A Comparison of White and Yellow Seminal Plasma Phosphoproteomes Obtained from Turkey (Meleagris gallopavo) Semen
Abstract
:1. Introduction
2. Result
2.1. Biochemical Parameters of White and Yellow Seminal Plasmas Derived from Turkey Semen
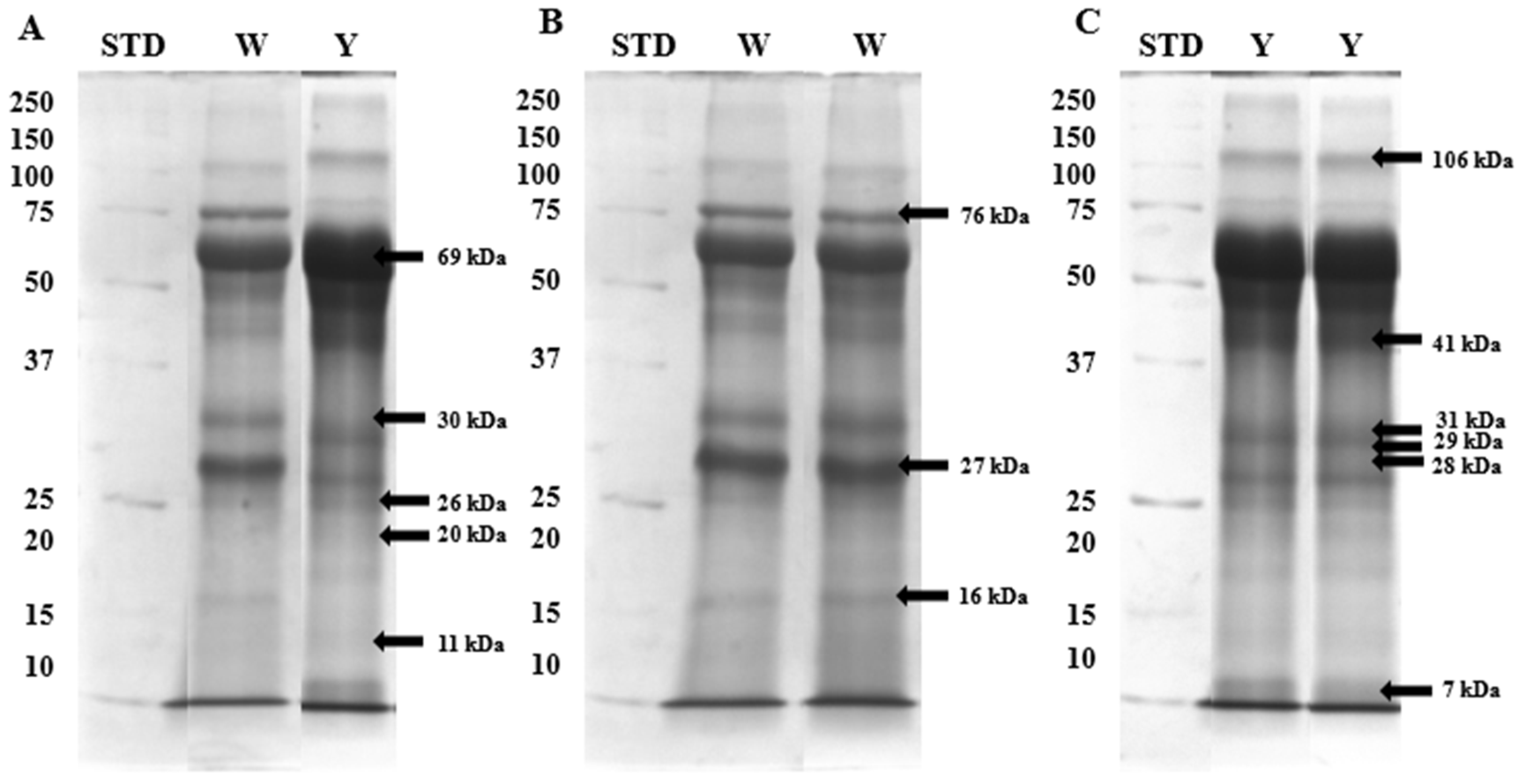
2.2. Identification of White and Yellow Seminal Plasma Phosphoproteins by Mass Spectrometry
2.3. Immunodetection of White and Yellow Seminal Plasma Phosphoproteins
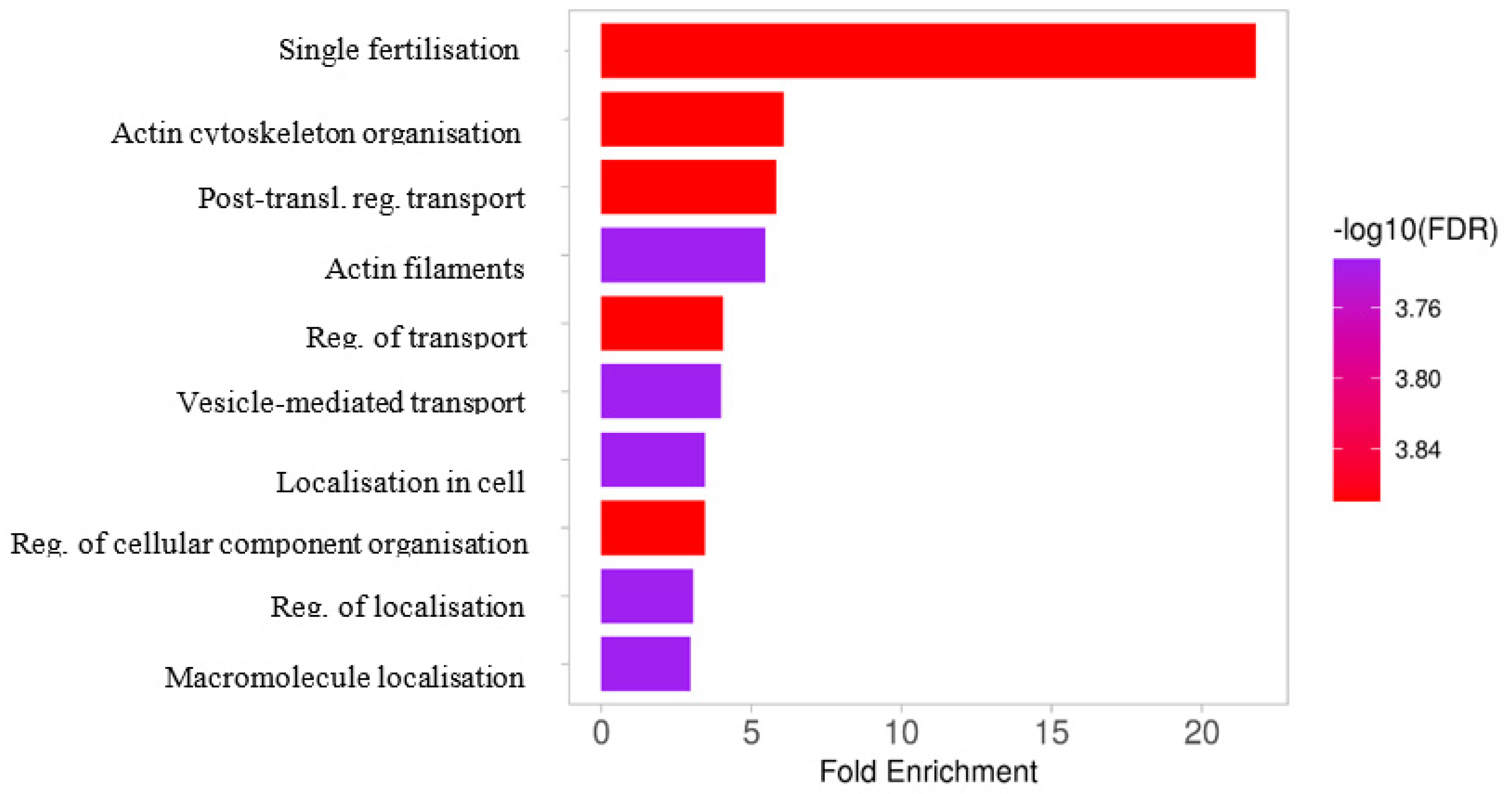
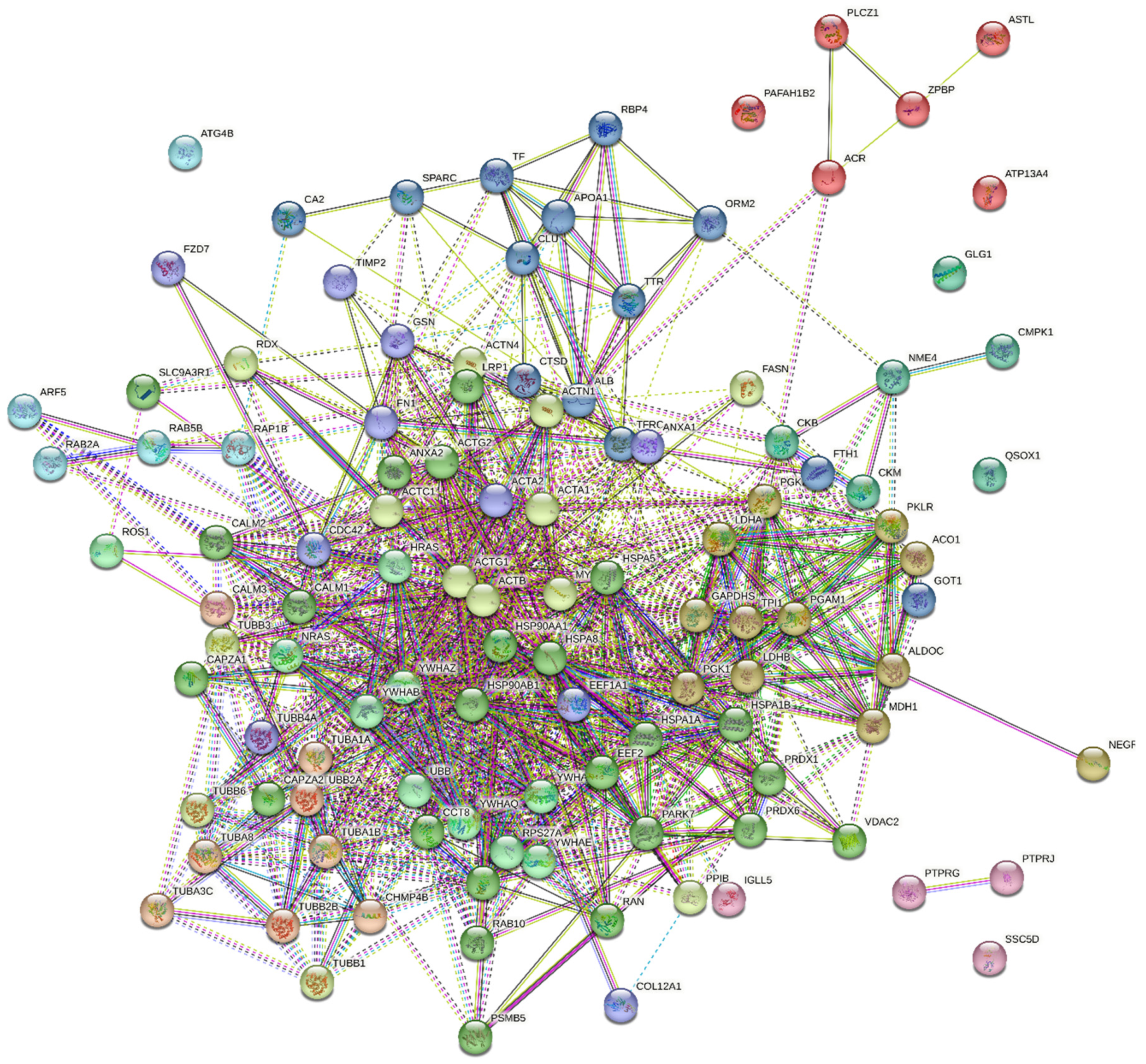
2.4. The Results of Functional Analysis of the Identified Phosphoproteins
3. Discussion
3.1. Differences in Biochemical Parameters Characterising White and Yellow Seminal Plasmas Derived from Turkey Semen
3.2. The Potential Importance of Canonical Phosphorylation in the Physiology of Turkey Semen
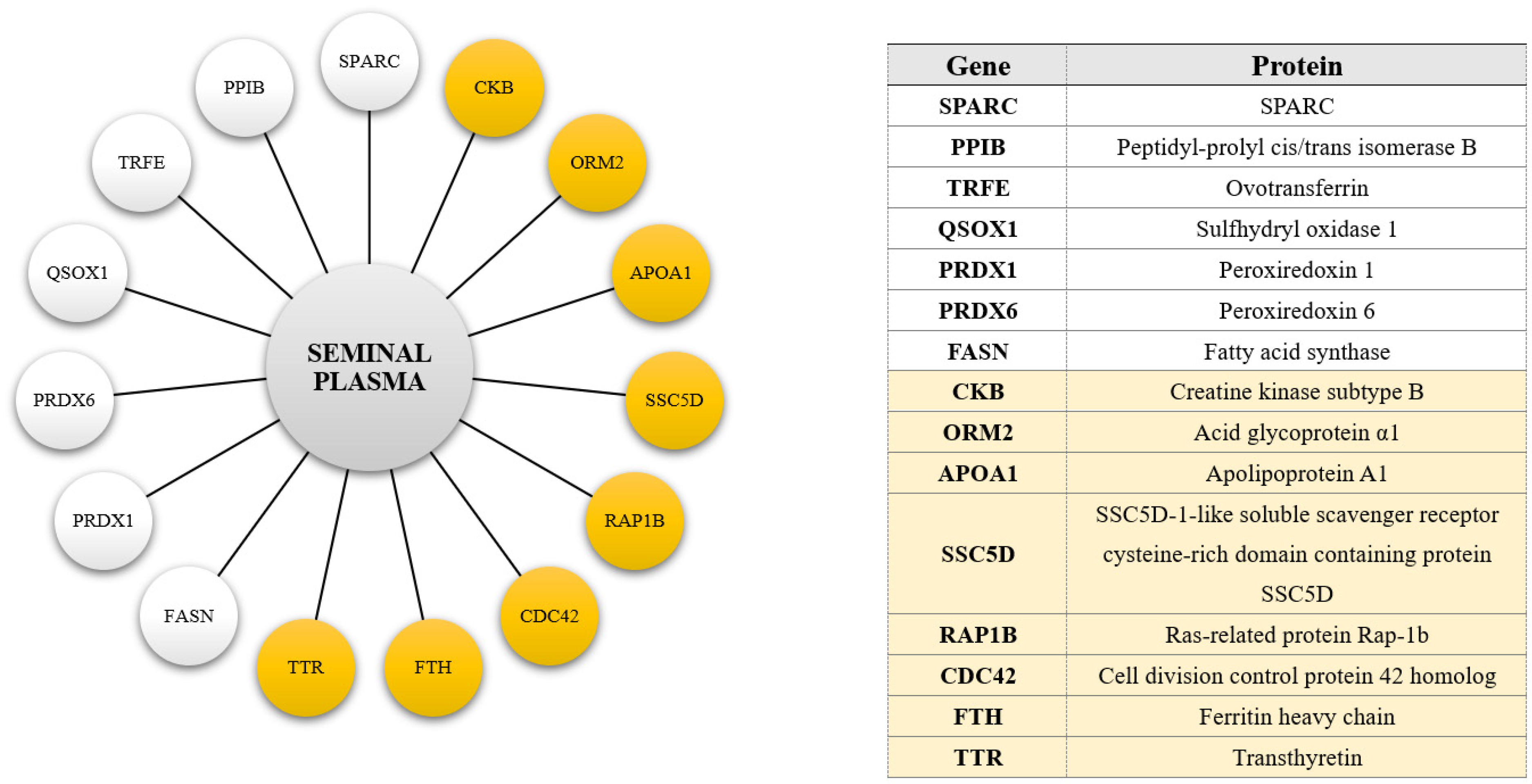
3.3. Proteins of Extracellular Origin Unveiled in the Phosphoproteome of Turkey Seminal Plasma
3.4. Parts of the Ubiquitinating System among the Phosphoproteins Identified in Turkey Seminal Plasma
3.5. Components of Protein Folding/Unfolding Apparatus Present in the Phosphoproteome of Turkey Seminal Plasma
3.6. The Phosphoproteome of Turkey Seminal Plasma Includes Some Regulatory Proteins
3.7. The Differences in White and Yellow Turkey Seminal Plasma Phosphoprotein Profiles
4. Materials and Methods
4.1. Determination of Total Protein Content
4.2. Determination of Alkaline (ALP) and Acid Phosphatase (ACP) Activity
4.3. Determination of Superoxide Dismutase (SOD) Activity
4.4. Determination of Glutathione Peroxidase (GPx) Activity
4.5. Determination of Catalase (CAT) Activity
4.6. Determination of Glutathione (GSH) Content
4.7. Determination of Malondialdehyde (MDA) Levels
4.8. Isolation of Phosphoproteins by Fe3+ Ion Affinity Chromatography on a PHOS-Select Iron Affinity Gel Bed
4.9. SDS-PAGE Electrophoresis of Seminal Plasma Phosphoproteins
4.10. Western Blotting and Immunodetection of Seminal Plasma Phosphoproteins
4.11. Identification of Seminal Plasma Phosphoproteins by Nano LC-MS/MS
4.12. Functional Analysis of the Identified Phosphoproteins
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Słowińska, M.; Kozłowski, K.; Jankowski, J.; Ciereszko, A. Proteomic analysis of white and yellow seminal plasma in turkeys (Meleagris gallopavo). J. Anim. Sci. 2015, 93, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Thurston, R.J.; Hess, R.A.; Froman, D.P. Elevated seminal plasma protein: A characteristic of yellow turkey semen. Poult. Sci. 1982, 61, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; Thurston, R.J. Detection and incidence of yellow turkey semen on commercial breeder farms. Poult. Sci. 1984, 63, 2084–2086. [Google Scholar] [CrossRef] [PubMed]
- Thurston, R.J.; Hess, R.A.; Korn, N. Seminal plasma protein concentration as a predictor of fertility and hatchability in large white domestic turkeys. J. Appl. Poult. Res. 1992, 1, 335–338. [Google Scholar] [CrossRef]
- Thurston, R.J.; Korn, N. Semen quality in the domestic turkey: The yellow semen syndrome. Avian. Poult. Biol. Rev. 1997, 8, 109–121. [Google Scholar]
- Iaffaldano, N.; Manchisi, A.; Rosato, M.P. The preservability of turkey semen quality during liquid storage in relation to strain and age of males. Anim. Reprod. Sci. 2008, 109, 266–273. [Google Scholar] [CrossRef]
- Zaniboni, L.; Cerolinim, S. Liquid storage of turkey semen: Changes in quality parameters, lipid composition and susceptibility to induced in vitro peroxidation in control, n-3 fatty acids and alpha-tocopherol rich spermatozoa. Anim. Reprod. Sci. 2009, 112, 51–65. [Google Scholar] [CrossRef]
- Marzoni, M.; Castillo, A.; Sagona, S.; Citti, L.; Rocchiccioli, S.; Romboli, I.; Felicioli, A. A proteomic approach to identify seminal plasma proteins in roosters (Gallus gallus domesticus). Anim. Reprod. Sci. 2013, 140, 216–223. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Saravia, F.; Wallagren, M.; Martinez, E.A.; Sanz, L.; Roca, J.; Vazqueaz, J.M.; Calvete, J.J. Spermadhesin PSP-I/PSP-II heterodimer induces migration of polymorphonuclear neutrophils into the uterine cavity of the sow. J. Reprod. Immunol. 2010, 84, 57–65. [Google Scholar] [CrossRef]
- Santiago-Moreno, J.; Blesbois, E. Functional aspects of seminal plasma in bird reproduction. Int. J. Mol. Sci. 2020, 21, 5664. [Google Scholar] [CrossRef]
- Druart, X.; de Graaf, S. Seminal plasma proteomes and sperm fertility. Anim. Reprod. Sci. 2018, 194, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. The origins of protein phosphorylation. Nat. Cell Biol. 2002, 4, E127–E130. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The protein kinase complement of the human genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed]
- Asano, A.; Tajima, A. Development and preservation of avian sperm. In Avian Reproduction, Advances in Experimental Medicine and Biology; Sasanami, T., Ed.; Springer Nature: Singapore, 2017; pp. 59–73. [Google Scholar]
- Słowińska, M.; Paukszto, Ł.; Jastrzębski, J.P.; Bukowska, J.; Kozłowski, K.; Jankowski, J.; Ciereszko, A. Transcriptome analysis of turkey (Meleagris gallopavo) reproductive tract revealed key pathways regulating spermatogenesis and post-testicular sperm maturation. Poult. Sci. 2020, 99, 6094–6118. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; Thurston, R.J.; Biellier, H.V. Morphology of the epididymal region of turkeys producing abnormal yellow semen. Poult. Sci. 1982, 61, 531–539. [Google Scholar] [CrossRef]
- Bucci, D.; Isani, G.; Giaretta, E.; Spinaci, M.; Tamanini, C.; Ferlizza, E.; Galeati, G. Alkaline phosphatase in boar sperm function. Andrology 2014, 21, 100–106. [Google Scholar] [CrossRef]
- Hess, R.A.; Thurston, R.J. Protein, cholesterol, acid phosphatase and aspartate aminotransaminase in the seminal plasma of turkeys (Meleagris gallopavo) producing normal white or abnormal yellow semen. Biol. Reprod. 1984, 31, 239–243. [Google Scholar] [CrossRef]
- Kotłowska, M.; Glogowski, J.; Dietrich, G.J.; Kozłowski, K.; Faruga, A.; Jankowski, J.; Ciereszko, A. Biochemical characteristics and sperm production of turkey semen in relation to strain and age of the males. Poult. Sci. 2005, 84, 1763–1768. [Google Scholar] [CrossRef]
- Słowińska, M.; Jankowski, J.; Dietrich, G.J.; Karol, H.; Liszewska, E.; Glogowski, J.; Kozłowski, K.; Sartowska, K.; Ciereszko, A. Effect of organic and irorganic forms of selenium in diets on turkey semen quality. Poult. Sci. 2011, 90, 181–190. [Google Scholar] [CrossRef]
- Słowińska, M.; Sallem, H.; Clench, M.R.; Ciereszko, A. Metabolomic analysis of white and yellow seminal plasma in turkeys (Meleagris gallopavo). Poult. Sci. 2018, 97, 1059–1065. [Google Scholar] [CrossRef]
- Słowińska, M.; Nynca, J.; Arnold, G.J.; Fröhlich, T.; Jankowski, J.; Kozłowski, K.; Mostek, A.; Ciereszko, A. Proteomic identification of turkey (Meleagris gallopavo) seminal plasma proteins. Poult. Sci. 2017, 96, 3422–3435. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Agarwal, A.; Mohanty, G.; Du Plessis, S.S.; Gopalan, B.; Willard, B. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod. Biol. Endocrinol. 2013, 11, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Zhang, H.-R.; Shi, H.-J.; Ma, D.; Zhao, H.-X.; Lin, B.; Li, R.-S. Proteomic analysis of seminal plasma from asthenozoospermia patients reveals proteins that affect oxidative stress responses and semen quality. Asian J. Androl. 2009, 11, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Peterson, S.E.; Loring, J.F. Protein post-translational modifications and regulation of pluripotency in human stem cells. Cell Res. 2014, 24, 143–160. [Google Scholar] [CrossRef]
- Adam, K.; Hunter, T. Histidine kinases and the missing phosphoproteome from prokaryotes to eukaryotes. Lab. Investig. 2018, 98, 233–247. [Google Scholar] [CrossRef]
- Beltrao, P.; Bork, P.; Krogan, N.J.; van Noort, V. Evolution and functional cross-talk of protein post-translational modifications. Mol. Syst. Biol. 2013, 9, 714. [Google Scholar] [CrossRef]
- Pandey, A.K.; Ganguly, H.K.; Sinha, S.K.; Daniels, K.E.; Yap, G.P.A.; Patel, S.; Zondlo, N.J. An inherent structural difference between serine and threonine phosphorylation: Phosphothreonine prefers an ordered, compact, cyclic conformation. ACS Chem. Biol. 2020, 18, 1938–1958. [Google Scholar] [CrossRef]
- Hunter, T. The genesis of tyrosine phosphorylation. Cold Spring Harb. Perspect. Biol. 2014, 6, a020644. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal plasma proteins: What role do they play? Am. J. Reprod. Immunol. 2011, 66, 11–22. [Google Scholar] [CrossRef]
- Siu, M.K.Y.; Cheng, C.Y. Extracellular matrix and its role in spermatogenesis. Adv. Exp. Med. Biol. 2008, 636, 74–91. [Google Scholar] [CrossRef]
- Yalak, G.; Shiu, J.Y.; Schoen, I.; Mitsi, M.; Vogel, V. Phosphorylated fibronectin enhances cell attachment and upregulates mechanical cell functions. PLoS ONE 2019, 14, e0218893. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.C.; Ekman, P. In vitro phosphorylation of serum albumin by two protein kinases: A potential pitfall in protein phosphorylation reactions. Anal. Biochem. 1986, 154, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Westbrook, V.A.; Chertihin, O.; Demarco, I.; Sleight, S.; Diekman, A.B. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J. Reprod. Immunol. 2002, 53, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Z.; Xue, C. The past and future of ovotransferrin: Physicochemical properties, assembly and applications. Trends Food Sci. Technol. 2021, 116, 47–62. [Google Scholar] [CrossRef]
- Chantananukul, W.; Panyim, S. In vitro specific phosphorylation of the human seminal proteins. Andrologia 1982, 14, 447–453. [Google Scholar] [CrossRef]
- Yalak, G.; Olsen, B.R. Proteomic database mining opens up avenues utilizing extracellular protein phosphorylation for novel therapeutic applications. J. Transl. Med. 2015, 13, 125. [Google Scholar] [CrossRef]
- Sutovsky, P.; Moreno, R.; Ramalho-Santos, J.; Dominko, T.; Thompson, W.E.; Schatten, G. A putative, ubiquitin-dependent mechanism for the recognition and elimination of defective spermatozoa in the mammalian epididymis. J. Cell Sci. 2001, 114, 1665–1675. [Google Scholar] [CrossRef]
- Richburg, J.H.; Myers, J.L.; Bratton, S.B. The role of E3 ligases in the ubiquitin-dependent regulation of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 27–35. [Google Scholar] [CrossRef]
- Parte, P.P.; Rao, P.; Redij, S.; Lobo, V.; D’Souza, S.J.; Gajbhiye, R.; Kulkarni, V. Sperm phosphoproteome profiling by ultra performance liquid chromatography followed by data independent analysis (LC-MS(E)) reveals altered proteomic signatures in asthenozoospermia. J. Proteom. 2012, 75, 5861–5871. [Google Scholar] [CrossRef]
- Bae, J.W.; Kim, S.H.; Kim, D.H.; Ha, J.J.; Yi, J.K.; Hwang, S.; Ryu, B.Y.; Pang, M.G.; Kwon, W.S. Ras-related proteins (Rab) are key proteins related to male fertility following a unique activation mechanism. Reprod. Biol. 2019, 19, 356–362. [Google Scholar] [CrossRef]
- Dun, M.D.; Aitken, R.J.; Nixon, B. The role of molecular chaperones in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum. Reprod. Update 2012, 18, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Bracke, A.; Peeters, K.; Punjabi, U.; Hoogewijs, D.; Dewilde, S. A search for molecular mechanisms underlying male idiopathic infertility. Reprod. Biomed. Online 2018, 36, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wu, Y.; Qin, Y. Effects of chronic heat stress on the expressions of heat shock proteins 60, 70, 90, A2, and HSC70 in the rabbit testis. Cell Stress Chaperones 2012, 17, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Rosyada, Z.N.A.; Ulum, M.F.; Tumbelaka, L.I.T.A.; Solihin, D.D.; Purwantara, B.; Memili, E. Implications of sperm heat shock protein 70-2 in bull fertility. Vet. World 2022, 15, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hidalgo, D.; Serrano, R.; Zaragoza, C.; Garcia-Marin, L.J.; Bragado, M.J. Human sperm phosphoproteome reveals differential phosphoprotein signatures that regulate human sperm motility. J. Proteom. 2020, 215, 103654. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, W.; Yu, W.; Niu, X.; Liu, F.; Zhou, T.; Zhang, H.; Li, Y.; Zhu, H.; Zhou, Z.; et al. Proteomics analysis of asthenozoospermia and identification of glucose-6-phosphate isomerase as an important enzyme for sperm motility. J. Proteom. 2019, 208, 103478. [Google Scholar] [CrossRef]
- Shan, S.; Xu, F.; Hirschfeld, M.; Brenig, B. Sperm lipid markers of male fertility in mammals. Int. J. Mol. Sci. 2021, 22, 8767. [Google Scholar] [CrossRef]
- Munuce, M.J.; Marini, P.E.; Teijeiro, J.M. Expression profile and distribution of Annexin A1, A2 and A5 in human semen. Andrologia 2019, 51, e13224. [Google Scholar] [CrossRef]
- Martins, A.D.; Selvam, M.K.P.; Agarwal, A.; Alves, M.G.; Baskaran, S. Alterations in seminal plasma proteomic profile in men with primary and secondary infertility. Sci. Rep. 2020, 10, 7539. [Google Scholar] [CrossRef]
- Intasqui, P.; Camargo, M.; Del Giudice, P.T.; Spaine, D.M.; Carvalho, V.M.; Cardozo, K.H.; Cadenho, A.P.; Bertolla, R.P. Unraveling the sperm proteome and post-genomic pathways associated with sperm nuclear DNA fragmentation. J. Assist. Reprod. Genet. 2013, 30, 1187–1202. [Google Scholar] [CrossRef]
- Grindheim, A.K.; Saraste, J.; Vedeler, A. Protein phosphorylation and its role in the regulation of Annexin A2 function. Biochim. Biophys. Acta Gen. Subj. 2017, 1861 Pt A, 2515–2529. [Google Scholar] [CrossRef] [PubMed]
- Ficarro, S.; Chertihin, O.; Westbrook, V.A.; White, F.; Jayes, F.; Kalab, P.; Marto, J.A.; Shabanowitz, J.; Herr, J.C.; Hunt, D.F.; et al. Phosphoproteome analysis of capacitated human sperm. J. Biol. Chem. 2003, 278, 11579–11589. [Google Scholar] [CrossRef] [PubMed]
- Brekken, R.A.; Sage, E.H. SPARC, a matricellular protein: At the crossroads of cell-matrix communication. Matrix Biol. 2001, 19, 816–827. [Google Scholar] [CrossRef]
- Yan, Q.; Sage, E.H. SPARC, a matricellular glycoprotein with important biological functions. J. Histochem. Cytochem. 1999, 47, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Sage, H.; Johnson, C.; Bornstein, P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J. Biol. Chem. 1984, 259, 3993–4007. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Yoon, H.; Kim, B.S.; Bae, H.; Shin, H.; Kim, W.J.; Yoon, W.; Lee, Y.S.; Woo, K.M.; Baek, J.H.; et al. Blood-testis barrier integrity depends on Pin1 expression in Sertoli cells. Sci. Rep. 2017, 7, 6977. [Google Scholar] [CrossRef]
- Kodali, V.K.; Thorpe, C. Oxidative protein folding and the Quiescin–sulfhydryl oxidase family of flavoproteins. Antioxid. Redox Signal. 2010, 13, 1217–1230. [Google Scholar] [CrossRef]
- Wang, T.E.; Yeh, L.Y.; Lee, R.K.K.; Lu, C.H.; Yang, T.H.; Kuo, Y.W.; Joshi, R.; Tsai, P.S.; Li, S.H. Secretory mouse quiescin sulfhydryl oxidase 1 aggregates defected human and mouse spermatozoa in vitro and in vivo. iScience 2021, 24, 103167. [Google Scholar] [CrossRef]
- O’Flaherty, C. Peroxiredoxin 6: The Protector of Male Fertility. Antioxidants 2018, 7, 173. [Google Scholar] [CrossRef]
- Woo, H.A.; Yim, S.H.; Shin, D.H.; Kang, D.; Yu, D.-Y.; Rhee, S.G. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signalling. Cell 2010, 140, 517–528. [Google Scholar] [CrossRef]
- Fisher, A.B. The phospholipase A2 activity of peroxiredoxin 6. J. Lipid Res. 2018, 59, 1132–1147. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Real, J.M.; Menendez, J.A.; Moreno-Navarrete, J.M.; Blüher, M.; Vazquez-Martin, A.; Vázquez, M.J.; Ortega, F.; Diéguez, C.; Diéguez, C.; Ricart, W.; et al. Extracellular fatty acid synthase: A possible surrogate biomarker of insulin resistance. Diabetes 2010, 59, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Finelli, R.; Darbandi, S.; Pushparaj, P.N.; Henkel, R.; Ko, E.; Agarwal, A. In silico sperm proteome analysis to investigate DNA repair mechanisms in varicocele patients. Front. Endocrinol. 2021, 12, 757592. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.; Steen, T.V.; Espinoza, I.; Venkatapoorna, C.M.K.; Hu, Z.; Silva, F.M.; Regan, K.; Cuyàs, E.; Meng, X.W.; Verdura, S.; et al. Fatty acid synthase (FASN) regulates the mitochondrial priming of cancer cells. Cell Death Dis. 2021, 12, 977. [Google Scholar] [CrossRef]
- Wallimann, T.; Moser, H.; Zurbriggen, B.; Wegmann, G.; Eppenberger, H.M. Creatine kinase isoenzymes in spermatozoa. J. Muscle Res. Cell Motil. 1986, 7, 25–34. [Google Scholar] [CrossRef]
- Tombes, R.M.; Shapiro, B.M. Metabolite channeling: A phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell 1985, 41, 325–334. [Google Scholar] [CrossRef]
- Nasrallah, F.; Hammami, M.B.; Omar, S.; Aribia, H.B.; Sanhaji, H.; Feki, M. Semen creatine and creatine kinase activity as an indicator of sperm quality. Clin. Lab. 2020, 66, 1751–1757. [Google Scholar] [CrossRef]
- Mahadevan, L.C.; Whatley, S.A.; Leung, T.K.; Lim, L. The brain isoform of a key ATP-regulating enzyme, creatine kinase, is a phosphoprotein. Biochem. J. 1984, 222, 139–144. [Google Scholar] [CrossRef]
- Wu, K.; Yan, M.; Liu, T.; Wang, Z.; Duan, Y.; Xia, Y.; Ji, G.; Shen, Y.; Wang, L.; Li, L.; et al. Creatine kinase B suppresses ferroptosis by phosphorylating GPX4 through a moonlighting function. Nat. Cell Biol. 2023, 25, 714–725. [Google Scholar] [CrossRef]
- Intasqui, P.; Camargo, M.; Giudice, P.T.D.; Spaine, D.M.; Carvalho, V.M.; Cardozo, K.H.M.; Zylbersztejn, D.S.; Bertolla, R.P. Sperm nuclear DNA fragmentation rate is associated with differential protein expression and enriched functions in human seminal plasma. BJU Int. 2013, 112, 835–843. [Google Scholar] [CrossRef]
- Akerlöf, E.; Jörnvall, H.; Slotte, H.; Pousette, A. Identification of apolipoprotein A1 and immunoglobulin as components of a serum complex that mediates activation of human sperm motility. Biochemistry 1991, 30, 8986–8990. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.S.; Rahman, M.S.; Lee, J.S.; Kim, J.; Yoon, S.J.; Park, Y.J.; You, Y.A.; Hwang, S.; Pang, M.G. A comprehensive proteomic approach to identifying capacitation related proteins in boar spermatozoa. BMC Genom. 2014, 15, 897. [Google Scholar] [CrossRef] [PubMed]
- Labas, V.; Grasseau, I.; Cahier, K.; Gargaros, A.; Harichaux, G.; Teixeira-Gomes, A.P.; Alves, S.; Bourin, M.; Gérard, N.; Blesbois, E. Qualitative and quantitative peptidomic and proteomic approaches to phenotyping chicken semen. J. Proteom. 2015, 112, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.C.; Bocková, M.; Santos, R.F.; Santos, A.M.; de Araújo, M.M.; Oliveira, L.; Homola, J.; Carmo, A.M. The Scavenger receptor SSc5D physically interacts with bacteria through the SRCR-containing N-terminal domain. Front. Immunol. 2016, 7, 416. [Google Scholar] [CrossRef]
- Aivatiadou, A.; Mattei, E.; Ceriani, M.; Tilia, L.; Berruti, G. Impaired fertility and spermiogenetic disorders with loss of cell adhesion in male mice expressing an interfering rap1 mutant. Mol. Biol. Cell. 2007, 18, 1530–1542. [Google Scholar] [CrossRef]
- Edreira, M.M.; Li, S.; Hochbaum, D.; Wong, S.; Gorfe, A.A.; Ribeiro-Neto, F.; Woods, W.L.; Altschuler, D.L. Phosphorylation-induced conformational changes in Rap1b. J. Biol Chem. 2009, 284, 27480–27486. [Google Scholar] [CrossRef]
- Heinrich, A.; Bhandary, B.; Potter, S.J.; Ratner, N.; DeFalco, T. Cdc42 activity in Sertoli cells is essential for maintenance of spermatogenesis. Cell Rep. 2021, 37, 109885. [Google Scholar] [CrossRef]
- Forget, M.-A.; Desrosiers, R.R.; Gingras, D.; Béliveau, R. Phosphorylation states of Cdc42 and RhoA regulate their interactions with Rho GDP dissociation inhibitor and their extraction from biological membranes. Biochem. J. 2002, 361 Pt 2, 243–254. [Google Scholar] [CrossRef]
- Xu, Q.L.; Kang, B.; Jiang, D.M. The mechanism of ferritin mediated apoptosis. Chin. J. Biochem. Mol. Biol. 2016, 32, 1213–1218. [Google Scholar]
- Li, R.; Luo, C.; Mines, M.; Zhang, J.; Fan, G.H. Chemokine CXCL12 induces binding of ferritin heavy chain to the chemokine receptor CXCR4, alters CXCR4 signaling, and induces phosphorylation and nuclear translocation of ferritin heavy chain. J. Biol. Chem. 2006, 281, 37616–37627. [Google Scholar] [CrossRef]
- Chung, S.S.W.; Wolgemuth, D.J. Role of retinoid signaling in the regulation of spermatogenesis. Cytogenet. Genome Res. 2004, 105, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Fong, V.H.; Vieira, A. Transthyretin aggregates induce production of reactive nitrogen species. Neurodegener. Dis. 2013, 11, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Genereux, J.C.; Suh, E.H.; Vartabedian, V.F.; Rius, B.; Qu, S.; Dendele, M.T.A.; Kelly, J.W.; Wiseman, R.L. Endoplasmic reticulum proteostasis influences the oligomeric state of an amyloidogenic protein secreted from mammalian cells. Cell Chem. Biol. 2016, 23, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Terazaki, H.; Ando, Y.; Suhr, O.; Ohlsson, P.; Obayashi, K.; Yamashita, T.; Yoshimatsu, S.; Suga, M.; Uchino, M.; Ando, M. Post-translational modification of transthyretin in plasma. Biochem. Biophys. Res. Commun. 1998, 249, 26–30. [Google Scholar] [CrossRef]
- Burrows, W.H.; Quinn, J.P. The Collection of Spermatozoa from the Domestic Fowl and Turkey. Poult. Sci. 1937, 16, 19–24. [Google Scholar] [CrossRef]
- Bessey, O.A.; Lowry, O.H.; Brock, M.J. A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J. Biol. Chem. 1946, 164, 321–329. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]

| Parameters | White | Yellow |
|---|---|---|
| Protein content (mg/mL) | 7.63 ± 0.42 A | 17.87 ± 0.34 B |
| ALP activity (U/mL) | 26.55 ± 2.32 B | 75.43 ± 4.89 A |
| ACP activity (U/mL) | 6918.98 ± 1284.72 b | 11,651.34 ± 1710.60 a |
| SOD activity (U/mL) | 2.19 ± 0.14 | 1.90 ± 0.21 |
| GPx activity (U/mL) | 0.39 ± 0.02 B | 0.65 ± 0.07 A |
| CAT activity (µM/min/mL) | 27.64 ± 4.65 | 29.74 ± 7.92 |
| GSH content (µM/mL) | 437.82 ± 34.14 B | 671.61 ± 47.57 A |
| MDA level (µM/mL) | 10.82 ± 1.69 B | 18.91 ± 2.08 A |
| Protein (kDa) | White | Yellow |
|---|---|---|
| 106 | 0.23 | 0.28 |
| 76 | 0.41 | 0.26 |
| 69 | 0.70 | 0.83 |
| 41 | 0.45 | 0.49 |
| 31 | 0.50 | 0.69 |
| 30 | 0.45 | 0.48 |
| 29 | 0.39 | 0.41 |
| 28 | 0.40 | 0.46 |
| 27 | 0.47 | 0.45 |
| 26 | 0.45 | 0.48 |
| 20 | 0.30 | 0.34 |
| 16 | 0.28 | 0.24 |
| 11 | 0.23 | 0.19 |
| 7 | 0.19 | 0.24 |
| Protein [kDa] | Molecular Weight by Multi-Analyst | Phosphorylated Residue | Type of Semen | Mean | ± SEM |
|---|---|---|---|---|---|
| 1 | 106 kDa | SER | W | 0.18 | 0.01 |
| Y | 0.09 | 0.01 | |||
| THR | W | 0.15 | 0.01 | ||
| Y | 0.12 | 0.01 | |||
| TYR | W | 0.16 | 0.01 | ||
| Y | 0.06 | 0.01 | |||
| 2 | 76 kDa | SER | W | 0.24 | 0.01 |
| Y | 0.27 | 0.01 | |||
| THR | W | 0.20 | 0.01 | ||
| Y | 0.16 | 0.01 | |||
| TYR | W | 0.27 | 0.01 | ||
| Y | 0.11 | 0.01 | |||
| 3 | 69 kDa | SER | W | 0.24 | 0.01 |
| Y | 0.21 | 0.01 | |||
| THR | W | 0.21 | 0.01 | ||
| Y | 0.18 | 0.00 | |||
| TYR | W | 0.27 | 0.01 | ||
| Y | 0.11 | 0.01 | |||
| 4 | 41 kDa | SER | W | 0.23 | 0.03 |
| Y | 0.26 | 0.02 | |||
| THR | W | 0.22 | 0.03 | ||
| Y | 0.25 | 0.03 | |||
| TYR | W | 0.23 | 0.02 | ||
| Y | 0.10 | 0.01 | |||
| 5 | 31 kDa | SER | W | 0.24 | 0.02 |
| Y | 0.22 | 0.02 | |||
| THR | W | 0.23 | 0.01 | ||
| Y | 0.17 | 0.01 | |||
| TYR | W | 0.24 | 0.02 | ||
| Y | 0.09 | 0.02 | |||
| 6 | 30 kDa | SER | W | 0.24 | 0.02 |
| Y | 0.24 | 0.02 | |||
| THR | W | 0.23 | 0.01 | ||
| Y | 0.20 | 0.01 | |||
| TYR | W | 0.22 | 0.02 | ||
| Y | 0.11 | 0.02 | |||
| 7 | 29 kDa | SER | W | 0.48 | 0.05 |
| Y | 0.34 | 0.04 | |||
| THR | W | 0.39 | 0.04 | ||
| Y | 0.35 | 0.06 | |||
| TYR | W | 0.45 | 0.06 | ||
| Y | 0.17 | 0.04 | |||
| 8 | 28 kDa | SER | W | 0.24 | 0.06 |
| Y | 0.20 | 0.04 | |||
| THR | W | 0.20 | 0.03 | ||
| Y | 0.21 | 0.05 | |||
| TYR | W | 0.24 | 0.04 | ||
| Y | 0.11 | 0.03 | |||
| 9 | 27 kDa | SER | W | 0.16 | 0.02 |
| Y | 0.17 | 0.01 | |||
| THR | W | 0.14 | 0.01 | ||
| Y | 0.16 | 0.01 | |||
| TYR | W | 0.15 | 0.01 | ||
| Y | 0.09 | 0.02 | |||
| 10 | 26 kDa | SER | W | 0.17 | 0.02 |
| Y | 0.18 | 0.01 | |||
| THR | W | 0.14 | 0.01 | ||
| Y | 0.16 | 0.01 | |||
| TYR | W | 0.15 | 0.01 | ||
| Y | 0.10 | 0.02 | |||
| 11 | 20 kDa | SER | W | 0.08 | 0.01 |
| Y | 0.12 | 0.02 | |||
| THR | W | 0.11 | 0.01 | ||
| Y | 0.13 | 0.01 | |||
| TYR | W | 0.06 | 0.02 | ||
| Y | 0.10 | 0.01 | |||
| 12 | 16 kDa | SER | W | 0.09 | 0.01 |
| Y | 0.10 | 0.01 | |||
| THR | W | 0.10 | 0.01 | ||
| Y | 0.12 | 0.02 | |||
| TYR | W | 0.06 | 0.00 | ||
| Y | 0.08 | 0.01 | |||
| 13 | 11 kDa | SER | W | 0.06 | 0.01 |
| Y | 0.06 | 0.01 | |||
| THR | W | 0.07 | 0.00 | ||
| Y | 0.07 | 0.01 | |||
| TYR | W | 0.05 | 0.01 | ||
| Y | 0.03 | 0.00 | |||
| 14 | 7 kDa | SER | W | 0.06 | 0.00 |
| Y | 0.05 | 0.01 | |||
| THR | W | 0.09 | 0.01 | ||
| Y | 0.07 | 0.01 | |||
| TYR | W | 0.05 | 0.01 | ||
| Y | 0.02 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafalska, K.T.; Orzołek, A.; Ner-Kluza, J.; Wysocki, P. A Comparison of White and Yellow Seminal Plasma Phosphoproteomes Obtained from Turkey (Meleagris gallopavo) Semen. Int. J. Mol. Sci. 2024, 25, 9941. https://doi.org/10.3390/ijms25189941
Rafalska KT, Orzołek A, Ner-Kluza J, Wysocki P. A Comparison of White and Yellow Seminal Plasma Phosphoproteomes Obtained from Turkey (Meleagris gallopavo) Semen. International Journal of Molecular Sciences. 2024; 25(18):9941. https://doi.org/10.3390/ijms25189941
Chicago/Turabian StyleRafalska, Katarzyna T., Aleksandra Orzołek, Joanna Ner-Kluza, and Paweł Wysocki. 2024. "A Comparison of White and Yellow Seminal Plasma Phosphoproteomes Obtained from Turkey (Meleagris gallopavo) Semen" International Journal of Molecular Sciences 25, no. 18: 9941. https://doi.org/10.3390/ijms25189941
APA StyleRafalska, K. T., Orzołek, A., Ner-Kluza, J., & Wysocki, P. (2024). A Comparison of White and Yellow Seminal Plasma Phosphoproteomes Obtained from Turkey (Meleagris gallopavo) Semen. International Journal of Molecular Sciences, 25(18), 9941. https://doi.org/10.3390/ijms25189941






