Predicting Biochemical and Physiological Parameters: Deep Learning from IgG Glycome Composition
Abstract
1. Introduction
2. Results
2.1. Population Characteristics
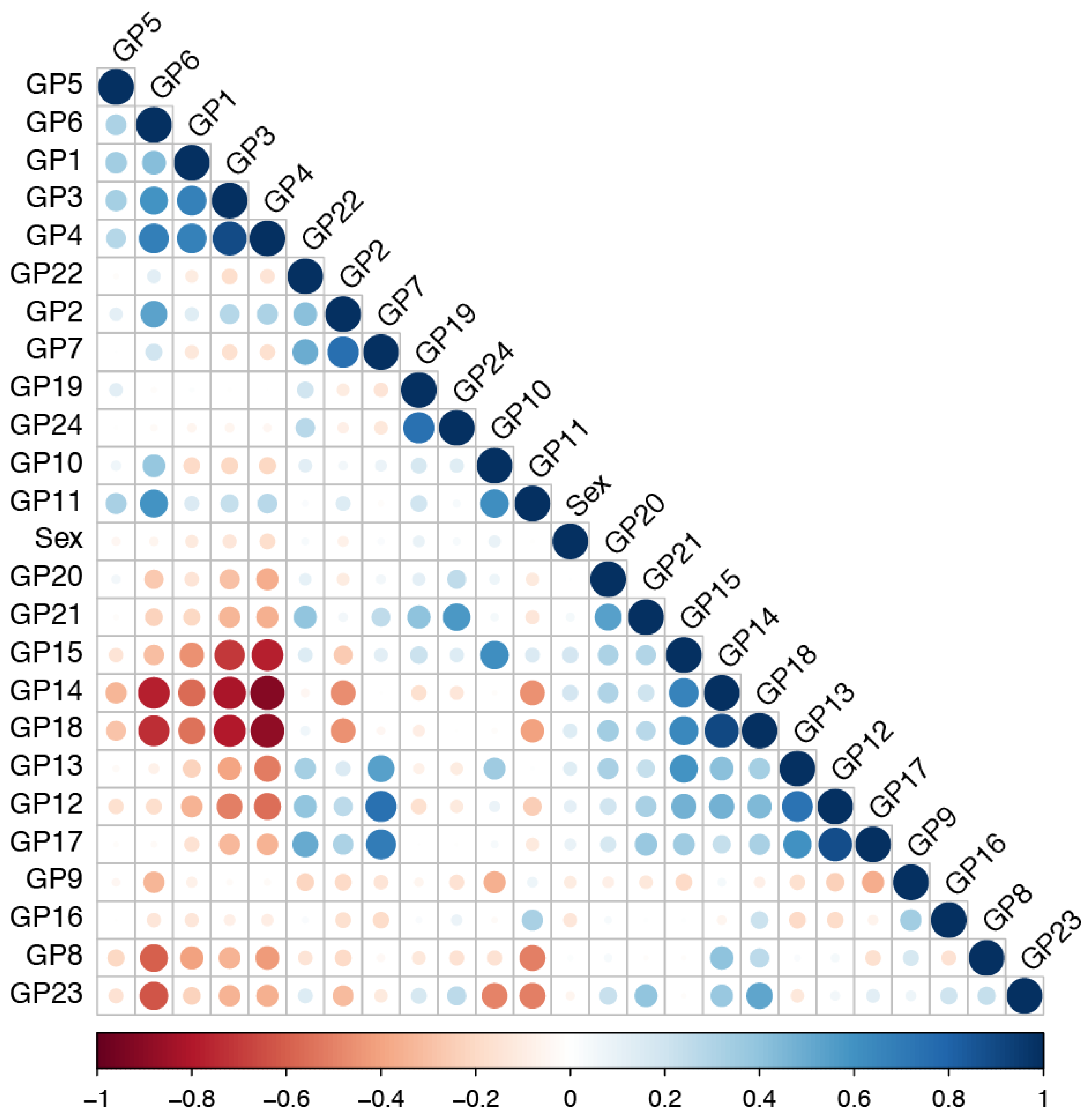
2.2. Glycomics Data Correlation
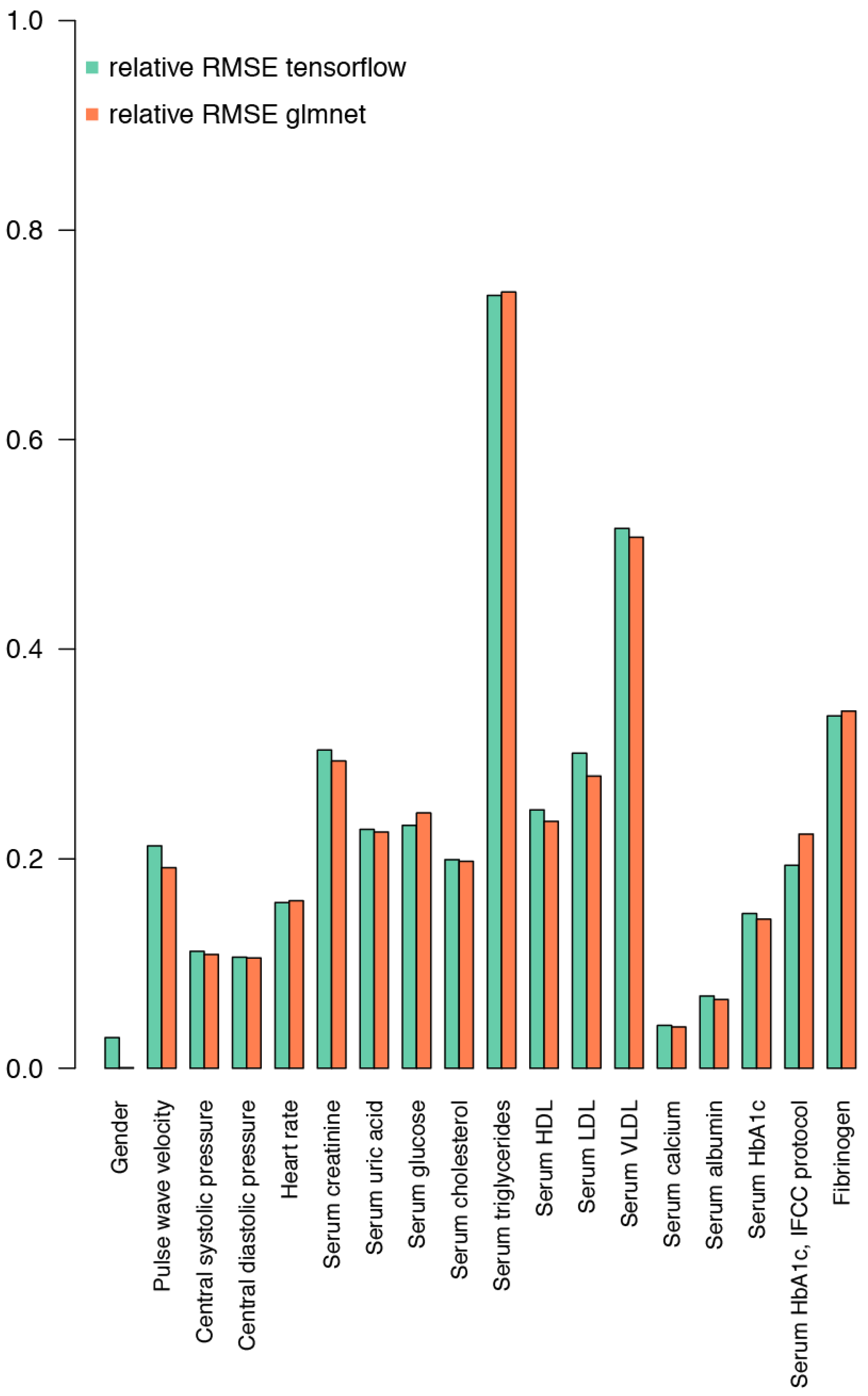
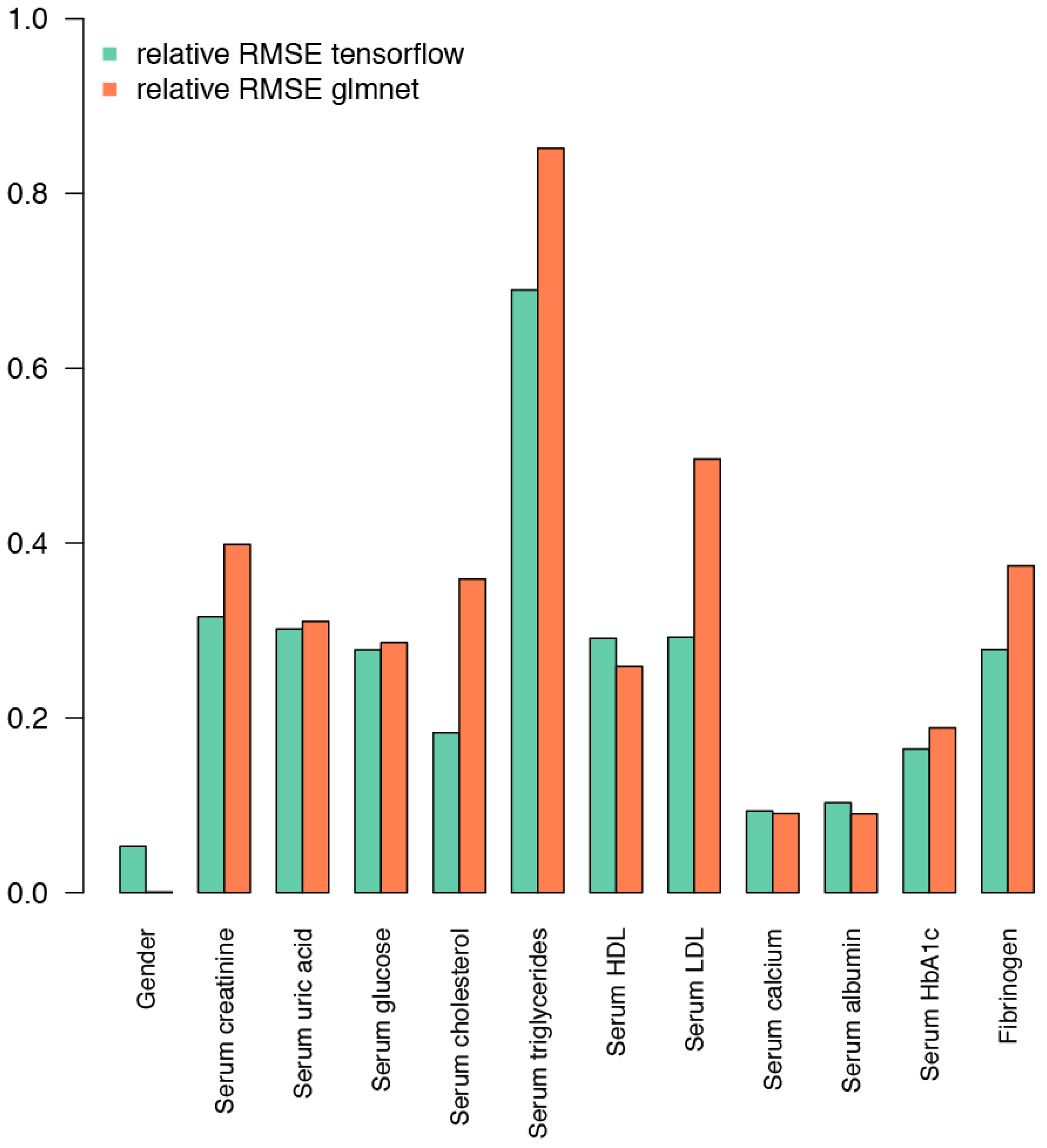
2.3. Prediction of Biochemical and Physiological Parameters from the IgG Glycome Composition
3. Discussion
4. Materials and Methods
4.1. Cohorts
4.2. Biochemical and Physiological Parameters
4.3. IgG Isolation and N-Glycan Analyses
4.4. Building of Predictive Models Using IgG Glycan Data
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shkunnikova, S.; Mijakovac, A.; Sironic, L.; Hanic, M.; Lauc, G.; Kavur, M.M. IgG Glycans in Health and Disease: Prediction, Intervention, Prognosis, and Therapy. Biotechnol. Adv. 2023, 67, 108169. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Yanaka, S.; Kato, K. Structure and Dynamics of Immunoglobulin G Glycoproteins. Adv. Exp. Med. Biol. 2018, 1104, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Krapp, S.; Mimura, Y.; Jefferis, R.; Huber, R.; Sondermann, P. Structural Analysis of Human IgG-Fc Glycoforms Reveals a Correlation between Glycosylation and Structural Integrity. J. Mol. Biol. 2003, 325, 979–989. [Google Scholar] [CrossRef]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; de Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Giddens, J.; Pincetic, A.; Lomino, J.V.; Ravetch, J.V.; Wang, L.-X.; Bjorkman, P.J. Structural Characterization of Anti-Inflammatory Immunoglobulin G Fc Proteins. J. Mol. Biol. 2014, 426, 3166–3179. [Google Scholar] [CrossRef] [PubMed]
- Gudelj, I.; Lauc, G.; Pezer, M. Immunoglobulin G Glycosylation in Aging and Diseases. Cell. Immunol. 2018, 333, 65–79. [Google Scholar] [CrossRef]
- Anumula, K.R. Quantitative Glycan Profiling of Normal Human Plasma Derived Immunoglobulin and Its Fragments Fab and Fc. J. Immunol. Methods 2012, 382, 167–176. [Google Scholar] [CrossRef]
- Zhu, D.; McCarthy, H.; Ottensmeier, C.H.; Johnson, P.; Hamblin, T.J.; Stevenson, F.K. Acquisition of Potential N-Glycosylation Sites in the Immunoglobulin Variable Region by Somatic Mutation is a Distinctive Feature of Follicular Lymphoma. Blood 2002, 99, 2562–2568. [Google Scholar] [CrossRef]
- Dunn-Walters, D.; Boursier, L.; Spencer, J. Effect of Somatic Hypermutation on Potential N-Glycosylation Sites in Human Immunoglobulin Heavy Chain Variable Regions. Mol. Immunol. 2000, 37, 107–113. [Google Scholar] [CrossRef]
- Zoldoš, V.; Horvat, T.; Lauc, G. Glycomics Meets Genomics, Epigenomics and Other High Throughput Omics for System Biology Studies. Curr. Opin. Chem. Biol. 2013, 17, 34–40. [Google Scholar] [CrossRef]
- Lauc, G.; Vojta, A.; Zoldoš, V. Epigenetic Regulation of Glycosylation Is the Quantum Mechanics of Biology. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Klarić, L.; Tsepilov, Y.A.; Stanton, C.M.; Mangino, M.; Sikka, T.T.; Esko, T.; Pakhomov, E.; Salo, P.; Deelen, J.; McGurnaghan, S.J.; et al. Glycosylation of Immunoglobulin G is Regulated by a Large Network of Genes Pleiotropic with Inflammatory Diseases. Sci. Adv. 2020, 6, eaax0301. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.F.; Camelo, J.S.; Molfetta, G.A.; Turcato, M.; Souza, C.F.M.; Porta, G.; Steiner, C.E.; Silva, W.A. Clinical Profile and Molecular Characterization of Galactosemia in Brazil: Identification of Seven Novel Mutations. BMC Med. Genet. 2016, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Chang, I.J.; He, M.; Lam, C.T. Congenital Disorders of Glycosylation. Ann. Transl. Med. 2018, 6, 477. [Google Scholar] [CrossRef] [PubMed]
- Krištić, J.; Vučković, F.; Menni, C.; Klarić, L.; Keser, T.; Beceheli, I.; Pučić-Baković, M.; Novokmet, M.; Mangino, M.; Thaqi, K.; et al. Glycans Are a Novel Biomarker of Chronological and Biological Ages. J. Gerontol. Ser. A 2013, 69, 779–789. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Kristic, J.; Dong, J.; Chu, X.; Ge, S.; Wang, H.; Fang, H.; Gao, Q.; Liu, D.; et al. Profiling IgG N-Glycans as Potential Biomarker of Chronological and Biological Ages: A Community-Based Study in a Han Chinese Population. Medicine 2016, 95, e4112. [Google Scholar] [CrossRef]
- Hou, H.; Xu, X.; Sun, F.; Zhang, X.; Dong, H.; Wang, L.; Ge, S.; An, K.; Sun, Q.; Li, Y.; et al. Hyperuricemia is associated with immunoglobulin G N-Glycosylation: A Community-Based Study of Glycan Biomarkers. OMICS J. Integr. Biol. 2019, 23, 660–667. [Google Scholar] [CrossRef]
- Liu, J.N.; Dolikun, M.; Stambuk, J.; Trbojevic-Akmacic, I.; Zhang, J.; Wang, H.; Zheng, D.Q.; Zhang, X.Y.; Peng, H.L.; Zhao, Z.Y.; et al. The Association Between Subclass-Specific IgG Fc N-Glycosylation Profiles and Hypertension in the Uygur, Kazak, Kirgiz, and Tajik Populations. J. Hum. Hypertens. 2018, 32, 555–563. [Google Scholar] [CrossRef]
- Štambuk, T.; Kifer, D.; Smirčić-Duvnjak, L.; Lovrenčić, M.V.; Gornik, O. Associations between Plasma Protein, IgG and IgA N-Glycosylation and Metabolic Health Markers in Pregnancy and Gestational Diabetes. PLoS ONE 2023, 18, e0284838. [Google Scholar] [CrossRef]
- Wang, Y.; Klarić, L.; Yu, X.; Thaqi, K.; Dong, J.; Novokmet, M.; Wilson, J.; Polasek, O.; Liu, Y.; Krištić, J.; et al. The Association between Glycosylation of Immunoglobulin G and Hypertension: A Multiple Ethnic Cross-Sectional Study. Medicine 2016, 95, e3379. [Google Scholar] [CrossRef]
- Plomp, R.; Ruhaak, L.R.; Uh, H.-W.; Reiding, K.R.; Selman, M.; Houwing-Duistermaat, J.J.; Slagboom, P.E.; Beekman, M.; Wuhrer, M. Subclass-Specific IgG Glycosylation is Associated with Markers of Inflammation and Metabolic Health. Sci. Rep. 2017, 7, 12325. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, M.N.; Bakovic, M.P.; Kristic, J.; Novokmet, M.; Huffman, J.E.; Vitart, V.; Hayward, C.; Rudan, I.; Wilson, J.F.; Campbell, H.; et al. The Association Between Galactosylation of Immunoglobulin G and Body Mass Index. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Q.; Dong, J.; Li, D.; Xu, X.; Xing, W.; Zhang, X.; Cao, W.; Hou, H.; Wang, H.; et al. The Association between Normal BMI with Central Adiposity and Proinflammatory Potential Immunoglobulin G N-Glycosylation. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2373–2385. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chu, X.; Wang, H.; Dong, J.; Ge, S.-Q.; Zhao, Z.-Y.; Peng, H.-L.; Sun, M.; Wu, L.-J.; Song, M.-S.; et al. The Changes of Immunoglobulin G N-Glycosylation in Blood Lipids and Dyslipidaemia. J. Transl. Med. 2018, 16, 235. [Google Scholar] [CrossRef]
- Gao, Q.; Dolikun, M.; Štambuk, J.; Wang, H.; Zhao, F.; Yiliham, N.; Wang, Y.; Trbojević-Akmačić, I.; Zhang, J.; Fang, H.; et al. Immunoglobulin G N-Glycans as Potential Postgenomic Biomarkers for Hypertension in the Kazakh Population. OMICS J. Integr. Biol. 2017, 21, 380–389. [Google Scholar] [CrossRef]
- Meng, X.; Cao, W.; Liu, D.; Elijah, I.M.; Xing, W.; Hou, H.; Xu, X.; Song, M.; Wang, Y. Bidirectional Causality Between Immunoglobulin G N-Glycosylation and Metabolic Traits: A Mendelian Randomization Study. Engineering 2023, 26, 74–88. [Google Scholar] [CrossRef]
- Kifer, D.; Louca, P.; Cvetko, A.; Deriš, H.; Cindrić, A.; Grallert, H.; Peters, A.; Polašek, O.; Gornik, O.; Mangino, M.; et al. N-Glycosylation of Immunoglobulin G Predicts Incident Hypertension. J. Hypertens. 2021, 39, 2527–2533. [Google Scholar] [CrossRef]
- Li, S.; Meng, J.; Xu, F.; Wang, Q.; Tian, X.; Li, M.; Zeng, X.; Hu, C.; Zheng, Y. IgG Glycosylation Profiling of Peripheral Artery Diseases with Lectin Microarray. J. Clin. Med. 2022, 11, 5727. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and Variable Selection via the Elastic Net. J. R. Stat. Soc. Stat. Methodol. Ser. B 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Chen, R.J.; Lu, M.Y.; Williamson, D.F.; Chen, T.Y.; Lipkova, J.; Noor, Z.; Shaban, M.; Shady, M.; Williams, M.; Joo, B.; et al. Pan-Cancer Integrative Histology-Genomic Analysis via Multimodal Deep Learning. Cancer Cell 2022, 40, 865–878.e6. [Google Scholar] [CrossRef]
- Din, N.M.U.; Dar, R.A.; Rasool, M.; Assad, A. Breast Cancer Detection Using Deep Learning: Datasets, Methods, and Challenges Ahead. Comput. Biol. Med. 2022, 149, 106073. [Google Scholar] [CrossRef] [PubMed]
- Pučić, M.; Knežević, A.; Vidič, J.; Adamczyk, B.; Novokmet, M.; Polašek, O.; Gornik, O.; Šupraha-Goreta, S.; Wormald, M.R.; Redžić, I.; et al. High Throughput Isolation and Glycosylation Analysis of IgG-Variability and Heritability of the IgG Glycome in Three Isolated Human Populations. Mol. Cell. Proteom. 2011, 10, M111.010090. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Gudelj, I.; Macdonald-Dunlop, E.; Mangino, M.; Zierer, J.; Bešić, E.; Joshi, P.K.; Trbojević-Akmačić, I.; Chowienczyk, P.J.; Spector, T.D.; et al. Glycosylation Profile of Immunoglobulin G Is Cross-Sectionally Associated with Cardiovascular Disease Risk Score and Subclinical Atherosclerosis in Two Independent Cohorts. Circ. Res. 2018, 122, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dolikun, M.; Štambuk, J.; Trbojević-Akmačić, I.; Zhang, J.; Zhang, J.; Wang, H.; Meng, X.; Razdorov, G.; Menon, D.; et al. Glycomics for Type 2 Diabetes Biomarker Discovery: Promise of Immunoglobulin G Subclass-Specific Fragment Crystallizable N-Glycosylation in the Uyghur Population. OMICS J. Integr. Biol. 2019, 23, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G. Pretreatment Serum Albumin as a Predictor of Cancer Survival: A Systematic Review of the Epidemiological Literature. Nutr. J. 2010, 9, 69. [Google Scholar] [CrossRef]
- Phillips, A.; Shaper, A.G.; Whincup, P. Association Between Serum Albumin and Mortality From Cardiovascular Disease, Cancer, and Other Causes. Lancet 1989, 334, 1434–1436. [Google Scholar] [CrossRef]
- Djoussé, L.; Rothman, K.J.; Cupples, L.A.; Levy, D.; Ellison, R.C. Serum Albumin and Risk of Myocardial Infarction and All-Cause Mortality in the Framingham Offspring Study. Circulation 2002, 106, 2919–2924. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Hu, H.-Y.; Huang, N.; Chou, Y.-C.; Li, C.-P.; Chou, Y.-J. Albumin Levels and Cause-Specific Mortality in Community-Dwelling Older Adults. Prev. Med. 2018, 112, 145–151. [Google Scholar] [CrossRef]
- Klonoff-Cohen, H.; Barrett-Connor, E.L.; Edelstein, S.L. Albumin Levels as a Predictor of Mortality in the Healthy Elderly. J. Clin. Epidemiol. 1992, 45, 207–212. [Google Scholar] [CrossRef]
- Štambuk, J.; Nakić, N.; Vučković, F.; Pučić-Baković, M.; Razdorov, G.; Trbojević-Akmačić, I.; Novokmet, M.; Keser, T.; Vilaj, M.; Štambuk, T.; et al. Global Variability of the Human IgG Glycome. Aging 2020, 12, 15222–15259. [Google Scholar] [CrossRef]
- Rudman, N.; Kifer, D.; Kaur, S.; Simunović, V.; Cvetko, A.; Pociot, F.; Morahan, G.; Gornik, O. Children at Onset of Type 1 Diabetes Show Altered N-Glycosylation of Plasma Proteins and IgG. Diabetologia 2022, 65, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- McEniery, C.M.; Cockcroft, J.R.; Roman, M.J.; Franklin, S.S.; Wilkinson, I.B. Central Blood Pressure: Current Evidence and Clinical Importance. Eur. Heart J. 2014, 35, 1719–1725. [Google Scholar] [CrossRef]
- Chen, J.; Chuang, S.; Chen, H.; Yeh, W.; Pan, W. Serum Uric Acid Level as an Independent Risk Factor for All-Cause, Cardiovascular, and Ischemic Stroke Mortality: A Chinese Cohort Study. Arthritis Care Res. 2009, 61, 225–232. [Google Scholar] [CrossRef]
- Dehghan, A.; van Hoek, M.; Sijbrands, E.J.; Hofman, A.; Witteman, J.C. High Serum Uric Acid as a Novel Risk Factor for Type 2 Diabetes. Diabetes Care 2008, 31, 361–362. [Google Scholar] [CrossRef]
- Barrios, C.; Zierer, J.; Gudelj, I.; Štambuk, J.; Ugrina, I.; Rodríguez, E.; Soler, M.J.; Pavić, T.; Šimurina, M.; Keser, T.; et al. Glycosylation Profile of IgG in Moderate Kidney Dysfunction. J. Am. Soc. Nephrol. 2016, 27, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Sun, S. A Survey of Multi-View Machine Learning. Neural Comput. Appl. 2013, 23, 2031–2038. [Google Scholar] [CrossRef]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.; Philbrick, K. Toolkits and Libraries for Deep Learning. J. Digit. Imaging 2017, 30, 400–405. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Yuan, H.; Ong, M.E.H.; Ning, Y.; Xie, F.; Saffari, S.E.; Shang, Y.; Volovici, V.; Chakraborty, B.; et al. Handling Missing Values in Healthcare Data: A Systematic Review of Deep Learning-Based Imputation Techniques. Artif. Intell. Med. 2023, 142, 102587. [Google Scholar] [CrossRef]
- Browning, S.R. Missing Data Imputation and Haplotype Phase Inference for Genome-Wide Association Studies. Hum. Genet. 2008, 124, 439–450. [Google Scholar] [CrossRef]
- Wang, A.; Yang, J.; An, N. Regularized Sparse Modelling for Microarray Missing Value Estimation. IEEE Access 2021, 9, 16899–16913. [Google Scholar] [CrossRef]
- Taylor, S.L.; Ruhaak, L.R.; Kelly, K.; Weiss, R.H.; Kim, K. Effects of Imputation on Correlation: Implications for Analysis of Mass Spectrometry Data from Multiple Biological Matrices. Brief. Bioinform. 2016, 18, 312–320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kang, H. The Prevention and Handling of the Missing Data. Korean J. Anesthesiol. 2013, 64, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-C.; Tsai, C.-F.; Zhong, J.R. Deep Learning for Missing Value Imputation of Continuous Data and the Effect of Data Discretization. Knowl.-Based Syst. 2022, 239, 108079. [Google Scholar] [CrossRef]
- Zemunik, T.; Boban, M.; Lauc, G.; Janković, S.; Rotim, K.; Vatavuk, Z.; Benčić, G.; Đogaš, Z.; Boraska, V.; Torlak, V.; et al. Genome-Wide Association Study of Biochemical Traits in Korcula Island, Croatia. Croat. Med. J. 2009, 50, 23–33. [Google Scholar] [CrossRef]
- Rudan, I.; Marušić, A.; Janković, S.; Rotim, K.; Boban, M.; Lauc, G.; Grković, I.; Đogaš, Z.; Zemunik, T.; Vatavuk, Z.; et al. “10 001 Dalmatians:” Croatia Launches Its National Biobank. Croat. Med. J. 2009, 50, 4–6. [Google Scholar] [CrossRef]
- Rudan, I.; Campbell, H.; Rudan, P. Genetic Epidemiological Studies of Eastern Adriatic Island Isolates, Croatia: Objectives and Strategies. Coll. Antropol. 1999, 23, 531–546. [Google Scholar]
- Rudan, I.; Biloglav, Z.; Vorko-Jović, A.; Kujundžić-Tiljak, M.; Stevanović, R.; Ropac, D.; Puntarić, D.; Čučević, B.; Salzer, B.; Campbell, H. Effects of Inbreeding, Endogamy, Genetic Admixture, and Outbreeding on Human Health: A “1001 Dalmatians” Study. Croat. Med. J. 2006, 47, 601–610. [Google Scholar]
- Trbojević-Akmačić, I.; Vučković, F.; Pribić, T.; Vilaj, M.; Černigoj, U.; Vidič, J.; Šimunović, J.; Kępka, A.; Kolčić, I.; Klarić, L.; et al. Comparative Analysis of Transferrin and IgG N-Glycosylation in Two Human Populations. Commun. Biol. 2023, 6, 312. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 14 July 2024).
- Wei, T.; Simko, V. R Package ‘corrplot’: Visualization of a Correlation Matrix (Version 0.92). 2021. Available online: https://github.com/taiyun/corrplot (accessed on 14 July 2024).
- Friedman, J.H.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Allaire, J.; Chollet, F. keras: R Interface to ‘Keras’ [R Package Version 2.13.0]. Available online: https://cran.r-project.org/web/packages/keras/index.html (accessed on 14 July 2024).
- Nematzadeh, S.; Kiani, F.; Torkamanian-Afshar, M.; Aydin, N. Tuning Hyperparameters of Machine Learning Algorithms and Deep Neural Networks Using Metaheuristics: A Bioinformatics Study on Biomedical and Biological Cases. Comput. Biol. Chem. 2021, 97, 107619. [Google Scholar] [CrossRef]
| Korčula Cohort (N = 950) | Vis Cohort (N = 888) | p Value | |
|---|---|---|---|
| Female | 615 (64.7%) | 521 (58.7%) | |
| Male | 335 (35.3%) | 367 (41.3%) | |
| Age (years) | 53.76 ± 16.97 | 56.30 ± 15.74 | |
| Pulse wave velocity | 8.64 ± 2.24 | / | |
| Central systolic pressure | 120.16 ± 16.72 | / | |
| Central diastolic pressure | 81.51 ± 9.20 | / | |
| Heart rate | 65.64 ± 10.34 | / | |
| Serum creatinine | 82.70 ± 17.37 | 87.73 ± 28.37 | 2.064 × 10−6 |
| Serum uric acid | 289.28 ± 79.20 | 310.83 ± 93.19 | 5.564 × 10−7 |
| Serum glucose | 5.68 ± 1.48 | 5.73 ± 1.55 | 4.201 × 10−1 |
| Serum cholesterol | 5.81 ± 1.22 | 6.06 ± 1.14 | 4.405 × 10−6 |
| Serum triglycerides | 1.39 ± 0.86 | 1.59 ± 1.01 | 7.655 × 10−7 |
| Serum HDL | 1.49 ± 0.36 | 1.60 ± 0.35 | 1.031 × 10−10 |
| Serum LDL | 3.70 ± 1.05 | 3.81 ± 1.05 | 2.736 × 10−2 |
| Serum VLDL | 0.63 ± 0.36 | / | |
| Serum calcium | 2.43 ± 0.10 | 2.33 ± 0.16 | 2.051 × 10−83 |
| Serum albumin | 45.04 ± 2.91 | 45.01 ± 3.64 | 2.660 × 10−1 |
| Serum HbA1c | 5.45 ± 0.77 | 5.46 ± 0.87 | 6.659 × 10−1 |
| Serum HbA1c, IFCC protocol | 36.14 ± 8.42 | / | |
| Fibrinogen | 2.79 ± 0.89 | 3.73 ± 0.99 | 1.921 × 10−89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vujić, A.; Klasić, M.; Lauc, G.; Polašek, O.; Zoldoš, V.; Vojta, A. Predicting Biochemical and Physiological Parameters: Deep Learning from IgG Glycome Composition. Int. J. Mol. Sci. 2024, 25, 9988. https://doi.org/10.3390/ijms25189988
Vujić A, Klasić M, Lauc G, Polašek O, Zoldoš V, Vojta A. Predicting Biochemical and Physiological Parameters: Deep Learning from IgG Glycome Composition. International Journal of Molecular Sciences. 2024; 25(18):9988. https://doi.org/10.3390/ijms25189988
Chicago/Turabian StyleVujić, Ana, Marija Klasić, Gordan Lauc, Ozren Polašek, Vlatka Zoldoš, and Aleksandar Vojta. 2024. "Predicting Biochemical and Physiological Parameters: Deep Learning from IgG Glycome Composition" International Journal of Molecular Sciences 25, no. 18: 9988. https://doi.org/10.3390/ijms25189988
APA StyleVujić, A., Klasić, M., Lauc, G., Polašek, O., Zoldoš, V., & Vojta, A. (2024). Predicting Biochemical and Physiological Parameters: Deep Learning from IgG Glycome Composition. International Journal of Molecular Sciences, 25(18), 9988. https://doi.org/10.3390/ijms25189988







