Involvement of Aryl Hydrocarbon Receptor in Longevity and Healthspan: Insights from Humans, Mice, and C. elegans
Abstract
1. Introduction
2. Results
2.1. Human Study: AHR Canonical Pathway Is Up-Regulated in Centenarians and Their Offspring
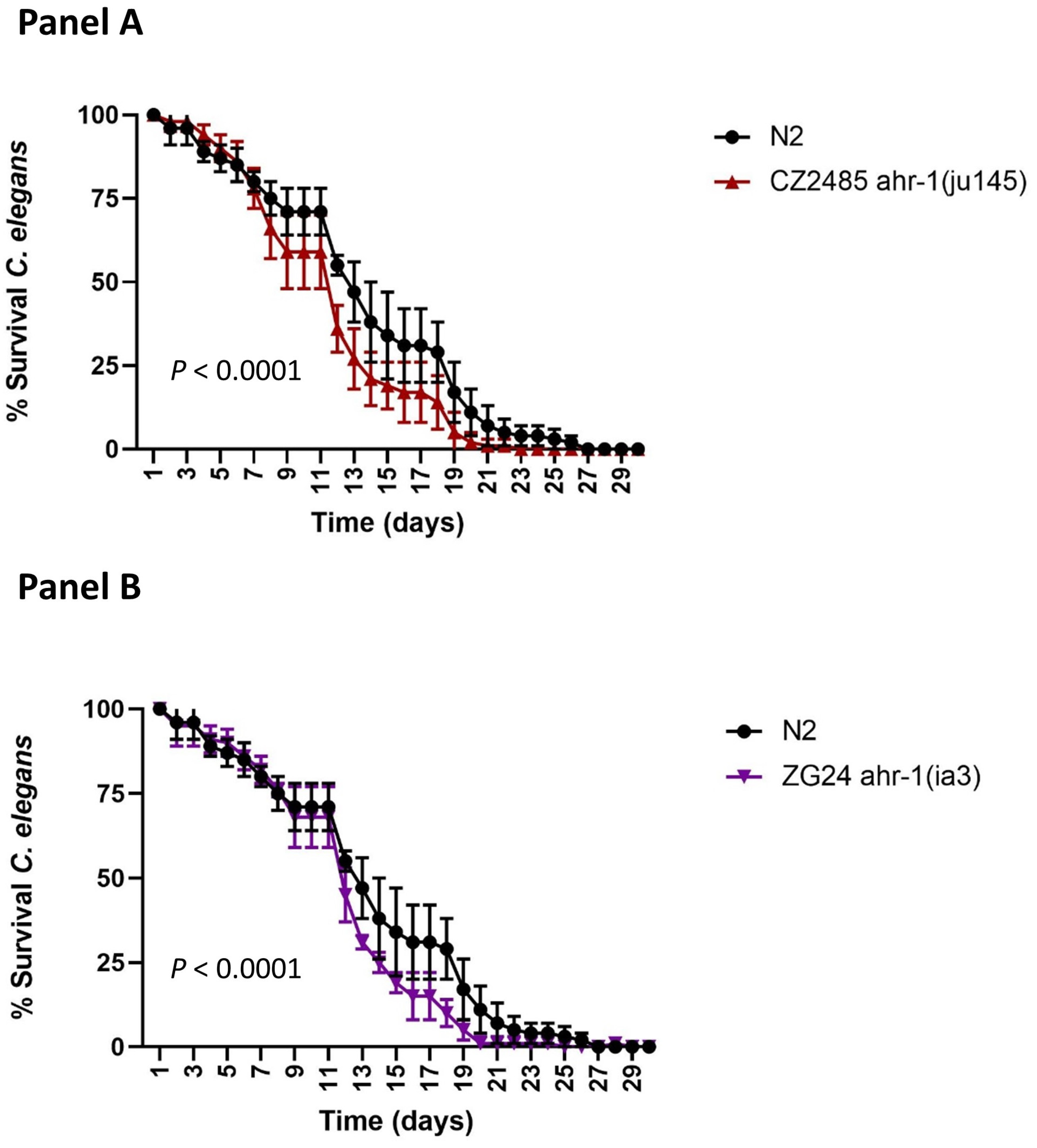
2.2. C. elegans Study: Involvement of AHR-1 in Lifespan
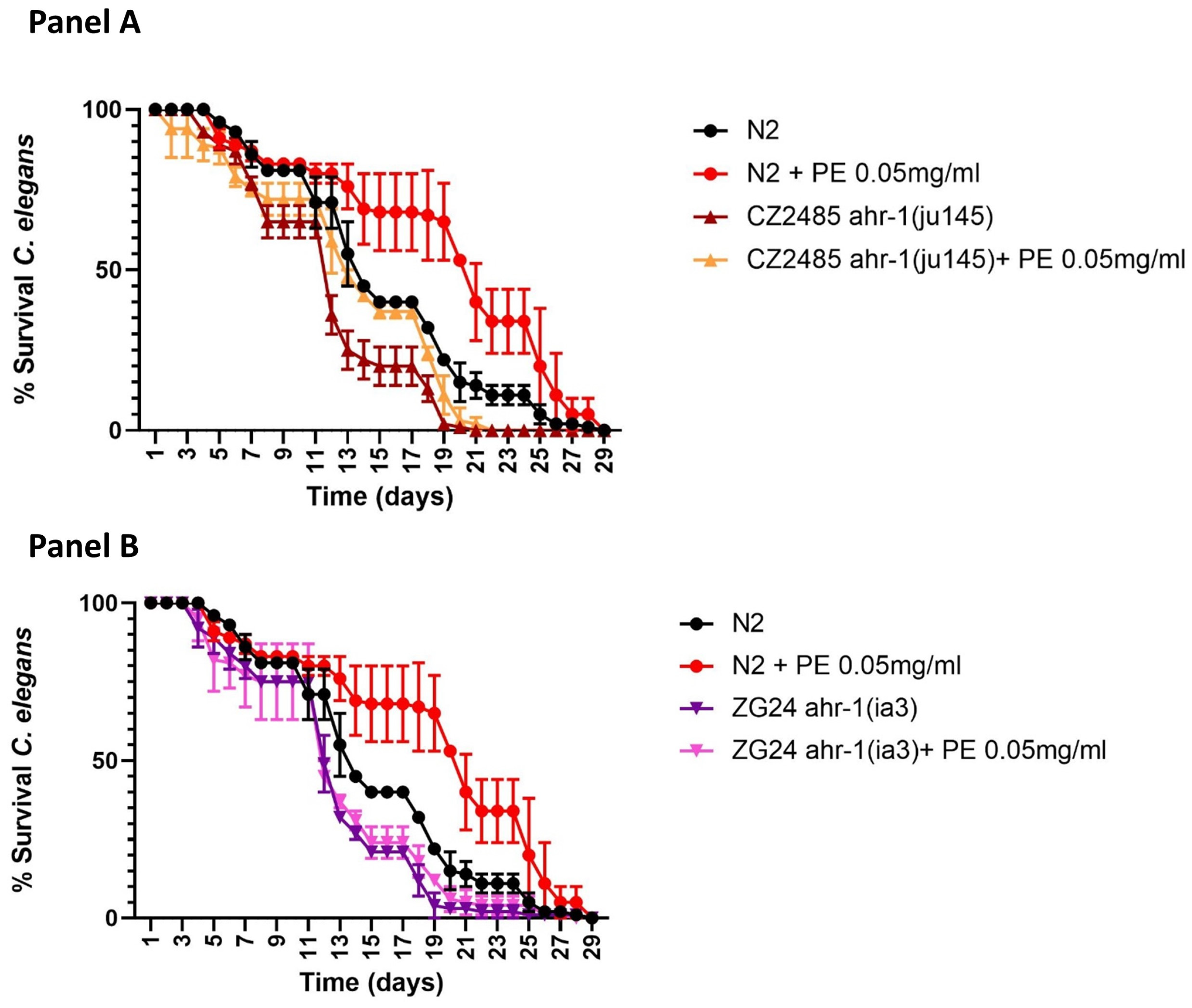
2.3. Nutritional Intervention with Pomegranate Extract on AHR Pathway to Promote Longevity in C. elegans
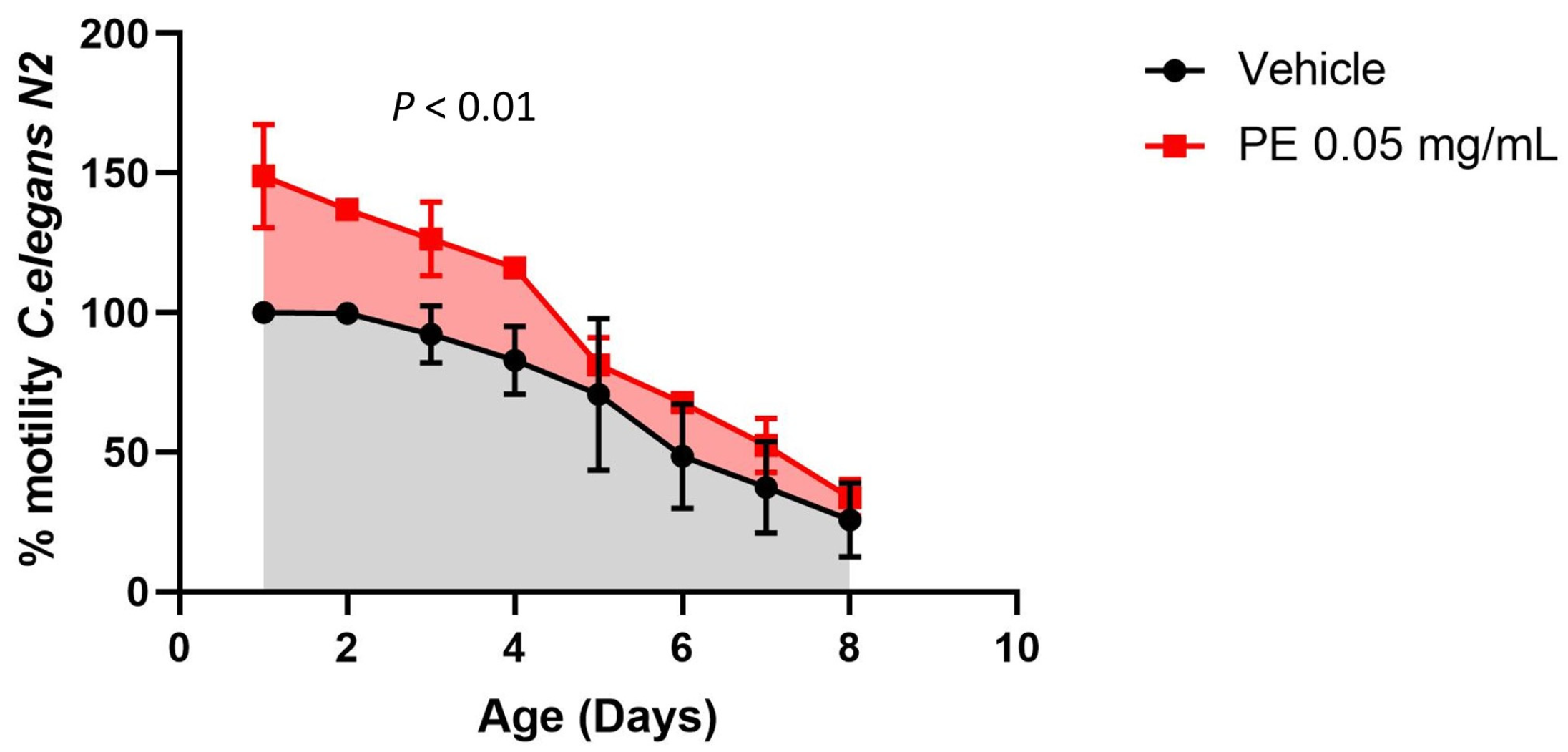
2.4. Effect of PE on Motility in C. elegans
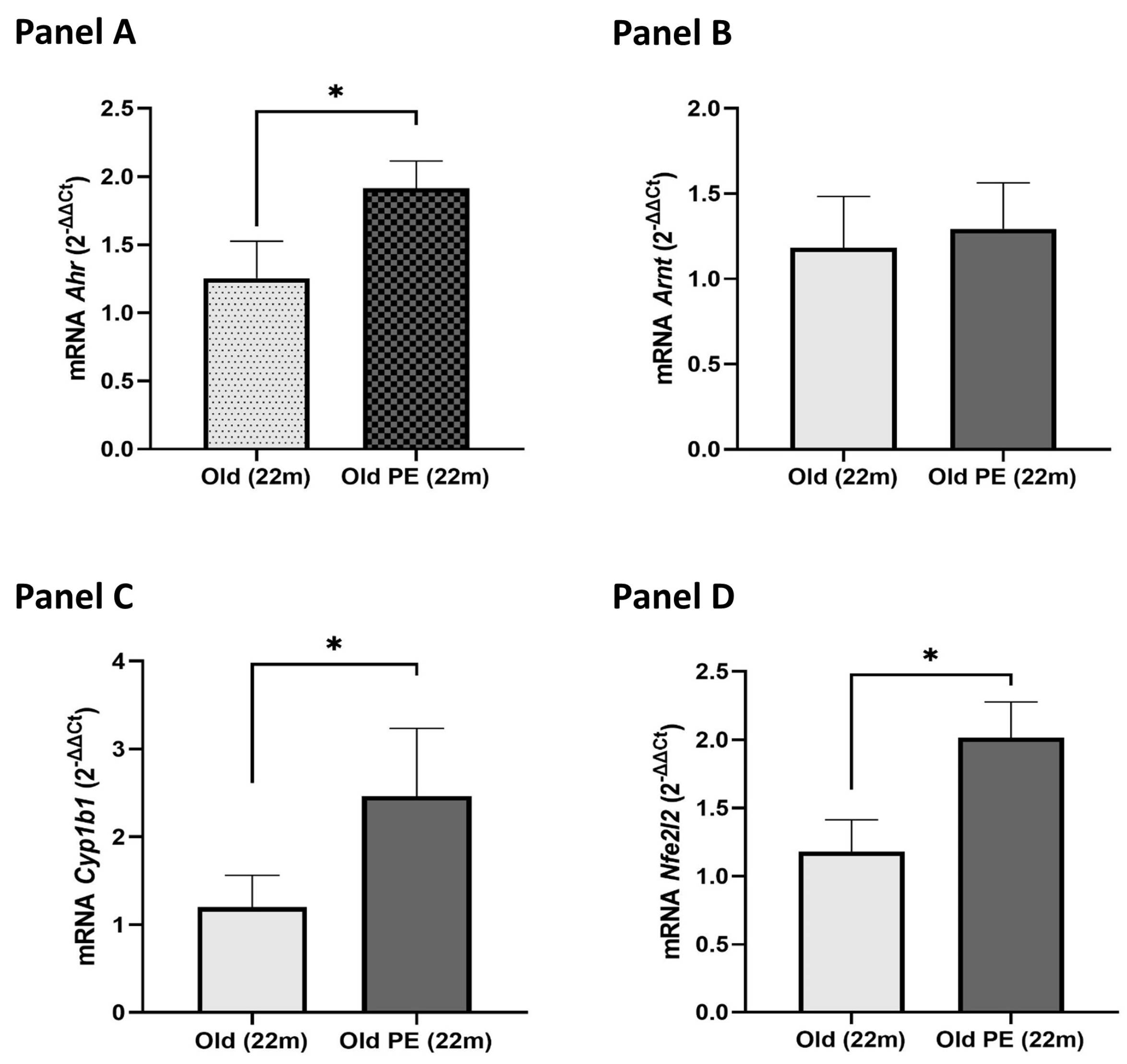
2.5. Nutritional Intervention with Pomegranate Extract on AHR Pathway Gene Expression in Mouse Liver
3. Discussion
Limitations of this Study
4. Materials and Methods
4.1. Human Study
4.1.1. Participants
4.1.2. Peripheral Blood Mononuclear Cell Isolation
4.1.3. Isolation of Total RNA from Peripheral Blood Mononuclear Cells
4.1.4. Gene Expression Profiling and Microarray Data Analysis
4.2. C. elegans Study
4.2.1. Lifespan Study
4.2.2. Assessment of Vitality in C. elegans
4.3. Mouse Study
4.3.1. Experimental Animals
4.3.2. Total RNA Extraction and Real-Time Polymerase Chain Reaction (RT-PCR) Studies
4.3.3. Statistical Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serna, E.; Cespedes, C.; Vina, J. Anti-Aging Physiological Roles of Aryl Hydrocarbon Receptor and Its Dietary Regulators. Int. J. Mol. Sci. 2020, 22, 374. [Google Scholar] [CrossRef] [PubMed]
- Okey, A.B.; Riddick, D.S.; Harper, P.A. The Ah Receptor: Mediator of the Toxicity of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and Related Compounds. Toxicol. Lett. 1994, 70, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sondermann, N.C.; Faßbender, S.; Hartung, F.; Hätälä, A.M.; Rolfes, K.M.; Vogel, C.F.A.; Haarmann-Stemmann, T. Functions of the Aryl Hydrocarbon Receptor (AHR) beyond the Canonical AHR/ARNT Signaling Pathway. Biochem. Pharmacol. 2023, 208, 115371. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Dai, W. Aryl Hydrocarbon Receptor: Its Roles in Physiology. Biochem. Pharmacol. 2021, 185, 114428. [Google Scholar] [CrossRef]
- Bravo-Ferrer, I.; Cuartero, M.I.; Medina, V.; Ahedo-Quero, D.; Peña-Martínez, C.; Pérez-Ruíz, A.; Fernández-Valle, M.E.; Hernández-Sánchez, C.; Fernández-Salguero, P.M.; Lizasoain, I.; et al. Lack of the Aryl Hydrocarbon Receptor Accelerates Aging in Mice. FASEB J. 2019, 33, 12644–12654. [Google Scholar] [CrossRef]
- Casado, F.L. The Aryl Hydrocarbon Receptor Relays Metabolic Signals to Promote Cellular Regeneration. Stem Cells Int. 2016, 2016, 4389802. [Google Scholar] [CrossRef]
- Hu, T.; Wang, D.; Yu, Q.; Li, L.; Mo, X.; Pan, Z.; Zouboulis, C.C.; Peng, L.; Xia, L.; Ju, Q. Aryl Hydrocarbon Receptor Negatively Regulates Lipid Synthesis and Involves in Cell Differentiation of SZ95 Sebocytes in Vitro. Chem. Biol. Interact. 2016, 258, 52–58. [Google Scholar] [CrossRef]
- Riemschneider, S.; Hoffmann, M.; Slanina, U.; Weber, K.; Hauschildt, S.; Lehmann, J. Indol-3-Carbinol and Quercetin Ameliorate Chronic DSS-Induced Colitis in C57BL/6 Mice by AhR-Mediated Anti-Inflammatory Mechanisms. Int. J. Environ. Res. Public Health 2021, 18, 2262. [Google Scholar] [CrossRef]
- Singh, K.P.; Casado, F.L.; Opanashuk, L.A.; Gasiewicz, T.A. The Aryl Hydrocarbon Receptor Has a Normal Function in the Regulation of Hematopoietic and Other Stem/Progenitor Cell Populations. Biochem. Pharmacol. 2009, 77, 577–587. [Google Scholar] [CrossRef]
- Kawajiri, K.; Fujii-Kuriyama, Y. Cytochrome P450 Gene Regulation and Physiological Functions Mediated by the Aryl Hydrocarbon Receptor. Arch. Biochem. Biophys. 2007, 464, 207–212. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The Aryl Hydrocarbon Receptor as a Target of Environmental Stressors—Implications for Pollution Mediated Stress and Inflammatory Responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Gates, E.J.; Bernath, A.K.; Klegeris, A. Modifying the Diet and Gut Microbiota to Prevent and Manage Neurodegenerative Diseases. Rev. Neurosci. 2022, 33, 767–787. [Google Scholar] [CrossRef] [PubMed]
- Verdú, D.; Valls, A.; Díaz, A.; Carretero, A.; Dromant, M.; Kuligowski, J.; Serna, E.; Viña, J. Pomegranate Extract Administration Reverses Loss of Motor Coordination and Prevents Oxidative Stress in Cerebellum of Aging Mice. Antioxidants 2023, 12, 1991. [Google Scholar] [CrossRef]
- Sharma, P.; McClees, S.F.; Afaq, F. Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef]
- Hosseini, A.; Razavi, B.M.; Hosseinzadeh, H. Protective Effects of Pomegranate (Punica Granatum) and Its Main Components against Natural and Chemical Toxic Agents: A Comprehensive Review. Phytomedicine 2023, 109, 154581. [Google Scholar] [CrossRef]
- Kothe, B.; Klein, S.; Petrosky, S.N. Urolithin A as a Potential Agent for Prevention of Age-Related Disease: A Scoping Review. Cureus 2023, 15, e42550. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Y.; Fan, S.; Huang, Y.; Su, X.; Lu, C. Urolithin A Alleviates Colitis in Mice by Improving Gut Microbiota Dysbiosis, Modulating Microbial Tryptophan Metabolism, and Triggering AhR Activation. J. Agric. Food Chem. 2023, 71, 7710–7722. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the Gut Barrier Integrity by a Microbial Metabolite through the Nrf2 Pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Eckers, A.; Jakob, S.; Heiss, C.; Haarmann-Stemmann, T.; Goy, C.; Brinkmann, V.; Cortese-Krott, M.M.; Sansone, R.; Esser, C.; Ale-Agha, N.; et al. The Aryl Hydrocarbon Receptor Promotes Aging Phenotypes across Species. Sci. Rep. 2016, 6, 19618. [Google Scholar] [CrossRef]
- Torgovnick, A.; Schiavi, A.; Maglioni, S.; Ventura, N. Healthy Aging: What Can We Learn from Caenorhabditis Elegans? Z. Gerontol. Geriatr. 2013, 46, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Giri, N.A.; Gaikwad, N.N.; Raigond, P.; Damale, R.; Marathe, R.A. Exploring the Potential of Pomegranate Peel Extract as a Natural Food Additive: A Review. Curr. Nutr. Rep. 2023, 12, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Heber, D.; Wang, M.; Gao, C.; Heymsfield, S.B.; Martin, R.J.; Greenway, F.L.; Finley, J.W.; Burton, J.H.; Johnson, W.D.; et al. Pomegranate Juice and Extract Extended Lifespan and Reduced Intestinal Fat Deposition in Caenorhabditis Elegans. Int. J. Vitam. Nutr. Res. 2017, 87, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Kılıçgün, H.; Arda, N.; Uçar, E.Ö. Identification of Longevity, Fertility and Growth-Promoting Properties of Pomegranate in Caenorhabditis Elegans. Pharmacogn. Mag. 2015, 11, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and Potential Health Benefits of Pomegranate: A Review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, M.; Wen, J.; Ren, F.; Yang, Z.; Jiang, X.; Chen, Y. The Bioactivity and Applications of Pomegranate Peel Extract: A Review. J. Food Biochem. 2022, 46, e14105. [Google Scholar] [CrossRef]
- Cerdá, B.; Espín, J.C.; Parra, S.; Martínez, P.; Tomás-Barberán, F.A. The Potent in Vitro Antioxidant Ellagitannins from Pomegranate Juice Are Metabolised into Bioavailable but Poor Antioxidant Hydroxy-6H-Dibenzopyran-6-One Derivatives by the Colonic Microflora of Healthy Humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of Urolithin a as a Metabolite Produced by Human Colon Microflora from Ellagic Acid and Related Compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef]
- Nuñez-Sánchez, M.A.; García-Villalba, R.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; Pastor-Quirante, F.A.; et al. Targeted Metabolic Profiling of Pomegranate Polyphenols and Urolithins in Plasma, Urine and Colon Tissues from Colorectal Cancer Patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; García-Villalba, R.; Martínez-Díaz, F.; Ocaña-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sánchez, A.; Abellán, B.; González-Sarrías, A.; Espín, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, e1801239. [Google Scholar] [CrossRef]
- García-Villalba, R.; Beltrán, D.; Espín, J.C.; Selma, M.V.; Tomás-Barberán, F.A. Time Course Production of Urolithins from Ellagic Acid by Human Gut Microbiota. J. Agric. Food Chem. 2013, 61, 8797–8806. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Gómez-Sánchez, M.B.; García-Talavera, N.V.; Gil-Izquierdo, A.; Sánchez-Alvarez, C.; Fontana-Compiano, L.O.; Morga-Egea, J.P.; Pastor-Quirante, F.A.; et al. Occurrence of Urolithins, Gut Microbiota Ellagic Acid Metabolites and Proliferation Markers Expression Response in the Human Prostate Gland upon Consumption of Walnuts and Pomegranate Juice. Mol. Nutr. Food Res. 2010, 54, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Caille, O.; Ferreira, D.; Francaux, M. Pomegranate Extract Prevents Skeletal Muscle of Mice against Wasting Induced by Acute TNF-α Injection. Mol. Nutr. Food Res. 2017, 61, 1600169. [Google Scholar] [CrossRef] [PubMed]
- Inglés, M.; Belenguer-Varea, A.; Serna, E.; Mas-Bargues, C.; Tarazona-Santabalbina, F.J.; Borrás, C.; Vina, J. Functional Transcriptomic Analysis of Centenarians’ Offspring Reveals a Specific Genetic Footprint That May Explain That They Are Less Frail Than Age-Matched Noncentenarians’ Offspring. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
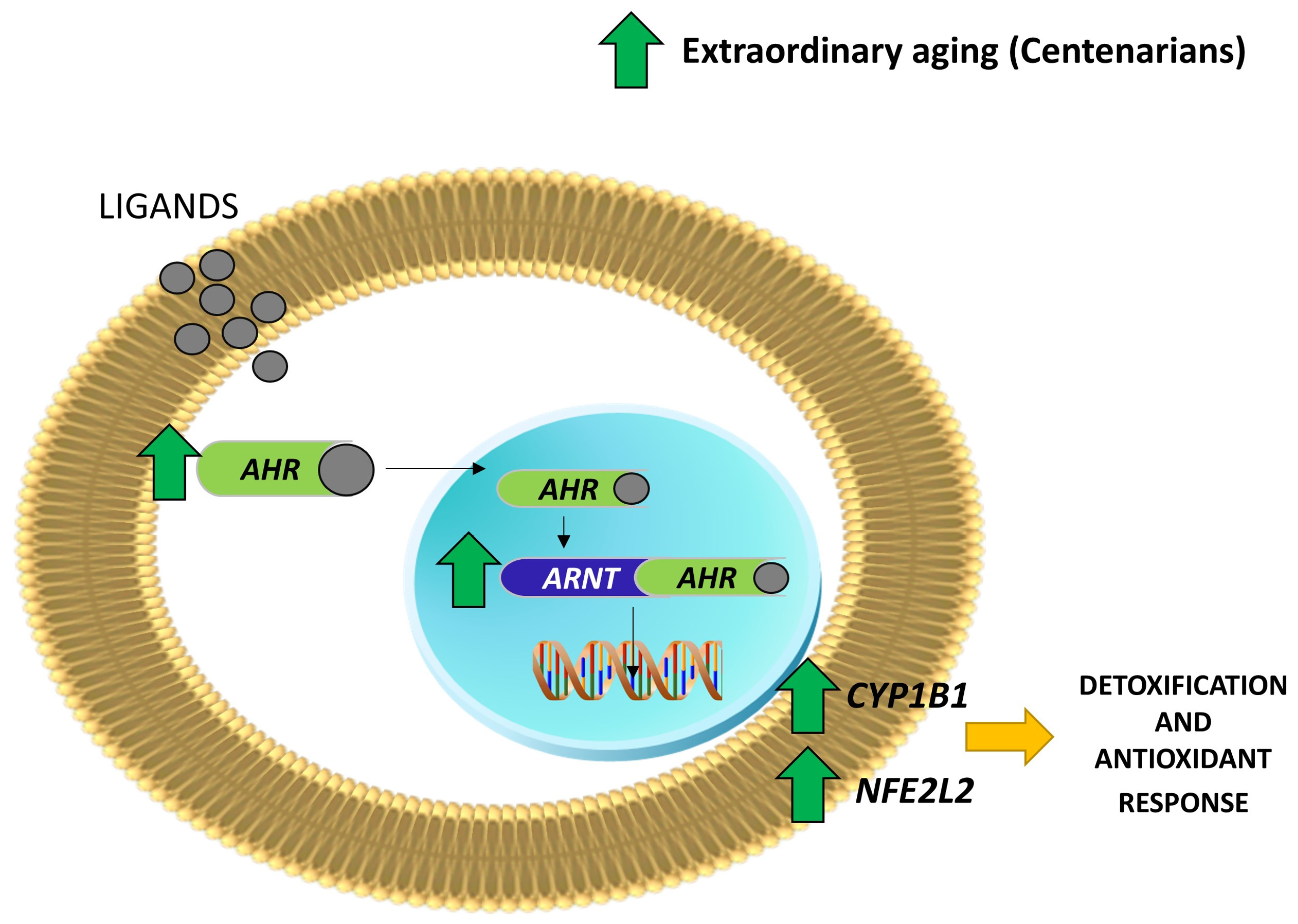
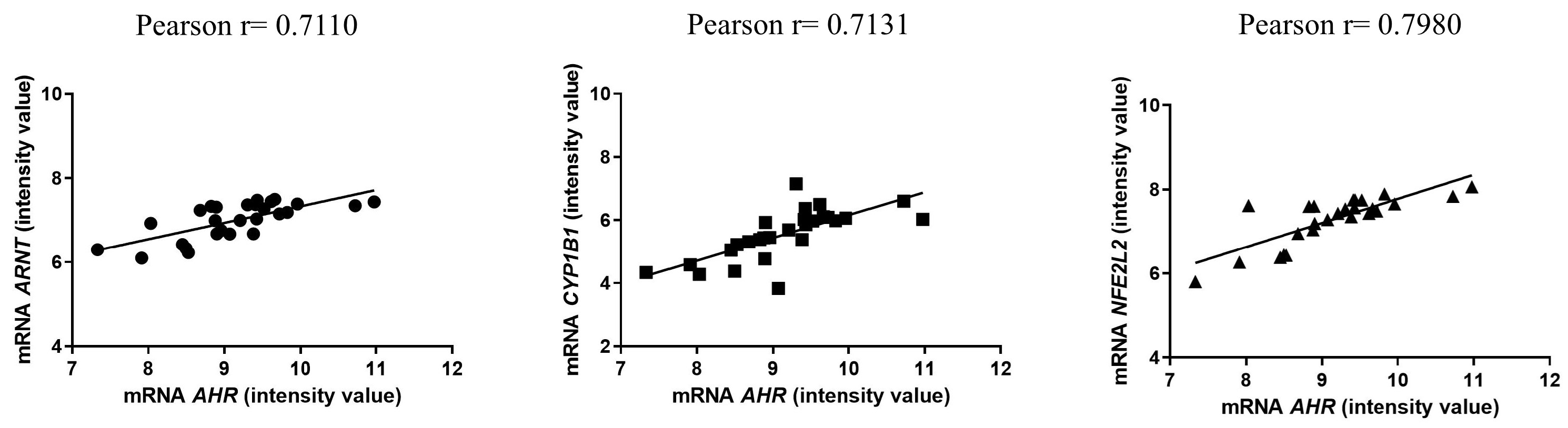
| AHR | ARNT | CYP1B1 | NFR2 | |
|---|---|---|---|---|
| C vs. S | 2.02 (p = 0.003) | 1.34 (p = 0.03) | 1.69 (p = 0.03) | 1.68 (p = 0.002) |
| C vs. D | 1.67 (p = 0.03) | 1.08 | 1.43 | 1.09 |
| D vs. S | 1.20 | 1.24 | 1.18 | 1.55 (p = 0.01) |
| PE Doses (mg/mL) | Positive Effect on Lifespan | Increment Half-Life vs. Vehicle (Days) | Increment Maximal Lifespan vs. Vehicle (Days) |
|---|---|---|---|
| 0.005 | p > 0.05 | 0 | 0 |
| 0.01 | p > 0.05 | 0 | 0 |
| 0.05 | p < 0.0001 | 6 | 2 |
| 0.1 | p < 0.0001 | 2 | 1 |
| 0.5 | p < 0.001 | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna, E.; Verdú, D.; Valls, A.; Belenguer-Varea, Á.; Tarazona-Santabalbina, F.J.; Borrás, C.; Viña, J. Involvement of Aryl Hydrocarbon Receptor in Longevity and Healthspan: Insights from Humans, Mice, and C. elegans. Int. J. Mol. Sci. 2024, 25, 9943. https://doi.org/10.3390/ijms25189943
Serna E, Verdú D, Valls A, Belenguer-Varea Á, Tarazona-Santabalbina FJ, Borrás C, Viña J. Involvement of Aryl Hydrocarbon Receptor in Longevity and Healthspan: Insights from Humans, Mice, and C. elegans. International Journal of Molecular Sciences. 2024; 25(18):9943. https://doi.org/10.3390/ijms25189943
Chicago/Turabian StyleSerna, Eva, David Verdú, Alicia Valls, Ángel Belenguer-Varea, Francisco José Tarazona-Santabalbina, Consuelo Borrás, and José Viña. 2024. "Involvement of Aryl Hydrocarbon Receptor in Longevity and Healthspan: Insights from Humans, Mice, and C. elegans" International Journal of Molecular Sciences 25, no. 18: 9943. https://doi.org/10.3390/ijms25189943
APA StyleSerna, E., Verdú, D., Valls, A., Belenguer-Varea, Á., Tarazona-Santabalbina, F. J., Borrás, C., & Viña, J. (2024). Involvement of Aryl Hydrocarbon Receptor in Longevity and Healthspan: Insights from Humans, Mice, and C. elegans. International Journal of Molecular Sciences, 25(18), 9943. https://doi.org/10.3390/ijms25189943










