Iodine Rearrangements of Tetraallylsilane and Synthesis of Silicon-Stereogenic Organosilanes
Abstract
:1. Introduction
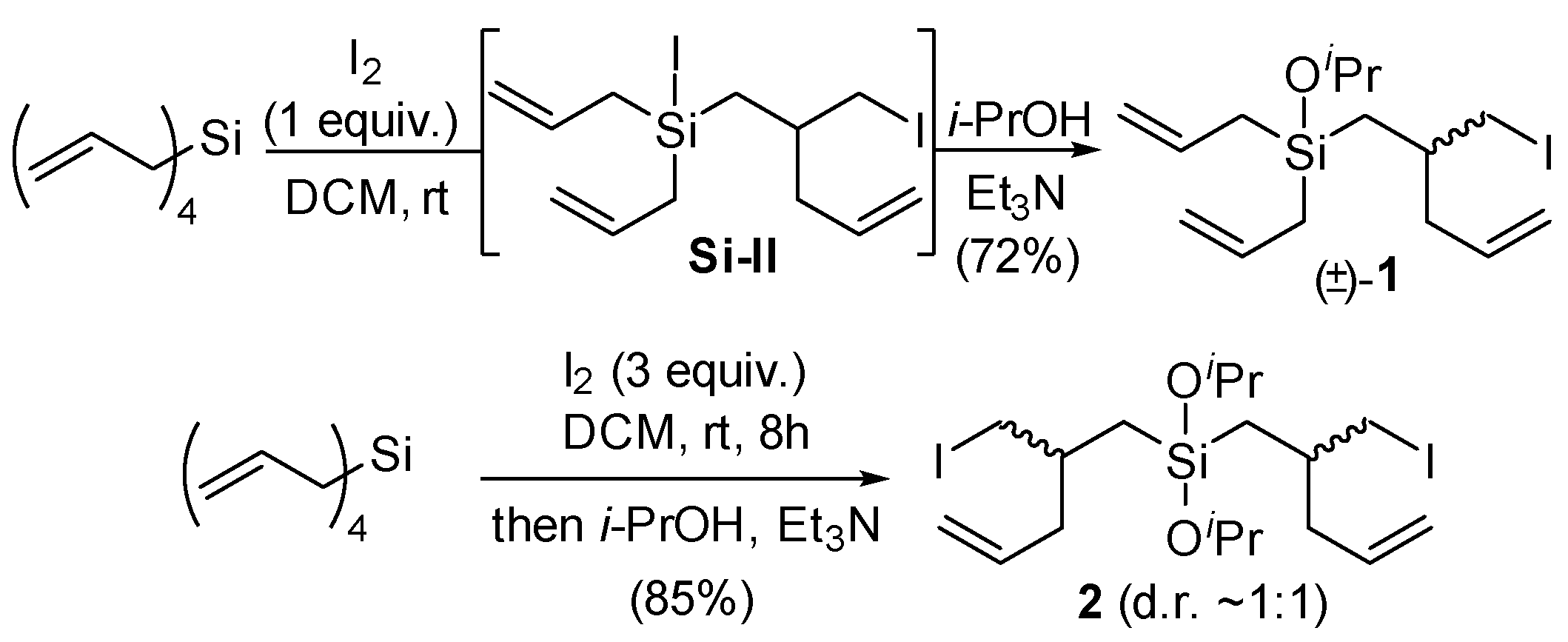
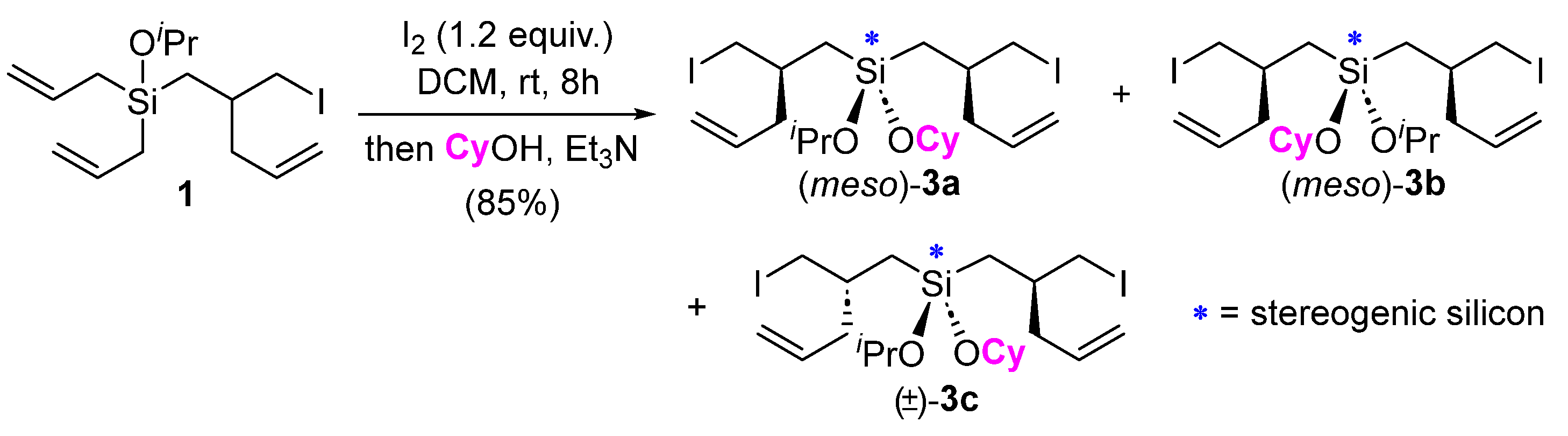
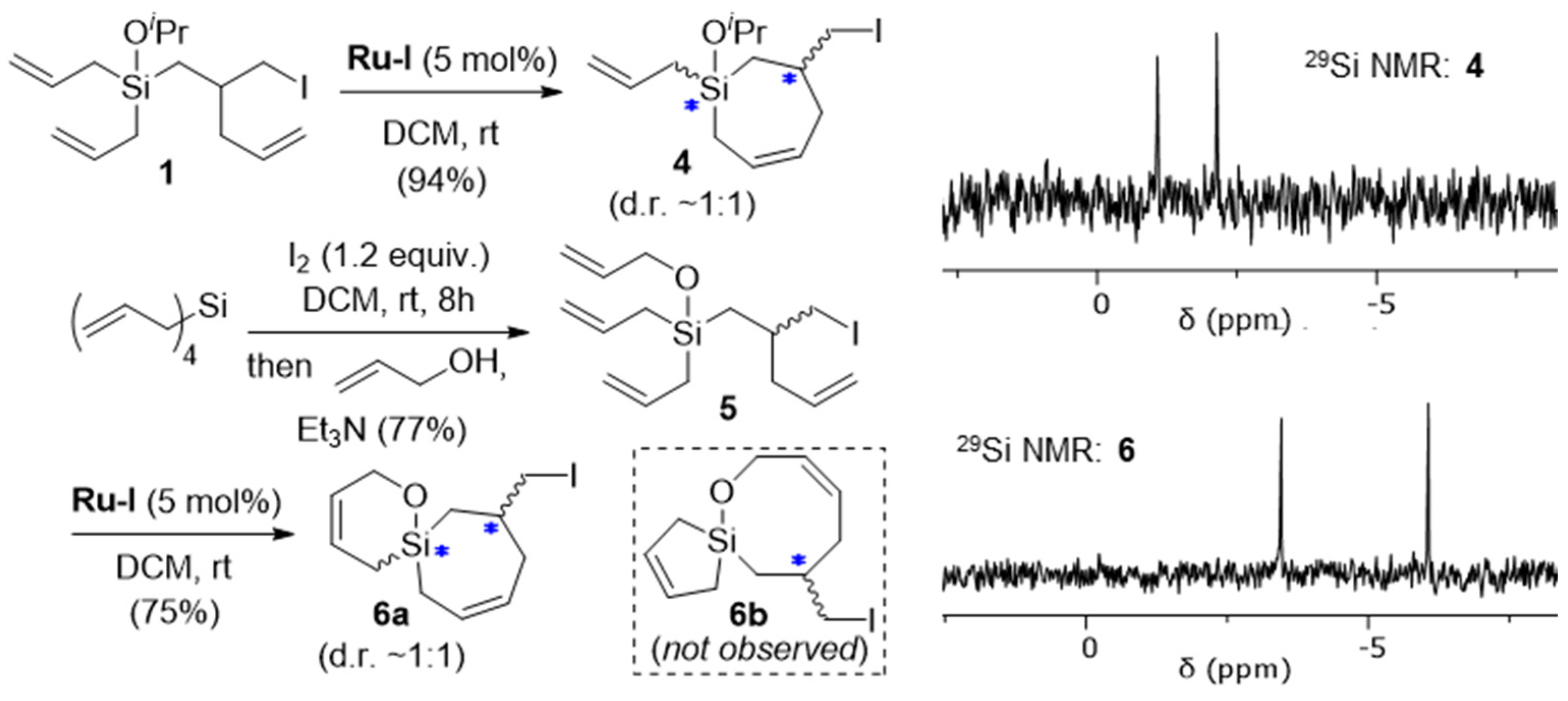
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Compound Syntheses and Spectral Data
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Neil, G.W.; Cummins, E.J. Iodine-mediated rearrangements of diallylsilanes. Tetrahedron Lett. 2017, 58, 3406–3409. [Google Scholar] [PubMed]
- Hosomi, A.; Sakurai, H. Protection of alcohols and acids with allylsilanes catalyzed by iodine or iodotrimethylsilane in chlorinated hydrocarbons. Chem. Lett. 1981, 10, 85–88. [Google Scholar]
- Jones, R.G.; Cragg, R.H.; Swain, A.C. Structure and mechanism in the cyclopolymerization of diallylsilanes. Eur. Polym. J. 1992, 28, 651–655. [Google Scholar]
- Suslova, E.N.; Albanov, B.A.; Shainyan, J. Unusual product of the Si-C bond cleavage in diallyldiphenylsilane. Russ. J. Gen. Chem. 2008, 78, 1016–1017. [Google Scholar]
- Suslova, E.N.; Albanov, B.A.; Shainyan, J. Transformations of diallylsilanes under the action of electrophilic reagents. J. Organomet. Chem. 2009, 694, 420–426. [Google Scholar]
- Bols, M.; Skrydstrup, T. Silicon-Tethered Reactions. Chem. Rev. 1995, 95, 1253–1277. [Google Scholar]
- Bracegirdle, S.; Anderson, E.A. Recent advances in the use of temporary silicon tethers in metal-mediated reactions. Chem. Soc. Rev. 2010, 39, 4114–4129. [Google Scholar]
- Moss, G.P. ‘stereogenic unit’. In IUPAC Compendium of Chemical Terminology, 3rd ed.; International Union of Pure and Applied Chemistry: Research Triangle Park, NC, USA, 2006; Online version 3.0.1, 2019. [Google Scholar] [CrossRef]
- Xu, L.-W.; Li, L.; Lai, G.-Q.; Jiang, J.-X. The recent synthesis and application of silicon-stereogenic silanes: A renewed and significant challenge in asymmetric synthesis. Chem. Soc. Rev. 2011, 40, 1777–1790. [Google Scholar]
- Wu, Y.; Zheng, L.; Wang, Y.; Wang, P. Catalytic asymmetric synthesis of silicon-stereogenic organosilanes. Chem 2023, 9, 3461–3514. [Google Scholar]
- Ye, Z.-T.; Wu, Z.-W.; Zhang, X.-X.; Zhou, J.; Yu, J.-S. Organocatalytic enantioselective construction of Si-stereocenters: Recent advances and perspectives. Chem. Soc. Rev. 2024, 53, 8546–8562. [Google Scholar]
- Tomooka, K.; Nakazaki, A.; Nakai, T. A Novel Aryl Migration from Silicon to Carbon: An Efficient Approach to Asymmetric Synthesis of r-Aryl â-Hydroxy Cyclic Amines and Silanols. J. Am. Chem. Soc. 2000, 122, 408–409. [Google Scholar]
- Igawa, K.; Takada, J.; Shimono, T.; Tomooka, K. Enantioselective Synthesis of Silanol. J. Am. Chem. Soc. 2008, 130, 16132–16133. [Google Scholar] [PubMed]
- Bauer, J.O.; Strohmann, C. Stereocontrol in Nucleophilic Substitution Reactions at Silicon: The Role of Permutation in Generating Silicon-Centered Chirality. J. Am. Chem. Soc. 2015, 137, 4304–4307. [Google Scholar]
- Bai, X.-F.; Zou, J.-F.; Chen, M.-Y.; Xu, Z.; Li, L.; Cui, Y.-M.; Zheng, Z.-J.; Xu, L.-W. Lewis-Base-Mediated Diastereoselective Silylations of Alcohols: Synthesis of Silicon-Stereogenic Dialkoxysilanes Controlled by Chiral Aryl BINMOLs. Chem. Asian J. 2017, 12, 1730–1735. [Google Scholar]
- Zhang, X.-X.; Gao, Y.; Zhang, Y.-X.; Zhou, J.; Yu, J.-S. Highly Enantioselective Construction of Multifunctional Silicon- Stereogenic Silacycles by Asymmetric Enamine Catalysis. Angew. Chem. Int. Ed. 2023, e202217724. [Google Scholar]
- Bains, W.; Tacke, R. Silicon chemistry as a novel source of chemical diversity in drug design. Curr. Opin. Drug Discovery Dev. 2003, 6, 526–543. [Google Scholar]
- Fotie, J.; Matherne, C.M.; Wroblewski, J.E. Silicon switch: Carbon-silicon Bioisosteric replacement as a strategy to modulate the selectivity, physicochemical, and drug-like properties in anticancer pharmacophores. Chem. Biol. Drug. Des. 2023, 102, 235–254. [Google Scholar]
- Kawakami, Y.; Kakihana, Y.; Ooi, O.; Oishi, M.; Suzuki, K.; Shinke, S.; Uenishi, K. Control of stereochemical structures of silicon-containing polymeric systems. Polym. Int. 2009, 58, 279–284. [Google Scholar]
- Fomina, I.A.; Myers, C.R.; Soumis, C.L.M.; Scheuermann, M.L.; McCarty, J.; Clark, T.B.; O’Neil, G.W. Regiodivergent Medium-Ring Oxasilacycle Synthesis from Diallylsilanes. Heterocycles 2022, 104, 1966–1993. [Google Scholar] [CrossRef]
- Ahmad, I.; Falck-Pederson, M.L.; Undheim, K. Synthesis of silacycloalkenes and silaspirenes by Ru(II)-catalyzed ring-closing metathesis reactions. J. Organomet. Chem. 2001, 625, 160–172. [Google Scholar]
- Mahieux, C.; Laguerre, M.; Landais, Y.; Pianet, I. Multinuclear magnetic resonance and molecular modeling investigations as unambiguous methods for the determination of silacycle 3D structures. Magn. Reson. Chem. 2004, 42, 467–473. [Google Scholar] [PubMed]
- Anhaus, J.T.; Gibson, V.C.; Clegg, W.; Collingwood, S.P. Silicon-Containing Macrocycles and Polymers via Metathesis: X-ray Crystal Structures of cis,cis- and trans,trans-1,1,6,6,Tetraphenyl-1,6-disilacyclodeca-3,8-diene. Organometallics 1993, 12, 1780–1798. [Google Scholar]
- Kulkarni, A.A.; Diver, S.T. Cycloheptadiene Ring Synthesis by Tandem Intermolecular Enyne Metathesis. Org. Lett. 2003, 5, 3463–3466. [Google Scholar]
- Shieh, P.; Nguyen, H.V.-T.; Johnson, J.A. Tailored silyl ether monomers enable backbond-degradable polynorbornene-based linear, bottlebrush and star copolymers through ROMP. Nature Chem. 2019, 11, 1124–1132. [Google Scholar]
- Morontsev, A.; Gringolts, M.; Lakhtin, V.; Finkelshtein, E. Synthesis of high-molecular weight poly(1,1-dimethyl-1-silapentene) by olefin metathesis polymerization in the presence of Grubbs catalysts. J. Organomet. Chem. 2020, 911, 121156. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, E.D.; Wier, K.E.; O’Neil, G.W. Iodine Rearrangements of Tetraallylsilane and Synthesis of Silicon-Stereogenic Organosilanes. Int. J. Mol. Sci. 2024, 25, 9996. https://doi.org/10.3390/ijms25189996
Tan ED, Wier KE, O’Neil GW. Iodine Rearrangements of Tetraallylsilane and Synthesis of Silicon-Stereogenic Organosilanes. International Journal of Molecular Sciences. 2024; 25(18):9996. https://doi.org/10.3390/ijms25189996
Chicago/Turabian StyleTan, Elliott D., Kerry E. Wier, and Gregory W. O’Neil. 2024. "Iodine Rearrangements of Tetraallylsilane and Synthesis of Silicon-Stereogenic Organosilanes" International Journal of Molecular Sciences 25, no. 18: 9996. https://doi.org/10.3390/ijms25189996





