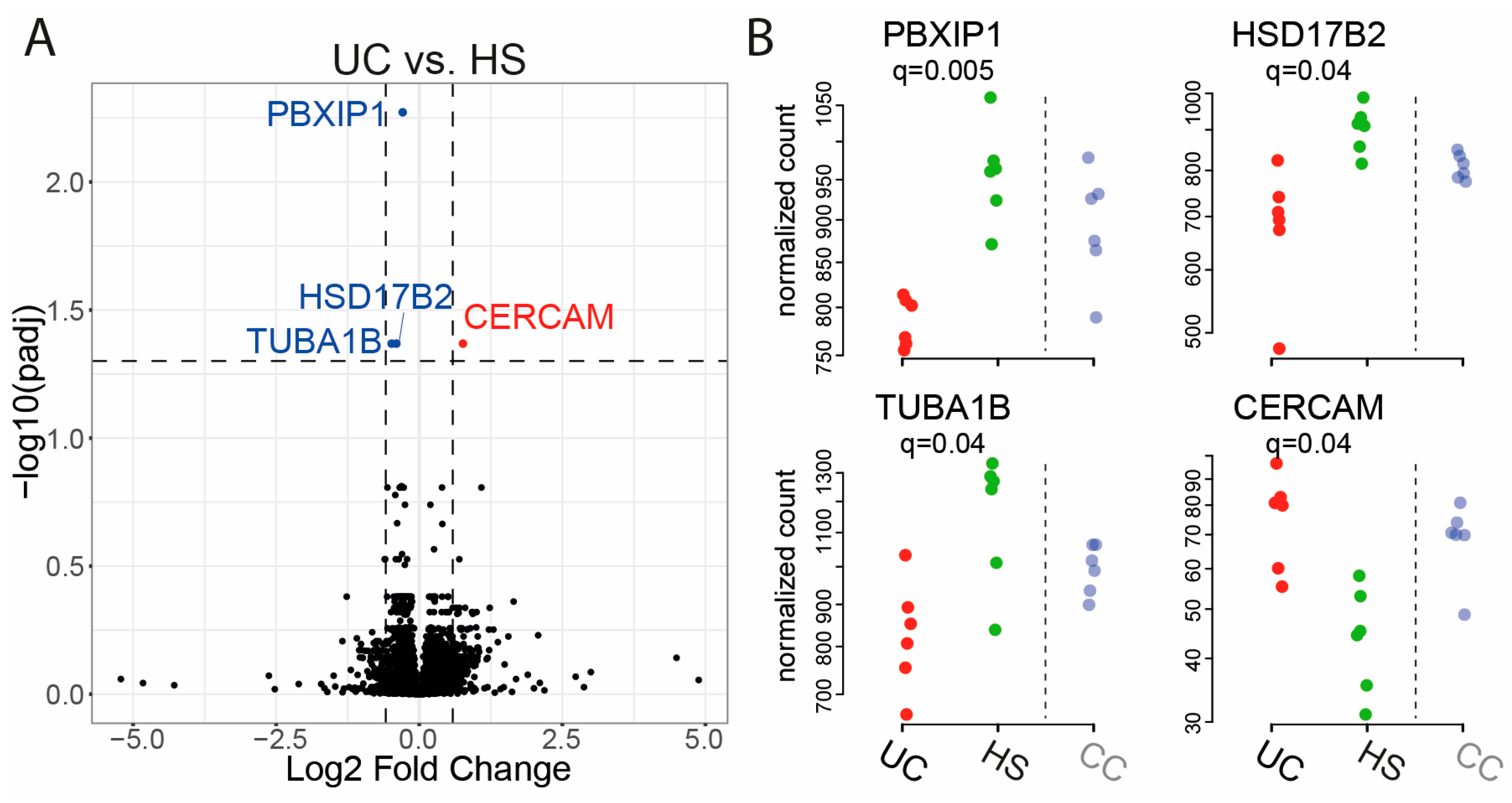
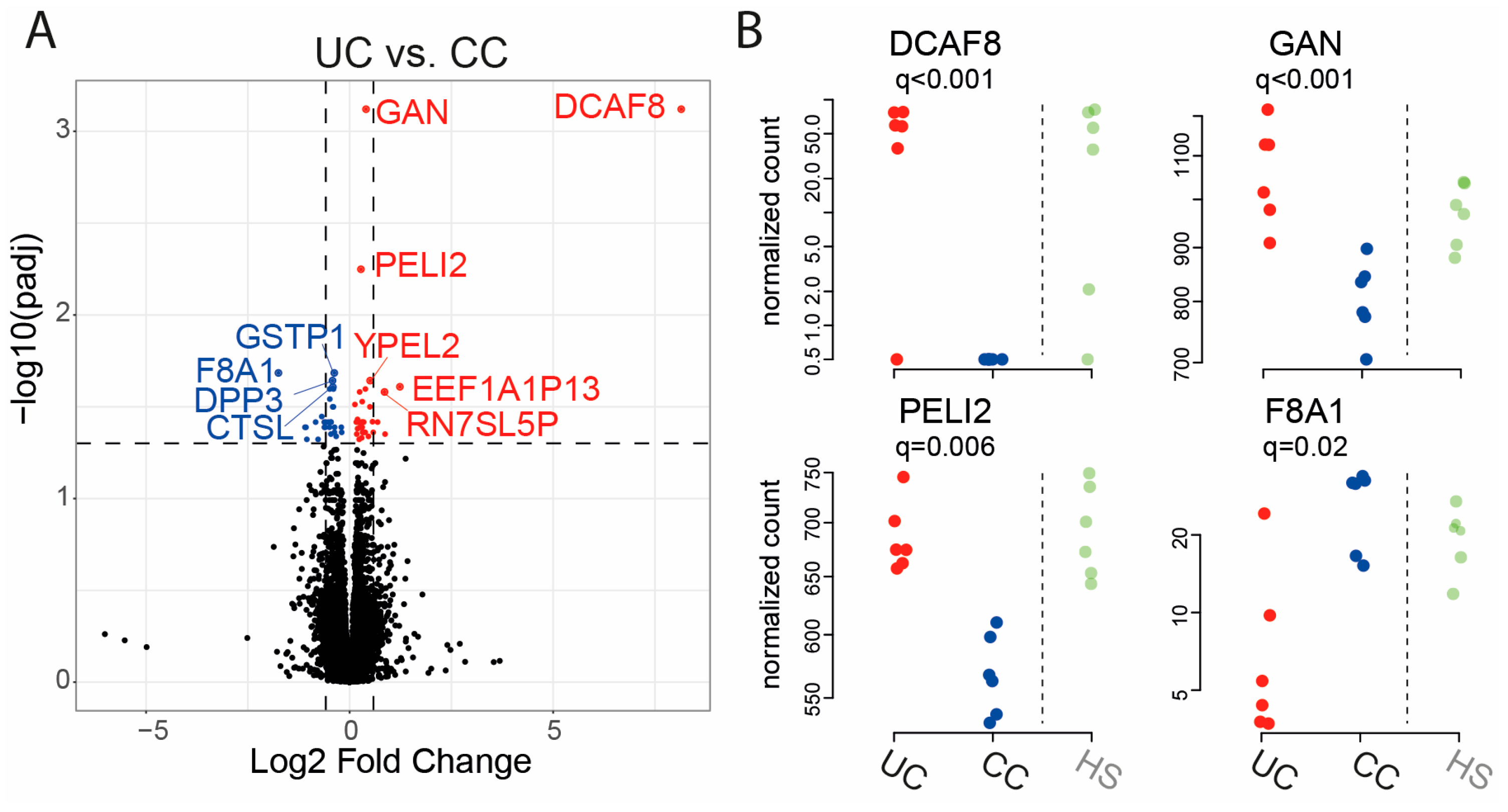
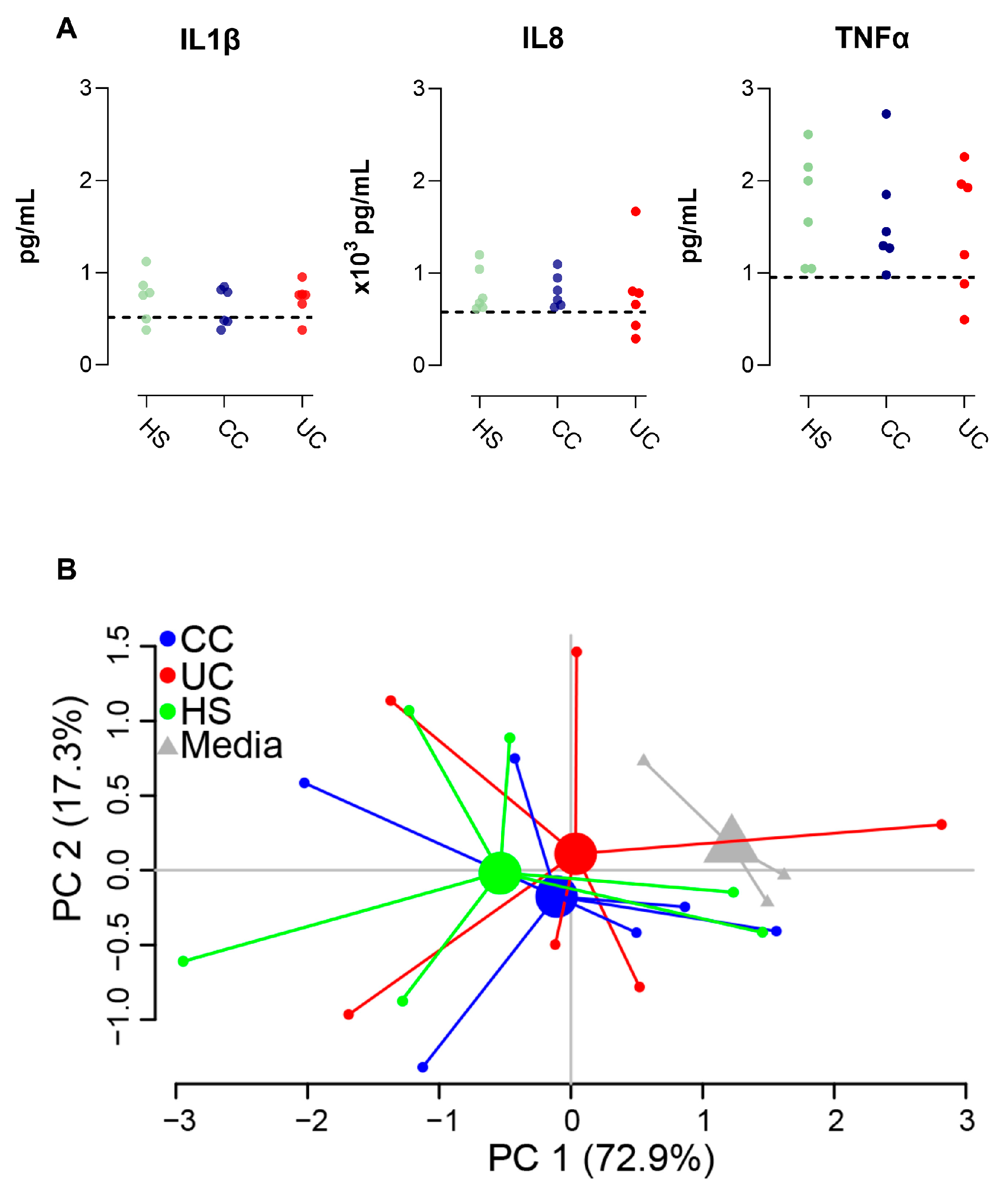
2.2. Gene Expression in Epithelial Monolayer Is Altered by Fecal Supernatants (FS) and Linked to Each Patient Group
2.3. Epithelial Monolayer Secretion of IL8 Is Altered by Fecal Supernatants
3. Discussion
4. Materials and Methods
4.1. Study Subjects and Sample Collection
4.2. Preparation of Fecal Supernatants
4.3. Liquid Chromatography−Mass Spectrometry
4.4. Stimulation of Colonoid Monolayers with Fecal Supernatants
4.5. Immunofluorescence and Imaging
4.6. Gene Expression Analysis
4.7. Cytokine Analysis
4.8. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial genes and pathways in inflammatory bowel disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef]
- Lechuga, S.; Braga-Neto, M.B.; Naydenov, N.G.; Rieder, F.; Ivanov, A.I. Understanding disruption of the gut barrier during inflammation: Should we abandon traditional epithelial cell lines and switch to intestinal organoids? Front. Immunol. 2023, 14, 1108289. [Google Scholar] [CrossRef] [PubMed]
- Ternet, C.; Kiel, C. Signaling pathways in intestinal homeostasis and colorectal cancer: KRAS at centre stage. Cell Commun. Signal 2021, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Genua, F.; Raghunathan, V.; Jenab, M.; Gallagher, W.M.; Hughes, D.J. The Role of Gut Barrier Dysfunction and Microbiome Dysbiosis in Colorectal Cancer Development. Front. Oncol. 2021, 11, 626349. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Khambhati, K.; Gohil, N.; Singh, P.; Gohil, J.; Gautam, H.; Maurya, R.; Chu, D.-T.; Ramakrishna, S.; Singh, V. Chapter Five-Gut microbiota in gastrointestinal diseases. In Progress in Molecular Biology and Translational Science; Das, B., Singh, V., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 191, pp. 141–151. [Google Scholar]
- Colgan, S.P.; Wang, R.X.; Hall, C.H.T.; Bhagavatula, G.; Lee, J.S. Revisiting the “starved gut” hypothesis in inflammatory bowel disease. Immunometabolism 2023, 5, e0016. [Google Scholar] [CrossRef]
- Lopez, L.R.; Ahn, J.H.; Alves, T.; Arthur, J.C. Microenvironmental factors that shape bacterial metabolites in inflammatory bowel disease. Front. Cell Infect. Microbiol. 2022, 12, 934619. [Google Scholar] [CrossRef]
- Dorrestein, P.C.; Mazmanian, S.K.; Knight, R. Finding the missing links among metabolites, microbes and the host. Immunity 2014, 40, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Speer, J.E.; Wang, Y.; Fallon, J.K.; Smith, P.C.; Allbritton, N.L. Evaluation of human primary intestinal monolayers for drug metabolizing capabilities. J. Biol. Eng. 2019, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; DiSalvo, M.; Gunasekara, D.B.; Dutton, J.; Proctor, A.; Lebhar, M.S.; Williamson, I.A.; Speer, J.; Howard, R.L.; Smiddy, N.M.; et al. Self-renewing monolayer of primary colonic or rectal epithelial cells. Cell Mol. Gastroenterol. Hepatol. 2017, 4, 165–182. [Google Scholar] [CrossRef]
- Holst, L.M.; Iribarren, C.; Sapnara, M.; Savolainen, O.; Tornblom, H.; Wettergren, Y.; Strid, H.; Simren, M.; Magnusson, M.K.; Ohman, L. Fecal Luminal factors from patients with gastrointestinal diseases alter gene expression profiles in Caco-2 cells and colonoids. Int. J. Mol. Sci. 2022, 23, 15505. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Nordlander, S.; Sundin, J.; Isaksson, S.; Savolainen, O.; Tornblom, H.; Magnusson, M.K.; Simren, M.; Ohman, L. Fecal luminal factors from patients with irritable bowel syndrome induce distinct gene expression of colonoids. Neurogastroenterol. Motil. 2022, 34, e14390. [Google Scholar] [CrossRef] [PubMed]
- Flood, P.; Hanrahan, N.; Nally, K.; Melgar, S. Human intestinal organoids: Modeling gastrointestinal physiology and immunopathology-current applications and limitations. Eur. J. Immunol. 2024, 54, e2250248. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Saeterstad, S.; Ostvik, A.E.; Royset, E.S.; Bakke, I.; Sandvik, A.K.; Granlund, A.V.B. Profound gene expression changes in the epithelial monolayer of active ulcerative colitis and Crohn’s disease. PLoS ONE 2022, 17, e0265189. [Google Scholar] [CrossRef]
- Asghari Alashti, F.; Goliaei, B.; Minuchehr, Z. Analyzing large scale gene expression data in colorectal cancer reveals important clues; CLCA1 and SELENBP1 downregulated in CRC not in normal and not in adenoma. Am. J. Cancer Res. 2022, 12, 371–380. [Google Scholar]
- Huang, D.; Liu, C.; Sun, X.; Sun, X.; Qu, Y.; Tang, Y.; Li, G.; Tong, T. CRL4(DCAF8) and USP11 oppositely regulate the stability of myeloid leukemia factors (MLFs). Biochem. Biophys. Res. Commun. 2020, 529, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Humphries, F.; Bergin, R.; Jackson, R.; Delagic, N.; Wang, B.; Yang, S.; Dubois, A.V.; Ingram, R.J.; Moynagh, P.N. The E3 ubiquitin ligase Pellino2 mediates priming of the NLRP3 inflammasome. Nat. Commun. 2018, 9, 1560. [Google Scholar] [CrossRef] [PubMed]
- Davidson, G.; Shen, J.; Huang, Y.L.; Su, Y.; Karaulanov, E.; Bartscherer, K.; Hassler, C.; Stannek, P.; Boutros, M.; Niehrs, C. Cell cycle control of wnt receptor activation. Dev. Cell. 2009, 17, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Khumukcham, S.S.; Manavathi, B. Two decades of a protooncogene HPIP/PBXIP1: Uncovering the tale from germ cell to cancer. Biochim. Biophys. Acta Rev. Cancer. 2021, 1876, 188576. [Google Scholar] [CrossRef] [PubMed]
- Vihko, P.; Isomaa, V.; Ghosh, D. Structure and function of 17beta-hydroxysteroid dehydrogenase type 1 and type 2. Mol. Cell Endocrinol. 2001, 171, 71–76. [Google Scholar] [CrossRef]
- Drzewiecka, H.; Jarmolowska-Jurczyszyn, D.; Kluk, A.; Galecki, B.; Dyszkiewicz, W.; Jagodzinski, P.P. Altered expression of 17-beta-hydroxysteroid dehydrogenase type 2 and its prognostic significance in non-small cell lung cancer. Int. J. Oncol. 2020, 56, 1352–1372. [Google Scholar] [CrossRef]
- Hu, J.; Han, C.; Zhong, J.; Liu, H.; Liu, R.; Luo, W.; Chen, P.; Ling, F. Dynamic network biomarker of pre-exhausted CD8(+) T cells Contributed to T cell exhaustion in colorectal Cancer. Front. Immunol. 2021, 12, 691142. [Google Scholar] [CrossRef]
- Pardini, B.; Ferrero, G.; Tarallo, S.; Gallo, G.; Francavilla, A.; Licheri, N.; Trompetto, M.; Clerico, G.; Senore, C.; Peyre, S.; et al. A fecal microRNA signature by small RNA sequencing accurately distinguishes colorectal cancers: Results from a multicenter study. Gastroenterology 2023, 165, 582–599.e588. [Google Scholar] [CrossRef]
- Kanaan, Z.; Roberts, H.; Eichenberger, M.R.; Billeter, A.; Ocheretner, G.; Pan, J.; Rai, S.N.; Jorden, J.; Williford, A.; Galandiuk, S. A Plasma microRNA Panel for detection of colorectal adenomas: A step toward more precise screening for colorectal cancer. Ann. Surg. 2013, 258, 400–408. [Google Scholar] [CrossRef]
- Le Gall, G.; Guttula, K.; Kellingray, L.; Tett, A.J.; Ten Hoopen, R.; Kemsley, E.K.; Savva, G.M.; Ibrahim, A.; Narbad, A. Metabolite quantification of faecal extracts from colorectal cancer patients and healthy controls. Oncotarget 2018, 9, 33278–33289. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Wang, L. Systematic review of metabolomic alterations in ulcerative colitis: Unveiling key metabolic signatures and pathways. Ther. Adv. Gastroenterol. 2024, 17, 17562848241239580. [Google Scholar] [CrossRef] [PubMed]
- Heidarian, F.; Alebouyeh, M.; Shahrokh, S.; Balaii, H.; Zali, M.R. Altered fecal bacterial composition correlates with disease activity in inflammatory bowel disease and the extent of IL8 induction. Curr. Res. Transl. Med. 2019, 67, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar]
- Iribarren, C.; Savolainen, O.; Sapnara, M.; Tornblom, H.; Simren, M.; Magnusson, M.K.; Ohman, L. Temporal stability of fecal metabolomic profiles in irritable bowel syndrome. Neurogastroenterol. Motil. 2024, 36, e14741. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Brierley, J.; Byrd, D.; Bosman, F.; Kehoe, S.; Kossary, C.; Piñeros, M.; Van Eycken, E.; Weir, H.K.; Gospodarowicz, M. The TNM classification of malignant tumours-towards common understanding and reasonable expectations. Lancet Oncol. 2017, 18, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Sturm, A.; Maaser, C.; Calabrese, E.; Annese, V.; Fiorino, G.; Kucharzik, T.; Vavricka, S.R.; Verstockt, B.; van Rheenen, P.; Tolan, D.; et al. ECCO-ESGAR guideline for diagnostic assessment in IBD Part 2: IBD scores and general principles and technical aspects. J. Crohns Colitis. 2019, 13, 273–284. [Google Scholar] [CrossRef]
- Zheng, R.; Brunius, C.; Shi, L.; Zafar, H.; Paulson, L.; Landberg, R.; Naluai, Å.T. Prediction and evaluation of the effect of pre-centrifugation sample management on the measurable untargeted LC-MS plasma metabolome. Anal. Chim. Acta 2021, 1182, 338968. [Google Scholar] [CrossRef]
- Stekhoven, D.J.; Bühlmann, P. MissForest--non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef]
- Wingett, S.W.; Andrews, S. FastQ Screen: A tool for multi-genome mapping and quality control. F1000Research 2018, 7, 1338. [Google Scholar] [CrossRef]
- Ewels, P.A.; Peltzer, A.; Fillinger, S.; Patel, H.; Alneberg, J.; Wilm, A.; Garcia, M.U.; Di Tommaso, P.; Nahnsen, S. The nf-core framework for community-curated bioinformatics pipelines. Nat. Biotechnol. 2020, 38, 276–278. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate-a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computin; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 21 April 2023).
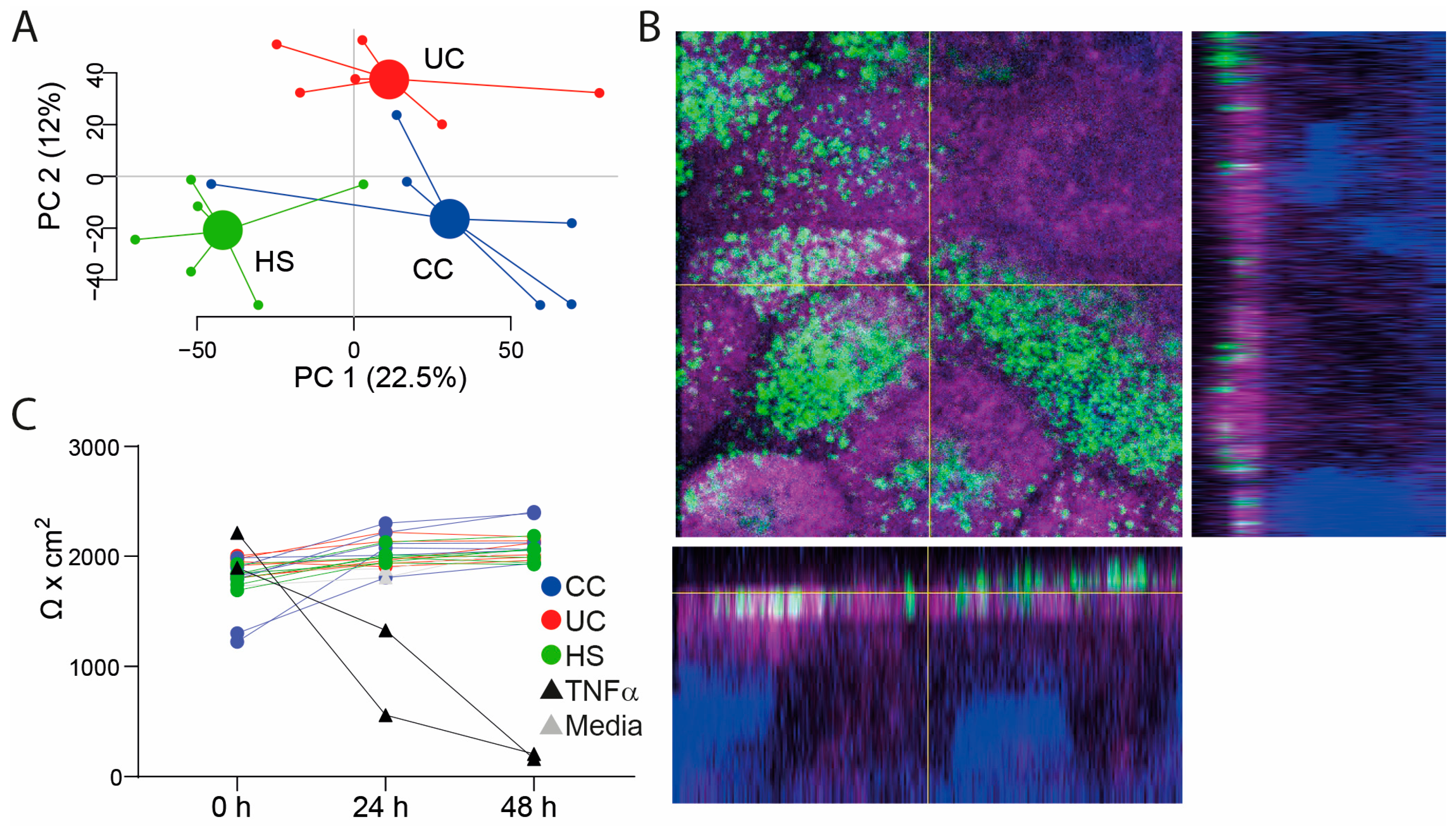
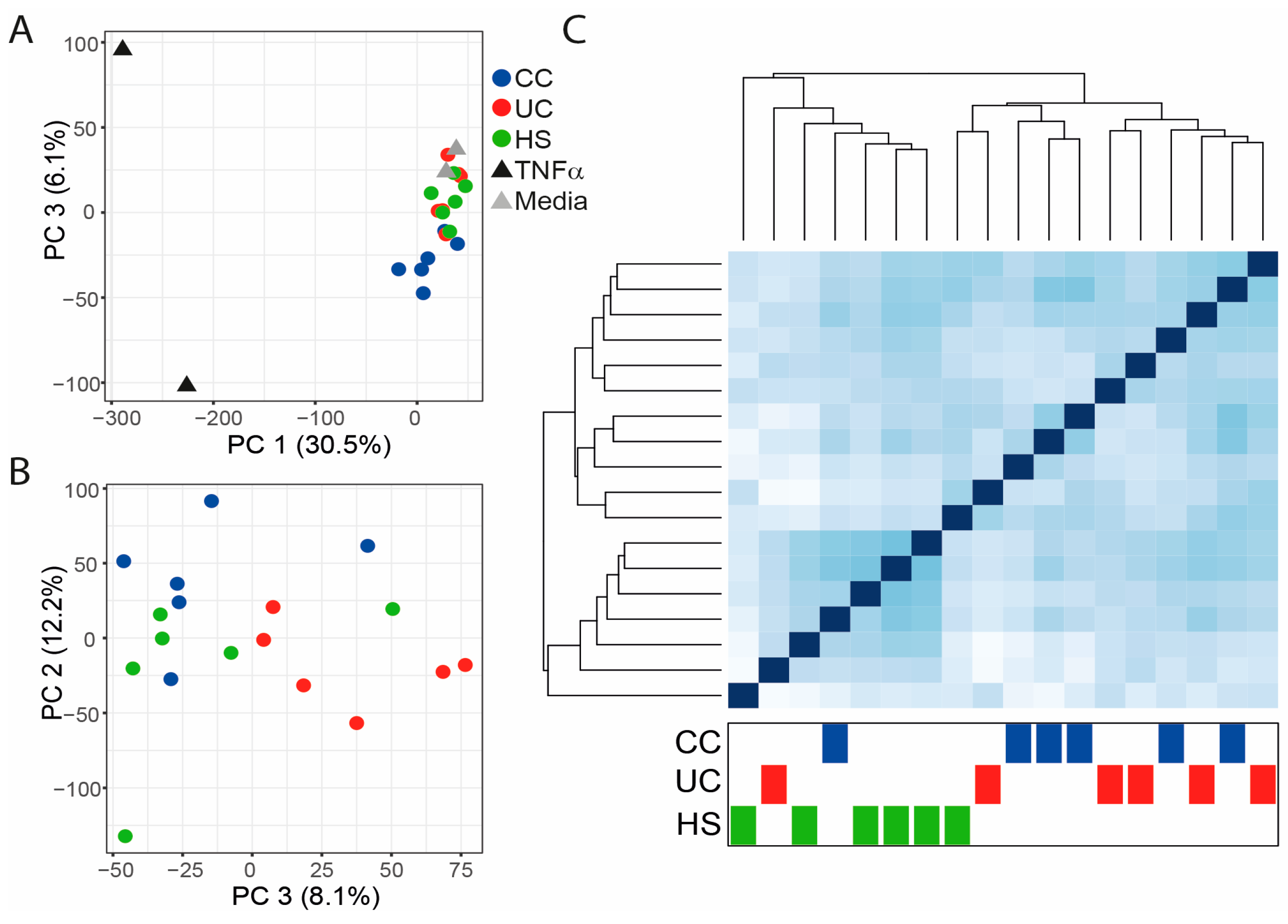

| Ulcerative Colitis (n = 6) | Colon Cancer (n = 6) | Healthy (n = 6) | |
|---|---|---|---|
| Age, median (range) | 49 (40–67) | 81 (68–91) | 32 (25–44) |
| Sex, female/male | 3/3 | 3/3 | 2/4 |
| Mayo score, median (range) | 9 (6–11) | N/A | N/A |
| Tumor stage 1, I/II/III/IV | N/A | 2/1/2/1 | N/A |
| Gene Name | Fold Change | q-Value (CC vs. HS) |
|---|---|---|
| CCNY | −0.3857 | 0.006 |
| CNST | −0.3386 | 0.033 |
| DCAF8 | −7.8589 | 0.003 |
| FAR1 | 0.3316 | 0.018 |
| FGFR2 | −0.6135 | 0.018 |
| FLRT3 | 0.9376 | 0.031 |
| GLDC | 0.6479 | 0.033 |
| GOPC | 0.2851 | 0.023 |
| KCNK5 | −0.3865 | 0.042 |
| LRP6 | −0.2310 | 0.006 |
| MBD1 | −0.3579 | 0.027 |
| MPZL3 | −0.3737 | 0.037 |
| NABP1 | 0.3126 | 0.019 |
| PCK1 | −1.0437 | 0.018 |
| PELI2 | −0.2855 | 0.004 |
| PNCK | −0.3762 | 0.037 |
| PPAP2B | −0.4411 | 0.023 |
| PTK2B | −0.1826 | 0.045 |
| RIN2 | −0.2263 | 0.026 |
| VEGFA | −0.3487 | 0.047 |
| YPEL2 | −0.5200 | 0.016 |
| ZFYVE27 | −0.2907 | 0.019 |
| Gene Name | Fold Change | q-Value (UC vs. HS) |
|---|---|---|
| CERCAM | 0.7624 | 0.043 |
| HSD17B2 | −0.4005 | 0.043 |
| PBXIP1 | −0.2895 | 0.005 |
| TUBA1B | −0.4825 | 0.043 |
| Gene Name | Fold Change | q-Value (UC vs. CC) |
|---|---|---|
| AL139819.1 | 0.3827 | 0.044 |
| ALDH1A3 | −0.5806 | 0.038 |
| APLF | −0.7745 | 0.048 |
| AVL9 | 0.1957 | 0.038 |
| CCL2 | −1.0974 | 0.041 |
| CCNY | 0.3068 | 0.041 |
| CD63 | −0.1948 | 0.044 |
| CHD4 | −0.2044 | 0.041 |
| CLIP1 | 0.1637 | 0.038 |
| CLN8 | 0.4625 | 0.046 |
| CRHR1-IT1 | 0.4987 | 0.032 |
| CTSL | −0.4901 | 0.025 |
| DCAF8 | 8.1459 | 0.001 |
| DNAJC11 | −0.4601 | 0.045 |
| DNAJC3 | 0.1745 | 0.045 |
| DPP3 | −0.4107 | 0.025 |
| DYNC1LI1 | −0.5180 | 0.038 |
| EDIL3 | −1.0717 | 0.041 |
| EEF1A1P13 | 1.2351 | 0.025 |
| ERN1 | 0.2983 | 0.041 |
| F8A1 | −1.7489 | 0.021 |
| FUCA2 | −0.3988 | 0.032 |
| GAN | 0.4025 | 0.001 |
| GBA | −0.3971 | 0.025 |
| GLDC | −0.6223 | 0.038 |
| GREM1 | −0.8350 | 0.038 |
| GSTP1 | −0.3766 | 0.021 |
| HPGD | −0.3718 | 0.044 |
| INHBA | −1.0509 | 0.048 |
| INSIG1 | 0.2298 | 0.038 |
| KANSL1 | 0.2560 | 0.041 |
| KLF7 | 0.2948 | 0.038 |
| KLHL28 | 0.3201 | 0.044 |
| LRIG1 | 0.5803 | 0.044 |
| LRP6 | 0.1937 | 0.037 |
| LXN | −0.4863 | 0.029 |
| MLLT4 | 0.1303 | 0.031 |
| MLXIPL | 0.6879 | 0.038 |
| MSL1 | 0.2348 | 0.048 |
| NCL | −0.4204 | 0.023 |
| NEAT1 | 0.5613 | 0.038 |
| PELI2 | 0.2772 | 0.006 |
| PLA2G4C | −0.5980 | 0.041 |
| PPP1R15B | 0.2462 | 0.026 |
| PRAP1 | −0.4953 | 0.041 |
| PTGES | −0.6840 | 0.036 |
| RAPGEFL1 | 0.3226 | 0.044 |
| RN7SL5P | 0.8572 | 0.026 |
| RP11-34P13.13 | 0.8704 | 0.045 |
| RP11-395B7.2 | 0.3212 | 0.038 |
| SEPP1 | −0.4565 | 0.038 |
| SH3D21 | 0.3871 | 0.025 |
| SLC25A25 | 0.1949 | 0.042 |
| SQSTM1 | −0.3686 | 0.041 |
| TM4SF20 | −0.4150 | 0.032 |
| TMEM176A | −0.4591 | 0.038 |
| TMEM176B | −0.3301 | 0.046 |
| TRIB3 | 0.3095 | 0.030 |
| VEGFA | 0.3470 | 0.038 |
| YPEL2 | 0.5035 | 0.023 |
| ZNF330 | −0.6198 | 0.041 |
| ZNF488 | 0.2957 | 0.047 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnusson, M.K.; Bas Forsberg, A.; Verveda, A.; Sapnara, M.; Lorent, J.; Savolainen, O.; Wettergren, Y.; Strid, H.; Simrén, M.; Öhman, L. Exposure of Colon-Derived Epithelial Monolayers to Fecal Luminal Factors from Patients with Colon Cancer and Ulcerative Colitis Results in Distinct Gene Expression Patterns. Int. J. Mol. Sci. 2024, 25, 9886. https://doi.org/10.3390/ijms25189886
Magnusson MK, Bas Forsberg A, Verveda A, Sapnara M, Lorent J, Savolainen O, Wettergren Y, Strid H, Simrén M, Öhman L. Exposure of Colon-Derived Epithelial Monolayers to Fecal Luminal Factors from Patients with Colon Cancer and Ulcerative Colitis Results in Distinct Gene Expression Patterns. International Journal of Molecular Sciences. 2024; 25(18):9886. https://doi.org/10.3390/ijms25189886
Chicago/Turabian StyleMagnusson, Maria K., Anna Bas Forsberg, Alexandra Verveda, Maria Sapnara, Julie Lorent, Otto Savolainen, Yvonne Wettergren, Hans Strid, Magnus Simrén, and Lena Öhman. 2024. "Exposure of Colon-Derived Epithelial Monolayers to Fecal Luminal Factors from Patients with Colon Cancer and Ulcerative Colitis Results in Distinct Gene Expression Patterns" International Journal of Molecular Sciences 25, no. 18: 9886. https://doi.org/10.3390/ijms25189886
APA StyleMagnusson, M. K., Bas Forsberg, A., Verveda, A., Sapnara, M., Lorent, J., Savolainen, O., Wettergren, Y., Strid, H., Simrén, M., & Öhman, L. (2024). Exposure of Colon-Derived Epithelial Monolayers to Fecal Luminal Factors from Patients with Colon Cancer and Ulcerative Colitis Results in Distinct Gene Expression Patterns. International Journal of Molecular Sciences, 25(18), 9886. https://doi.org/10.3390/ijms25189886






