NMR-Based Metabolomic Analysis of Biotic Stress Responses in the Traditional Korean Landrace Red Pepper (Capsicum annuum var. annuum, cv. Subicho)
Abstract
:1. Introduction
2. Results and Discussion
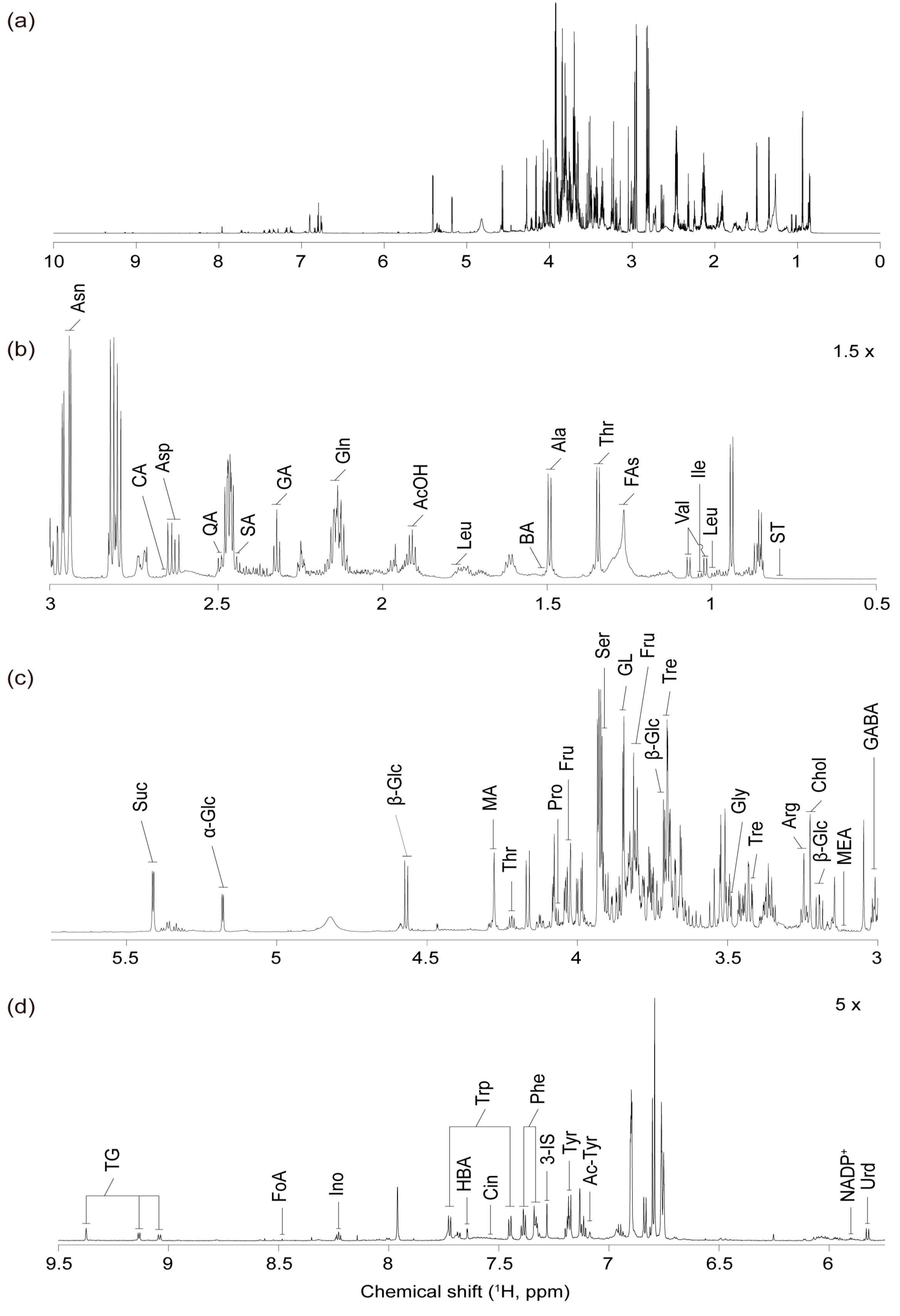
2.1. Identification of Metabolites in Red Pepper Extracts Using 1D and 2D NMR Spectroscopy
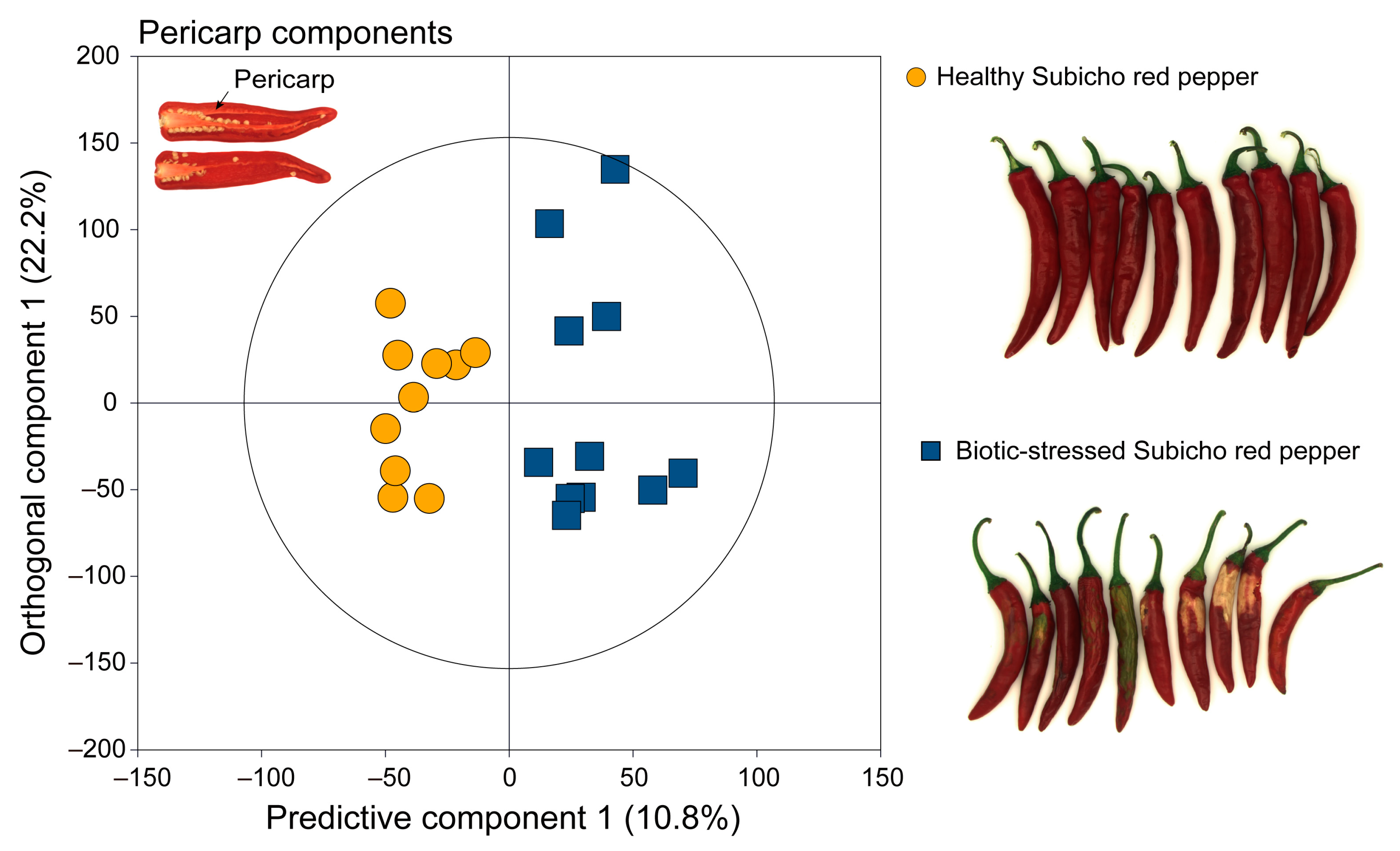
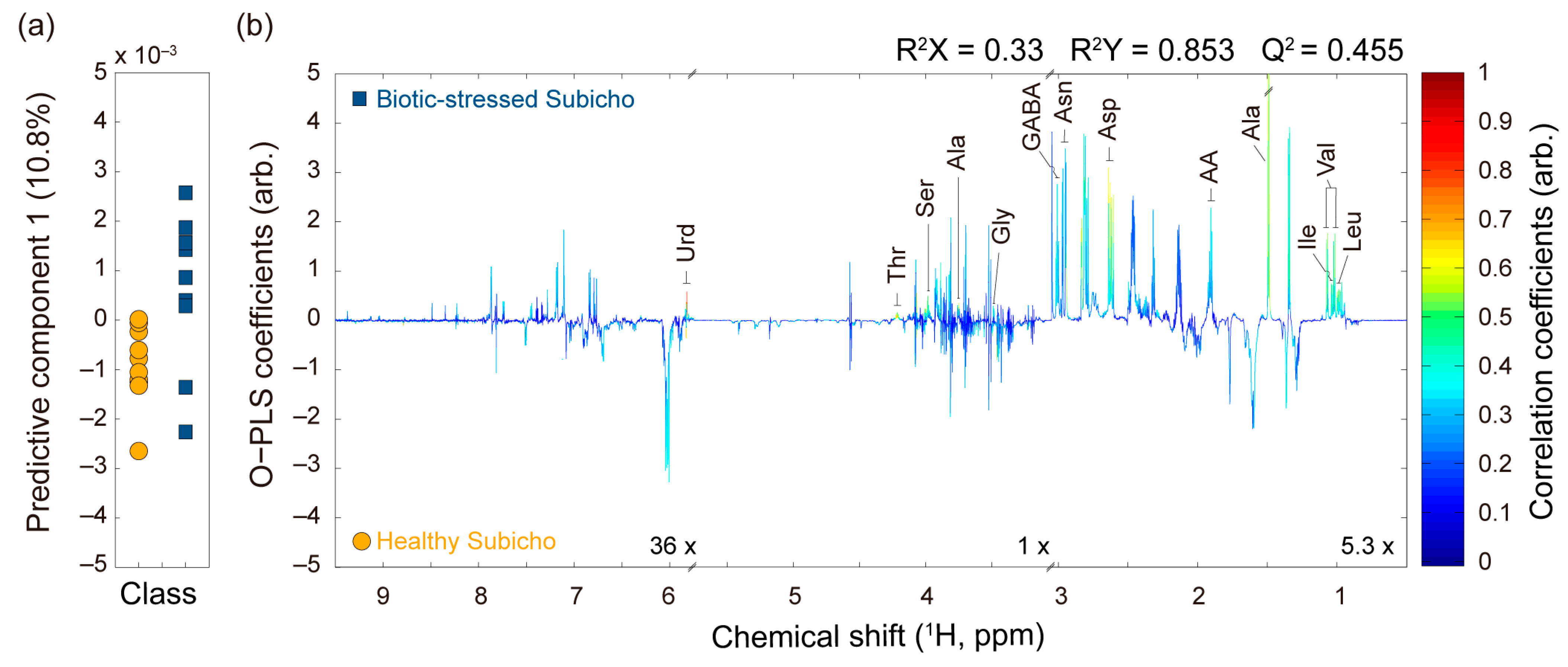
2.2. Multivariate Statistical Analysis of the 1H NMR Spectra
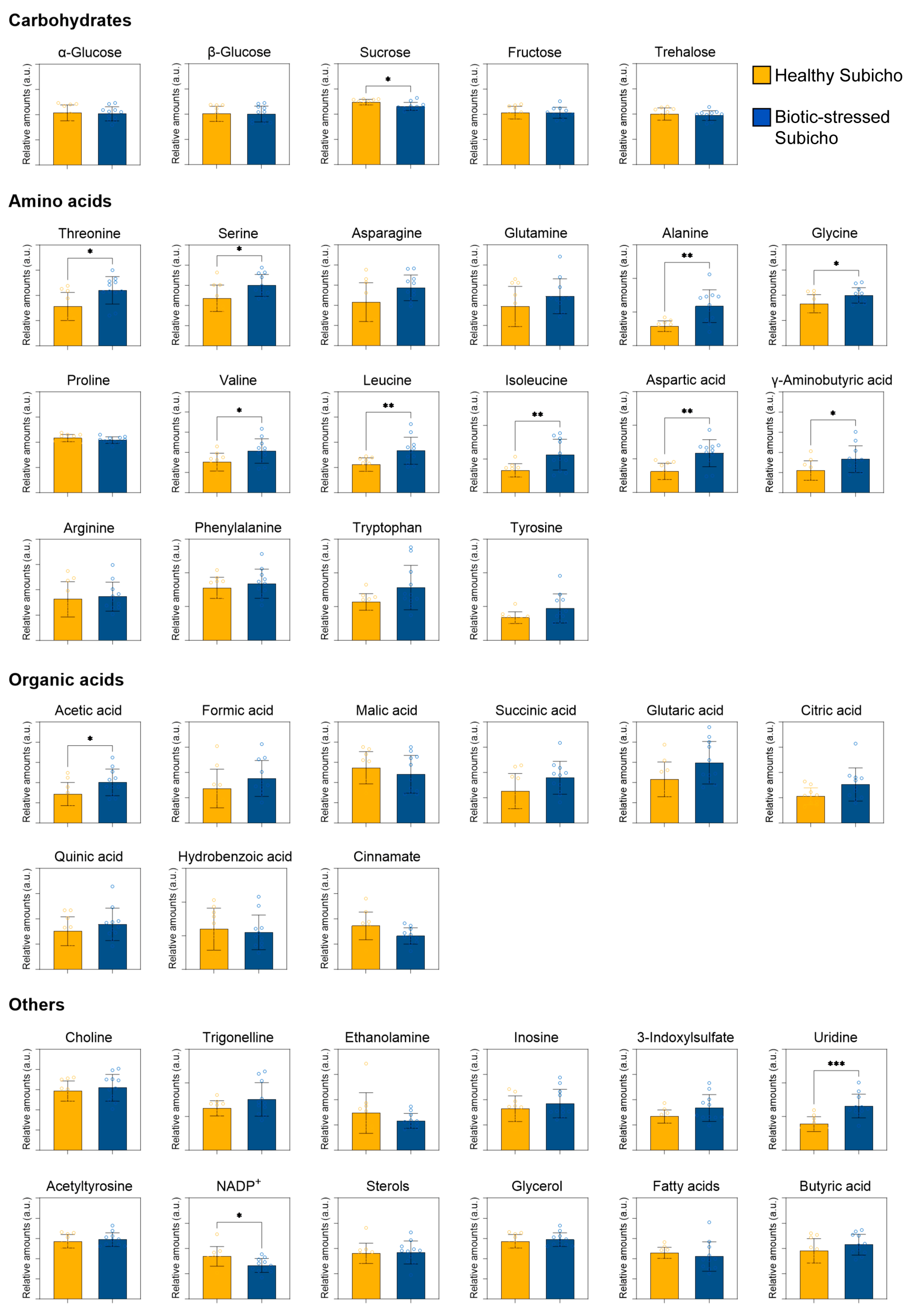
2.3. Metabolomics in Red Peppers Using 1H NMR Spectroscopy
3. Materials and Methods
3.1. Plant Source and Sample Preparation
3.2. 1H NMR Spectroscopic Analysis of the Red Pepper Extracts
3.3. NMR Data Processing and Multivariate Statistical Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duranova, H.; Valkova, V.; Gabriny, L. Chili peppers (Capsicum spp.): The spice not only for cuisine purposes: An update on current knowledge. Phytochem. Rev. 2022, 21, 1379–1413. [Google Scholar] [CrossRef]
- Zou, Z.; Zou, X. Geographical and ecological differences in pepper cultivation and consumption in China. Front. Nutr. 2021, 8, 718517. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.; Ponnam, N.; Reddy, A.C.; Reddy, D.C.L.; Saha, K.; Acharya, G.C.; Reddy, K.M. Breeding peppers for industrial uses: Progress and prospects. Ind. Crop. Prod. 2022, 178, 114626. [Google Scholar] [CrossRef]
- Jang, H.; Choi, M.; Jang, K.-S. Comprehensive phytochemical profiles and antioxidant activity of Korean local cultivars of red chili pepper (Capsicum annuum L.). Front. Plant Sci. 2024, 15, 1333035. [Google Scholar] [CrossRef]
- Yun, S.; Kim, H. Insight into the phylogenetic relationships and evolutionary history of pepper cultivars (Capsicum annuum L.) through comparative analyses of plastomes. Horticulturae 2023, 9, 1092. [Google Scholar] [CrossRef]
- Jung, S.-J.; Chae, S.-W.; Shin, D.-H. Fermented foods of Korea and their functionalities. Fermentation 2022, 8, 645. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Mujumdar, A.S.; Liu, Y. Recent developments in key processing techniques for oriental spices/herbs and condiments: A review. Food Rev. Int. 2022, 38, 1791–1811. [Google Scholar] [CrossRef]
- Ridzuan, R.; Rafii, M.Y.; Ismail, S.I.; Mohammad Yusoff, M.; Miah, G.; Usman, M. Breeding for anthracnose disease resistance in chili: Progress and prospects. Int. J. Mol. Sci. 2018, 19, 3122. [Google Scholar] [CrossRef]
- Parisi, M.; Alioto, D.; Tripodi, P. Overview of biotic stresses in pepper (Capsicum spp.): Sources of genetic resistance, molecular breeding and genomics. Int. J. Mol. Sci. 2020, 21, 2587. [Google Scholar] [CrossRef]
- Po, L.G.; Siddiq, M.; Shahzad, T. Chili, peppers, and paprika. In Handbook of Vegetables and Vegetable Processing; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 633–660. [Google Scholar]
- Jalgaonkar, K.; Mahawar, M.K.; Girijal, S.; Hp, G. Post-harvest profile, processing and value addition of dried red chillies (Capsicum annum L.). J. Food Sci. Technol. 2024, 61, 201–219. [Google Scholar] [CrossRef]
- Cao, Z.-X.; Zhou, L.-Y.; Bi, J.-F.; Yi, J.-Y.; Chen, Q.-Q.; Wu, X.-Y.; Zheng, J.-K.; Li, S.-R. Effect of different drying technologies on drying characteristics and quality of red pepper (Capsicum frutescens L.): A comparative study. J. Sci. Food Agric. 2016, 96, 3596–3603. [Google Scholar] [CrossRef] [PubMed]
- Aranha, B.C.; Hoffmann, J.F.; Barbieri, R.L.; Rombaldi, C.V.; Chaves, F.C. Untargeted metabolomic analysis of Capsicum spp. by GC–MS. Phytochem. Anal. 2017, 28, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Álvarez-Martínez, F.J.; Borrás, F.; Pérez, D.; Herrero, N.; Ruiz, J.J.; Micol, V. Metabolomic analysis of the effects of a commercial complex biostimulant on pepper crops. Food Chem. 2020, 310, 125818. [Google Scholar] [CrossRef]
- Becerra-Martínez, E.; Florentino-Ramos, E.; Pérez-Hernández, N.; Gerardo Zepeda-Vallejo, L.; Villa-Ruano, N.; Velázquez-Ponce, M.; García-Mendoza, F.; Bañuelos-Hernández, A.E. 1H NMR-based metabolomic fingerprinting to determine metabolite levels in serrano peppers (Capsicum annum L.) grown in two different regions. Food Res. Int. 2017, 102, 163–170. [Google Scholar] [CrossRef]
- Taiti, C.; Costa, C.; Migliori, C.A.; Comparini, D.; Figorilli, S.; Mancuso, S. Correlation between volatile compounds and spiciness in domesticated and wild fresh chili peppers. Food Bioproc. Tech. 2019, 12, 1366–1380. [Google Scholar] [CrossRef]
- Belmonte-Sánchez, E.; Romero-González, R.; Garrido Frenich, A. Applicability of high-resolution NMR in combination with chemometrics for the compositional analysis and quality control of spices and plant-derived condiments. J. Sci. Food Agric. 2021, 101, 3541–3550. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Raftery, D. Can NMR solve some significant challenges in metabolomics? J. Magn. Reson. 2015, 260, 144–160. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Meraz, M.; Méndez-Aguilar, R.; Zepeda-Vallejo, L.G.; Hernández-Guerrero, C.J.; Hidalgo-Martínez, D.; Becerra-Martínez, E. Exploring the chemical diversity of Capsicum chinense cultivars using NMR-based metabolomics and machine learning methods. Food Res. Int. 2024, 178, 113796. [Google Scholar] [CrossRef]
- Yun, B.H.; Yu, H.-Y.; Kim, H.; Myoung, S.; Yeo, N.; Choi, J.; Chun, H.S.; Kim, H.; Ahn, S. Geographical discrimination of Asian red pepper powders using 1H NMR spectroscopy and deep learning-based convolution neural networks. Food Chem. 2024, 439, 138082. [Google Scholar] [CrossRef]
- Yun, D.-Y.; Kang, Y.-G.; Kim, M.; Kim, D.; Kim, E.-H.; Hong, Y.-S. Metabolomic understanding of pod removal effect in soybean plants and potential association with their health benefit. Food Res. Int. 2020, 138, 109797. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, G.; Zhang, J.; Zhang, M.; Fu, M.; Xiang, K.; Zhang, M.; Chen, X. Identification of phenolic compounds and active antifungal ingredients of walnut in response to anthracnose (Colletotrichum gloeosporioides). Postharvest Biol. Technol. 2022, 192, 112019. [Google Scholar] [CrossRef]
- Huang, L.; Zeng, X.; Ye, Y.; Cheng, L.; Pan, D.; He, J.; Dang, Y. NMR-based metabolomics profiling of no-added-nitrite Chinese bacon (unsmoked) during processing. J. Food Sci. 2020, 85, 1027–1036. [Google Scholar] [CrossRef]
- Liu, J.; Shi, X.; Lin, H.; He, C.; Li, Q.; Shen, G.; Feng, J. Geographical origin identification and quality comparison of Ningxia goji berries (Lycium barbarum L.) by NMR-based techniques. J. Food Compost. Anal. 2023, 119, 105258. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar signaling during fruit ripening. Front. Plant Sci. 2020, 11, 564917. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.; Van den Ende, W. Sweet immunity in the plant circadian regulatory network. J. Exp. Bot. 2013, 64, 1439–1449. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The role of sugars in plant responses to stress and their regulatory function during development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Jacobs, M.; Thompson, S.; Platts, A.E.; Body, M.J.A.; Kelsey, A.; Saad, A.; Abeli, P.; Teresi, S.J.; Schilmiller, A.; Beaudry, R.; et al. Uncovering genetic and metabolite markers associated with resistance against anthracnose fruit rot in northern highbush blueberry. Hortic. Res. 2023, 10, uhad169. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, Q.; Zhu, H.; Zhou, Y.; Jiang, Y.; Gao, H.; Yun, Z. Comparative transcriptomic and metabolic analysis reveals the effect of melatonin on delaying anthracnose incidence upon postharvest banana fruit peel. BMC Plant Biol. 2019, 19, 289. [Google Scholar] [CrossRef]
- Hounsome, N.; Hounsome, B.; Tomos, D.; Edwards-Jones, G. Plant metabolites and nutritional quality of vegetables. J. Food Sci. 2008, 73, R48–R65. [Google Scholar] [CrossRef]
- Máthé, C.; Garda, T.; Freytag, C.; M-Hamvas, M. The role of serine-threonine protein phosphatase PP2A in plant oxidative stress signaling—Facts and hypotheses. Int. J. Mol. Sci. 2019, 20, 3028. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Guo, Z.; Gong, J.; Luo, S.; Zuo, Y.; Shen, Y. Role of gamma-aminobutyric acid in plant defense response. Metabolites 2023, 13, 741. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.; Son, H.; Baek, J.-H. Tricarboxylic acid (TCA) cycle intermediates: Regulators of immune responses. Life 2021, 11, 69. [Google Scholar] [CrossRef]
- Vo, K.T.X.; Rahman, M.M.; Rahman, M.M.; Trinh, K.T.T.; Kim, S.T.; Jeon, J.-S. Proteomics and metabolomics studies on the biotic stress responses of rice: An Update. Rice 2021, 14, 30. [Google Scholar] [CrossRef]
- Lai, S.-H.; Chye, M.-L. Plant Acyl-CoA-binding proteins—Their lipid and protein interactors in abiotic and biotic stresses. Cells 2021, 10, 1064. [Google Scholar] [CrossRef]
- Ullah, S.; Khan, M.N.; Lodhi, S.S.; Ahmed, I.; Tayyab, M.; Mehmood, T.; Din, I.U.; Khan, M.; Sohail, Q.; Akram, M. Targeted metabolomics reveals fatty acid abundance adjustments as playing a crucial role in drought-stress response and post-drought recovery in wheat. Front. Genet. 2022, 13, 972696. [Google Scholar] [CrossRef] [PubMed]
- Raveendar, S.; Jeon, Y.-A.; Lee, J.-R.; Lee, G.-A.; Lee, K.J.; Cho, G.-T.; Ma, K.-H.; Lee, S.-Y.; Chung, J.-W. The complete chloroplast genome sequence of Korean landrace “Subicho” pepper (Capsicum annuum var. annuum). Plant Breed. Biotechnol. 2015, 3, 88–94. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Sardari, H. Defect detection in fruit and vegetables by using machine vision systems and image processing. Food Eng. Rev. 2022, 14, 353–379. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; Rossi, C.; Grande-Tovar, C.D.; Chaves-López, C. Green management of postharvest anthracnose caused by Colletotrichum gloeosporioides. J. Fungi 2023, 9, 623. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Seong, G.-U.; Yun, D.-Y.; Shin, D.-H.; Cho, J.-S.; Lee, G.; Choi, J.H.; Park, K.-J.; Ku, K.-H.; Lim, J.-H. Comparative 1H NMR-based metabolomics of traditional landrace and disease-resistant chili peppers (Capsicum annuum L.). Foods 2024, 13, 1966. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Pérez, A.; Romero-González, R.; Garrido Frenich, A. A metabolomics approach based on 1H NMR fingerprinting and chemometrics for quality control and geographical discrimination of black pepper. J. Food Compost. Anal. 2022, 105, 104235. [Google Scholar] [CrossRef]
- Savorani, F.; Tomasi, G.; Engelsen, S.B. icoshift: A versatile tool for the rapid alignment of 1D NMR spectra. J. Magn. Reson. 2010, 202, 190–202. [Google Scholar] [CrossRef]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seong, G.-U.; Yun, D.-Y.; Shin, D.-H.; Cho, J.-S.; Park, S.-K.; Choi, J.H.; Park, K.-J.; Lim, J.-H. NMR-Based Metabolomic Analysis of Biotic Stress Responses in the Traditional Korean Landrace Red Pepper (Capsicum annuum var. annuum, cv. Subicho). Int. J. Mol. Sci. 2024, 25, 9903. https://doi.org/10.3390/ijms25189903
Seong G-U, Yun D-Y, Shin D-H, Cho J-S, Park S-K, Choi JH, Park K-J, Lim J-H. NMR-Based Metabolomic Analysis of Biotic Stress Responses in the Traditional Korean Landrace Red Pepper (Capsicum annuum var. annuum, cv. Subicho). International Journal of Molecular Sciences. 2024; 25(18):9903. https://doi.org/10.3390/ijms25189903
Chicago/Turabian StyleSeong, Gi-Un, Dae-Yong Yun, Dong-Hyeok Shin, Jeong-Seok Cho, Seul-Ki Park, Jeong Hee Choi, Kee-Jai Park, and Jeong-Ho Lim. 2024. "NMR-Based Metabolomic Analysis of Biotic Stress Responses in the Traditional Korean Landrace Red Pepper (Capsicum annuum var. annuum, cv. Subicho)" International Journal of Molecular Sciences 25, no. 18: 9903. https://doi.org/10.3390/ijms25189903






