Metabolomics-Based Study of the Protective Effect of 4-Hydroxybenzyl Alcohol on Ischemic Astrocytes
Abstract
:1. Introduction
2. Results
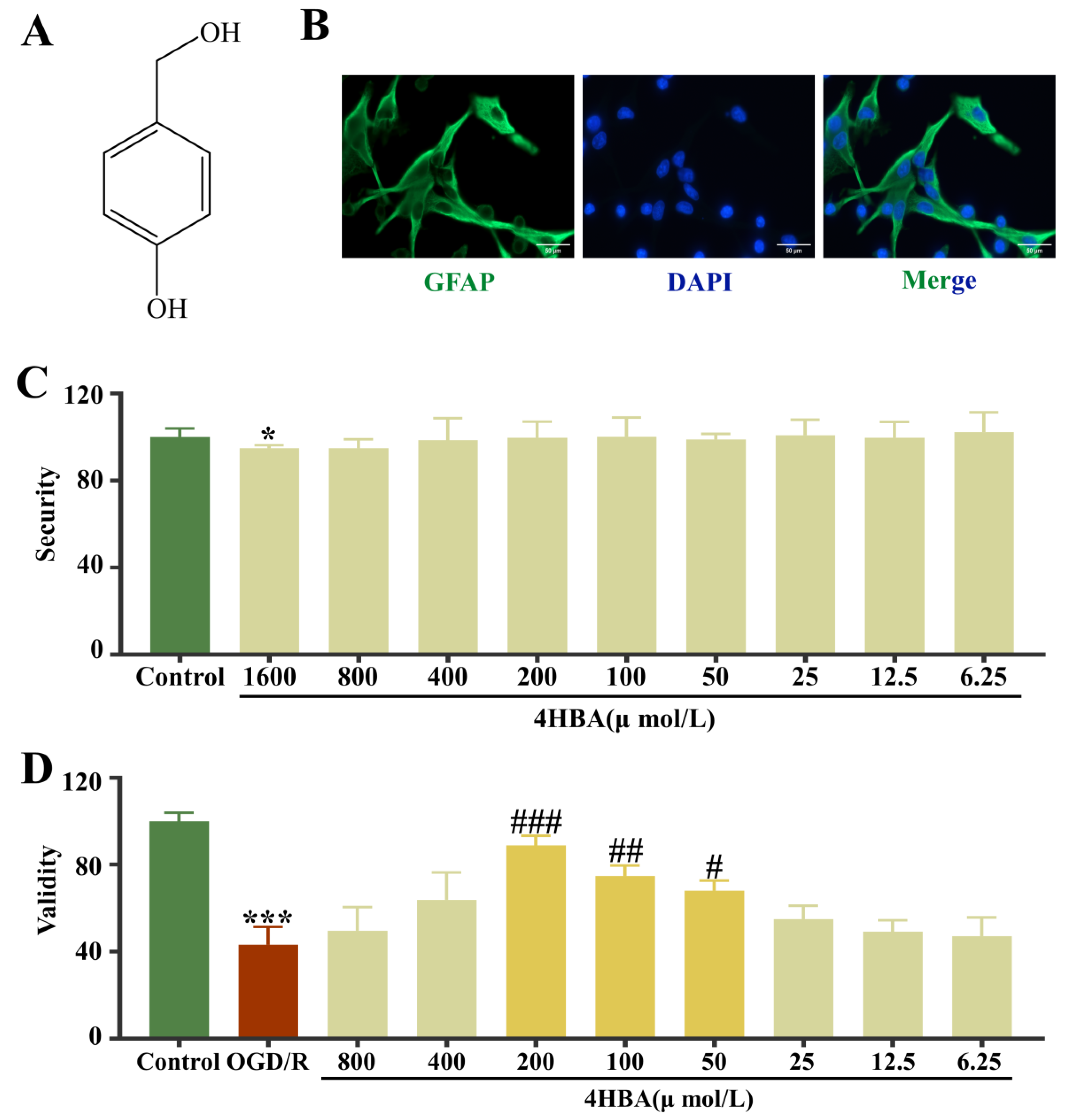
2.1. Identification of ASs and Screening of 4HBA Safety and Efficacy in ASs
2.2. Effect of 4-HBA on CAT, MPTP, and Ca2+ Levels in ASs
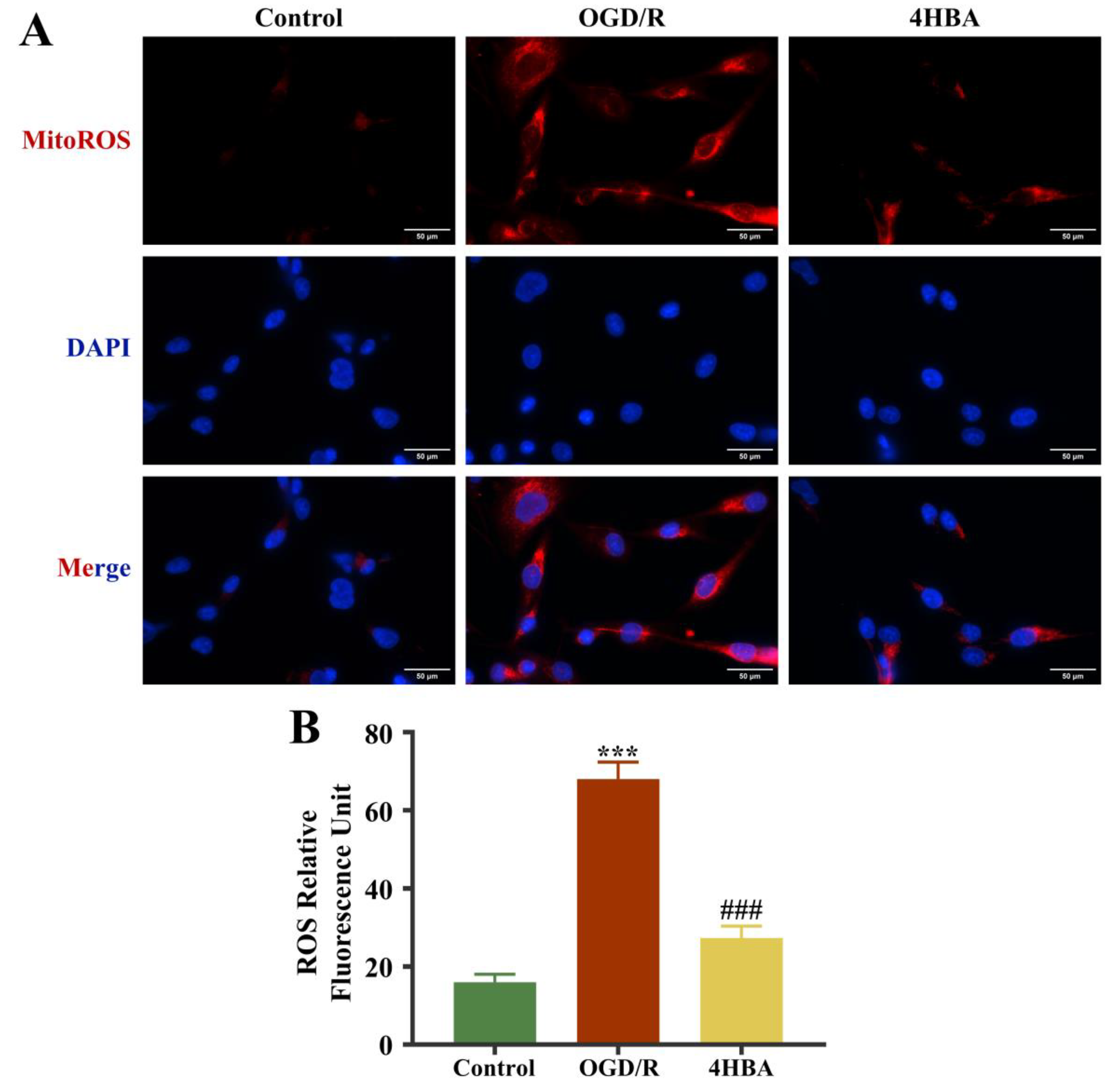
2.3. Effect of 4HBA on ROS Content in ASs
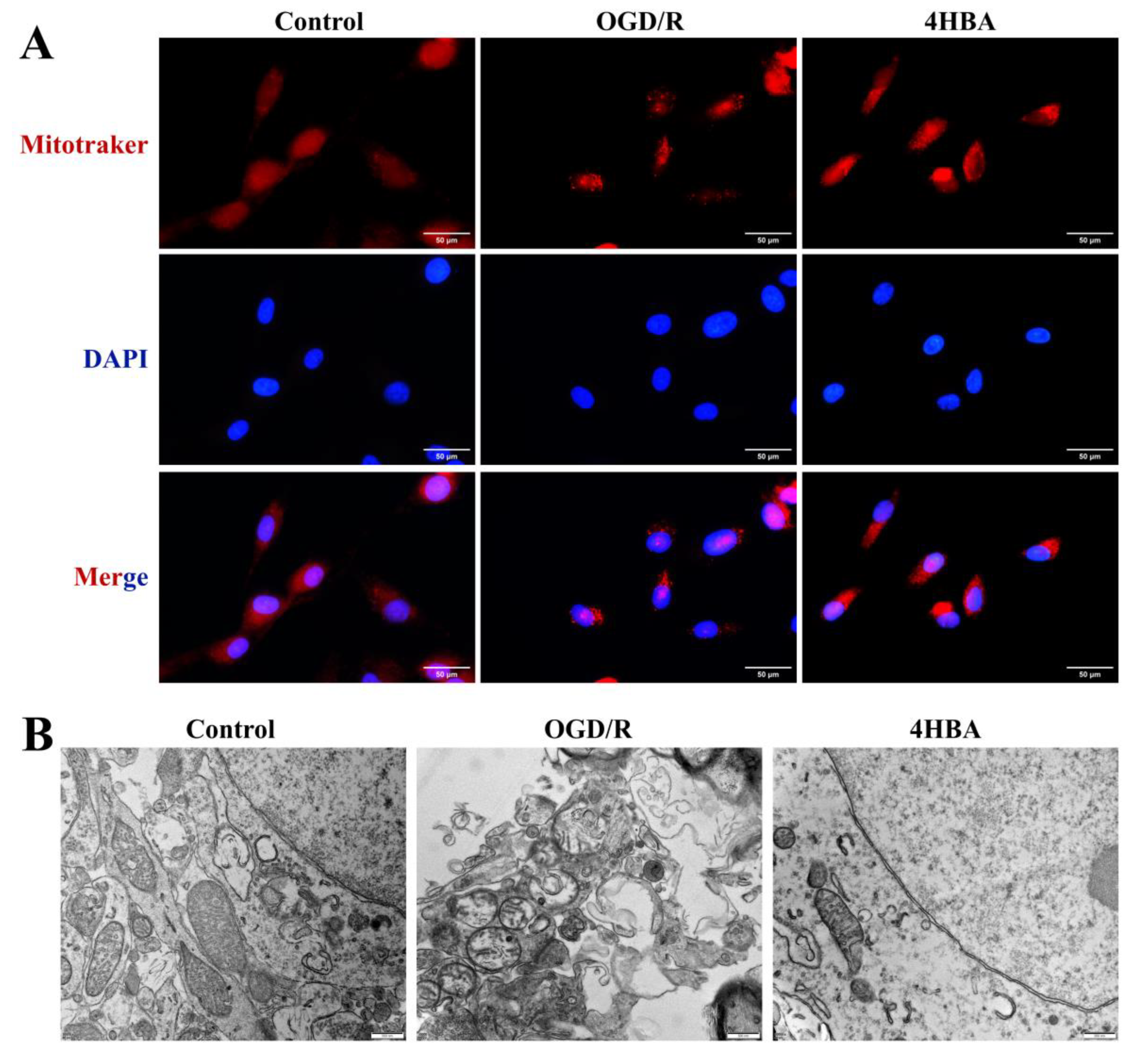
2.4. Effect of 4HBA on Mitochondrial Morphology in ASs
2.5. Screening for Differential Metabolites in ASs
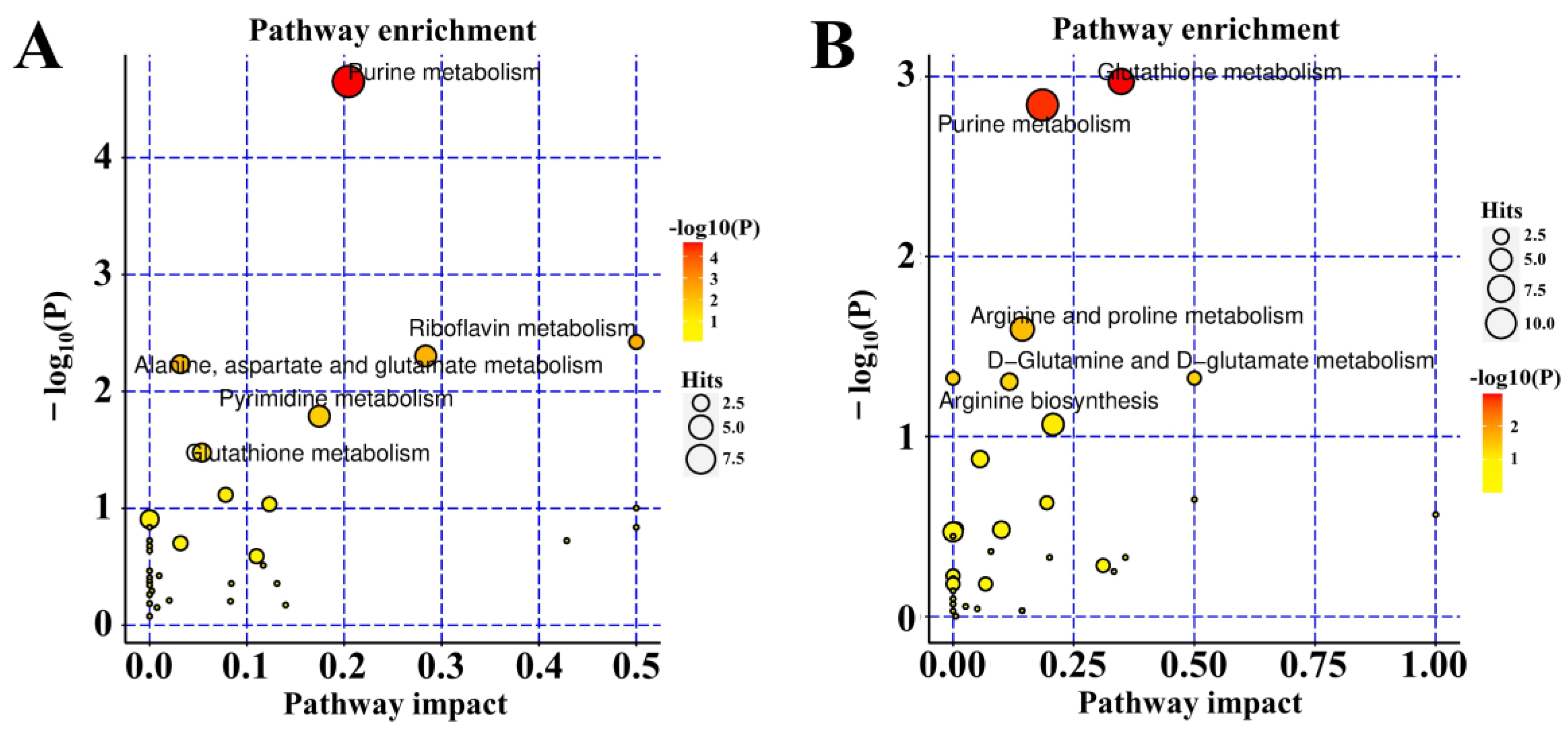
2.6. KEGG Enrichment of Metabolic Pathways
2.7. Metabolomic Validation in ASs
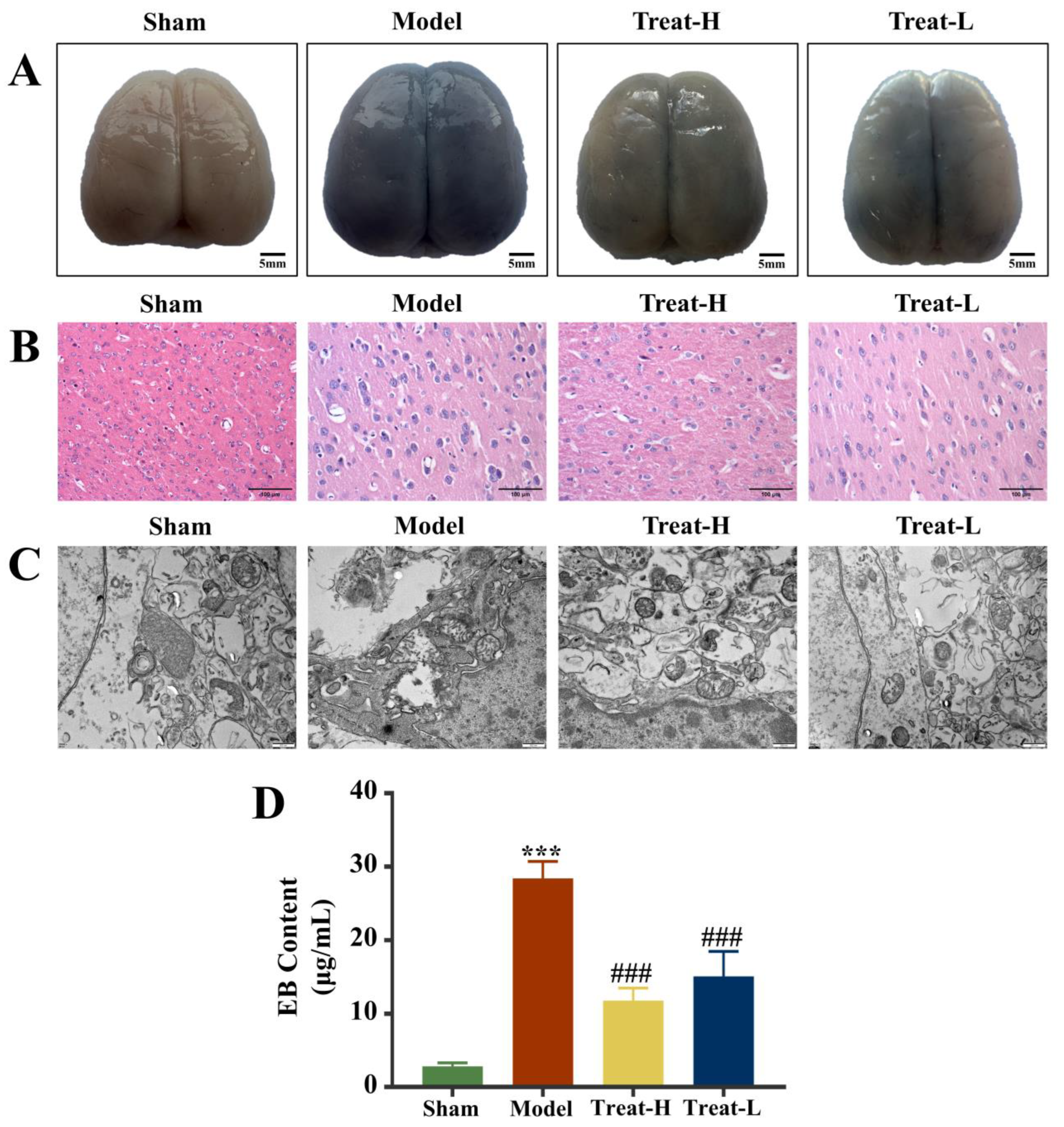
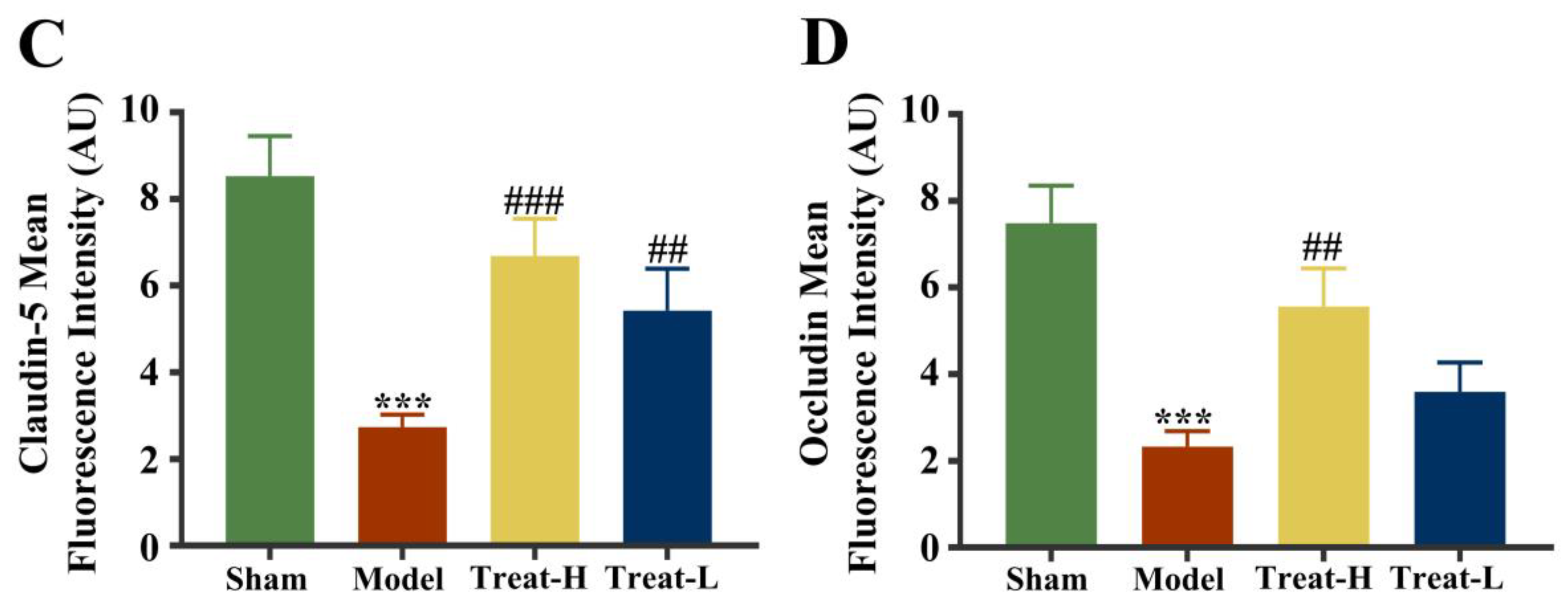
2.8. Effect of 4HBA on BBB Exudation in 2VO Rats
2.9. Effect of 4HBA on Brain Tissue and Mitochondria in 2VO Rats
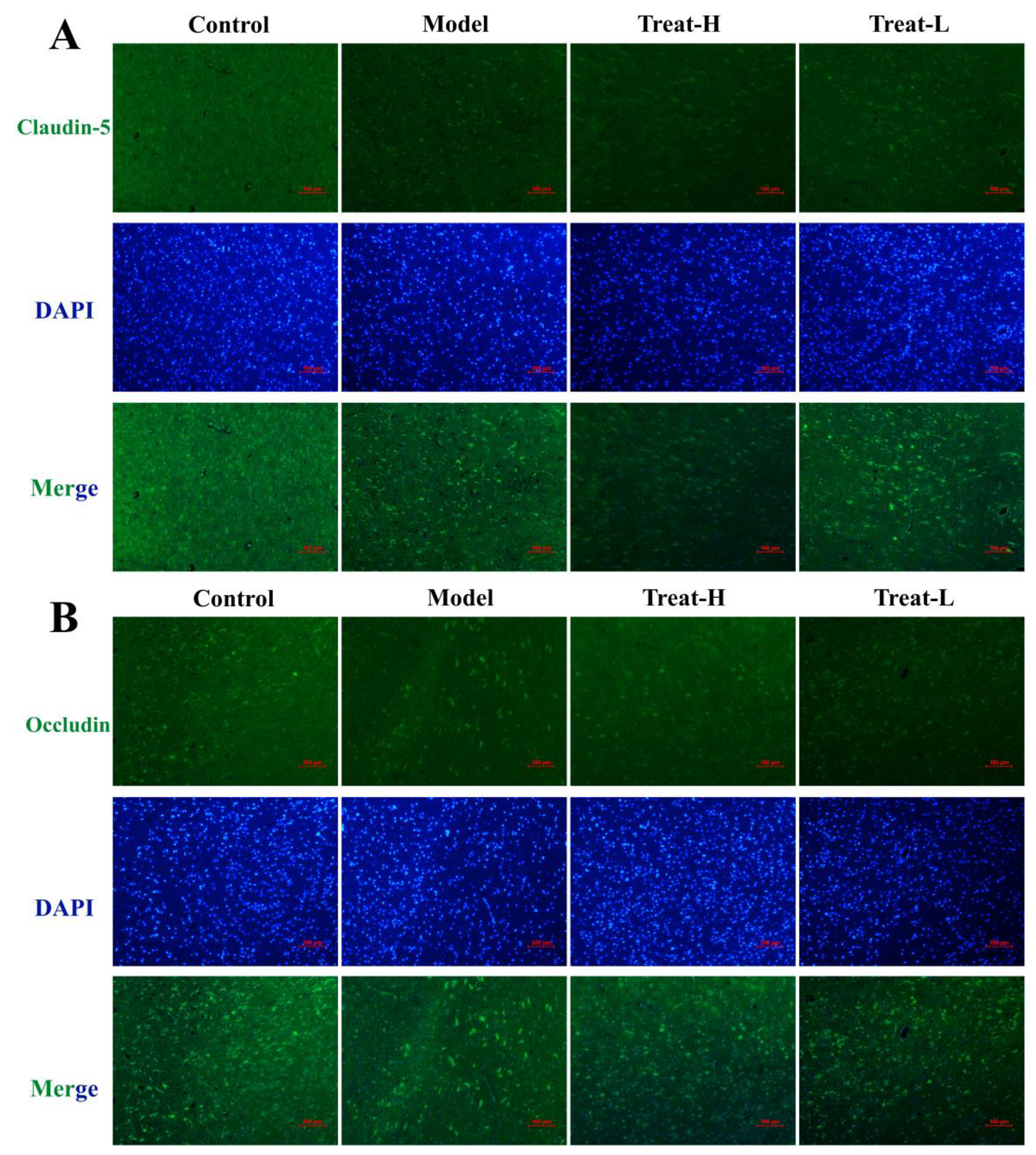
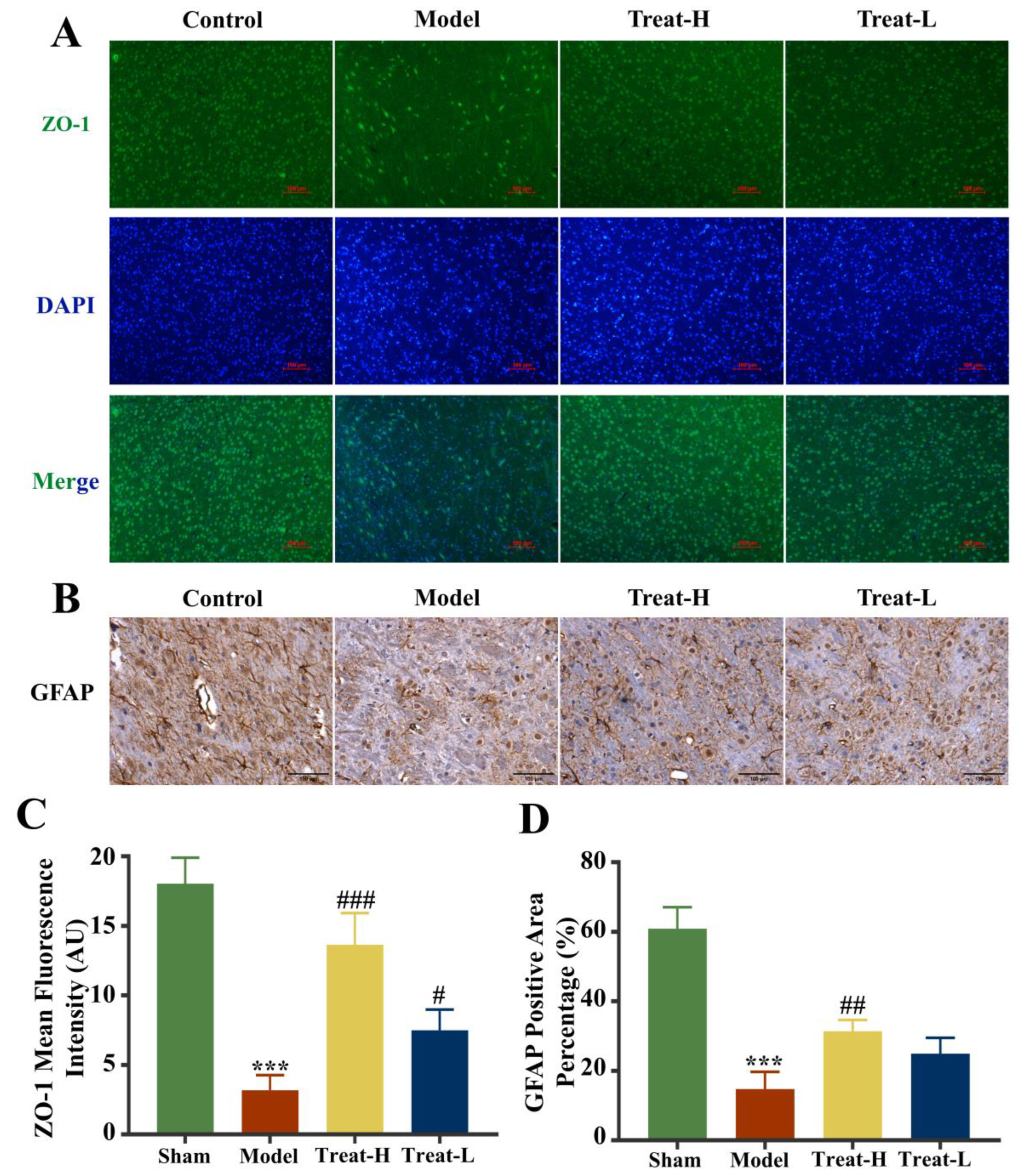
2.10. Effect of 4HBA Connexin Protein in 2VO Rats
2.11. Effects of 4HBA on the Expression of GFAP, AQP4 and BDNF in 2VO Rats
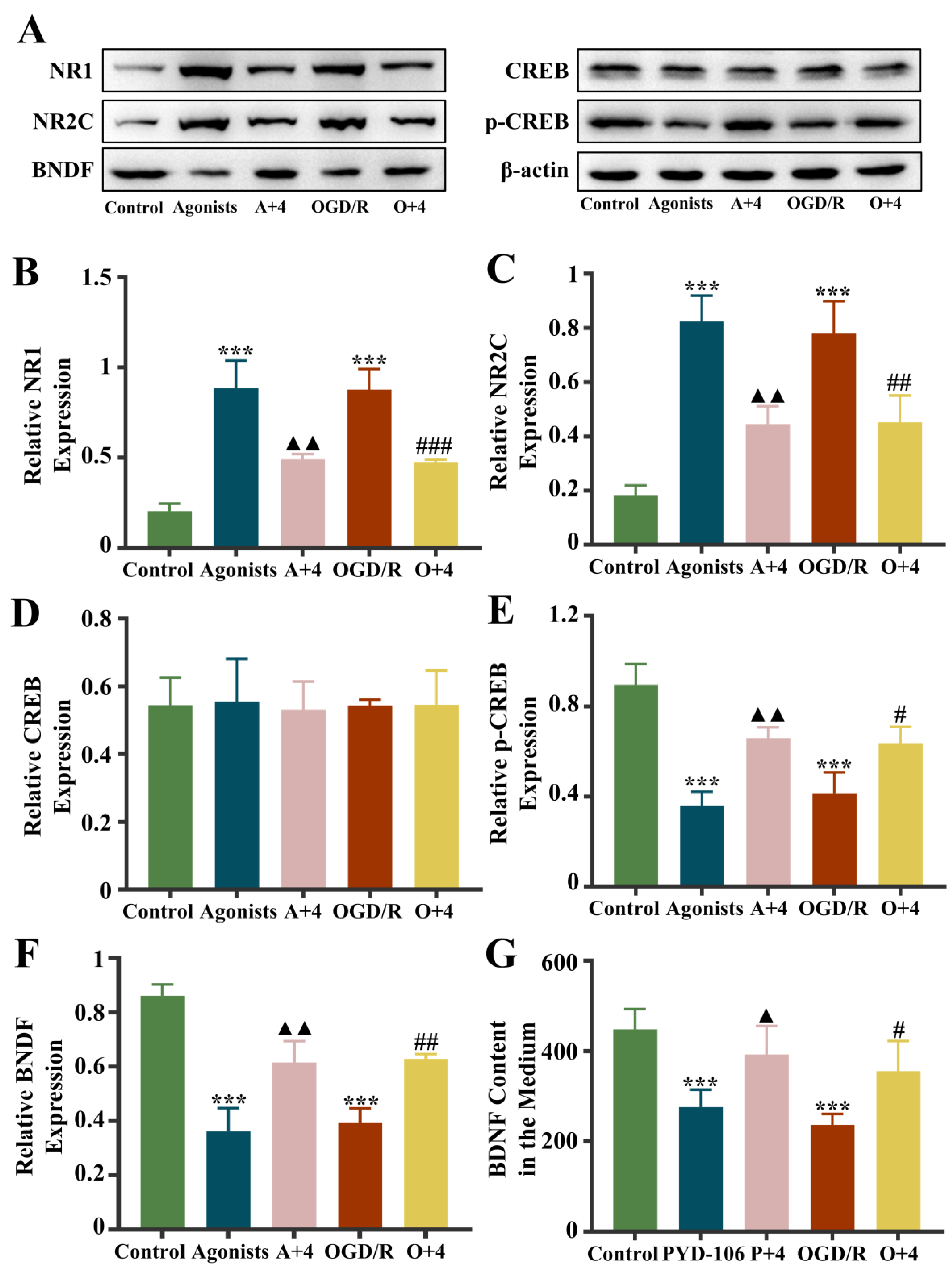
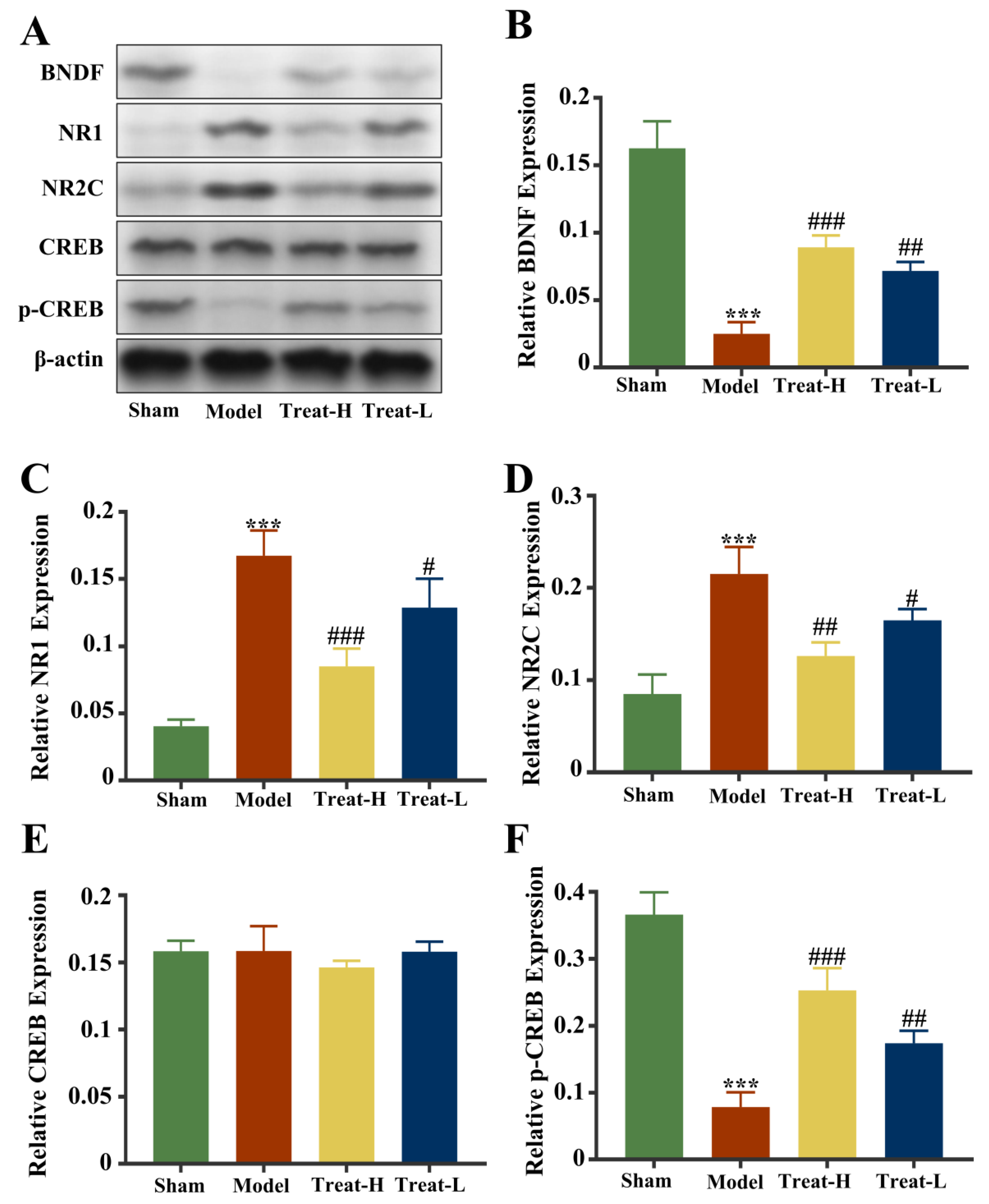
2.12. Effect of 4HBA on NR1/NR2C-CREB-BNDF Pathway in 2VO Rats
3. Discussion
4. Materials and Methods
4.1. Animal
4.2. Drugs and Cell Culture Conditions
4.3. Extraction of Rat Cortical Primary ASs
4.4. Drug Administration and Model Replication in Groups
4.5. Cell Viability Assay for Drug Safety and Efficacy
4.6. Detection of Intracellular CAT, MPTP, and Ca2+ Content in ASs and Ca2+ Content in Mitochondria
4.7. Flow Cytometry to Detect Mitochondrial Membrane Potential
4.8. MitoTracker Red Staining to Measure Changes in Mitochondrial Morphology
4.9. MitoROS Levels Detected by Mitochondrial Superoxide Red Fluorescent Probe
4.10. Metabolomics Assay
4.11. BDNF Content in Culture Medium Was Detected
4.12. EB Experiment
4.13. HE Staining
4.14. Immunofluorescence Detection
4.15. Immunohistochemistry Assay
4.16. Electron Microscopy
4.17. Western Blotting
4.18. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ak, C.; Gupta, R.; Warkade, V. Study of Clinical Profile and Risk Factors for Ischemic Stroke in Young Adults. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar]
- Paun, O.; Cercel, R.A.; Radu, R.I.; Raicea, V.C.; Pirscoveanu, D.F.V.; Hontaru, S.O.; Zorila, M.V.; Mogoanta, L. Histopathological lesions induced by stroke in the encephalon. Rom. J. Morphol. Embryol. 2023, 64, 389–398. [Google Scholar] [CrossRef]
- Sun, K.; Fan, J.; Han, J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm. Sin. B 2015, 5, 8–24. [Google Scholar] [CrossRef]
- Yang, L.; Yang, B.C.; Zhang, C.X.; Tong, J. Protective effects of acupuncture and LGNHFD on expressions of vascular endothelial growth factor, basic fibroblast growth factor, and cluster of differentiation 34 in rats with cerebral ischemia-reperfusion injury. J. Tradit. Chin. Med. 2021, 41, 463–470. [Google Scholar] [CrossRef]
- Brandao, M.; Simon, T.; Critchley, G.; Giamas, G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 2019, 67, 779–790. [Google Scholar] [CrossRef]
- Dzyubenko, E.; Prazuch, W.; Pillath-Eilers, M.; Polanska, J.; Hermann, D.M. Analysing Intercellular Communication in Astrocytic Networks Using “Astral”. Front. Cell Neurosci. 2021, 15, 689268. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Gu, T.; Yu, D.; Shi, Y.; Gao, Z.; Wang, Z.; Liu, W.; Fan, Z.; Hou, W.; et al. Astrocytic glycogen mobilization participates in salvianolic acid B-mediated neuroprotection against reperfusion injury after ischemic stroke. Exp. Neurol. 2022, 349, 113966. [Google Scholar] [CrossRef]
- Vilchez, D.; Ros, S.; Cifuentes, D.; Pujadas, L.; Valles, J.; Garcia-Fojeda, B.; Criado-Garcia, O.; Fernandez-Sanchez, E.; Medrano-Fernandez, I.; Dominguez, J.; et al. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 2007, 10, 1407–1413. [Google Scholar] [CrossRef]
- Voloboueva, L.A.; Suh, S.W.; Swanson, R.A.; Giffard, R.G. Inhibition of mitochondrial function in astrocytes: Implications for neuroprotection. J. Neurochem. 2007, 102, 1383–1394. [Google Scholar] [CrossRef]
- Garcia, J.H.; Yoshida, Y.; Chen, H.; Li, Y.; Zhang, Z.G.; Lian, J.; Chen, S.; Chopp, M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am. J. Pathol. 1993, 142, 623–635. [Google Scholar]
- Yuan, W.Q.; Huang, W.P.; Jiang, Y.C.; Xu, H.; Duan, C.S.; Chen, N.H.; Liu, Y.J.; Fu, X.M. The function of astrocytes and their role in neurological diseases. Eur. J. Neurosci. 2023, 58, 3932–3961. [Google Scholar] [CrossRef]
- Zhang, R.; Meng, J.; Wang, X.; Pu, L.; Zhao, T.; Huang, Y.; Han, L. Metabolomics of ischemic stroke: Insights into risk prediction and mechanisms. Metab. Brain Dis. 2022, 37, 2163–2180. [Google Scholar] [CrossRef]
- Li, W.; Shao, C.; Li, C.; Zhou, H.; Yu, L.; Yang, J.; Wan, H.; He, Y. Metabolomics: A useful tool for ischemic stroke research. J. Pharm. Anal. 2023, 13, 968–983. [Google Scholar] [CrossRef]
- Guo, X.; Li, Z.; Zhou, Y.; Yu, S.; Yang, H.; Zheng, L.; Liu, Y.; Sun, Y. Metabolic Profile for Prediction of Ischemic Stroke in Chinese Hypertensive Population. J. Stroke Cerebrovasc. Dis. 2019, 28, 1062–1069. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Feng, Y.; Sun, G.; Sun, X. Classical Active Ingredients and Extracts of Chinese Herbal Medicines: Pharmacokinetics, Pharmacodynamics, and Molecular Mechanisms for Ischemic Stroke. Oxid. Med. Cell Longev. 2021, 2021, 8868941. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, H.; Shen, D.; Liu, S.; Kong, W.; Zheng, K.; Yang, J.; Ge, L. Artemisinin attenuated ischemic stroke induced pyroptosis by inhibiting ROS/TXNIP/NLRP3/Caspase-1 signaling pathway. Biomed. Pharmacother. 2024, 177, 116894. [Google Scholar] [CrossRef]
- Sun, X.; Jia, B.; Sun, J.; Lin, J.; Lu, B.; Duan, J.; Li, C.; Wang, Q.; Zhang, X.; Tan, M.; et al. Gastrodia elata Blume: A review of its mechanisms and functions on cardiovascular systems. Fitoterapia 2023, 167, 105511. [Google Scholar] [CrossRef]
- Yu, X.; Luo, Y.; Yang, L.; Chen, P.; Duan, X. P-hydroxybenzyl alcohol ameliorates neuronal cerebral ischemia-reperfusion injury by activating mitochondrial autophagy through SIRT1. Mol. Med. Rep. 2023, 27, 68. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Zhang, D.; Zhang, Q.; Zhao, Y.; He, Z.M.; Gao, Y.G.; Zhang, L.X. 4-Hydroxybenzyl alcohol derivatives and their sedative-hypnotic activities. RSC Adv. 2018, 8, 19539–19550. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, J.H. Vanillin and 4-hydroxybenzyl alcohol attenuate cognitive impairment and the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus in a mouse model of scopolamine-induced amnesia. Anat. Cell Biol. 2017, 50, 143–151. [Google Scholar] [CrossRef]
- Ma, T.; Chen, P.; Dong, H.; Wang, X. Identification of key anti-neuroinflammatory components in Gastrodiae Rhizoma based on spectrum-effect relationships and its mechanism exploration. J. Pharm. Biomed. Anal. 2024, 248, 116266. [Google Scholar] [CrossRef]
- Chen, W.C.; Lai, Y.S.; Lin, S.H.; Lu, K.H.; Lin, Y.E.; Panyod, S.; Ho, C.T.; Sheen, L.Y. Anti-depressant effects of Gastrodia elata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling. J. Ethnopharmacol. 2016, 182, 190–199. [Google Scholar] [CrossRef]
- Jia, F.; Yu, B.; Li, J.; Cai, F.; Fu, G.; Jin, Q.; Ji, J. Supramolecular Nano-Assembly of Caffeate-Strengthened Phenylboronic Ester with Multistep ROS Scavenging Ability for Targeted Therapy of Acute Kidney Injury. Adv. Healthc. Mater. 2023, 12, e2301615. [Google Scholar] [CrossRef]
- Yu, S.S.; Zhao, J.; Lei, S.P.; Lin, X.M.; Wang, L.L.; Zhao, Y. 4-hydroxybenzyl alcohol ameliorates cerebral injury in rats by antioxidant action. Neurochem. Res. 2011, 36, 339–346. [Google Scholar] [CrossRef]
- Luo, L.; Kim, S.W.; Lee, H.K.; Kim, I.D.; Lee, H.; Lee, J.K. Anti-Zn(2+)-Toxicity of 4-Hydroxybenzyl Alcohol in Astrocytes and Neurons Contribute to a Robust Neuroprotective Effects in the Postischemic Brain. Cell Mol. Neurobiol. 2018, 38, 615–626. [Google Scholar] [CrossRef]
- Descamps, E.; Petrault-Laprais, M.; Maurois, P.; Pages, N.; Bac, P.; Bordet, R.; Vamecq, J. Experimental stroke protection induced by 4-hydroxybenzyl alcohol is cancelled by bacitracin. Neurosci. Res. 2009, 64, 137–142. [Google Scholar] [CrossRef]
- Abbott, N.J.; Ronnback, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Falkowska, A.; Gutowska, I.; Goschorska, M.; Nowacki, P.; Chlubek, D.; Baranowska-Bosiacka, I. Energy Metabolism of the Brain, Including the Cooperation between Astrocytes and Neurons, Especially in the Context of Glycogen Metabolism. Int. J. Mol. Sci. 2015, 16, 25959–25981. [Google Scholar] [CrossRef]
- Gurer, G.; Gursoy-Ozdemir, Y.; Erdemli, E.; Can, A.; Dalkara, T. Astrocytes are more resistant to focal cerebral ischemia than neurons and die by a delayed necrosis. Brain Pathol. 2009, 19, 630–641. [Google Scholar] [CrossRef]
- Marina, N.; Turovsky, E.; Christie, I.N.; Hosford, P.S.; Hadjihambi, A.; Korsak, A.; Ang, R.; Mastitskaya, S.; Sheikhbahaei, S.; Theparambil, S.M.; et al. Brain metabolic sensing and metabolic signaling at the level of an astrocyte. Glia 2018, 66, 1185–1199. [Google Scholar] [CrossRef]
- Narzt, M.S.; Kremslehner, C.; Golabi, B.; Nagelreiter, I.M.; Malikovic, J.; Hussein, A.M.; Plasenzotti, R.; Korz, V.; Lubec, G.; Gruber, F.; et al. Molecular species of oxidized phospholipids in brain differentiate between learning- and memory impaired and unimpaired aged rats. Amino Acids 2022, 54, 1311–1326. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Diaz-Vivancos, P.; de Simone, A.; Kiddle, G.; Foyer, C.H. Glutathione–linking cell proliferation to oxidative stress. Free Radic. Biol. Med. 2015, 89, 1154–1164. [Google Scholar] [CrossRef]
- Iskusnykh, I.Y.; Zakharova, A.A.; Pathak, D. Glutathione in Brain Disorders and Aging. Molecules 2022, 27, 324. [Google Scholar] [CrossRef]
- Dringen, R.; Gutterer, J.M.; Hirrlinger, J. Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur. J. Biochem. 2000, 267, 4912–4916. [Google Scholar] [CrossRef]
- Steele, M.L.; Fuller, S.; Patel, M.; Kersaitis, C.; Ooi, L.; Munch, G. Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol. 2013, 1, 441–445. [Google Scholar] [CrossRef]
- Gessner, P.; Lum, J.; Frenguelli, B.G. The mammalian purine salvage pathway as an exploitable route for cerebral bioenergetic support after brain injury. Neuropharmacology 2023, 224, 109370. [Google Scholar] [CrossRef]
- Bortoluzzi, V.T.; Ribeiro, R.T.; Pinheiro, C.V.; Castro, E.T.; Tavares, T.Q.; Leipnitz, G.; Sass, J.O.; Castilho, R.F.; Amaral, A.U.; Wajner, M. N-Acetylglutamate and N-acetylmethionine compromise mitochondrial bioenergetics homeostasis and glutamate oxidation in brain of developing rats: Potential implications for the pathogenesis of ACY1 deficiency. Biochem. Biophys. Res. Commun. 2023, 684, 149123. [Google Scholar] [CrossRef]
- Aoyama, K. Glutathione in the Brain. Int. J. Mol. Sci. 2021, 22, 5010. [Google Scholar] [CrossRef]
- Cherry, K.M.; Lenze, E.J.; Lang, C.E. Combining d-cycloserine with motor training does not result in improved general motor learning in neurologically intact people or in people with stroke. J. Neurophysiol. 2014, 111, 2516–2524. [Google Scholar] [CrossRef]
- Flores-Soto, M.E.; Chaparro-Huerta, V.; Escoto-Delgadillo, M.; Vazquez-Valls, E.; Gonzalez-Castaneda, R.E.; Beas-Zarate, C. [Structure and function of NMDA-type glutamate receptor subunits]. Neurologia 2012, 27, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Schuler, T.; Mesic, I.; Madry, C.; Bartholomaus, I.; Laube, B. Formation of NR1/NR2 and NR1/NR3 heterodimers constitutes the initial step in N-methyl-D-aspartate receptor assembly. J. Biol. Chem. 2008, 283, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiong, L.; Yu, Z.; Zhang, L.; Liu, G.; Su, G.; Huang, C.; Huang, Z.; Li, L. Icaritin alleviates cerebral ischemia–reperfusion injury by regulating NMDA receptors through ERK signaling. Eur. J. Pharmacol. 2023, 941, 175492. [Google Scholar] [CrossRef] [PubMed]
- Dravid, S.M.; Burger, P.B.; Prakash, A.; Geballe, M.T.; Yadav, R.; Le, P.; Vellano, K.; Snyder, J.P.; Traynelis, S.F. Structural determinants of D-cycloserine efficacy at the NR1/NR2C NMDA receptors. J. Neurosci. 2010, 30, 2741–2754. [Google Scholar] [CrossRef]
- Ravikrishnan, A.; Gandhi, P.J.; Shelkar, G.P.; Liu, J.; Pavuluri, R.; Dravid, S.M. Region-specific Expression of NMDA Receptor GluN2C Subunit in Parvalbumin-Positive Neurons and Astrocytes: Analysis of GluN2C Expression using a Novel Reporter Model. Neuroscience 2018, 380, 49–62. [Google Scholar] [CrossRef]
- Pivovarova, N.B.; Andrews, S.B. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 2010, 277, 3622–3636. [Google Scholar] [CrossRef]
- Alshial, E.E.; Abdulghaney, M.I.; Wadan, A.S.; Abdellatif, M.A.; Ramadan, N.E.; Suleiman, A.M.; Waheed, N.; Abdellatif, M.; Mohammed, H.S. Mitochondrial dysfunction and neurological disorders: A narrative review and treatment overview. Life Sci. 2023, 334, 122257. [Google Scholar] [CrossRef]
- Holmes, A.; Zhou, N.; Donahue, D.L.; Balsara, R.; Castellino, F.J. A deficiency of the GluN2C subunit of the N-methyl-D-aspartate receptor is neuroprotective in a mouse model of ischemic stroke. Biochem. Biophys. Res. Commun. 2018, 495, 136–144. [Google Scholar] [CrossRef]
- Rao, J.; Li, H.; Zhang, H.; Xiang, X.; Ding, X.; Li, L.; Geng, F.; Qi, H. Periplaneta americana (L.) extract activates the ERK/CREB/BDNF pathway to promote post-stroke neuroregeneration and recovery of neurological functions in rats. J. Ethnopharmacol. 2024, 321, 117400. [Google Scholar] [CrossRef]
- Ishii, T.; Warabi, E.; Mann, G.E. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic. Biol. Med. 2019, 133, 169–178. [Google Scholar] [CrossRef]
- Chou, T.H.; Kang, H.; Simorowski, N.; Traynelis, S.F.; Furukawa, H. Structural insights into assembly and function of GluN1-2C, GluN1-2A-2C, and GluN1-2D NMDARs. Mol. Cell 2022, 82, 4548–4563. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, J.; Duchen, M.R. Mitochondrial oxidative stress and cell death in astrocytes--requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci. 2002, 115 Pt 6, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Ludhiadch, A.; Sharma, R.; Muriki, A.; Munshi, A. Role of Calcium Homeostasis in Ischemic Stroke: A Review. CNS Neurol. Disord. Drug Targets 2022, 21, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Liu, C.; Hsu, J.W.; Chacko, S.; Minard, C.; Jahoor, F.; Sekhar, R.V. Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Results of a pilot clinical trial. Clin. Transl. Med. 2021, 11, e372. [Google Scholar] [CrossRef]
- Wang, A.R.; Mi, L.F.; Zhang, Z.L.; Hu, M.Z.; Zhao, Z.Y.; Liu, B.; Li, Y.B.; Zheng, S. Corrigendum to “Saikosaponin A improved depression-like behavior and inhibited hippocampal neuronal apoptosis after cerebral ischemia through p-CREB/BDNF pathway”. Behav. Brain Res. 2021, 403, 113138, Erratum in Behav. Brain Res.2023, 452, 114596. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, J.M.; Wang, Y.S.; Hao, Y.J.; Li, Y.X.; Li, N.; Wang, J.; Niu, Y.; Sun, T.; Yu, J.Q. Neuroprotection of Cytisine Against Cerebral Ischemia-Reperfusion Injury in Mice by Regulating NR2B-ERK/CREB Signal Pathway. Neurochem. Res. 2018, 43, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Potokar, M.; Morita, M.; Wiche, G.; Jorgacevski, J. The Diversity of Intermediate Filaments in Astrocytes. Cells 2020, 9, 1604. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhan, J.; Cai, Q.; Xu, F.; Chai, R.; Lam, K.; Luan, Z.; Zhou, G.; Tsang, S.; Kipp, M.; et al. The Water Transport System in Astrocytes-Aquaporins. Cells 2022, 11, 2564. [Google Scholar] [CrossRef]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799. [Google Scholar] [CrossRef]
- Sun, W.; Liu, Z.; Jiang, X.; Chen, M.B.; Dong, H.; Liu, J.; Sudhof, T.C.; Quake, S.R. Spatial transcriptomics reveal neuron-astrocyte synergy in long-term memory. Nature 2024, 627, 374–381. [Google Scholar] [CrossRef]
- Yao, S.Q.; Ye, Y.; Li, Q.; Wang, X.Y.; Yan, L.; Huo, X.M.; Pan, C.S.; Fu, Y.; Liu, J.; Han, J.Y. YangXueQingNaoWan attenuated blood brain barrier disruption after thrombolysis with tissue plasminogen activator in ischemia stroke. J. Ethnopharmacol. 2024, 318 Pt B, 117024. [Google Scholar] [CrossRef]
- Sasson, E.; Anzi, S.; Bell, B.; Yakovian, O.; Zorsky, M.; Deutsch, U.; Engelhardt, B.; Sherman, E.; Vatine, G.; Dzikowski, R.; et al. Nano-scale architecture of blood-brain barrier tight-junctions. Elife 2021, 10, e63253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, B.; Zhao, T.; Quan, X.; Han, Y.; Cheng, Y.; Chen, Y.; Shen, X.; Zheng, Y.; Zhao, Y. Comparative study of extracellular vesicles derived from mesenchymal stem cells and brain endothelial cells attenuating blood-brain barrier permeability via regulating Caveolin-1-dependent ZO-1 and Claudin-5 endocytosis in acute ischemic stroke. J. Nanobiotechnol. 2023, 21, 70. [Google Scholar] [CrossRef] [PubMed]




| Compound | VIP | OGD/R vs. Control | 4HBA vs. OGD/R | p-Value | KEGG | ||
|---|---|---|---|---|---|---|---|
| FC | Change | FC | Change | ||||
| Cycloserine | 1.346 | 44.196 | up | 0.146 | down | 0.005 | C08057 |
| Betaine aldehyde | 1.413 | 5.5120 | up | 1.100 | up | 0.001 | C00576 |
| D-Lactic acid | 1.813 | 5.083 | up | 1.402 | up | 0.000 | C00256 |
| Beta-Sitosterol | 1.198 | 1.994 | up | 0.564 | down | 0.019 | C01753 |
| Cyclic AMP | 1.683 | 1.837 | up | 2.863 | up | 0.022 | C00575 |
| L-Phenylalanine | 1.441 | 1.722 | up | 1.405 | up | 0.021 | C00079 |
| 4-hydroxy-2H-chromen-2-one | 1.231 | 1.648 | up | 1.165 | up | 0.031 | C20414 |
| Xanthurenic acid | 1.512 | 1.313 | up | 1.262 | up | 0.031 | C02470 |
| (3S,4S)-3-hydroxytetradecane-1,3,4-tricarboxylic acid | 1.394 | 1.268 | up | 0.780 | down | 0.013 | C04529 |
| Phosphocreatine | 1.841 | 1.070 | up | 1.355 | up | 0.012 | C02305 |
| D-Ribulose 5-phosphate | 1.938 | 1.068 | up | 0.765 | down | 0.005 | C00199 |
| 2′-Deoxyinosine triphosphate | 1.608 | 1.064 | up | 1.531 | up | 0.039 | C01345 |
| Azelaic acid | 1.844 | 1.015 | up | 0.771 | down | 0.008 | C08261 |
| Aniline | 1.839 | 1.013 | up | 0.580 | down | 0.011 | C00292 |
| 16-Hydroxy hexadecanoic acid | 1.835 | 1.010 | up | 1.793 | up | 0.013 | C18218 |
| Gamma-Tocopherol | 1.651 | 1.000 | down | 0.787 | down | 0.029 | C02483 |
| Oxidized glutathione | 1.737 | 0.962 | down | 1.317 | up | 0.031 | C00127 |
| Glutathione | 1.971 | 0.927 | down | 1.497 | up | 0.006 | C00051 |
| Uridine diphosphate glucose | 1.675 | 0.907 | down | 1.438 | up | 0.042 | C00029 |
| Hexadecanedioic acid | 1.376 | 0.905 | down | 0.899 | down | 0.035 | C19615 |
| Cysteinylglycine | 2.091 | 0.872 | down | 1.645 | up | 0.002 | C01419 |
| L-Glutamine | 1.377 | 0.508 | down | 1.839 | up | 0.006 | C00064 |
| Spermine | 1.309 | 0.507 | down | 1.503 | up | 0.000 | C00750 |
| 5′-Methylthioadenosine | 1.252 | 0.496 | down | 1.464 | up | 0.000 | C00170 |
| Cyclic AMP | 1.986 | 0.494 | down | 2.584 | up | 0.000 | C00575 |
| Citicoline | 1.198 | 0.488 | down | 1.377 | up | 0.001 | C00307 |
| Succinic acid | 1.163 | 0.486 | down | 1.531 | up | 0.004 | C00042 |
| Uridine 5′-monophosphate | 1.321 | 0.483 | down | 1.643 | up | 0.000 | C00105 |
| L-Tyrosine | 1.30 | 0.471 | down | 1.387 | up | 0.000 | C00082 |
| L-Leucine | 1.292 | 0.466 | down | 1.480 | up | 0.000 | C00123 |
| Albanin G | 1.341 | 0.453 | down | 1.781 | up | 0.001 | C10100 |
| Creatinine | 1.242 | 0.437 | down | 1.400 | up | 0.000 | C00791 |
| NADH | 1.244 | 0.434 | down | 2.133 | up | 0.026 | C00004 |
| ADP | 1.493 | 0.431 | down | 2.078 | up | 0.001 | C00008 |
| Spermidine | 1.455 | 0.423 | down | 2.030 | up | 0.000 | C00315 |
| S-Adenosylhomocysteine | 1.047 | 0.423 | down | 1.269 | up | 0.010 | C00021 |
| N-Acetylglutamic acid | 1.301 | 0.380 | down | 1.152 | up | 0.000 | C00624 |
| Biotin | 1.299 | 0.319 | down | 2.100 | up | 0.000 | C00120 |
| 2′-Deoxyguanosine 5′-monophosphate | 1.368 | 0.303 | down | 2.427 | up | 0.000 | C00362 |
| N-Acetylputrescine | 1.558 | 0.300 | down | 1.231 | up | 0.000 | C02714 |
| Cytidine | 1.099 | 0.282 | down | 2.052 | up | 0.004 | C00475 |
| Cycloserine | 1.346 | 44.196 | up | 0.146 | down | 0.005 | C08057 |
| Betaine aldehyde | 1.413 | 5.5120 | up | 1.100 | up | 0.001 | C00576 |
| D-Lactic acid | 1.813 | 5.083 | up | 1.402 | up | 0.000 | C00256 |
| Beta-Sitosterol | 1.198 | 1.994 | up | 0.564 | down | 0.019 | C01753 |
| Cyclic AMP | 1.683 | 1.837 | up | 2.863 | up | 0.022 | C00575 |
| L-Phenylalanine | 1.441 | 1.722 | up | 1.405 | up | 0.021 | C00079 |
| 4-hydroxy-2H-chromen-2-one | 1.231 | 1.648 | up | 1.165 | up | 0.031 | C20414 |
| Xanthurenic acid | 1.512 | 1.313 | up | 1.262 | up | 0.031 | C02470 |
| (3S,4S)-3-hydroxytetradecane-1,3,4-tricarboxylic acid | 1.394 | 1.268 | up | 0.780 | down | 0.013 | C04529 |
| Phosphocreatine | 1.841 | 1.070 | up | 1.355 | up | 0.012 | C02305 |
| D-Ribulose 5-phosphate | 1.938 | 1.068 | up | 0.765 | down | 0.005 | C00199 |
| 2′-Deoxyinosine triphosphate | 1.608 | 1.064 | up | 1.531 | up | 0.039 | C01345 |
| Azelaic acid | 1.844 | 1.015 | up | 0.771 | down | 0.008 | C08261 |
| Aniline | 1.839 | 1.013 | up | 0.580 | down | 0.011 | C00292 |
| 16-Hydroxy hexadecanoic acid | 1.835 | 1.010 | up | 1.793 | up | 0.013 | C18218 |
| Gamma-Tocopherol | 1.651 | 1.000 | down | 0.787 | down | 0.029 | C02483 |
| Oxidized glutathione | 1.737 | 0.962 | down | 1.317 | up | 0.031 | C00127 |
| Glutathione | 1.971 | 0.927 | down | 1.497 | up | 0.006 | C00051 |
| Uridine diphosphate glucose | 1.675 | 0.907 | down | 1.438 | up | 0.042 | C00029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, T.; Yu, X.; Tao, J.; Yang, L.; Duan, X. Metabolomics-Based Study of the Protective Effect of 4-Hydroxybenzyl Alcohol on Ischemic Astrocytes. Int. J. Mol. Sci. 2024, 25, 9907. https://doi.org/10.3390/ijms25189907
Xiao T, Yu X, Tao J, Yang L, Duan X. Metabolomics-Based Study of the Protective Effect of 4-Hydroxybenzyl Alcohol on Ischemic Astrocytes. International Journal of Molecular Sciences. 2024; 25(18):9907. https://doi.org/10.3390/ijms25189907
Chicago/Turabian StyleXiao, Tian, Xingzhi Yu, Jie Tao, Liping Yang, and Xiaohua Duan. 2024. "Metabolomics-Based Study of the Protective Effect of 4-Hydroxybenzyl Alcohol on Ischemic Astrocytes" International Journal of Molecular Sciences 25, no. 18: 9907. https://doi.org/10.3390/ijms25189907







