Dysregulated S100A9 Expression Impairs Matrix Deposition in Chronic Wounds
Abstract
1. Introduction
2. Results
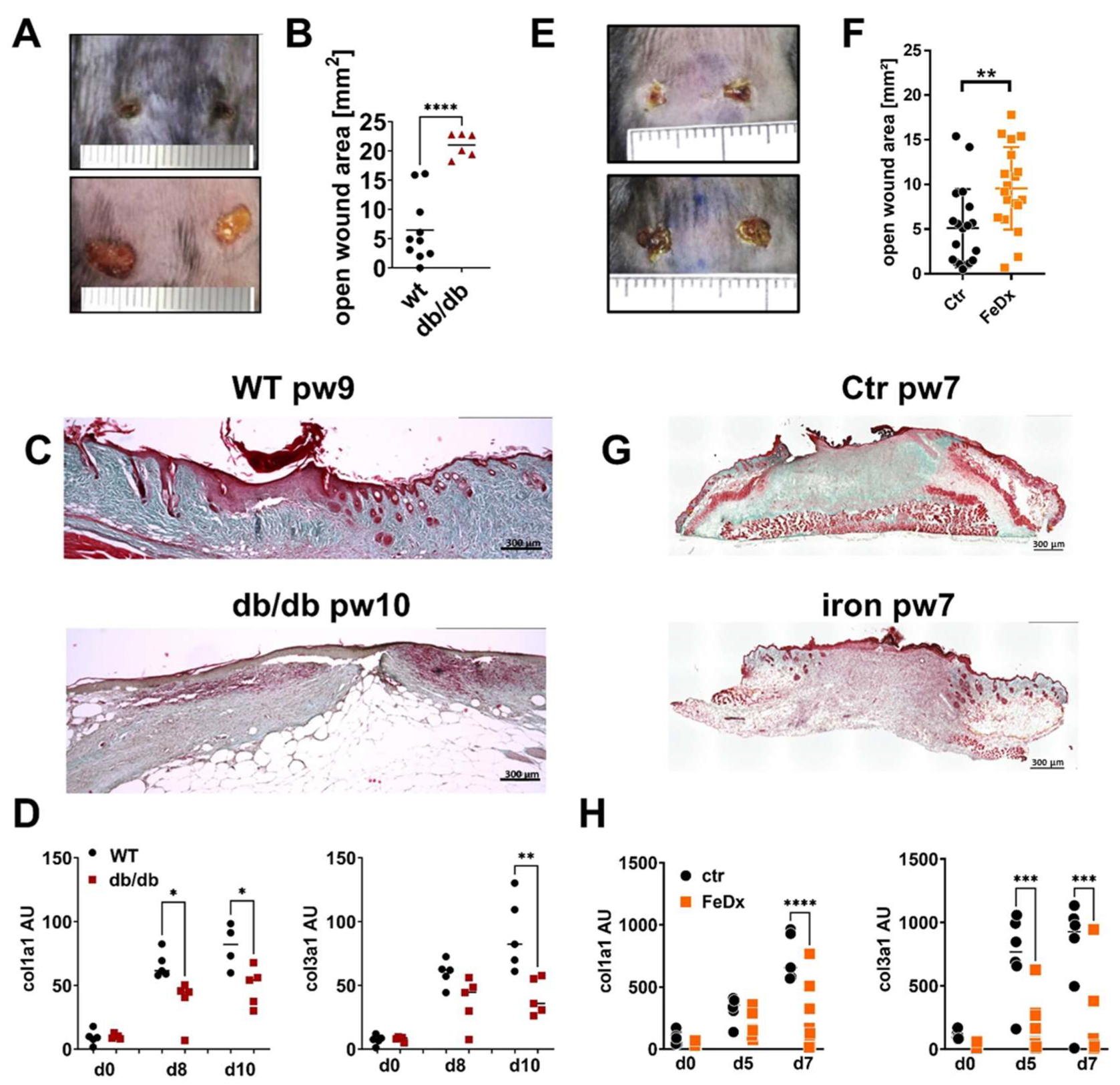
2.1. Production and Deposition of ECM Is Attenuated in Impaired Wound Healing
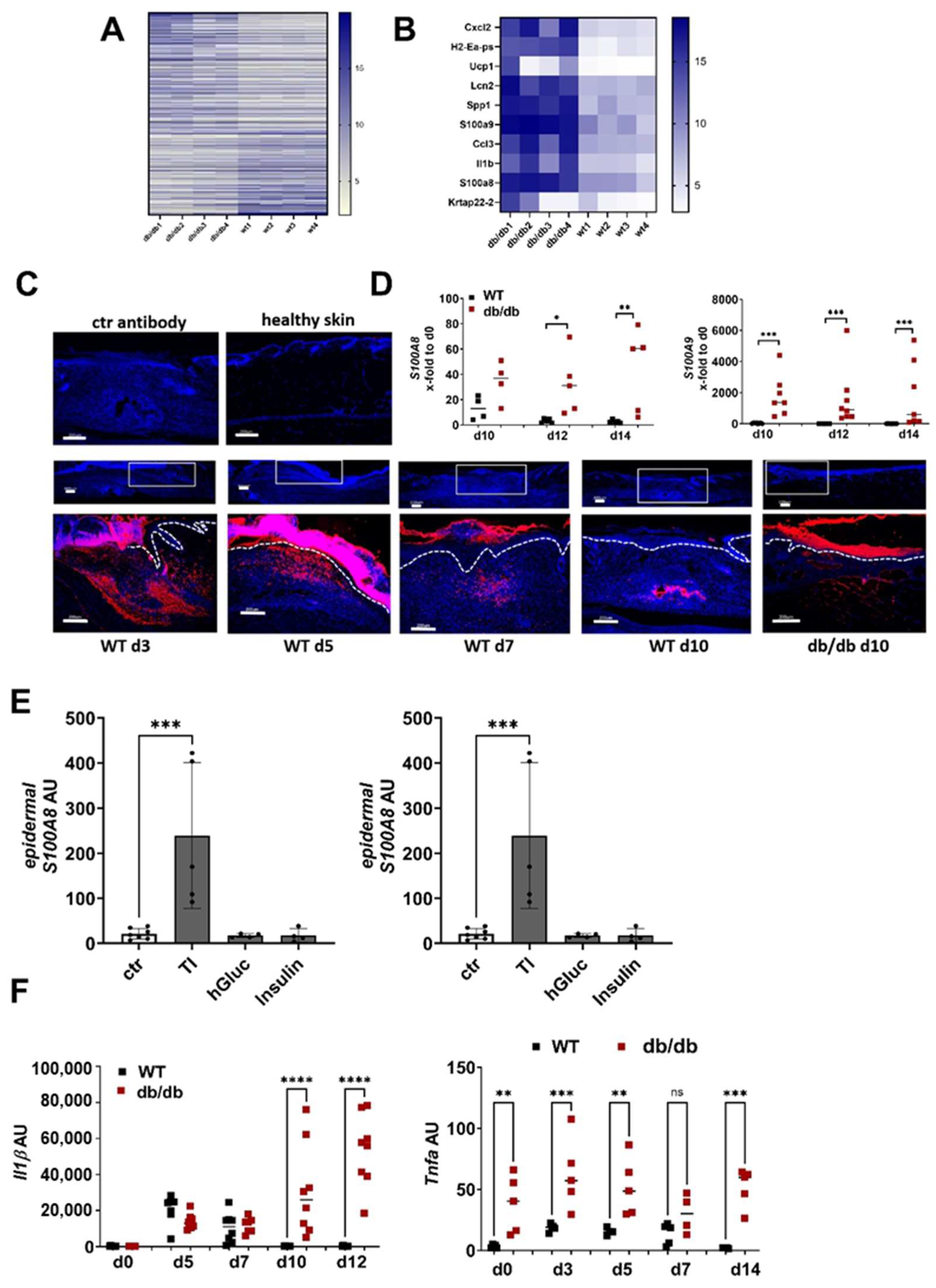
2.2. Overexpression of S100A8 and S100A9 during Impaired Wound Healing Is Associated with Diminished ECM Production
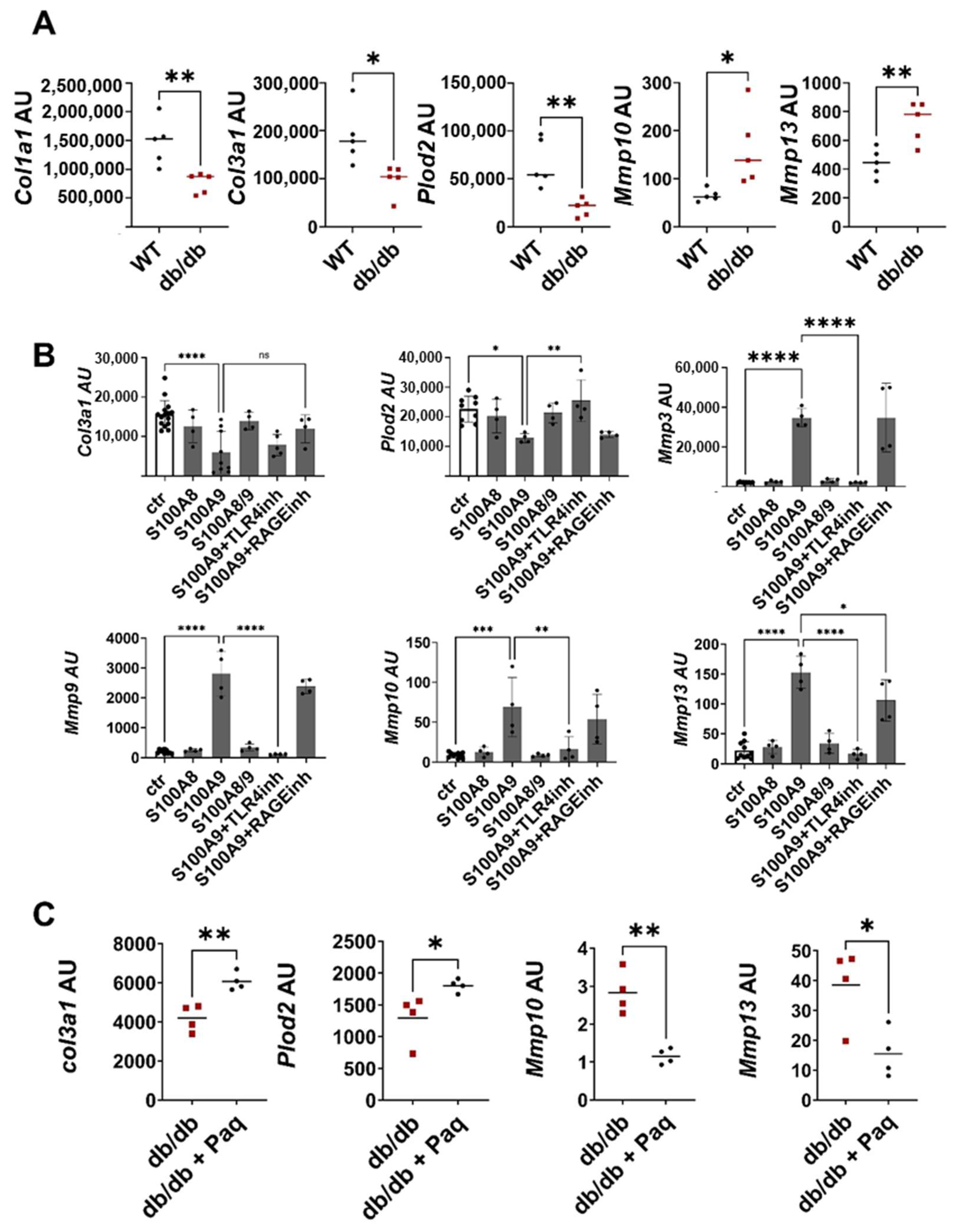
2.3. S100A9 Directly Affects the Deposition of ECM by Dermal Fibroblasts during Impaired Wound Healing
3. Discussion
4. Materials and Methods
4.1. Human Studies
4.2. Mouse Studies
4.3. Isolation of Skin Cells from Wounds
4.4. Cell Culture
4.5. Ex-Vivo Skin Culture
4.6. RNA Preparation and Quantitative Real-Time PCR
4.7. Genome-Wide Expression Analysis
4.8. Tissue Staining
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.A.; Martin, P. Cellular and Molecular Mechanisms of Skin Wound Healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef]
- Wang, J. Neutrophils in Tissue Injury and Repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef]
- Berberich, B.; Thriene, K.; Gretzmeier, C.; Kühl, T.; Bayer, H.; Athanasiou, I.; Rafei-Shamsabadi, D.A.; Bruckner-Tuderman, L.; Nyström, A.; Kiritsi, D.; et al. Proteomic Profiling of Fibroblasts Isolated from Chronic Wounds Identifies Disease-Relevant Signaling Pathways. J. Investig. Dermatol. 2020, 140, 2280–2290.e4. [Google Scholar] [CrossRef]
- Huang, Y.; Kyriakides, T.R. The Role of Extracellular Matrix in the Pathophysiology of Diabetic Wounds. Matrix Biol. Plus 2020, 6, 100037. [Google Scholar] [CrossRef]
- Sutcliffe, J.E.S.; Thrasivoulou, C.; Serena, T.E.; Madden, L.; Richards, T.; Phillips, A.R.J.; Becker, D.L. Changes in the Extracellular Matrix Surrounding Human Chronic Wounds Revealed by 2-photon Imaging. Int. Wound J. 2017, 14, 1225–1236. [Google Scholar] [CrossRef]
- Eming, S.A.; Koch, M.; Krieger, A.; Brachvogel, B.; Kreft, S.; Bruckner-Tuderman, L.; Krieg, T.; Shannon, J.D.; Fox, J.W. Differential Proteomic Analysis Distinguishes Tissue Repair Biomarker Signatures in Wound Exudates Obtained from Normal Healing and Chronic Wounds. J. Proteome Res. 2010, 9, 4758–4766. [Google Scholar] [CrossRef]
- Lim, D.X.E.; Richards, T.; Kanapathy, M.; Sudhaharan, T.; Wright, G.D.; Phillips, A.R.J.; Becker, D.L. Extracellular Matrix and Cellular Senescence in Venous Leg Ulcers. Sci. Rep. 2021, 11, 20168. [Google Scholar] [CrossRef]
- Vogl, T.; Tenbrock, K.; Ludwig, S.; Leukert, N.; Ehrhardt, C.; van Zoelen, M.A.D.; Nacken, W.; Foell, D.; van der Poll, T.; Sorg, C.; et al. Mrp8 and Mrp14 Are Endogenous Activators of Toll-like Receptor 4, Promoting Lethal, Endotoxin-Induced Shock. Nat. Med. 2007, 13, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Stratis, A.; Wixler, V.; Völler, T.; Thurainayagam, S.; Jorch, S.K.; Zenker, S.; Dreiling, A.; Chakraborty, D.; Fröhling, M.; et al. Autoinhibitory Regulation of S100A8/S100A9 Alarmin Activity Locally Restricts Sterile Inflammation. J. Clin. Investig. 2018, 128, 1852–1866. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, C.; Voss, A.; Scholzen, T.E.; Averill, M.M.; Zänker, K.S.; Bornfeldt, K.E. Novel Insights into the Role of S100A8/A9 in Skin Biology. Exp. Dermatol. 2012, 21, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1208. [Google Scholar] [CrossRef]
- Thorey, I.S.; Roth, J.; Regenbogen, J.; Halle, J.-P.; Bittner, M.; Vogl, T.; Kaesler, S.; Bugnon, P.; Reitmaier, B.; Durka, S.; et al. The Ca2+-Binding Proteins S100A8 and S100A9 Are Encoded by Novel Injury-Regulated Genes. J. Biol. Chem. 2001, 276, 35818–35825. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, H.; Huang, T.; Shi, J.; Wei, T.; Wang, Y. S100A9 Blockade Improves the Functional Recovery after Spinal Cord Injury via Mediating Neutrophil Infiltration. Exp. Ther. Med. 2022, 23, 291. [Google Scholar] [CrossRef]
- Marinković, G.; Koenis, D.S.; De Camp, L.; Jablonowski, R.; Graber, N.; De Waard, V.; De Vries, C.J.; Goncalves, I.; Nilsson, J.; Jovinge, S.; et al. S100A9 Links Inflammation and Repair in Myocardial Infarction. Circ. Res. 2020, 127, 664–676. [Google Scholar] [CrossRef]
- Franz, S.; Ertel, A.; Engel, K.M.; Simon, J.C.; Saalbach, A. Overexpression of S100A9 in Obesity Impairs Macrophage Differentiation via TLR4-NFkB-Signaling Worsening Inflammation and Wound Healing. Theranostics 2022, 12, 1659–1682. [Google Scholar] [CrossRef]
- Schonthaler, H.B.; Guinea-Viniegra, J.; Wculek, S.K.; Ruppen, I.; Ximenez-Embun, P.; Guio-Carrion, A.; Navarro, R.; Hogg, N.; Ashman, K.; Wagner, E.F. S100A8-S100A9 Protein Complex Mediates Psoriasis by Regulating the Expression of Complement Factor C3. Immunity. 2013, 39, 1171–1181. [Google Scholar] [CrossRef]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An Unrestrained Proinflammatory M1 Macrophage Population Induced by Iron Impairs Wound Healing in Humans and Mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef]
- Björk, P.; Björk, A.; Vogl, T.; Stenström, M.; Liberg, D.; Olsson, A.; Roth, J.; Ivars, F.; Leanderson, T. Identification of Human S100A9 as a Novel Target for Treatment of Autoimmune Disease via Binding to Quinoline-3-Carboxamides. PLoS Biol. 2009, 7, 0800–0812. [Google Scholar] [CrossRef] [PubMed]
- Eckes, B.; Zigrino, P.; Kessler, D.; Holtkötter, O.; Shephard, P.; Mauch, C.; Krieg, T. Fibroblast-Matrix Interactions in Wound Healing and Fibrosis. Matrix Biol. 2000, 19, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, M.; Kakpenova, A.; Simon, J.C.; Franz, S. Modulation of Macrophage Functions by ECM-Inspired Wound Dressings—A Promising Therapeutic Approach for Chronic Wounds. Biol. Chem. 2021, 402, 1289–1307. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Tecilazich, F.; Kafanas, A.; Doupis, J.; Gnardellis, C.; Leal, E.; Tellechea, A.; Pradhan, L.; Lyons, T.E.; Giurini, J.M.; et al. Mechanisms Involved in the Development and Healing of Diabetic Foot Ulceration. Diabetes 2012, 61, 2937–2947. [Google Scholar] [CrossRef]
- Yager, D.R.; Nwomeh, B.C. The Proteolytic Environment of Chronic Wounds. Wound Repair. Regen. 1999, 7, 433–441. [Google Scholar] [CrossRef]
- Krisp, C.; Jacobsen, F.; McKay, M.J.; Molloy, M.P.; Steinstraesser, L.; Wolters, D.A. Proteome Analysis Reveals Antiangiogenic Environments in Chronic Wounds of Diabetes Mellitus Type 2 Patients. Proteomics 2013, 13, 2670–2681. [Google Scholar] [CrossRef]
- Shabani, F.; Farasat, A.; Mahdavi, M.; Gheibi, N. Calprotectin (S100A8/100A9): A Key Protein between Inflammation and Cancer. Inflamm. Res. 2018, 67, 801–812. [Google Scholar] [CrossRef]
- Arias, J.L.; Funes, S.C.; Blas, R.; Callegari, E.; Eliçabe, R.J.; Páez, M.D.; Munarriz, A.; Pardo-Hidalgo, R.; Tamashiro, H.; Di Genaro, M.S. S100A8 Alarmin Supports IL-6 and Metalloproteinase-9 Production by Fibroblasts in the Synovial Microenvironment of Peripheral Spondyloarthritis. Front. Immunol. 2023, 13, 1077914. [Google Scholar] [CrossRef]
- Crowe, L.A.N.; McLean, M.; Kitson, S.M.; Melchor, E.G.; Patommel, K.; Cao, H.M.; Reilly, J.H.; Leach, W.J.; Rooney, B.P.; Spencer, S.J.; et al. S100A8 & S100A9: Alarmin Mediated Inflammation in Tendinopathy. Sci. Rep. 2019, 9, 1463. [Google Scholar] [CrossRef]
- Hibino, T.; Sakaguchi, M.; Miyamoto, S.; Yamamoto, M.; Motoyama, A.; Hosoi, J.; Shimokata, T.; Ito, T.; Tsuboi, R.; Huh, N.H. S100A9 Is a Novel Ligand of EMMPRIN That Promotes Melanoma Metastasis. Cancer Res. 2013, 73, 172–183. [Google Scholar] [CrossRef]
- Qi, Y.; Xu, R. Roles of PLODs in Collagen Synthesis and Cancer Progression. Front. Cell Dev. Biol. 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, H.; Zhu, X.; Ma, Y.; Liu, Q.; Xue, Y.; Chu, H.; Wu, W.; Wang, J.; Zou, H. S100A9 Promotes Human Lung Fibroblast Cells Activation through Receptor for Advanced Glycation End-Product-Mediated Extracellular-Regulated Kinase 1/2, Mitogen-Activated Protein-Kinase and Nuclear Factor-ΚB-Dependent Pathways. Clin. Exp. Immunol. 2013, 173, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Y.; Du, J.; Liu, W.; Dong, C.; Huang, Z.; Zhang, Z.; Yang, L.; Wang, T.; Xiong, S.; et al. S100 Calcium-Binding Protein A9 Promotes Skin Regeneration through Toll-like Receptor 4 during Tissue Expansion. Burn. Trauma. 2023, 11, tkad030. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.; Csondor, R.; Šulskis, D.; Baronaitė, I.; Smirnovas, V.; Maheswaran, L.; Horrocks, J.; Munro, R.; Georgiadou, C.; Horvath, I.; et al. The Stabilization of S100A9 Structure by Calcium Inhibits the Formation of Amyloid Fibrils. Int. J. Mol. Sci. 2023, 24, 13200. [Google Scholar] [CrossRef]
- Chen, L.; DiPietro, L.A. Toll-Like Receptor Function in Acute Wounds. Adv. Wound Care 2017, 6, 344–355. [Google Scholar] [CrossRef]
- Davis, F.M.; DenDekker, A.; Kimball, A.; Joshi, A.D.; El Azzouny, M.; Wolf, S.J.; Obi, A.T.; Lipinski, J.; Gudjonsson, J.E.; Xing, X.; et al. Epigenetic Regulation of TLR4 in Diabetic Macrophages Modulates Immunometabolism and Wound Repair. J. Immunol. 2020, 204, 2503–2513. [Google Scholar] [CrossRef]
- Källberg, E.; Tahvili, S.; Ivars, F.; Leanderson, T. Induction of S100A9 Homodimer Formation in Vivo. Biochem. Biophys. Res. Commun. 2018, 500, 564–568. [Google Scholar] [CrossRef]
- Signor, L.; Paris, T.; Mas, C.; Picard, A.; Lutfalla, G.; Boeri Erba, E.; Yatime, L. Divalent Cations Influence the Dimerization Mode of Murine S100A9 Protein by Modulating Its Disulfide Bond Pattern. J. Struct. Biol. 2021, 213, 107689. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, Definitions, and Functions in Health and Disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Schiopu, A.; Cotoi, O.S. S100A8 and S100A9: DAMPs at the Crossroads between Innate Immunity, Traditional Risk Factors, and Cardiovascular Disease. Mediators Inflamm. 2013, 2013, 828354. [Google Scholar] [CrossRef]
- Hesselstrand, R.; Distler, J.H.W.; Riemekasten, G.; Wuttge, D.M.; Törngren, M.; Nyhlén, H.C.; Andersson, F.; Eriksson, H.; Sparre, B.; Tuvesson, H.; et al. An Open-Label Study to Evaluate Biomarkers and Safety in Systemic Sclerosis Patients Treated with Paquinimod. Arthritis Res. Ther. 2021, 23, 204. [Google Scholar] [CrossRef] [PubMed]
- Herbert, D.; Franz, S.; Popkova, Y.; Anderegg, U.; Schiller, J.; Schwede, K.; Lorz, A.; Simon, J.C.; Saalbach, A. High-Fat Diet Exacerbates Early Psoriatic Skin Inflammation Independent of Obesity: Saturated Fatty Acids as Key Players. J. Investig. Dermatol. 2018, 138, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Hauck, S.; Zager, P.; Halfter, N.; Wandel, E.; Torregrossa, M.; Kakpenova, A.; Rother, S.; Ordieres, M.; Räthel, S.; Berg, A.; et al. Collagen/Hyaluronan Based Hydrogels Releasing Sulfated Hyaluronan Improve Dermal Wound Healing in Diabetic Mice via Reducing Inflammatory Macrophage Activity. Bioact. Mater. 2021, 6, 4342–4359. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franz, S.; Torregrossa, M.; Anderegg, U.; Ertel, A.; Saalbach, A. Dysregulated S100A9 Expression Impairs Matrix Deposition in Chronic Wounds. Int. J. Mol. Sci. 2024, 25, 9980. https://doi.org/10.3390/ijms25189980
Franz S, Torregrossa M, Anderegg U, Ertel A, Saalbach A. Dysregulated S100A9 Expression Impairs Matrix Deposition in Chronic Wounds. International Journal of Molecular Sciences. 2024; 25(18):9980. https://doi.org/10.3390/ijms25189980
Chicago/Turabian StyleFranz, Sandra, Marta Torregrossa, Ulf Anderegg, Anastasia Ertel, and Anja Saalbach. 2024. "Dysregulated S100A9 Expression Impairs Matrix Deposition in Chronic Wounds" International Journal of Molecular Sciences 25, no. 18: 9980. https://doi.org/10.3390/ijms25189980
APA StyleFranz, S., Torregrossa, M., Anderegg, U., Ertel, A., & Saalbach, A. (2024). Dysregulated S100A9 Expression Impairs Matrix Deposition in Chronic Wounds. International Journal of Molecular Sciences, 25(18), 9980. https://doi.org/10.3390/ijms25189980




