Correlating Ultrastructural Changes in the Invasion Area of Colorectal Cancer with CT and MRI Imaging
Abstract
:1. Introduction—General Features of Cancer Invasion
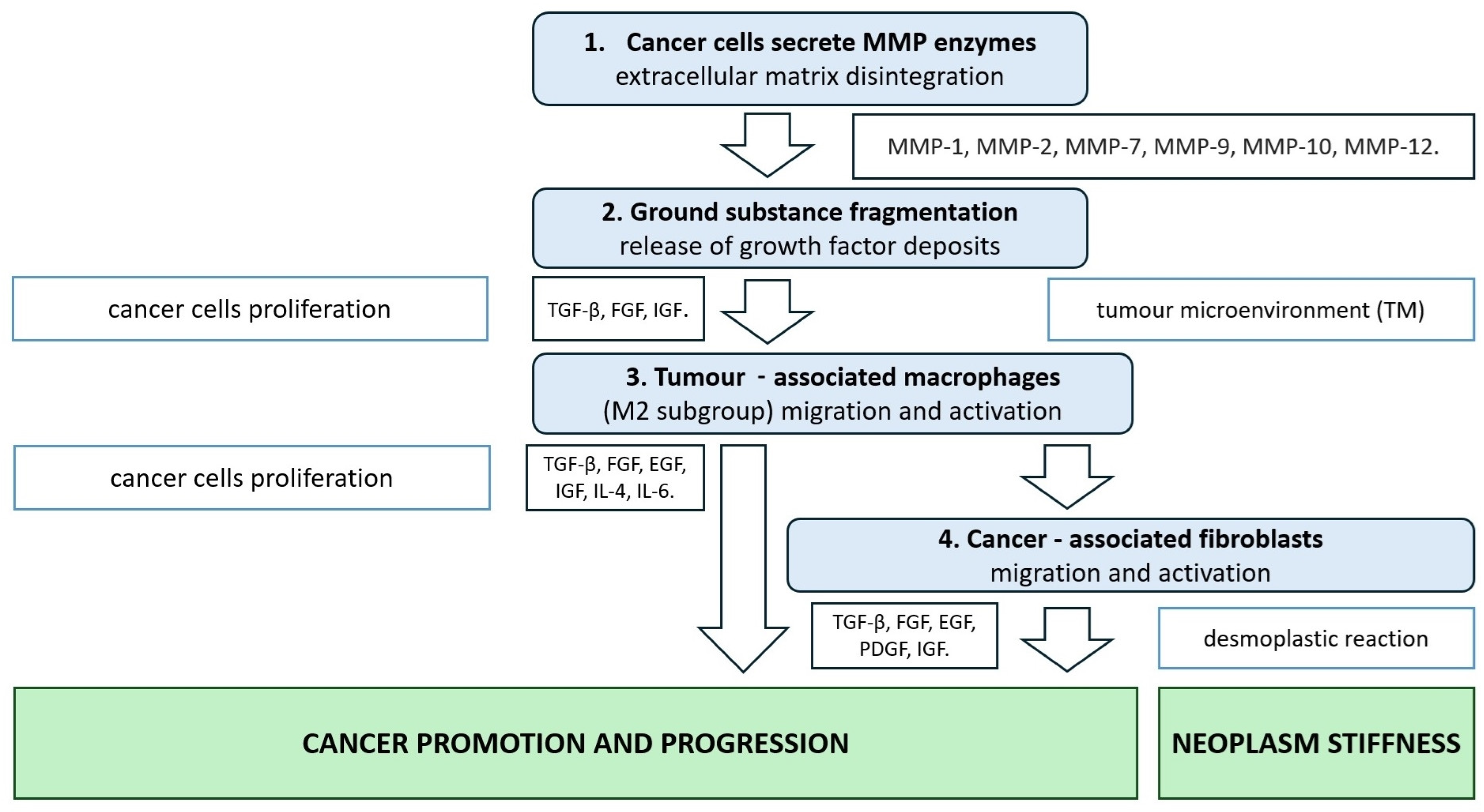
2. CRC Extracellular Matrix Disintegration Due to Metalloproteinase Activity
3. CRC Tumour Microenvironment
3.1. TAMs—Tumour-Associated Macrophages
3.2. CAFs—Cancer-Associated Fibroblasts
4. Neoplasm Tumour Organisation and Stiffness
5. Cancer Invasion and Desmoplastic Reaction
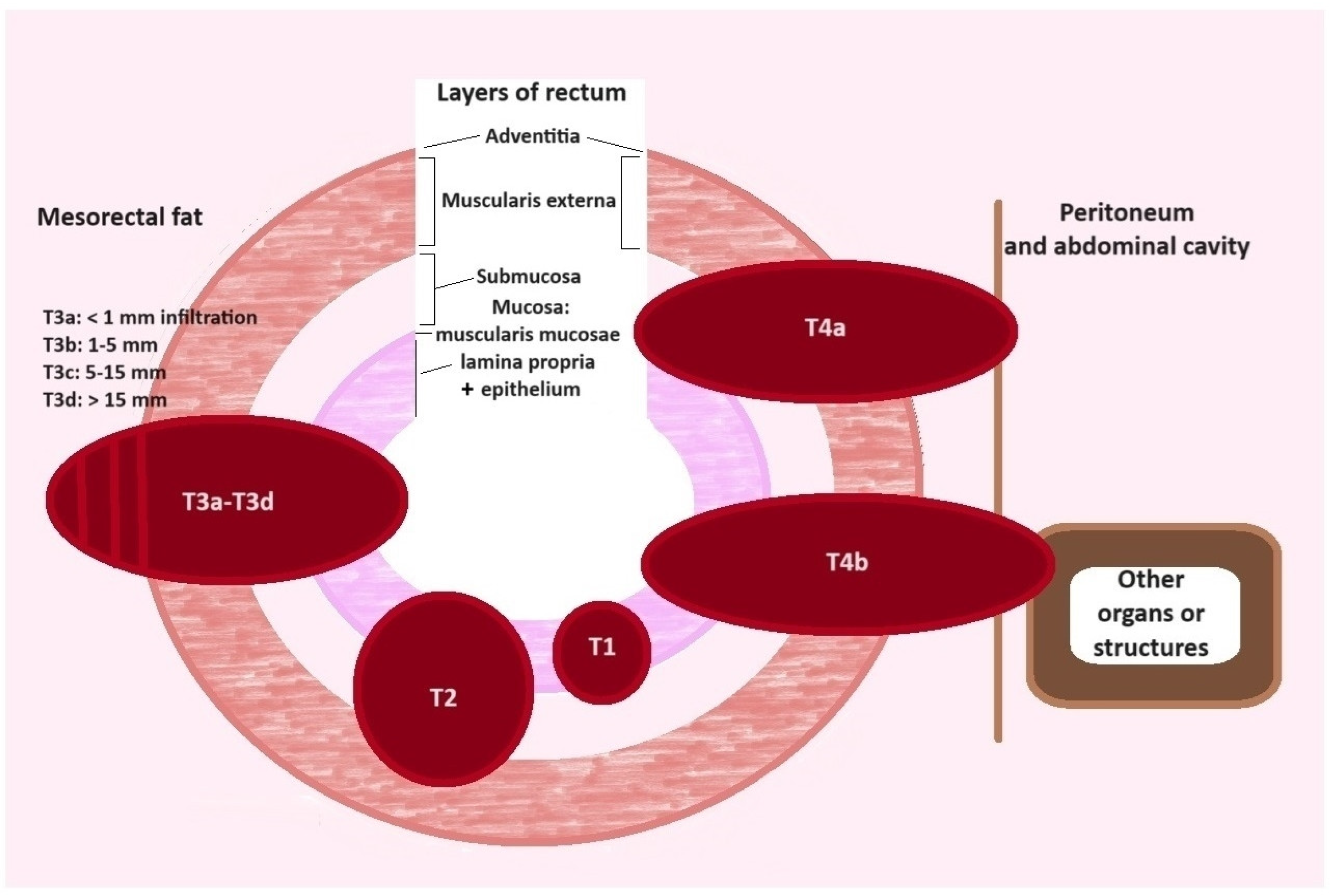
6. Modalities in Modern Radiology of Colorectal Cancer
7. Computer Tomography (CT) in CRC
8. Magnetic Resonance Imaging (MRI) and CRC
9. Radiomics and CRC
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Conlon, G.A.; Murray, G.I. Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2019, 247, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Biel, C.; Faber, K.N.; Bank, R.A.; Olinga, P. Matrix metalloproteinases in intestinal fibrosis. J. Crohn’s Colitis 2024, 18, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Schmidtmann, M.; D’Souza-Schorey, C. Extracellular Vesicles: Biological Packages That Modulate Tumor Cell Invasion. Cancers 2023, 15, 5617. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, J.; Ito, M.; Nakayama, T.; Nakashima, M.; Kohno, S.; Sekine, I. Expression of Matrix Metalloproteinase-1 in Human Colorectal Carcinoma. Mod. Pathol. 2000, 13, 925–933. [Google Scholar] [CrossRef]
- Parsons, S.L.; Watson, S.A.; Collins, H.M.; Griffin, N.R.; Clarke, P.A.; Steele, R.J. Gelatinase (MMP-2 and -9) expression in gastrointestinal malignancy. Br. J. Cancer 1998, 78, 1495–1502. [Google Scholar] [CrossRef]
- Zeng, Z.S.; Cohen, A.M.; Guliem, J.G. Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during colorectal tumorigenesis. Carcinogenesis 1999, 20, 749–755. [Google Scholar] [CrossRef]
- Tutton, M.G.; George, M.L.; Eccles, S.A.; Burton, S.; Swift, R.I.; Abulafi, M.A. Use of plasma MMP-2 and MM-9 levels as a surrogate for tumour expression in colorectal cancer patients. Int. J. Cancer 2003, 107, 541–550. [Google Scholar] [CrossRef]
- Herszényi, L.; Sipos, F.; Galamb, O.; Solymosi, N.; Hritz, I.; Miheller, P.; Berczi, L.; Molnár, B.; Tulassay, Z. Matrix metalloproteinase-9 expression in the normal mucosa-adenoma-dysplasia-adenocarcinoma sequence of the colon. Pathol. Oncol. Res. 2008, 14, 31–37. [Google Scholar] [CrossRef]
- Guzińska-Ustymowicz, K. MMP-9 and cathepsin B expression in tumor budding as an indicator of a more aggressive phenotype of colorectal cancer (CRC). Anticancer Res. 2006, 26, 1589–1594. [Google Scholar]
- Pezeshkian, Z.; Nobili, S.; Peyravian, N.; Shojaee, B.; Nazari, H.; Soleimani, H.; Asadzadeh-Aghdaei, H.; Bonab, M.A.; Nazemalhosseni-Mojarad, E.; Mini, E. Insights into the Role of Matrix Metalloproteinases in Precancerous Condition and in Colorectal Cancer. Cancers 2021, 13, 6226. [Google Scholar] [CrossRef] [PubMed]
- Bendardaf, R.; Buhmeida, A.; Hilska, M.; Laato, M.; Syrjänen, S.; Syrjänen, K.; Collan, Y.; Pyrhönen, S. MMP-9 (gelatinase B) expression is associated with disease-free survival and disease-specific survival in colorectal cancer patients. Cancer Investig. 2010, 28, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Herszényi, L.; Barabás, L.; Hritz, I.; István, G.; Tulassay, Z. Impact of proteolytic enzymes in colorectal cancer development and progression. World J. Gastroenterol. 2014, 20, 13246–13257. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.; Kamal, I.; Al-Maghrabi, J.; Abuzenadah, A.; Peer-Zada, A.A.; Qari, Y.; Al-Ahwal, M.; Al-Qahtani, M.; Buhmeida, A. High expression of matrix metalloproteinases: MMP-2 and MMP-9 predicts poor survival outcome in colorectal carcinoma. Future Oncol. 2016, 12, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.U.; Sturm, A. Peptide growth factors in the intestine. Eur. J. Gastroenterol. Hepatol. 2001, 13, 763–770. [Google Scholar] [CrossRef]
- Nunes, Q.M.; Li, Y.; Sun, C.; Kinnunen, T.K.; Fernig, D.G. Fibroblast growth factors as tissue repair and regeneration therapeutics. PeerJ 2016, 4, e1535. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health disease and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176. [Google Scholar] [CrossRef]
- Achyut, B.R.; Yang, L. Transforming growth factor-β in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology 2011, 141, 1167–1178. [Google Scholar] [CrossRef]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef]
- Krishnaswamy, V.R.; Mintz, D.; Sagi, I. Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Khalil, R.A. Matrix Metalloproteinase Inhibitors as Investigational and Therapeutic Tools in Unrestrained Tissue Remodeling and Pathological Disorders. Prog. Mol. Biol. Transl. Sci. 2017, 148, 355–420. [Google Scholar] [CrossRef] [PubMed]
- Niewiarowska, K.; Pryczynicz, A.; Dymicka-Piekarska, V.; Gryko, M.; Cepowicz, D.; Famulski, W.; Kemona, A.; Guzińska-Ustymowicz, K. Diagnostic significance of TIMP-1 level in serum and its immunohistochemical expression in colorectal cancer patients. Pol. J. Pathol. 2014, 65, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Sica, A.; Bronte, V. Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Investig. 2007, 117, 1155–1166. [Google Scholar] [CrossRef]
- Yahaya, M.A.F.; Lila, M.A.M.; Ismail, S.; Zainol, M.; Afizan, N. Tumour-Associated Macrophages (TAMs) in Colon Cancer and How to Reeducate Them. J. Immunol. Res. 2019, 2019, 2368249. [Google Scholar] [CrossRef]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Inagaki, K.; Kunisho, S.; Takigawa, H.; Yuge, R.; Oka, S.; Tanaka, S.; Shimamoto, F.; Chayama, K.; Kitadai, Y. Role of tumor-associated macrophages at the invasive front of human colorectal cancer progression. Cancer Sci. 2021, 112, 2692–2704. [Google Scholar] [CrossRef]
- Rieder, F.; Brenmoehl, J.; Leeb, S.; Schölmerich, J.; Rogler, G. Wound healing and fibrosis in intestinal disease. Gut 2007, 56, 130–139. [Google Scholar] [CrossRef]
- Koliaraki, V.; Pallangyo, C.K.; Greten, F.R.; Kollias, G. Mesenchymal Cells in Colon Cancer. Gastroenterology 2017, 152, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Simmons, J.G.; Pucilowska, J.B.; Keku, T.O.; Key Lund, P. IGF-I and TGF-beta1 have distinct effects on phenotype and proliferation of intestinal fibroblast. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G809–G818. [Google Scholar] [CrossRef] [PubMed]
- Räsänen, K.; Vaheri, A. Activation of fibroblast in cancer stroma. Exp. Cell Res. 2010, 316, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Hawinkels, L.J.A.C.; Paauwe, M.; Verspaget, H.W.; Wiercinska, E.; van der Zon, J.M.; van der Ploeg, K.; Koelink, P.J.; Lindeman, J.H.N.; Mesker, W.; Dijke, P.T.; et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer associated fibroblasts. Oncogene 2014, 33, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Roulis, M.; Flavell, R.A. Fibroblast and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 2016, 92, 116–131. [Google Scholar] [CrossRef]
- Tommelein, J.; Verset, L.; Boterberg, T.; Demetter, P.; Bracke, M.; De Wever, O. Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer. Front. Oncol. 2015, 5, 63. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Inamoto, S.; Yamamoto, T.; Ogawa, R.; Taketo, M.M.; Sakai, Y. The Role of Chemokines in Promoting Colorectal Cancer Invasion/Metastasis. Int. J. Mol. Sci. 2016, 17, E643. [Google Scholar] [CrossRef]
- Shi, Y.; Massagué, J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Jung, B.; Staudacher, J.J.; Beauchamp, D. Transforming Growth Factor β Superfamily Signaling in Development of Colorectal Cancer. Gastroenterology 2017, 152, 36–52. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Sakai, Y. Transforming Growth Factor-β Signaling Pathway in Colorectal Cancer and Its Tumor Microenvironment. Int. J. Mol. Sci. 2019, 20, E5822. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular matrix and its therapeutic potential for cancer treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Brauchle, E.; Kasper, J.; Daum, R.; Schierbaum, N.; Falch, C.; Kirschniak, A.; Shäffer, T.; Schenke-Layland, K. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol. 2018, 68–69, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Romero-López, M.; Trinh, A.L.; Sobrino, A.; Hatch, M.M.; Keating, M.T.; Fimbres, C.; Lewis, D.E.; Gershon, P.D.; Botvinick, E.L.; Digman, M.; et al. Recapitulating the human tumor microenvironment: Colon tumor-derived extracellular matrix promotes angiogenesis and tumor cell growth. Biomaterials 2017, 116, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Castro-Abril, H.; Heras, J.; Del Barrio, J.; Paz, L.; Alcaine, C.; Aliacar, M.P.; Garzon-Alvaro, D.; Doblare, M.; Ochoa, I. The Role of Mechanical Properties and Structure Type I Collagen Hydrogels on Colorectal Cell Migration. Macromol. Biosci. 2023, 23, e23000108. [Google Scholar] [CrossRef]
- Cooper, J.; Gianconti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell. 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Zhao, J.; Guan, J.-L. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009, 28, 35–49. [Google Scholar] [CrossRef]
- Wierzbicki, M.P.; Rybarczyk, A. The Hippo pathway in colorectal cancer. Folia Histochem. Cytobiol. 2015, 52, 105–119. [Google Scholar] [CrossRef]
- Nagelkerke, A.; Bussink, J.; Rowan, A.E.; Span, N.P. The mechanical microenvironment in cancer: How physics affects tumours. Semin. Cancer Biol. 2015, 35, 62–70. [Google Scholar] [CrossRef]
- Lopez-Crapez, E.; Costa, L.; Tosato, G.; Ramos, J.; Mazard, T.; Guiramand, J.; Thiery, A.; Colinge, J.; Milhiet, P.-E.; Benistant, C. Mechanical signatures of human colon cancer. Sci. Rep. 2022, 12, 12475. [Google Scholar] [CrossRef]
- Jass, J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007, 50, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Dawson, H.; Lugli, A. Molecular and pathogenetic aspects of tumor budding in colorectal cancer. Front. Med. 2015, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Bonetti, L.R.; Ieni, A.; Caruso, R.A.; Tuccari, G. Histological grading in colorectal cancer: New insights and perspectives. Histol. Histopathol. 2015, 30, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Pavlic, A.; Urh, K.; Bostjancic, E.; Zidar, N. Analyzing the invasive front of colorectal cancer–By punching tissue block or laser capture microdissection? Pathol. -Res. Pract. 2023, 248, 154727. [Google Scholar] [CrossRef] [PubMed]
- Koelzer, V.H.; Zlobec, I.; Lugli, A. Tumor budding in colorectal cancer–ready for diagnostic practice? Hum. Pathol. 2016, 47, 4–19. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Flejou, J.F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef]
- Ueno, H.; Kajiwara, Y.; Ajioka, Y.; Sugai, T.; Sekine, S.; Ishiguro, M.; Takashima, A.; Kanemitsu, Y. Histopathological atlas of desmoplastic reaction characterization in colorectal cancer. Jpn. J. Clin. Oncol. 2021, 51, 1004–1012. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ishida, M.; Miki, H.; Hatta, M.; Hamada, M.; Hirose, Y.; Sekimoto, M. Significance of desmoplastic reaction on tumor deposits in patients with colorectal cancer. Oncol. Lett. 2022, 25, 1. [Google Scholar] [CrossRef]
- Swiderska, M.; Choromańska, B.; Dąbrowska, E.; Konarzewska-Duchnowska, E.; Choromańska, K.; Szczurko, G.; Myśliwiec, P.; Dadan, J.; Ladny, J.R.; Zwierz, K. The diagnostics of colorectal cancer. Contemp. Oncol. 2014, 18, 1–6. [Google Scholar] [CrossRef]
- Lee, S.; Surabhi, V.R.; Kassam, Z.; Chang, K.J.; Kaur, H. Imaging of colon and rectal cancer. Curr. Probl. Cancer 2023, 47, 100970. [Google Scholar] [CrossRef]
- Kolligs, F.T. Diagnostics and Epidemiology of Colorectal Cancer. Visc. Med. 2016, 32, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Horvat, N.; Carlos Tavares Rocha, C.; Clemente de Oliveira, B.; Petkovska, I.; Gollub, M.J. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics 2019, 39, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Mazonakis, M.; Damilakis, J. Computed tomography: What and how does it measure? Eur. J. Radiol. 2016, 85, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Maino, C.; Bianco, I.; Drago, S.G.; Piazza, R.; Tamini, N.; Nespoli, L.; Giandola, T.; Sironi, S. The usefulness of preoperative CT in colon cancer staging: Impact of radiologists’ experience. Abdom. Radiol. 2023, 48, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Mou, A.; Li, H.; Chen, X.L.; Fan, Y.H.; Pu, H. Tumor size measured by multidetector CT in resectable colon cancer: Correlation with regional lymph node metastasis and N stage. World J. Surg. Oncol. 2021, 19, 179. [Google Scholar] [CrossRef]
- Huh, J.W.; Jeong, Y.Y.; Kim, H.R.; Kim, Y.J. Prognostic value of preoperative radiological staging assessed by computed tomography in patients with nonmetastatic colon cancer. Ann. Oncol. 2012, 23, 1198–1206. [Google Scholar] [CrossRef]
- Goiffon, R.J.; O’Shea, A.; Harisinghani, M.G. Advances in radiological staging of colorectal cancer. Clin. Radiol. 2021, 76, 879–888. [Google Scholar] [CrossRef]
- Macari, M.; Balthazar, E.J. CT of bowel wall thickening: Significance and pitfalls of interpretation. Am. J. Roentgenol. 2001, 176, 1105–1116. [Google Scholar] [CrossRef]
- Korngold, E.K.; Moreno, C.; Kim, D.H.; Fowler, K.J.; Cash, B.D.; Chang, K.J.; Gage, K.L.; Gajjar, A.H.; Garcia, E.M.; Kambadakone, A.R.; et al. ACR Appropriateness Criteria® Staging of Colorectal Cancer 2021 Update. J. Am. Coll. Radiol. 2022, 19, S208–S222. [Google Scholar] [CrossRef]
- Nerad, E.; Lahaye, M.J.; Maas, M.; Nelemans, P.; Bakers, F.C.; Beets, G.L.; Beets-Tan, R.G. Diagnostic Accuracy of CT for Local Staging of Colon Cancer: A Systematic Review and Meta-Analysis. AJR Am. J. Roentgenol. 2016, 207, 984–995. [Google Scholar] [CrossRef]
- van de Weerd, S.; Hong, E.; Berg, I.v.D.; Wijlemans, J.W.; van Vooren, J.; Prins, M.W.; Wessels, F.J.; Heeres, B.C.; Roberti, S.; Nederend, J.; et al. Accurate staging of non-metastatic colon cancer with CT: The importance of training and practice for experienced radiologists and analysis of incorrectly staged cases. Abdom. Radiol. 2022, 47, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Kadari, M.; Subhan, M.; Saji Parel, N.; Krishna, P.V.; Gupta, A.; Uthayaseelan, K.; Uthayaseelan, K.; Sunkara, N.A.B.S. CT Colonography and Colorectal Carcinoma: Current Trends and Emerging Developments. Cureus 2022, 14, e24916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Han, Z.; Dou, F.; Yan, T. Pre-colectomy location and TNM staging of colon cancer by the computed tomography colonography: A diagnostic performance study. World J. Surg. Oncol. 2021, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, N.; Sakamoto, T.; Hida, K.; Sakai, Y. Diagnostic accuracy of computed tomography colonography for tumor depth in colorectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2019, 30, 126–130. [Google Scholar] [CrossRef]
- Berger, A. Magnetic resonance imaging. BMJ 2002, 324, 35. [Google Scholar] [CrossRef]
- Deng, B.; Wang, Q.; Liu, Y.; Yang, Y.; Gao, X.; Dai, H. A nomogram based on MRI radiomics features of mesorectal fat for diagnosing T2- and T3-stage rectal cancer. Abdom. Radiol. 2024, 49, 1850–1860. [Google Scholar] [CrossRef]
- Curvo-Semedo, L. Colon Cancer Staging: When Does High Resolution MRI Have a Role? Curr. Color. Cancer Rep. 2019, 15, 170–174. [Google Scholar] [CrossRef]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Caseiro-Alves, F.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: Recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 2013, 23, 2522–2531. [Google Scholar] [CrossRef]
- Beets-Tan, R.G.H.; Lambregts, D.M.J.; Maas, M.; Bipat, S.; Barbaro, B.; Curvo-Semedo, L.; Fenlon, H.M.; Gollub, M.J.; Gourtsoyianni, S.; Halligan, S.; et al. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 2018, 28, 1465–1475. [Google Scholar] [CrossRef]
- Bogveradze, N.; Snaebjornsson, P.; Grotenhuis, B.A.; van Triest, B.; Lahaye, M.J.; Maas, M.; Beets, G.L.; Beets-Tan, R.G.H.; Lambregts, D.M.J. MRI anatomy of the rectum: Key concepts important for rectal cancer staging and treatment planning. Insights Imaging 2023, 14, 13. [Google Scholar] [CrossRef]
- Taylor, F.G.; Swift, R.I.; Blomqvist, L.; Brown, G. A systematic approach to the interpretation of preoperative staging MRI for rectal cancer. Am. J. Roentgenol. 2008, 191, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.; Kirkham, A.; Williams, G.T.; Bourne, M.; Radcliffe, A.G.; Sayman, J.; Newell, R.; Sinnatamby, C.; Heald, R.J. High-resolution MRI of the anatomy important in total mesorectal excision of the rectum. AJR Am. J. Roentgenol. 2004, 182, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.; Eveno, C.; Hoeffel, C.; Boudiaf, M.; Bazeries, P.; Foucher, R.; Pocard, M.; Dohan, A.; Soyer, P. Diffusion-weighted magnetic resonance imaging in colorectal cancer. J. Visc. Surg. 2016, 153, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Yacheva, A.; Dardanov, D.; Zlatareva, D. The Multipurpose Usage of Diffusion-Weighted MRI in Rectal Cancer. Medicina 2023, 59, 2162. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, J.S.; Park, S.I.; Kim, N.K.; Kim, J.H.; Moon, H.J.; Park, Y.N.; Kim, W.H. Accuracy in differentiation of mucinous and nonmucinous rectal carcinoma on MR imaging. J. Comput. Assist. Tomogr. 2003, 27, 48–55. [Google Scholar] [CrossRef]
- Liu, L.H.; Lv, H.; Wang, Z.C.; Rao, S.X.; Zeng, M.S. Performance comparison between MRI and CT for local staging of sigmoid and descending colon cancer. Eur. J. Radiol. 2019, 121, 108741. [Google Scholar] [CrossRef]
- Chia, C.S.; Wong, L.C.K.; Hennedige, T.P.; Ong, W.S.; Zhu, H.Y.; Tan, G.H.C.; Kwek, J.W.; Seo, C.J.; Wong, J.S.M.; Ong, C.J.; et al. Prospective Comparison of the Performance of MRI Versus CT in the Detection and Evaluation of Peritoneal Surface Malignancies. Cancers 2022, 14, 3179. [Google Scholar] [CrossRef]
- Inchingolo, R.; Maino, C.; Cannella, R.; Vernuccio, F.; Cortese, F.; Dezio, M.; Pisani, A.R.; Giandola, T.; Gatti, M.; Giannini, V.; et al. Radiomics in colorectal cancer patients. World J. Gastroenterol. 2023, 29, 2888–2904. [Google Scholar] [CrossRef]
- Limkin, E.J.; Sun, R.; Dercle, L.; Zacharaki, E.I.; Robert, C.; Reuzé, S.; Schernberg, A.; Paragios, N.; Deutsch, E.; Ferté, C. Promises and challenges for the implementation of computational medical imaging (radiomics) in oncology. Ann. Oncol. 2017, 28, 1191–1206. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, P.; Wang, J.; Shen, L.; Xia, F.; Qing, G.; Hu, W.; Zhang, Z.; Xin, C.; Peng, W.; et al. Radiomic features of pretreatment MRI could identify T stage in patients with rectal cancer: Preliminary findings. J. Magn. Reson. Imaging 2018, 48, 615–621. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging tumour hypoxia with oxygen-enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642. [Google Scholar] [CrossRef] [PubMed]
- Dinapoli, N.; Barbaro, B.; Gatta, R.; Chiloiro, G.; Casà, C.; Masciocchi, C.; Damiani, A.; Boldrini, L.; Gambacorta, M.A.; Dezio, M.; et al. Magnetic Resonance, Vendor-independent, Intensity Histogram Analysis Predicting Pathologic Complete Response After Radiochemotherapy of Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 765–774. [Google Scholar] [CrossRef] [PubMed]

| Potential Biomarker | Pathomorphological Features Related with Colorectal Cancer Invasion |
|---|---|
| Elevated expression of metalloproteinase enzymes: MMP-1, MMP-2, MMP-7, MMP-9, MMP-10, and MMP-12 | Correlation with tumour size, lymphatic dissemination, higher risk of metastases, and shorter survival [5,10,12,13,14]. |
| M2 macrophages (mainly M2C subgroup) accumulation | Correlation with more invasive cancer potential and the presence of cancer-distant metastases [25,29]. |
| The infiltrative tumour border arrangement and the tumour budding presence | Association with blood vessel invasion, lymph node metastases, and distant spread into internal organs. These features are correlated with shorter survival [55,56]. |
| The immature type of desmoplastic reaction in the front of cancer invasion | Correlation with tumour size, lymphatic and blood vessels invasion, regional lymph node dissemination, and poor prognosis [58]. |
| CT with Intravenous Contrast Agent | MRI (with or without Intravenous Contrast Agent) | |
|---|---|---|
| X-ray radiation [63] | present | absent |
| Vulnerability to motion artifacts of small and large intestine [60] | less vulnerable | more vulnerable |
| Time of the study [60] | shorter | longer |
| Toleration by the patients [60] | better tolerated | worse tolerated |
| Soft-tissue contrast resolution (visibility of the rectal wall layers) [60,62,67] | (−) | (+) |
| Calcifications presence evaluation [60] | (+) | (−) |
| Image acquisition in the plane perpendicular to the tumour [60] | (−) | (+) |
| Primary T-stage evaluation (tumour location and size, depth of invasion, EMVI, mesorectal fascia status, anal sphincter involvement) [60,62] | (−) | (+) |
| Initial local disease assessment (mesorectal and lateral pelvic lymph node involvement) [87] | (−/+) | (+) |
| Presence of distant metastasis [88] | Lung metastases (+) Liver metastases below 1 cm (−/+) Lymph nodes 15 mm in short axis (+) Peritoneal spread (+) | Lung metastases (−) Liver metastases below 1 cm (+) Lymph nodes 15 mm in short axis (+) Peritoneal spread (+) |
| Differentiation of mucinous and nonmucinous tumours [62] | (−) | (+) |
| Differentiation between fibrosis and residual tumour on restating after CTH/RTH [62] | (−) | (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbaniec-Stompór, J.; Michalak, M.; Godlewski, J. Correlating Ultrastructural Changes in the Invasion Area of Colorectal Cancer with CT and MRI Imaging. Int. J. Mol. Sci. 2024, 25, 9905. https://doi.org/10.3390/ijms25189905
Urbaniec-Stompór J, Michalak M, Godlewski J. Correlating Ultrastructural Changes in the Invasion Area of Colorectal Cancer with CT and MRI Imaging. International Journal of Molecular Sciences. 2024; 25(18):9905. https://doi.org/10.3390/ijms25189905
Chicago/Turabian StyleUrbaniec-Stompór, Joanna, Maciej Michalak, and Janusz Godlewski. 2024. "Correlating Ultrastructural Changes in the Invasion Area of Colorectal Cancer with CT and MRI Imaging" International Journal of Molecular Sciences 25, no. 18: 9905. https://doi.org/10.3390/ijms25189905





