Exploring the Potential Effects of Cryopreservation on the Biological Characteristics and Cardiomyogenic Differentiation of Rat Adipose-Derived Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results
2.1. Isolation and Morphology of Rat AD-MSCs
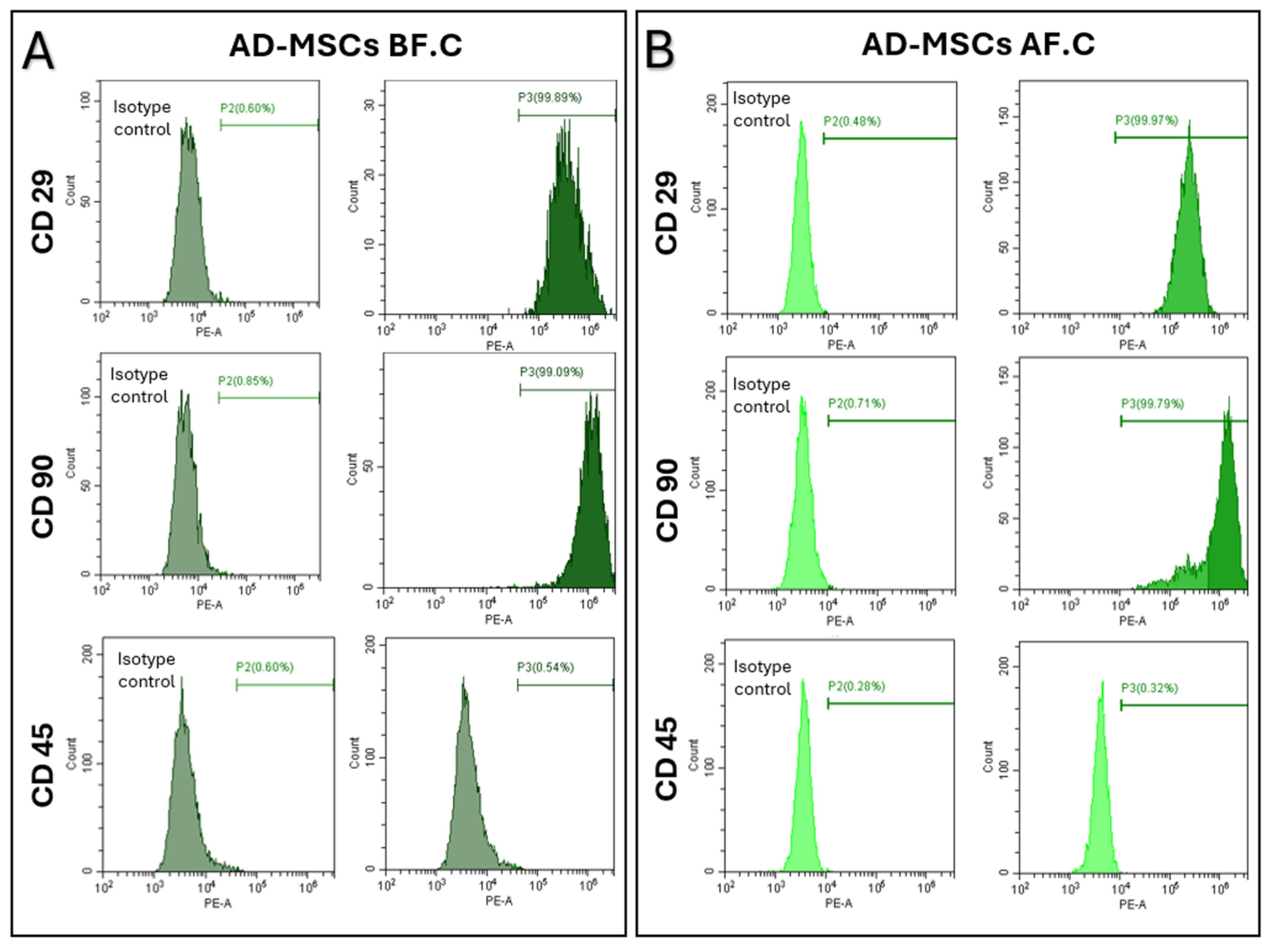
2.2. Surface Marker Expression of MSCs, Determined Using Flow Cytometry
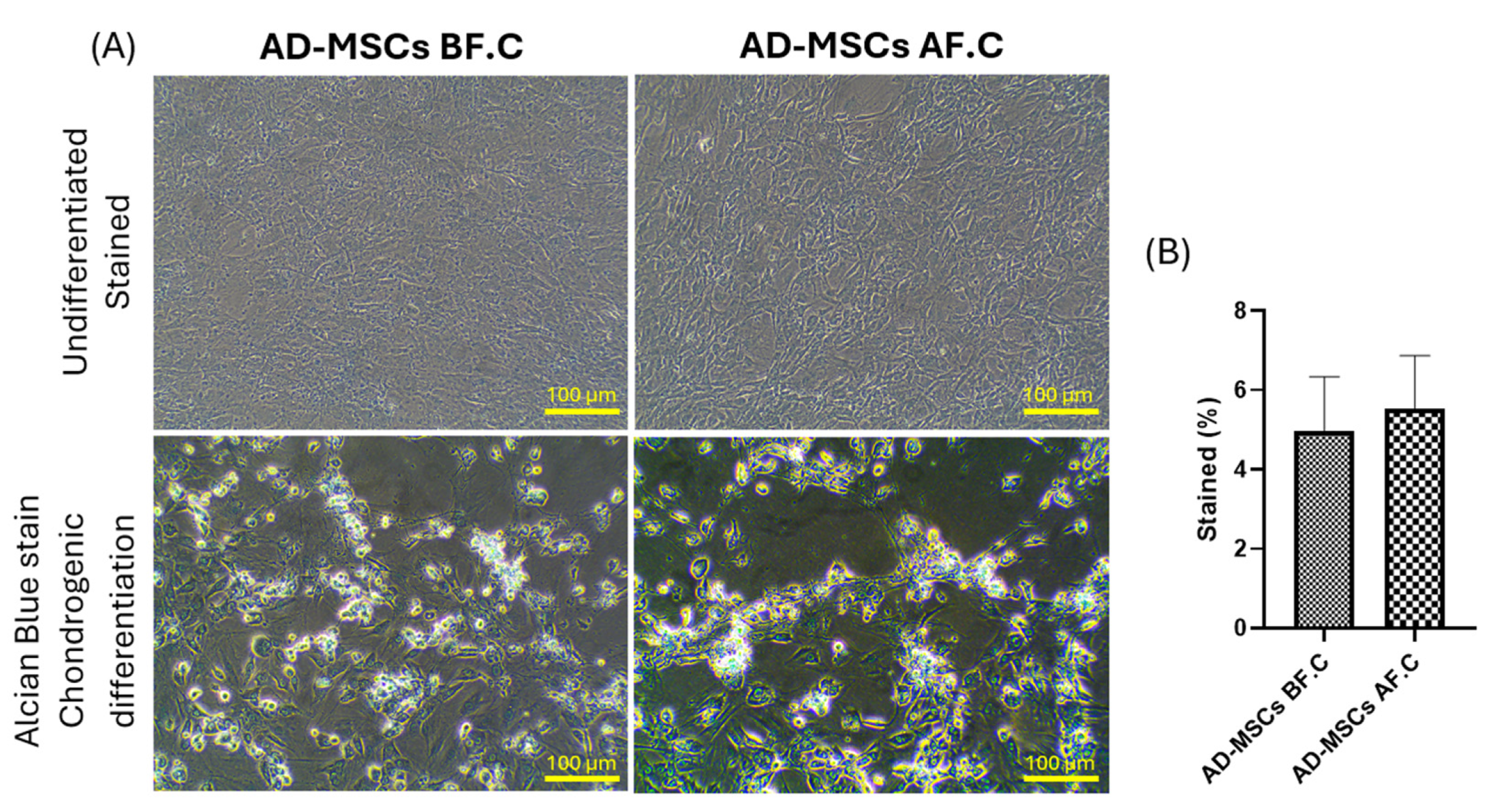
2.3. Differentiation Potential of Rat AD-MSCs
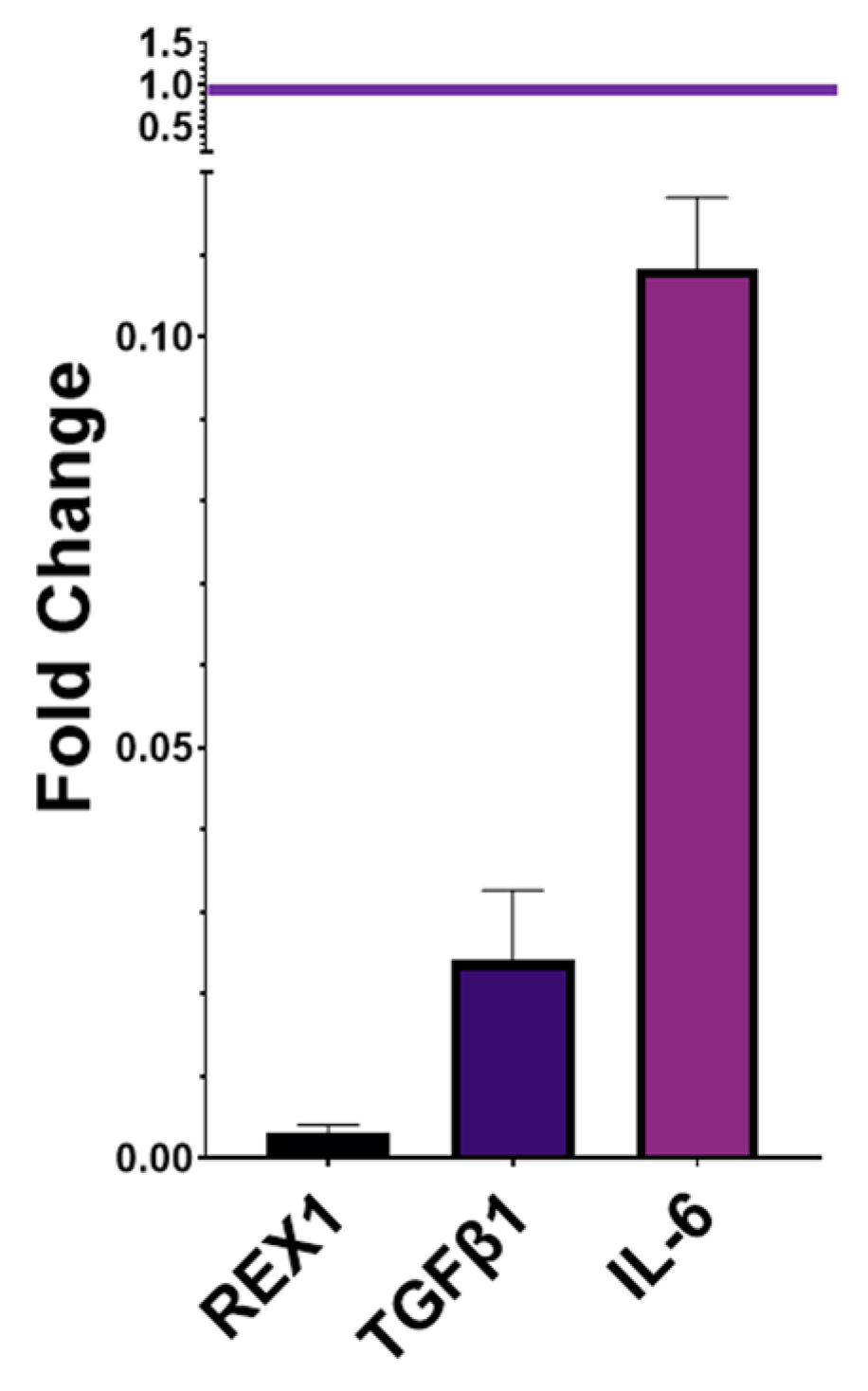
2.4. Gene Expression of Pluripotency and Immunomodulatory Markers
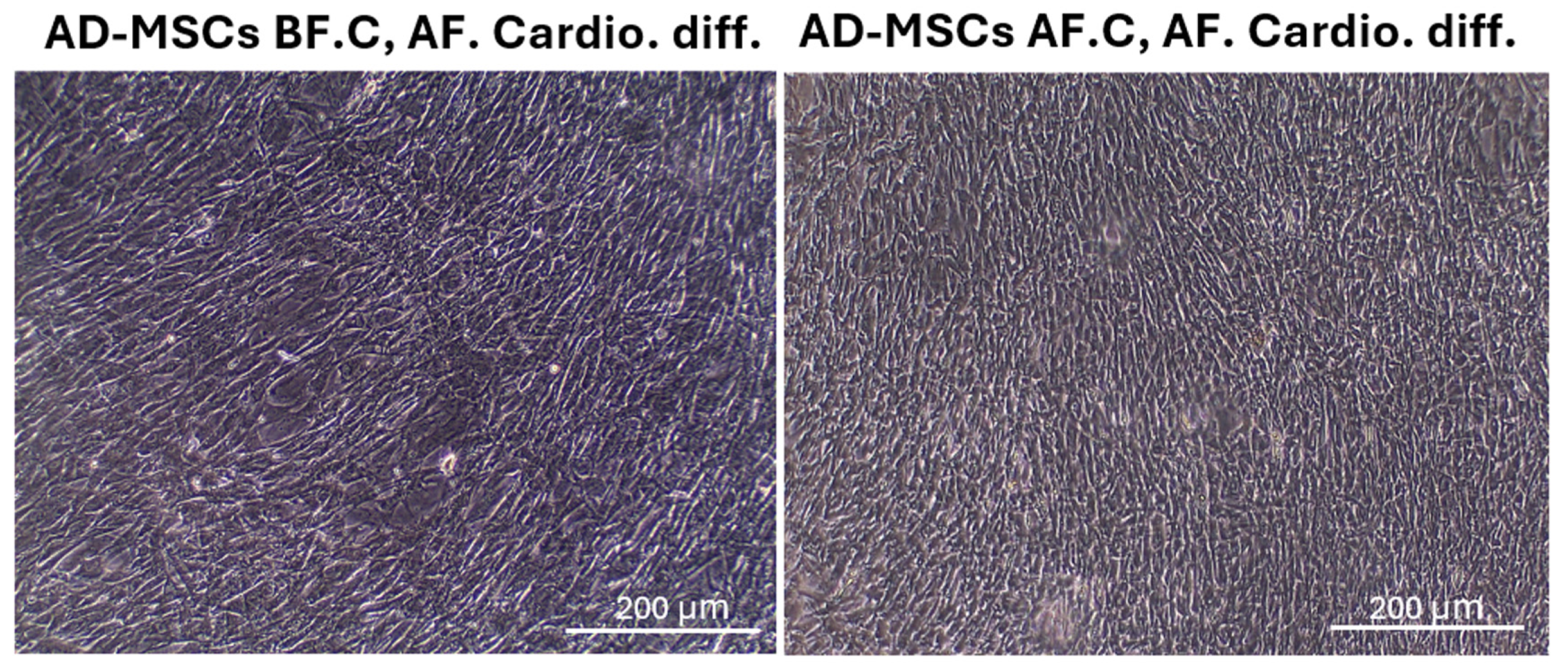
2.5. Cardiomyocyte Differentiation and Morphology after Differentiation
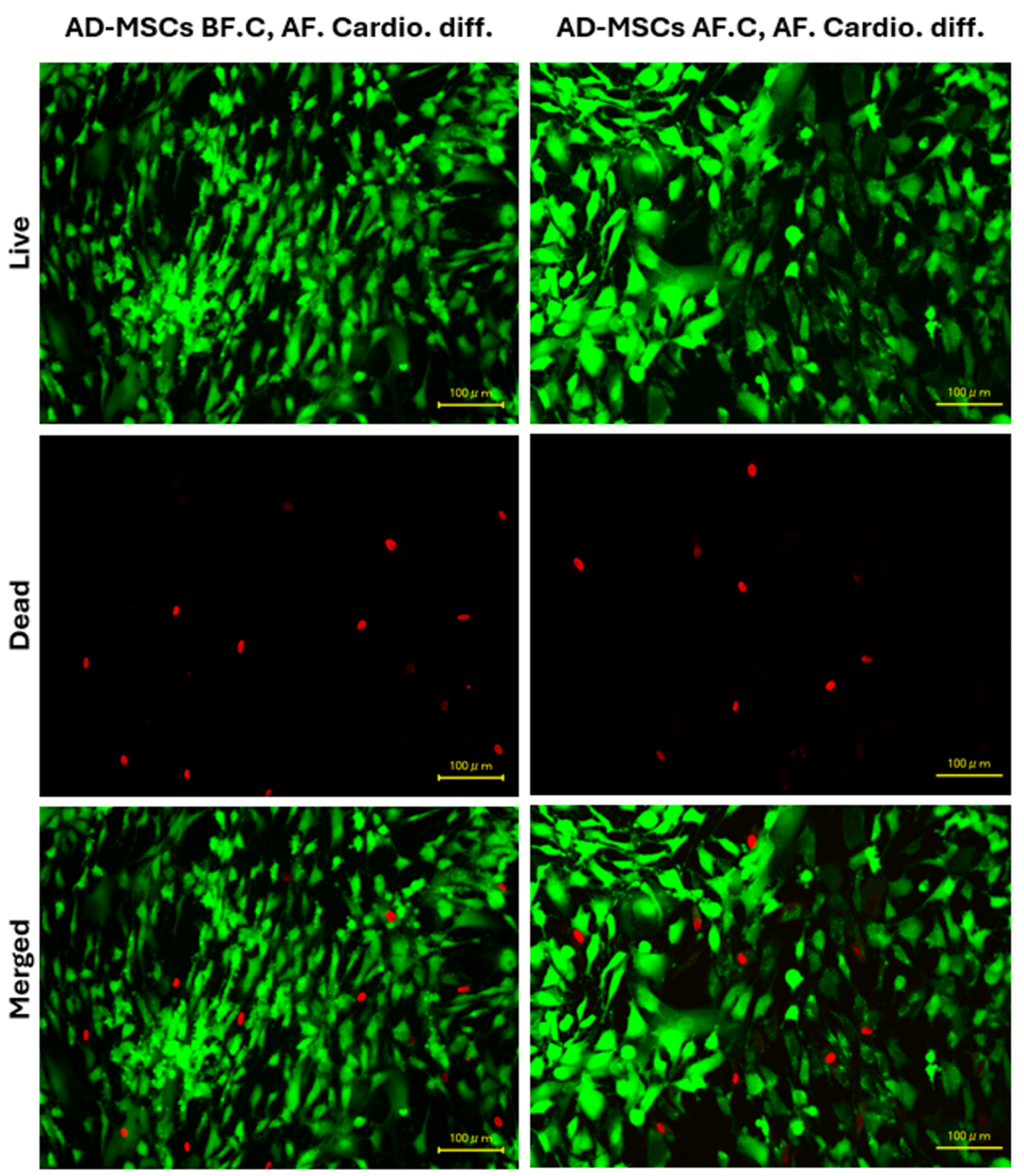
2.6. Cell Viability
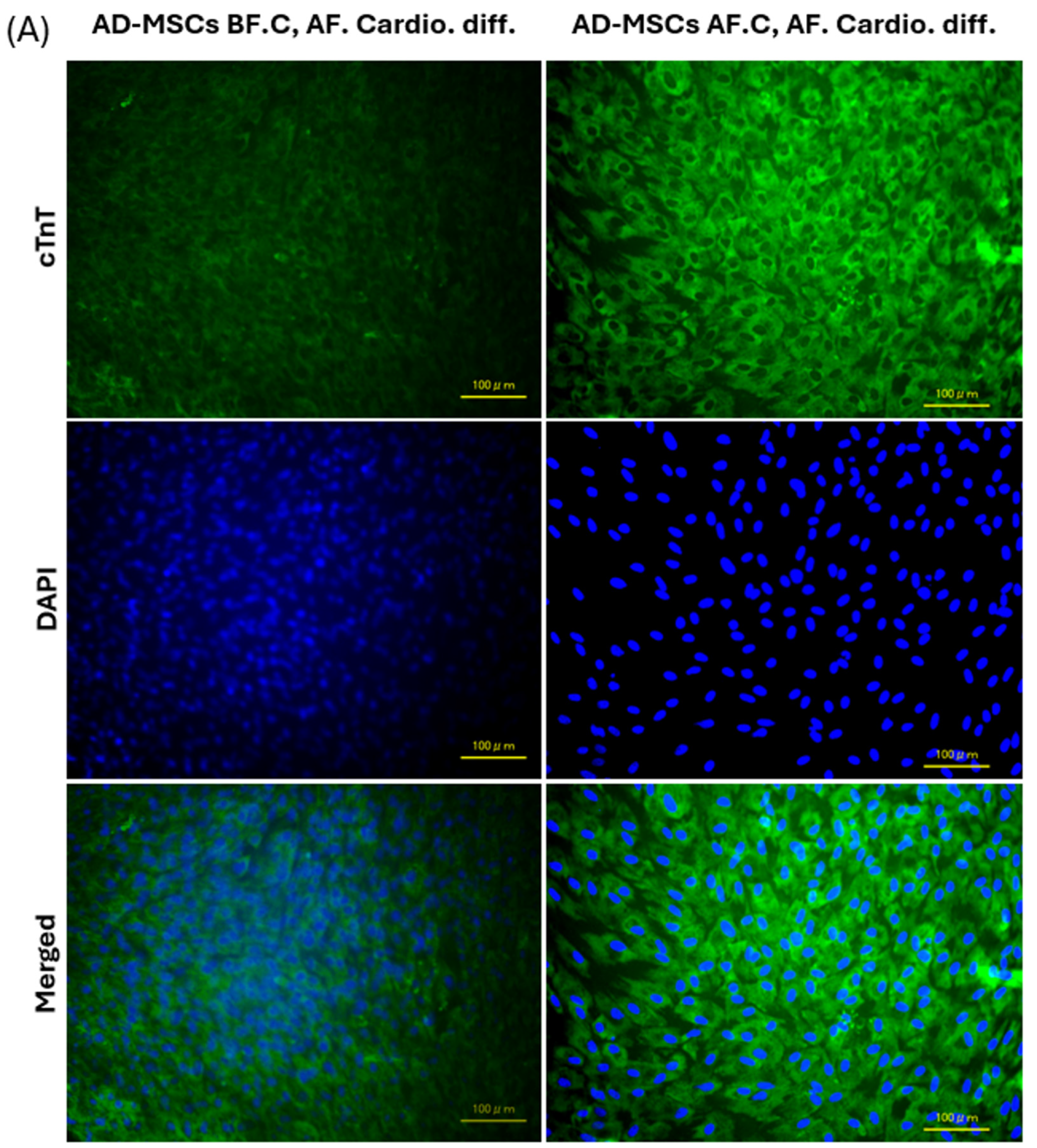
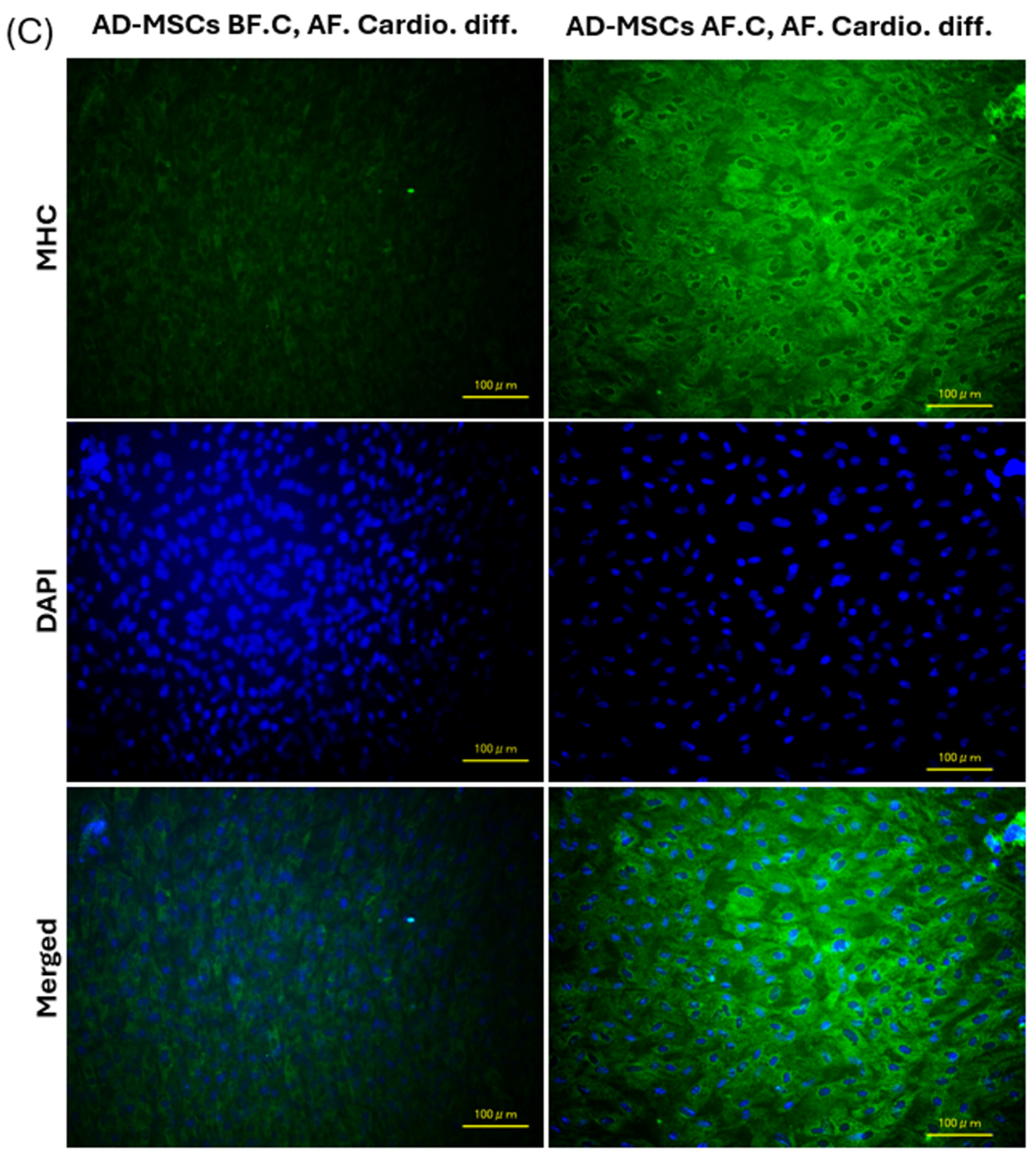
2.7. Expression of Cardiac-Specific Proteins by Immunofluorescence Staining
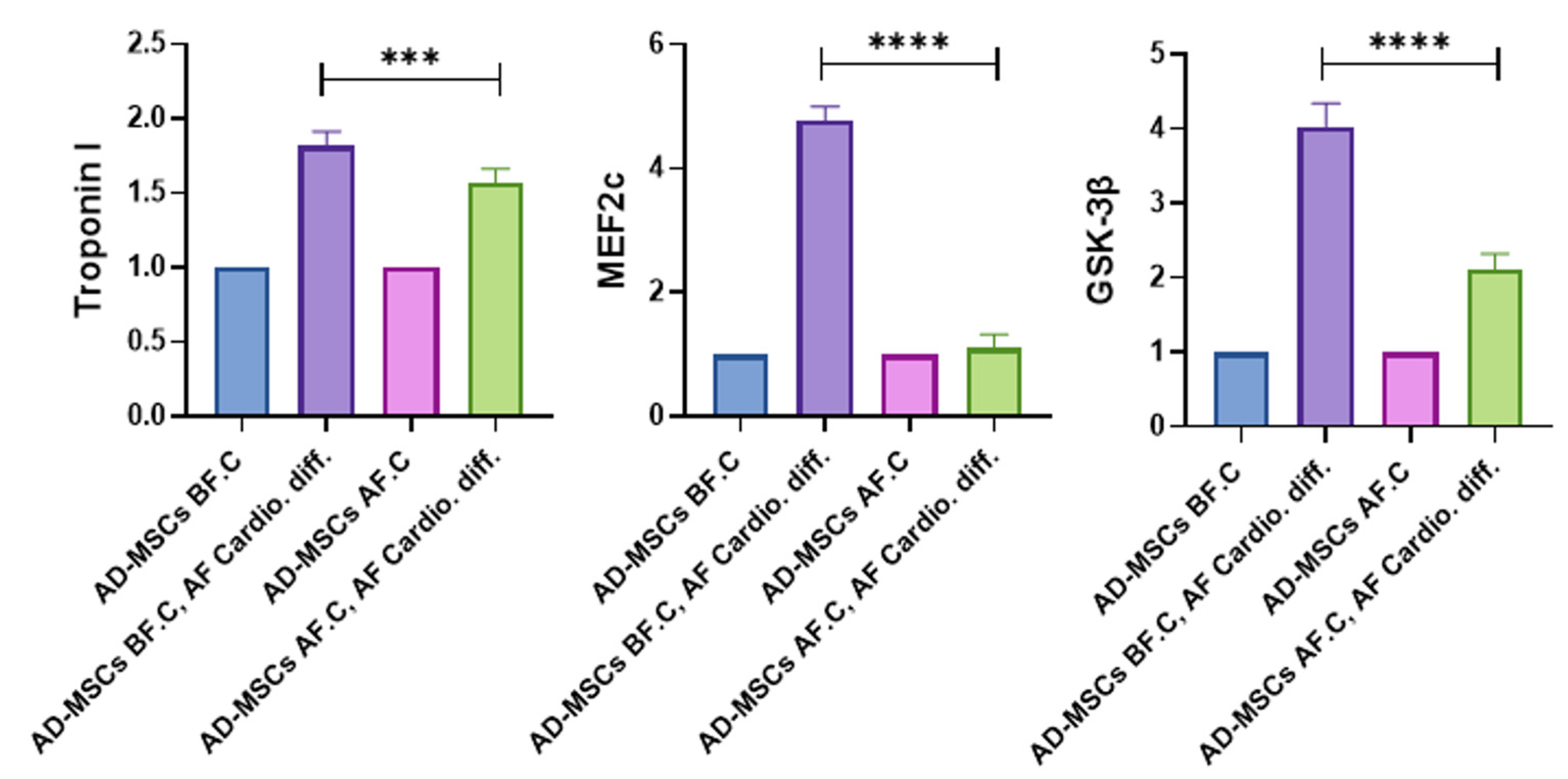
2.8. Quantitative Analysis of Cardiac-Specific Genes
3. Discussion
4. Materials and Methods
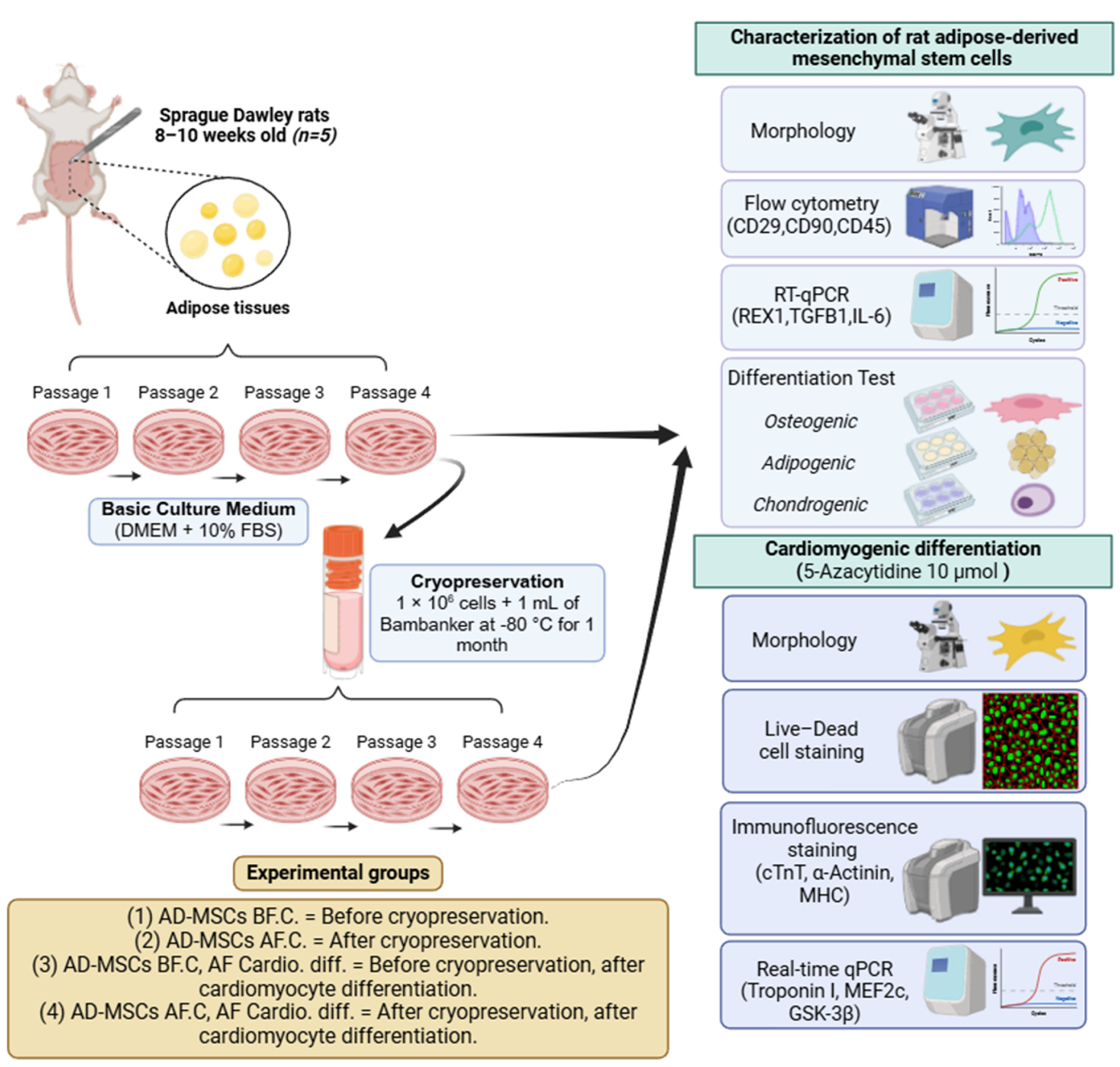
4.1. Design of Experimental Groups
4.2. Isolation and In Vitro Culture of AD-MSCs
4.3. Cryopreservation and Recovery of AD-MSCs
4.4. Cell Morphological and Immunophenotypic Characterization of AD-MSCs
4.5. AD-MSCs’ Differentiation (Adipogenesis, Osteogenesis, and Chondrogenesis)
4.5.1. Adipogenic Induction
4.5.2. Osteogenic Induction
4.5.3. Chondrogenic Induction
4.6. Gene Expression of Cultured AD-MSCs
4.7. Cardiomyogenic Differentiation
4.8. Morphological Alterations
4.9. Cell Viability Assay
4.10. Immunofluorescence Staining
4.11. Quantitative Assessment of Cardiac-Specific Genes
4.12. Statistical Analysis
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farag, A.; Mandour, A.S.; Hendawy, H.; Elhaieg, A.; Elfadadny, A.; Tanaka, R. A Review on Experimental Surgical Models and Anesthetic Protocols of Heart Failure in Rats. Front. Vet. Sci. 2023, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Mandour, A.S.; Hamabe, L.; Yoshida, T.; Shimada, K.; Tanaka, R. Novel Protocol to Establish the Myocardial Infarction Model in Rats Using a Combination of Medetomidine-Midazolam-Butorphanol (MMB) and Atipamezole. Front. Vet. Sci. 2022, 9, 1880. [Google Scholar] [CrossRef]
- Elhaieg, A.; Farag, A.; Elfadadny, A.; Yokoi, A.; Hendawy, H.; Mandour, A.S.; Tanaka, R. Effect of Experimental Periodontitis on Cardiac Functions: A Comprehensive Study Using Echocardiography, Hemodynamic Analysis, and Histopathological Evaluation in a Rat Model. Front. Vet. Sci. 2023, 10, 1327484. [Google Scholar] [CrossRef]
- Farag, A.; Mandour, A.S.; Kaneda, M.; Elfadadny, A.; Elhaieg, A.; Shimada, K.; Tanaka, R. Effect of Trehalose on Heart Functions in Rats Model after Myocardial Infarction: Assessment of Novel Intraventricular Pressure and Heart Rate Variability. Front. Cardiovasc. Med. 2023, 10, 1182628. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Elfadadny, A.; Mandour, A.S.; Ngeun, S.K.; Aboubakr, M.; Kaneda, M.; Tanaka, R. Potential Protective Effects of L-Carnitine against Myocardial Ischemia/Reperfusion Injury in a Rat Model. Environ. Sci. Pollut. Res. 2024, 31, 18813–18825. [Google Scholar] [CrossRef]
- Motoike, S.; Kajiya, M.; Komatsu, N.; Takewaki, M.; Horikoshi, S.; Matsuda, S.; Ouhara, K.; Iwata, T.; Takeda, K.; Fujita, T. Cryopreserved Clumps of Mesenchymal Stem Cell/Extracellular Matrix Complexes Retain Osteogenic Capacity and Induce Bone Regeneration. Stem Cell Res. Ther. 2018, 9, 73. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Minteer, D.; Marra, K.G.; Rubin, J.P. Adipose-Derived Mesenchymal Stem Cells: Biology and Potential Applications. In Mesenchymal Stem Cells-Basics and Clinical Application I; Springer: Berlin/Heidelberg, Germany, 2013; pp. 59–71. [Google Scholar]
- Farag, A.; Koung Ngeun, S.; Kaneda, M.; Aboubakr, M.; Tanaka, R. Optimizing Cardiomyocyte Differentiation: Comparative Analysis of Bone Marrow and Adipose-Derived Mesenchymal Stem Cells in Rats Using 5-Azacytidine and Low-Dose FGF and IGF Treatment. Biomedicines 2024, 12, 1923. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. [Google Scholar] [CrossRef]
- Abazari, A.; Hawkins, B.; Clarke, D.; Mathew, A. Biopreservation Best Practices: A Cornerstone in the Supply Chain of Cell-Based Therapies–MSC Model Case Study. Cell Gene Ther. Insights 2018, 3, 853–871. [Google Scholar] [CrossRef]
- Woods, E.J.; Thirumala, S.; Badhe-Buchanan, S.S.; Clarke, D.; Mathew, A.J. Off the Shelf Cellular Therapeutics: Factors to Consider during Cryopreservation and Storage of Human Cells for Clinical Use. Cytotherapy 2016, 18, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Mendicino, M.; Bailey, A.M.; Wonnacott, K.; Puri, R.K.; Bauer, S.R. MSC-Based Product Characterization for Clinical Trials: An FDA Perspective. Cell Stem Cell 2014, 14, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Bahsoun, S.; Coopman, K.; Akam, E.C. The Impact of Cryopreservation on Bone Marrow-Derived Mesenchymal Stem Cells: A Systematic Review. J. Transl. Med. 2019, 17, 397. [Google Scholar] [CrossRef]
- Coopman, K. Large-scale Compatible Methods for the Preservation of Human Embryonic Stem Cells: Current Perspectives. Biotechnol. Prog. 2011, 27, 1511–1521. [Google Scholar] [CrossRef]
- Marquez-Curtis, L.A.; Janowska-Wieczorek, A.; McGann, L.E.; Elliott, J.A.W. Mesenchymal Stromal Cells Derived from Various Tissues: Biological, Clinical and Cryopreservation Aspects. Cryobiology 2015, 71, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Garcia, M.A.; Sakurai, Y.; Lam, W.A.; Kirk, A.D.; Galipeau, J.; Copland, I.B. Actin Cytoskeletal Disruption Following Cryopreservation Alters the Biodistribution of Human Mesenchymal Stromal Cells in Vivo. Stem Cell Rep. 2014, 3, 60–72. [Google Scholar] [CrossRef]
- Moll, G.; Geißler, S.; Catar, R.; Ignatowicz, L.; Hoogduijn, M.J.; Strunk, D.; Bieback, K.; Ringdén, O. Cryopreserved or Fresh Mesenchymal Stromal Cells: Only a Matter of Taste or Key to Unleash the Full Clinical Potential of MSC Therapy? In Biobanking and Cryopreservation of Stem Cells. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; pp. 77–98. [Google Scholar]
- Shim, H.; Gutiérrez-Adán, A.; Chen, L.-R.; BonDurant, R.H.; Behboodi, E.; Anderson, G.B. Isolation of Pluripotent Stem Cells from Cultured Porcine Primordial Germ Cells. Biol. Reprod. 1997, 57, 1089–1095. [Google Scholar] [CrossRef]
- Robertson, E.J. Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. In Embryo-Derived Stem Cell Lines; IRL Press: Oxford, UK, 1987; pp. 71–112. [Google Scholar]
- Grout, B.; Morris, J.; McLellan, M. Cryopreservation and the Maintenance of Cell Lines. Trends Biotechnol. 1990, 8, 293–297. [Google Scholar] [CrossRef]
- Ji, L.; de Pablo, J.J.; Palecek, S.P. Cryopreservation of Adherent Human Embryonic Stem Cells. Biotechnol. Bioeng. 2004, 88, 299–312. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Pera, M.F.; Vajta, G.; Trounson, A.O. Effective Cryopreservation of Human Embryonic Stem Cells by the Open Pulled Straw Vitrification Method. Hum. Reprod. 2001, 16, 2187–2194. [Google Scholar] [CrossRef]
- Richards, M.; Fong, C.; Tan, S.; Chan, W.; Bongso, A. An Efficient and Safe Xeno-free Cryopreservation Method for the Storage of Human Embryonic Stem Cells. Stem Cells 2004, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]
- François, M.; Copland, I.B.; Yuan, S.; Romieu-Mourez, R.; Waller, E.K.; Galipeau, J. Cryopreserved Mesenchymal Stromal Cells Display Impaired Immunosuppressive Properties as a Result of Heat-Shock Response and Impaired Interferon-γ Licensing. Cytotherapy 2012, 14, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Alm, J.J.; Davies, L.C.; von Bahr, L.; Heldring, N.; Stenbeck-Funke, L.; Hamad, O.A.; Hinsch, R.; Ignatowicz, L.; Locke, M. Do Cryopreserved Mesenchymal Stromal Cells Display Impaired Immunomodulatory and Therapeutic Properties? Stem Cells 2014, 32, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Koung Ngeun, S.; Shimizu, M.; Kaneda, M. Characterization of Rabbit Mesenchymal Stem/Stromal Cells after Cryopreservation. Biology 2023, 12, 1312. [Google Scholar] [CrossRef]
- Agata, H.; Sumita, Y.; Hidaka, T.; Iwatake, M.; Kagami, H.; Asahina, I. Intra-bone Marrow Administration of Mesenchymal Stem/Stromal Cells Is a Promising Approach for Treating Osteoporosis. Stem Cells Int. 2019, 2019, 4214281. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Yang, J.-C.; Wang, C.-W.; Lee, S.-Y. Dental Stem Cells and Tooth Banking for Regenerative Medicine. J. Exp. Clin. Med. 2010, 2, 111–117. [Google Scholar] [CrossRef]
- Hernández, Y.G.; Fischer, R.W. Serum-Free Culturing of Mammalian Cells-Adaptation to and Cryopreservation in Fully Defined Media. ALTEX-Altern. Anim. Exp. 2007, 24, 110–116. [Google Scholar]
- Linkova, D.D.; Rubtsova, Y.P.; Egorikhina, M.N. Cryostorage of Mesenchymal Stem Cells and Biomedical Cell-Based Products. Cells 2022, 11, 2691. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Cryoprotectants and Their Usage in Cryopreservation Process. In Cryopreservation Biotechnology in Biomedical and Biological Sciences; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Pal, R.; Mamidi, M.K.; Das, A.K.; Bhonde, R. Diverse Effects of Dimethyl Sulfoxide (DMSO) on the Differentiation Potential of Human Embryonic Stem Cells. Arch. Toxicol. 2012, 86, 651–661. [Google Scholar] [CrossRef]
- McCullough, J.; Haley, R.; Clay, M.; Hubel, A.; Lindgren, B.; Moroff, G. Long-term Storage of Peripheral Blood Stem Cells Frozen and Stored with a Conventional Liquid Nitrogen Technique Compared with Cells Frozen and Stored in a Mechanical Freezer. Transfusion 2010, 50, 808–819. [Google Scholar] [CrossRef]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R.T. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transplant. 2021, 30, 0963689721999617. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Pingguan-Murphy, B.; Wan Abas, W.A.B.; Yong, K.W.; Poon, C.T.; Noor Azmi, M.A.; Omar, S.Z.; Chua, K.H.; Xu, F.; Wan Safwani, W.K.Z. In Situ Normoxia Enhances Survival and Proliferation Rate of Human Adipose Tissue-Derived Stromal Cells without Increasing the Risk of Tumourigenesis. PLoS ONE 2015, 10, e0115034. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- De Rosa, A.; De Francesco, F.; Tirino, V.; Ferraro, G.A.; Desiderio, V.; Paino, F.; Pirozzi, G.; D’Andrea, F.; Papaccio, G. A New Method for Cryopreserving Adipose-Derived Stem Cells: An Attractive and Suitable Large-Scale and Long-Term Cell Banking Technology. Tissue Eng. Part C Methods 2009, 15, 659–667. [Google Scholar] [CrossRef]
- Al-Saqi, S.H.; Saliem, M.; Quezada, H.C.; Ekblad, Å.; Jonasson, A.F.; Hovatta, O.; Götherström, C. Defined Serum-and Xeno-Free Cryopreservation of Mesenchymal Stem Cells. Cell Tissue Bank. 2015, 16, 181–193. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Chen, H.; Zhao, H.; Dai, Y.-W.; Xu, R.-X. Neural Stem Cells Differentiation Ability of Human Umbilical Cord Mesenchymal Stromal Cells Is Not Altered by Cryopreservation. Neurosci. Lett. 2011, 487, 118–122. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, F.; Li, H.; Zheng, Y.; Xiao, Y.; Yan, F. Evaluation of Canine Bone Marrow-Derived Mesenchymal Stem Cells after Long-Term Cryopreservation. Zoolog. Sci. 2013, 30, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Choi, J.R.; Pingguan-Murphy, B.; Abas, W.A.B.W.; Azmi, M.A.N.; Omar, S.Z.; Chua, K.H.; Safwani, W.K.Z.W. Hypoxia Promotes Growth and Viability of Human Adipose-Derived Stem Cells with Increased Growth Factors Secretion. J. Asian Sci. Res. 2014, 4, 328. [Google Scholar]
- Janz, F.d.L.; Debes, A.d.A.; Cavaglieri, R.d.C.; Duarte, S.A.; Romão, C.M.; Morón, A.F.; Zugaib, M.; Bydlowski, S.P. Evaluation of Distinct Freezing Methods and Cryoprotectants for Human Amniotic Fluid Stem Cells Cryopreservation. Biomed. Res. Int. 2012, 2012, 649353. [Google Scholar] [CrossRef]
- Davies, O.G.; Smith, A.J.; Cooper, P.R.; Shelton, R.M.; Scheven, B.A. The Effects of Cryopreservation on Cells Isolated from Adipose, Bone Marrow and Dental Pulp Tissues. Cryobiology 2014, 69, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Naaldijk, Y.; Staude, M.; Fedorova, V.; Stolzing, A. Effect of Different Freezing Rates during Cryopreservation of Rat Mesenchymal Stem Cells Using Combinations of Hydroxyethyl Starch and Dimethylsulfoxide. BMC Biotechnol. 2012, 12, 49. [Google Scholar] [CrossRef]
- Ginis, I.; Grinblat, B.; Shirvan, M.H. Evaluation of Bone Marrow-Derived Mesenchymal Stem Cells after Cryopreservation and Hypothermic Storage in Clinically Safe Medium. Tissue Eng. Part C Methods 2012, 18, 453–463. [Google Scholar] [CrossRef]
- Angelo, P.C.; Ferreira, A.C.; Fonseca, V.D.; Frade, S.P.; Ferreira, C.S.; Malta, F.S.; Pereira, A.K.; Leite, H.V.; Brum, A.P.; Pardini, V.C. Cryopreservation Does Not Alter Karyotype, Multipotency, or NANOG/SOX2 Gene Expression of Amniotic Fluid Mesenchymal Stem Cells. Genet. Mol. Res. 2012, 11, 1002–1012. [Google Scholar] [CrossRef]
- Minonzio, G.; Corazza, M.; Mariotta, L.; Gola, M.; Zanzi, M.; Gandolfi, E.; De Fazio, D.; Soldati, G. Frozen Adipose-Derived Mesenchymal Stem Cells Maintain High Capability to Grow and Differentiate. Cryobiology 2014, 69, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Sayago, J.M.; Fernandez-Arcas, N.; Benito, C.; Reyes-Engel, A.; Herrero, J.R.; Alonso, A. Evaluation of a Low Cost Cryopreservation System on the Biology of Human Amniotic Fluid-Derived Mesenchymal Stromal Cells. Cryobiology 2012, 64, 160–166. [Google Scholar] [CrossRef]
- Lauterboeck, L.; Saha, D.; Chatterjee, A.; Hofmann, N.; Glasmacher, B. Xeno-Free Cryopreservation of Bone Marrow-Derived Multipotent Stromal Cells from Callithrix Jacchus. Biopreserv. Biobank. 2016, 14, 530–538. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Ma, X.; Liu, J.; Cui, Z. Effect of Various Freezing Solutions on Cryopreservation of Mesenchymal Stem Cells from Different Animal Species. CryoLetters 2011, 32, 425–435. [Google Scholar] [PubMed]
- Hirose, M.; Kotobuki, N.; Machida, H.; Kitamura, S.; Ohgushi, H.; Tateishi, T. Osteogenic Potential of Cryopreserved Human Bone Marrow-Derived Mesenchymal Cells after Thawing in Culture. Mater. Sci. Eng. C 2004, 24, 355–359. [Google Scholar] [CrossRef]
- Bruder, S.P.; Jaiswal, N.; Haynesworth, S.E. Growth Kinetics, Self-renewal, and the Osteogenic Potential of Purified Human Mesenchymal Stem Cells during Extensive Subcultivation and Following Cryopreservation. J. Cell. Biochem. 1997, 64, 278–294. [Google Scholar] [CrossRef]
- Mamidi, M.K.; Nathan, K.G.; Singh, G.; Thrichelvam, S.T.; Mohd Yusof, N.A.N.; Fakharuzi, N.A.; Zakaria, Z.; Bhonde, R.; Das, A.K.; Majumdar, A. Sen Comparative Cellular and Molecular Analyses of Pooled Bone Marrow Multipotent Mesenchymal Stromal Cells during Continuous Passaging and after Successive Cryopreservation. J. Cell. Biochem. 2012, 113, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, K.; Hayashi, F.; Nagashima, T.; Hyon, S.H. Long-Term Cryopreservation of Human Mesenchymal Stem Cells Using Carboxylated Poly-l-Lysine without the Addition of Proteins or Dimethyl Sulfoxide. J. Biomater. Sci. Polym. Ed. 2013, 24, 1484–1497. [Google Scholar] [CrossRef]
- James, A.W.; Levi, B.; Nelson, E.R.; Peng, M.; Commons, G.W.; Lee, M.; Wu, B.; Longaker, M.T. Deleterious Effects of Freezing on Osteogenic Differentiation of Human Adipose-Derived Stromal Cells in Vitro and in Vivo. Stem Cells Dev. 2011, 20, 427–439. [Google Scholar] [CrossRef]
- Heino, T.J.; Alm, J.J.; Moritz, N.; Aro, H.T. Comparison of the Osteogenic Capacity of Minipig and Human Bone Marrow-derived Mesenchymal Stem Cells. J. Orthop. Res. 2012, 30, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Tokumoto, S.; Sotome, S.; Torigoe, I.; Omura, K.; Shinomiya, K. Effects of Cryopreservation on Bone Marrow Derived Mesenchymal Cells of a Nonhuman Primate. J. Med. Dent. Sci. 2008, 55, 137–143. [Google Scholar]
- Chua, K.-H.; Safwani, W.K.Z.W.; Hamid, A.A.; Shuhup, S.K.; Haflah, N.H.M.; Yahaya, N.H.M. Retropatellar Fat Pad–Derived Stem Cells from Older Osteoarthritic Patients Have Lesser Differentiation Capacity and Expression of Stemness Genes. Cytotherapy 2014, 16, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R.; Pingguan-Murphy, B.; Abas, W.A.B.W.; Azmi, M.A.N.; Omar, S.Z.; Chua, K.H.; Safwani, W.K.Z.W. Impact of Low Oxygen Tension on Stemness, Proliferation and Differentiation Potential of Human Adipose-Derived Stem Cells. Biochem. Biophys. Res. Commun. 2014, 448, 218–224. [Google Scholar] [CrossRef]
- Izadpanah, R.; Trygg, C.; Patel, B.; Kriedt, C.; Dufour, J.; Gimble, J.M.; Bunnell, B.A. Biologic Properties of Mesenchymal Stem Cells Derived from Bone Marrow and Adipose Tissue. J. Cell. Biochem. 2006, 99, 1285–1297. [Google Scholar] [CrossRef]
- Yong, K.W.; Pingguan-Murphy, B.; Xu, F.; Abas, W.A.B.W.; Choi, J.R.; Omar, S.Z.; Azmi, M.A.N.; Chua, K.H.; Safwani, W.K.Z.W. Phenotypic and Functional Characterization of Long-Term Cryopreserved Human Adipose-Derived Stem Cells. Sci. Rep. 2015, 5, 9596. [Google Scholar] [CrossRef]
- Pierantozzi, E.; Gava, B.; Manini, I.; Roviello, F.; Marotta, G.; Chiavarelli, M.; Sorrentino, V. Pluripotency Regulators in Human Mesenchymal Stem Cells: Expression of NANOG but Not of OCT-4 and SOX-2. Stem Cells Dev. 2011, 20, 915–923. [Google Scholar] [CrossRef]
- Han, S.; Zhao, Y.; Xiao, Z.; Han, J.; Chen, B.; Chen, L.; Dai, J. The Three-Dimensional Collagen Scaffold Improves the Stemness of Rat Bone Marrow Mesenchymal Stem Cells. J. Genet. Genom. 2012, 39, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Bárcia, R.N.; Santos, J.M.; Filipe, M.; Teixeira, M.; Martins, J.P.; Almeida, J.; Água-Doce, A.; Almeida, S.C.P.; Varela, A.; Pohl, S. What Makes Umbilical Cord Tissue-derived Mesenchymal Stromal Cells Superior Immunomodulators When Compared to Bone Marrow Derived Mesenchymal Stromal Cells? Stem Cells Int. 2015, 2015, 583984. [Google Scholar] [CrossRef] [PubMed]
- Di Trapani, M.; Bassi, G.; Ricciardi, M.; Fontana, E.; Bifari, F.; Pacelli, L.; Giacomello, L.; Pozzobon, M.; Féron, F.; De Coppi, P. Comparative Study of Immune Regulatory Properties of Stem Cells Derived from Different Tissues. Stem Cells Dev. 2013, 22, 2990–3002. [Google Scholar] [CrossRef]
- Luetzkendorf, J.; Nerger, K.; Hering, J.; Moegel, A.; Hoffmann, K.; Hoefers, C.; Mueller-Tidow, C.; Mueller, L.P. Cryopreservation Does Not Alter Main Characteristics of Good Manufacturing Process–Grade Human Multipotent Mesenchymal Stromal Cells Including Immunomodulating Potential and Lack of Malignant Transformation. Cytotherapy 2015, 17, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Semenov, O.V.; Koestenbauer, S.; Riegel, M.; Zech, N.; Zimmermann, R.; Zisch, A.H.; Malek, A. Multipotent Mesenchymal Stem Cells from Human Placenta: Critical Parameters for Isolation and Maintenance of Stemness after Isolation. Am. J. Obstet. Gynecol. 2010, 202, 193.e1–193.e13. [Google Scholar] [CrossRef]
- Bobis, S.; Jarocha, D.; Majka, M. Mesenchymal Stem Cells: Characteristics and Clinical Applications. Folia Histochem. Cytobiol. 2006, 44, 215–230. [Google Scholar]
- Rastegar, F.; Shenaq, D.; Huang, J.; Zhang, W.; Zhang, B.-Q.; He, B.-C.; Chen, L.; Zuo, G.-W.; Luo, Q.; Shi, Q. Mesenchymal Stem Cells: Molecular Characteristics and Clinical Applications. World J. Stem Cells 2010, 2, 67. [Google Scholar]
- Antebi, B.; Asher, A.M.; Rodriguez, L.A.; Moore, R.K.; Mohammadipoor, A.; Cancio, L.C. Cryopreserved Mesenchymal Stem Cells Regain Functional Potency Following a 24-h Acclimation Period. J. Transl. Med. 2019, 17, 297. [Google Scholar] [CrossRef]
- Zhou, B.; Shi, X.; Tang, X.; Zhao, Q.; Wang, L.; Yao, F.; Hou, Y.; Wang, X.; Feng, W.; Wang, L. Functional Isolation, Culture and Cryopreservation of Adult Human Primary Cardiomyocytes. Signal Transduct. Target. Ther. 2022, 7, 254. [Google Scholar] [CrossRef]
- Kuroda, Y.; Wakao, S.; Kitada, M.; Murakami, T.; Nojima, M.; Dezawa, M. Isolation, Culture and Evaluation of Multilineage-Differentiating Stress-Enduring (Muse) Cells. Nat. Protoc. 2013, 8, 1391–1415. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed]
- Thirumala, S.; Gimble, J.M.; Devireddy, R. V Evaluation of Methylcellulose and Dimethyl Sulfoxide as the Cryoprotectants in a Serum-Free Freezing Media for Cryopreservation of Adipose-Derived Adult Stem Cells. Stem Cells Dev. 2010, 19, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, T. Bioprocessing of Cryopreservation for Large-Scale Banking of Human Pluripotent Stem Cells. Biores. Open Access 2012, 1, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Linch, D.C.; Knott, L.J.; Patterson, K.G.; Cowan, D.A.; Harper, P.G. Bone Marrow Processing and Cryopreservation. J. Clin. Pathol. 1982, 35, 186–190. [Google Scholar] [CrossRef]
- Hammarsten, O.; Mair, J.; Möckel, M.; Lindahl, B.; Jaffe, A.S. Possible Mechanisms behind Cardiac Troponin Elevations. Biomarkers 2018, 23, 725–734. [Google Scholar] [CrossRef]
- Fan, X.; Hughes, B.G.; Ali, M.A.M.; Cho, W.J.; Lopez, W.; Schulz, R. Dynamic Alterations to α-Actinin Accompanying Sarcomere Disassembly and Reassembly during Cardiomyocyte Mitosis. PLoS ONE 2015, 10, e0129176. [Google Scholar] [CrossRef]
- Ban, K.; Wile, B.; Kim, S.; Park, H.-J.; Byun, J.; Cho, K.-W.; Saafir, T.; Song, M.-K.; Yu, S.P.; Wagner, M. Purification of Cardiomyocytes from Differentiating Pluripotent Stem Cells Using Molecular Beacons That Target Cardiomyocyte-Specific MRNA. Circulation 2013, 128, 1897–1909. [Google Scholar] [CrossRef]
- Fu, X.; Xu, B.; Jiang, J.; Du, X.; Yu, X.; Yan, Y.; Li, S.; Inglis, B.M.; Ma, H.; Wang, H. Effects of Cryopreservation and Long-Term Culture on Biological Characteristics and Proteomic Profiles of Human Umbilical Cord-Derived Mesenchymal Stem Cells. Clin. Proteomics 2020, 17, 15. [Google Scholar] [CrossRef]
- Di Giuseppe, F.; Pierdomenico, L.; Eleuterio, E.; Sulpizio, M.; Lanuti, P.; Riviello, A.; Bologna, G.; Gesi, M.; Di Ilio, C.; Miscia, S. Cryopreservation Effects on Wharton’s Jelly Stem Cells Proteome. Stem Cell Rev. Rep. 2014, 10, 429–446. [Google Scholar] [CrossRef]
- Bissoyi, A.; Nayak, B.; Pramanik, K.; Sarangi, S.K. Targeting Cryopreservation-Induced Cell Death: A Review. Biopreserv. Biobank. 2014, 12, 23–34. [Google Scholar] [CrossRef]
- Nynca, J.; Arnold, G.J.; Fröhlich, T.; Ciereszko, A. Cryopreservation-induced Alterations in Protein Composition of Rainbow Trout Semen. Proteomics 2015, 15, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Eisen, H.N. Affinity Enhancement of Antibodies: How Low-Affinity Antibodies Produced Early in Immune Responses Are Followed by High-Affinity Antibodies Later and in Memory B-Cell Responses. Cancer Immunol. Res. 2014, 2, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.; Koshkin, A.; Carido, M.; Espinha, N.; Šarić, T.; Lima, P.A.; Serra, M.; Alves, P.M. Effective Hypothermic Storage of Human Pluripotent Stem Cell-Derived Cardiomyocytes Compatible with Global Distribution of Cells for Clinical Applications and Toxicology Testing. Stem Cells Transl. Med. 2016, 5, 658–669. [Google Scholar] [CrossRef]
- van den Brink, L.; Brandão, K.O.; Yiangou, L.; Mol, M.P.H.; Grandela, C.; Mummery, C.L.; Verkerk, A.O.; Davis, R.P. Cryopreservation of Human Pluripotent Stem Cell-Derived Cardiomyocytes Is Not Detrimental to Their Molecular and Functional Properties. Stem Cell Res. 2020, 43, 101698. [Google Scholar] [CrossRef]
- Lin, Q.; Schwarz, J.; Bucana, C.; Olson, E.N. Control of Mouse Cardiac Morphogenesis and Myogenesis by Transcription Factor MEF2C. Science 1997, 276, 1404–1407. [Google Scholar] [CrossRef]
- Hardt, S.E.; Sadoshima, J. Glycogen Synthase Kinase-3β: A Novel Regulator of Cardiac Hypertrophy and Development. Circ. Res. 2002, 90, 1055–1063. [Google Scholar] [CrossRef]
- Lee, T.; Hwang, S.; Seo, D.; Cho, S.; Yang, S.; Kim, H.; Kim, J.; Uh, Y. Comparative Analysis of Biological Signatures between Freshly Preserved and Cryo-Preserved Bone Marrow Mesenchymal Stem Cells. Cells 2023, 12, 2355. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Cui, Z.F. Effects of Cryopreservation on Human Mesenchymal Stem Cells Attached to Different Substrates. J. Tissue Eng. Regen. Med. 2014, 8, 664–672. [Google Scholar] [CrossRef]
- Yong, K.W.; Wan Safwani, W.K.Z.; Xu, F.; Wan Abas, W.A.B.; Choi, J.R.; Pingguan-Murphy, B. Cryopreservation of Human Mesenchymal Stem Cells for Clinical Applications: Current Methods and Challenges. Biopreserv. Biobank. 2015, 13, 231–239. [Google Scholar] [CrossRef]
- Siennicka, K.; Zołocińska, A.; Dębski, T.; Pojda, Z. Comparison of the Donor Age-Dependent and in Vitro Culture-Dependent Mesenchymal Stem Cell Aging in Rat Model. Stem Cells Int. 2021, 2021, 6665358. [Google Scholar] [CrossRef]
- Tapp, H.; Deepe, R.; Ingram, J.A.; Kuremsky, M.; Hanley, E.N.; Gruber, H.E. Adipose-Derived Mesenchymal Stem Cells from the Sand Rat: Transforming Growth Factor Beta and 3D Co-Culture with Human Disc Cells Stimulate Proteoglycan and Collagen Type I Rich Extracellular Matrix. Arthritis Res. Ther. 2008, 10, R89. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ye, Z.; Zhou, Y.; Tan, W.-S. Comparative Study of Mesenchymal Stem Cells from Rat Bone Marrow and Adipose Tissue. Turkish J. Biol. 2018, 42, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, X.; Zhang, C.; Li, R.; Zeng, Q.; Guo, C.; Zhang, Y. Effects of Mechanical Stimulus on Mesenchymal Stem Cells Differentiation toward Cardiomyocytes. Asian Biomed. 2011, 5, 655–661. [Google Scholar]
- Xinyun, C.; Zhi, Z.; Bin, Z.; Li, R.; Yucheng, C.; Yafei, Y.; Tingjie, Z.; Shengfu, L. Effects of Cardiotrophin-1 on Differentiation and Maturation of Rat Bone Marrow Mesenchymal Stem Cells Induced with 5-Azacytidine in Vitro. Int. J. Cardiol. 2010, 143, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ouyang, Y.; Chang, Y.; Luo, C.; Xu, J.; Zhang, C.; Huang, W. Evaluation of the Proliferation and Differentiation Behaviors of Mesenchymal Stem Cells with Partially Converted Borate Glass Containing Different Amounts of Strontium in Vitro. Mol. Med. Rep. 2013, 7, 1129–1136. [Google Scholar] [CrossRef]
- Haleem-Smith, H.; Argintar, E.; Bush, C.; Hampton, D.; Postma, W.F.; Chen, F.H.; Rimington, T.; Lamb, J.; Tuan, R.S. Biological Responses of Human Mesenchymal Stem Cells to Titanium Wear Debris Particles. J. Orthop. Res. 2012, 30, 853–863. [Google Scholar] [CrossRef]
- Yi, Q.; Xu, H.; Yang, K.; Wang, Y.; Tan, B.; Tian, J.; Zhu, J. Islet-1 Induces the Differentiation of Mesenchymal Stem Cells into Cardiomyocyte-like Cells through the Regulation of Gcn5 and DNMT-1. Mol. Med. Rep. 2017, 15, 2511–2520. [Google Scholar] [CrossRef]
- Grafton, F.; Ho, J.; Ranjbarvaziri, S.; Farshidfar, F.; Budan, A.; Steltzer, S.; Maddah, M.; Loewke, K.E.; Green, K.; Patel, S. Deep Learning Detects Cardiotoxicity in a High-Content Screen with Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Elife 2021, 10, e68714. [Google Scholar] [CrossRef]
- Zhang, J.; Ho, J.C.; Chan, Y.; Lian, Q.; Siu, C.; Tse, H. Overexpression of Myocardin Induces Partial Transdifferentiation of Human-induced Pluripotent Stem Cell-derived Mesenchymal Stem Cells into Cardiomyocytes. Physiol. Rep. 2014, 2, e00237. [Google Scholar] [CrossRef]
- Hao, W.; Shi, S.; Zhou, S.; Wang, X.; Nie, S. Aspirin Inhibits Growth and Enhances Cardiomyocyte Differentiation of Bone Marrow Mesenchymal Stem Cells. Eur. J. Pharmacol. 2018, 827, 198–207. [Google Scholar] [CrossRef]
- Liu, B.-H.; Yeh, H.-Y.; Lin, Y.-C.; Wang, M.-H.; Chen, D.C.; Lee, B.-H.; Hsu, S. Spheroid Formation and Enhanced Cardiomyogenic Potential of Adipose-Derived Stem Cells Grown on Chitosan. Biores. Open Access 2013, 2, 28–39. [Google Scholar] [CrossRef] [PubMed]




| Name | Direction | Primer Sequences (5′–3′) | |
|---|---|---|---|
| Pluripotent marker gene | REX1 | Forward | GCTCCGGCGGAATCGAGTGG |
| Reverse | GCACGTGTTGCTTGGCGACC | ||
| Immunomodulatory marker genes | TGFβ1 | Forward | ATGCCAACTTCTGTCTGGGG |
| Reverse | GGTTGTAGAGGGCAAGGACC | ||
| IL-6 | Forward | CCACCCACAACAGACCAGTA | |
| Reverse | TCTGACAGTGCATCATCGCT | ||
| Cardiogenic marker genes | Troponin I | Forward | GAGCTTCAGGACCTATGCCG |
| Reverse | GAGAGTGGGCCGCTTAAACT | ||
| MEF2c | Forward | ATGCGGCTCTCTGAAGGATG | |
| Reverse | TAGCACACACACACACTGCA | ||
| GSK-3β | Forward | GGATGATGGCCGAGACTCTG | |
| Reverse | AGGCTCCCTCCAAGATCCAT | ||
| Housekeeping gene | Beta-actin | Forward | CCCATCTATGAGGGTTACGC |
| Reverse | TTTAATGTCACGCACGATTTC | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, A.; Ngeun, S.K.; Kaneda, M.; Aboubakr, M.; Elhaieg, A.; Hendawy, H.; Tanaka, R. Exploring the Potential Effects of Cryopreservation on the Biological Characteristics and Cardiomyogenic Differentiation of Rat Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 9908. https://doi.org/10.3390/ijms25189908
Farag A, Ngeun SK, Kaneda M, Aboubakr M, Elhaieg A, Hendawy H, Tanaka R. Exploring the Potential Effects of Cryopreservation on the Biological Characteristics and Cardiomyogenic Differentiation of Rat Adipose-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2024; 25(18):9908. https://doi.org/10.3390/ijms25189908
Chicago/Turabian StyleFarag, Ahmed, Sai Koung Ngeun, Masahiro Kaneda, Mohamed Aboubakr, Asmaa Elhaieg, Hanan Hendawy, and Ryou Tanaka. 2024. "Exploring the Potential Effects of Cryopreservation on the Biological Characteristics and Cardiomyogenic Differentiation of Rat Adipose-Derived Mesenchymal Stem Cells" International Journal of Molecular Sciences 25, no. 18: 9908. https://doi.org/10.3390/ijms25189908









