Pinpointing Novel Plasma and Brain Proteins for Common Ocular Diseases: A Comprehensive Cross-Omics Integration Analysis
Abstract
1. Introduction
2. Results
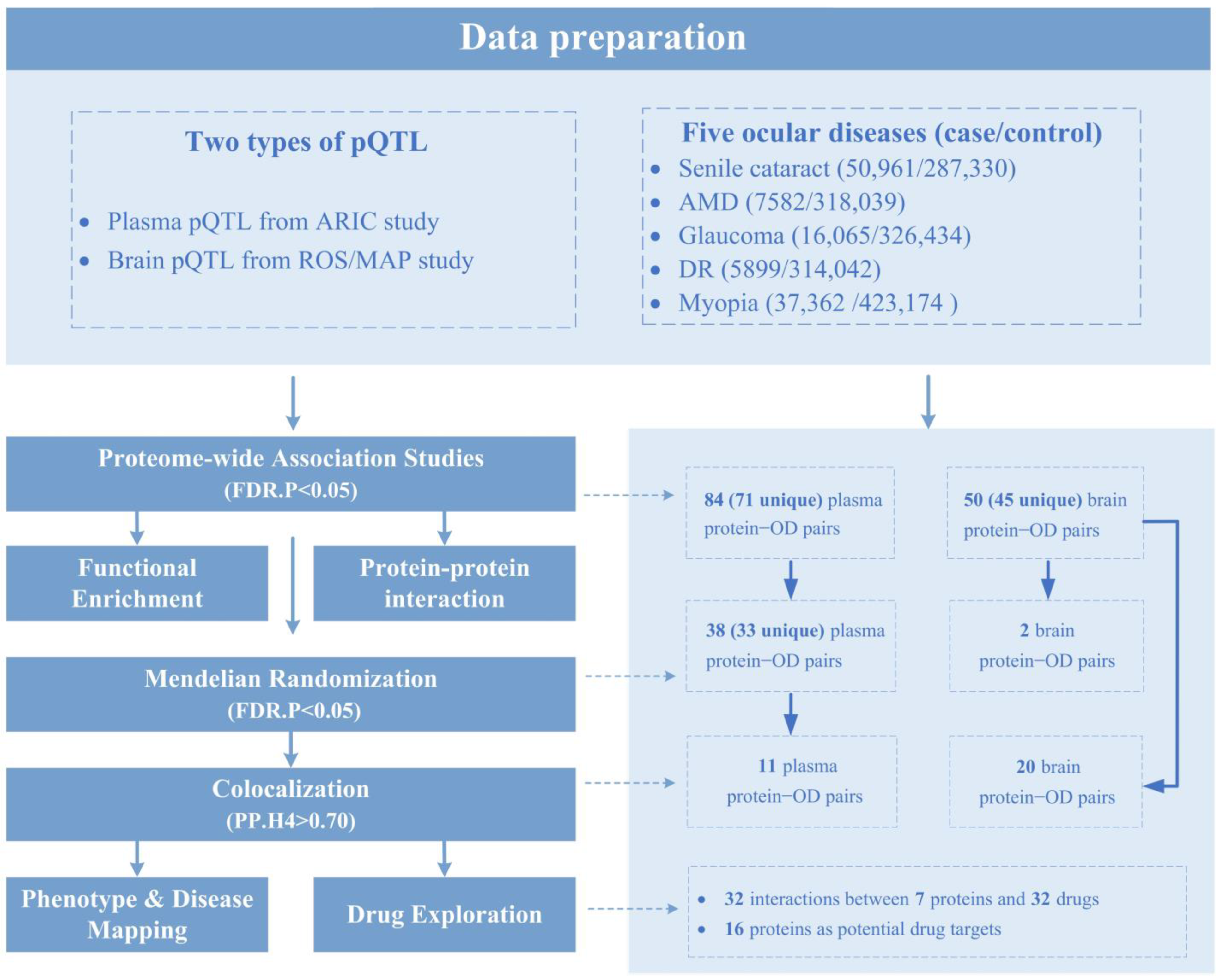
2.1. PWAS Identified 134 Protein–OD Pairs
2.2. MR Identified 40 Protein–OD Pairs
2.3. COLOC Validated Causal Relationships of 11 Plasma-Protein–OD Pairs
2.4. COLOC Validated 20 Out of 50 Brain-Protein–OD Pairs Identified by PWAS
2.5. Phenotype-Disease Mapping Analysis for COLOC-Associated Proteins
2.6. Candidate Druggable Targets
3. Discussion
4. Materials and Methods
4.1. Data Sources
4.1.1. GWAS Summary Data
4.1.2. Human Plasma pQTL Data
4.1.3. Human Brain pQTL Data
4.2. Statistical Analysis
4.2.1. Proteome-Wide Association Studies
4.2.2. Functional Enrichment Analysis and Protein–Protein Interaction Network
4.2.3. Mendelian Randomization Analysis
4.2.4. Bayesian Colocalization Analysis
4.2.5. Phenotype and Disease Mapping Analysis
4.2.6. Druggable Targets Exploration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Assi, L.; Chamseddine, F.; Ibrahim, P.; Sabbagh, H.; Rosman, L.; Congdon, N.; Evans, J.; Ramke, J.; Kuper, H.; Burton, M.J.; et al. A Global Assessment of Eye Health and Quality of Life: A Systematic Review of Systematic Reviews. JAMA Ophthalmol. 2021, 139, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Baird, P.N.; Saw, S.-M.; Lanca, C.; Guggenheim, J.A.; Smith, E.L., III; Zhou, X.; Matsui, K.-O.; Wu, P.-C.; Sankaridurg, P.; Chia, A.; et al. Myopia. Nat. Rev. Dis. Primers 2020, 6, 99. [Google Scholar] [CrossRef]
- Stuart, K.V.; Pasquale, L.R.; Kang, J.H.; Foster, P.J.; Khawaja, A.P. Towards Modifying the Genetic Predisposition for Glaucoma: An Overview of the Contribution and Interaction of Genetic and Environmental Factors. Mol. Asp. Med. 2023, 93, 101203. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.C.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A Large Genome-Wide Association Study of Age-Related Macular Degeneration Highlights Contributions of Rare and Common Variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Choquet, H.; Melles, R.B.; Anand, D.; Yin, J.; Cuellar-Partida, G.; Wang, W.; 23andMe Research Team; Hoffmann, T.J.; Nair, K.S.; Hysi, P.G.; et al. A Large Multiethnic GWAS Meta-Analysis of Cataract Identifies New Risk Loci and Sex-Specific Effects. Nat. Commun. 2021, 12, 3595. [Google Scholar] [CrossRef] [PubMed]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segrè, A.V.; Rouhana, J.M.; et al. Genome-Wide Meta-Analysis Identifies 127 Open-Angle Glaucoma Loci with Consistent Effect across Ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef]
- Ni, J.; Yuan, X.; Gu, J.; Yue, X.; Gu, X.; Nagaraj, R.H.; Crabb, J.W. Plasma Protein Pentosidine and Carboxymethyllysine, Biomarkers for Age-Related Macular Degeneration. Mol. Cell. Proteom. 2009, 8, 1921–1933. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Ridker, P.M.; Glynn, R.J.; Christen, W.G.; Dana, M.R.; Hennekens, C.H. High Levels of Plasma C-Reactive Protein and Future Risk of Age-Related Cataract. Ann. Epidemiol. 1999, 9, 166–171. [Google Scholar] [CrossRef]
- Fiedorowicz, E.; Cieślińska, A.; Kuklo, P.; Grzybowski, A. Protein Biomarkers in Glaucoma: A Review. J. Clin. Med. 2021, 10, 5388. [Google Scholar] [CrossRef]
- Richardson, M.R.; Price, M.O.; Price, F.W.; Pardo, J.C.; Grandin, J.C.; You, J.; Wang, M.; Yoder, M.C. Proteomic Analysis of Human Aqueous Humor Using Multidimensional Protein Identification Technology. Mol. Vis. 2009, 15, 2740–2750. [Google Scholar]
- Giannaccare, G.; Carnevali, A.; Senni, C.; Logozzo, L.; Scorcia, V. Umbilical Cord Blood and Serum for the Treatment of Ocular Diseases: A Comprehensive Review. Ophthalmol. Ther. 2020, 9, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Wang, S.Y.; Yoo, C.; Singh, K.; Lin, S.C. Association Between Serum Ferritin and Glaucoma in the South Korean Population. JAMA Ophthalmol. 2014, 132, 1414–1420. [Google Scholar] [CrossRef] [PubMed]
- Preservation of Vision after CaMKII-Mediated Protection of Retinal Ganglion Cells—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8530265/ (accessed on 27 December 2023).
- Santos, P.F.; Fazendeiro, B.; Luca, F.C.; Ambrósio, A.F.; Léger, H. The NDR/LATS Protein Kinases in Neurobiology: Key Regulators of Cell Proliferation, Differentiation and Migration in the Ocular and Central Nervous System. Eur. J. Cell Biol. 2023, 102, 151333. [Google Scholar] [CrossRef] [PubMed]
- Klöcker, N.; Kermer, P.; Weishaupt, J.H.; Labes, M.; Ankerhold, R.; Bähr, M. Brain-Derived Neurotrophic Factor-Mediated Neuroprotection of Adult Rat Retinal Ganglion Cells In Vivo Does Not Exclusively Depend on Phosphatidyl-Inositol-3′-Kinase/Protein Kinase B Signaling. J. Neurosci. 2000, 20, 6962–6967. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.-L.; Liu, Y.-F.; Wang, G.; Shao, Y.-Q.; Yu, C.-X.; Yang, Z.; Zhou, Z.-R.; Han, X.; Gong, X.; Qian, K.-W.; et al. The Role of ipRGCs in Ocular Growth and Myopia Development. Sci. Adv. 2022, 8, eabm9027. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Fan, Z.; Wu, Z.; Shu, J.; Yang, X.; Yang, Y.; Wang, X.; Li, B.; Wang, X.; et al. Eye-Brain Connections Revealed by Multimodal Retinal and Brain Imaging Genetics in the UK Biobank. medRxiv 2023, 2023.02.16.23286035. [Google Scholar] [CrossRef]
- Wingo, A.P.; Liu, Y.; Gerasimov, E.S.; Gockley, J.; Logsdon, B.A.; Duong, D.M.; Dammer, E.B.; Robins, C.; Beach, T.G.; Reiman, E.M.; et al. Integrating Human Brain Proteomes with Genome-Wide Association Data Implicates New Proteins in Alzheimer’s Disease Pathogenesis. Nat. Genet. 2021, 53, 143–146. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Jiang, F.; Wang, L.; Xiao, Q.; Han, F.; Chen, J.; Yuan, S.; Wei, J.; Larsson, S.C.; et al. Identification of Novel Protein Biomarkers and Drug Targets for Colorectal Cancer by Integrating Human Plasma Proteome with Genome. Genome Med. 2023, 15, 75. [Google Scholar] [CrossRef]
- Jung, J.; Lu, Z.; de Smith, A.; Mancuso, N. Novel Insight into the Etiology of Ischemic Stroke Gained by Integrative Transcriptome-Wide Association Study. medRxiv 2023, 2023.03.30.23287918. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q.; et al. Mendelian Randomization. Nat. Rev. Methods Primers 2022, 2, 6. [Google Scholar] [CrossRef]
- Armento, A.; Ueffing, M.; Clark, S.J. The Complement System in Age-Related Macular Degeneration. Cell. Mol. Life Sci. 2021, 78, 4487–4505. [Google Scholar] [CrossRef]
- Park, D.H.; Connor, K.M.; Lambris, J.D. The Challenges and Promise of Complement Therapeutics for Ocular Diseases. Front. Immunol. 2019, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Apellis Pharmaceuticals Announces 18-Month Results of Phase 2 Study (FILLY) of APL-2 in Geographic Atrophy; Apellis Pharmaceuticals, Inc.: Waltham, MA, USA, 2018.
- Munemasa, Y.; Kwong, J.M.K.; Kim, S.H.; Ahn, J.H.; Caprioli, J.; Piri, N. Thioredoxins 1 and 2 Protect Retinal Ganglion Cells from Pharmacologically Induced Oxidative Stress, Optic Nerve Transection and Ocular Hypertension. Adv. Exp. Med. Biol. 2010, 664, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Léveillard, T. Modulating Antioxidant Systems as a Therapeutic Approach to Retinal Degeneration. Redox Biol. 2022, 57, 102510. [Google Scholar] [CrossRef]
- Cui, J.; Gong, R.; Hu, S.; Cai, L.; Chen, L. Gambogic Acid Ameliorates Diabetes-Induced Proliferative Retinopathy through Inhibition of the HIF-1α/VEGF Expression via Targeting PI3K/AKT Pathway. Life Sci. 2018, 192, 293–303. [Google Scholar] [CrossRef]
- Li, B.; Zhou, J.; Zhang, G.; Wang, Y.; Kang, L.; Wu, J.; Chen, J.; Guan, H. Relationship between the Altered Expression and Epigenetics of GSTM3 and Age-Related Cataract. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4721–4732. [Google Scholar] [CrossRef]
- Coronado, B.N.L.; da Cunha, F.B.S.; de Oliveira, R.M.; Nóbrega, O.D.; Ricart, C.A.O.; Fontes, W.; de Sousa, M.V.; de Ávila, M.P.; Martins, A.M.A. Novel Possible Protein Targets in Neovascular Age-Related Macular Degeneration: A Pilot Study Experiment. Front. Med. 2022, 8, 692272. [Google Scholar] [CrossRef]
- de Jong, S.; Gagliardi, G.; Garanto, A.; de Breuk, A.; Lechanteur, Y.T.E.; Katti, S.; van den Heuvel, L.P.; Volokhina, E.B.; den Hollander, A.I. Implications of Genetic Variation in the Complement System in Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2021, 84, 100952. [Google Scholar] [CrossRef]
- Banc, A.; Bianchi Marzoli, S. Parapapillary Atrophy in Optic Neuropathies: Histology and Clinical Relevance. Eur. J. Ophthalmol. 2022, 32, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, S.; Zhang, X.; Hu, Y.; Shang, X.; Zhu, Z.; Huang, Y.; Wu, G.; Xiao, Y.; Du, Z.; et al. Shared Genetic Architecture between the Two Neurodegenerative Diseases: Alzheimer’s Disease and Glaucoma. Front. Aging Neurosci. 2022, 14, 880576. [Google Scholar] [CrossRef]
- Hysi, P.G.; Cheng, C.-Y.; Springelkamp, H.; Macgregor, S.; Bailey, J.N.C.; Wojciechowski, R.; Vitart, V.; Nag, A.; Hewitt, A.W.; Höhn, R.; et al. Genome-Wide Analysis of Multi-Ancestry Cohorts Identifies New Loci Influencing Intraocular Pressure and Susceptibility to Glaucoma. Nat. Genet. 2014, 46, 1126–1130. [Google Scholar] [CrossRef]
- Livingstone, I.; Uversky, V.N.; Furniss, D.; Wiberg, A. The Pathophysiological Significance of Fibulin-3. Biomolecules 2020, 10, 1294. [Google Scholar] [CrossRef]
- Shi, W.-Q.; Wan, T.; Li, B.; Li, T.; Zhou, X.-D. EFEMP1 Is a Potential Biomarker of Choroid Thickness Change in Myopia. Front. Neurosci. 2023, 17, 1144421. [Google Scholar] [CrossRef]
- Fan, Q.; Verhoeven, V.J.M.; Wojciechowski, R.; Barathi, V.A.; Hysi, P.G.; Guggenheim, J.A.; Höhn, R.; Vitart, V.; Khawaja, A.P.; Yamashiro, K.; et al. Meta-Analysis of Gene–Environment-Wide Association Scans Accounting for Education Level Identifies Additional Loci for Refractive Error. Nat. Commun. 2016, 7, 11008. [Google Scholar] [CrossRef] [PubMed]
- Himabindu, P.; Vishnupriya, S.; Shravankumar, K.P.; Rao Vittal, V.; Sunder Shyam, P. Association of Erythrocyte Acid Phosphatase Phenotypes with Myopia. Indian J. Hum. Genet. 2005, 11, 27–30. [Google Scholar]
- Zou, X.; Ye, S.; Tan, Y. Potential Disease Biomarkers for Diabetic Retinopathy Identified through Mendelian Randomization Analysis. Front. Endocrinol. 2024, 14, 1339374. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, S.U.; Sandahl, K.; Nielsen, L.B.; Broe, R.; Rasmussen, M.L.; Peto, T.; Grauslund, J.; Andersen, M.L.M.; Mortensen, H.B.; Pociot, F.; et al. Polymorphisms in the CTSH Gene May Influence the Progression of Diabetic Retinopathy: A Candidate-Gene Study in the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987). Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 1959–1965. [Google Scholar] [CrossRef]
- Yazdanpanah, N.; Yazdanpanah, M.; Wang, Y.; Forgetta, V.; Pollak, M.; Polychronakos, C.; Richards, J.B.; Manousaki, D. Clinically Relevant Circulating Protein Biomarkers for Type 1 Diabetes: Evidence From a Two-Sample Mendelian Randomization Study. Diabetes Care 2022, 45, 169–177. [Google Scholar] [CrossRef]
- Barrett, J.C.; Clayton, D.G.; Concannon, P.; Akolkar, B.; Cooper, J.D.; Erlich, H.A.; Julier, C.; Morahan, G.; Nerup, J.; Nierras, C.; et al. Genome-Wide Association Study and Meta-Analysis Find That over 40 Loci Affect Risk of Type 1 Diabetes. Nat. Genet. 2009, 41, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Fløyel, T.; Brorsson, C.; Nielsen, L.B.; Miani, M.; Bang-Berthelsen, C.H.; Friedrichsen, M.; Overgaard, A.J.; Berchtold, L.A.; Wiberg, A.; Poulsen, P.; et al. CTSH Regulates β-Cell Function and Disease Progression in Newly Diagnosed Type 1 Diabetes Patients. Proc. Natl. Acad. Sci. USA 2014, 111, 10305–10310. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.B.; Lee, K.H.; Kim, J.W.; Shiba, K.; Jo, Y.J.; Kim, S. Interaction between Human tRNA Synthetases Involves Repeated Sequence Elements. Proc. Natl. Acad. Sci. USA 1996, 93, 10128–10133. [Google Scholar] [CrossRef] [PubMed]
- Otani, A.; Slike, B.M.; Dorrell, M.I.; Hood, J.; Kinder, K.; Ewalt, K.L.; Cheresh, D.; Schimmel, P.; Friedlander, M. A Fragment of Human TrpRS as a Potent Antagonist of Ocular Angiogenesis. Proc. Natl. Acad. Sci. USA 2002, 99, 178–183. [Google Scholar] [CrossRef]
- Tu, C.; Li, H.; Liu, X.; Wang, Y.; Li, W.; Meng, L.; Wang, W.; Li, Y.; Li, D.; Du, J.; et al. TDRD7 Participates in Lens Development and Spermiogenesis by Mediating Autophagosome Maturation. Autophagy 2021, 17, 3848–3864. [Google Scholar] [CrossRef]
- Zhang, S.S.-M.; Wei, J.; Li, C.; Barnstable, C.J.; Fu, X.-Y. Expression and Activation of STAT Proteins during Mouse Retina Development. Exp. Eye Res. 2003, 76, 421–431. [Google Scholar] [CrossRef]
- Turner, M.J.; DaSilva-Arnold, S.; Luo, N.; Hu, X.; West, C.C.; Sun, L.; Hill, C.; Bradish, J.; Kaplan, M.H.; Travers, J.B.; et al. STAT6-Mediated Keratitis and Blepharitis: A Novel Murine Model of Ocular Atopic Dermatitis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3803–3808. [Google Scholar] [CrossRef]
- Han, X.; Qassim, A.; An, J.; Marshall, H.; Zhou, T.; Ong, J.-S.; Hassall, M.M.; Hysi, P.G.; Foster, P.J.; Khaw, P.T.; et al. Genome-Wide Association Analysis of 95 549 Individuals Identifies Novel Loci and Genes Influencing Optic Disc Morphology. Hum. Mol. Genet. 2019, 28, 3680–3690. [Google Scholar] [CrossRef]
- He, W.; Han, X.; Ong, J.-S.; Hewitt, A.W.; Mackey, D.A.; Gharahkhani, P.; MacGregor, S.; International Glaucoma Genetics Consortium. Association of Novel Loci with Keratoconus Susceptibility in a Multitrait Genome-Wide Association Study of the UK Biobank Database and Canadian Longitudinal Study on Aging. JAMA Ophthalmol. 2022, 140, 568–576. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen Provides Genetic Insights from a Well-Phenotyped Isolated Population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef]
- Zhang, J.; Dutta, D.; Köttgen, A.; Tin, A.; Schlosser, P.; Grams, M.E.; Harvey, B.; Yu, B.; Boerwinkle, E.; Coresh, J.; et al. Plasma Proteome Analyses in Individuals of European and African Ancestry Identify cis-pQTLs and Models for Proteome-Wide Association Studies. Nat. Genet. 2022, 54, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Gusev, A.; Ko, A.; Shi, H.; Bhatia, G.; Chung, W.; Penninx, B.W.J.H.; Jansen, R.; de Geus, E.J.C.; Boomsma, D.I.; Wright, F.A.; et al. Integrative Approaches for Large-Scale Transcriptome-Wide Association Studies. Nat. Genet. 2016, 48, 245–252. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Guo, P.; Wang, Y.; Liu, L.; Zhao, J.; Gao, L.; Yuan, Z.; Xue, F.; Zhao, J. Mendelian Randomisation Highlights Hypothyroidism as a Causal Determinant of Idiopathic Pulmonary Fibrosis. EBioMedicine 2021, 73, 103669. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Gong, W.; Guo, P.; Xue, F.; Zhou, X.; Wang, S.; Yuan, Z. Protein-Centric Omics Integration Analysis Identifies Candidate Plasma Proteins for Multiple Autoimmune Diseases. Hum. Genet. 2023, in press. [Google Scholar] [CrossRef]
- Gong, W.; Guo, P.; Li, Y.; Liu, L.; Yan, R.; Liu, S.; Wang, S.; Xue, F.; Zhou, X.; Yuan, Z. Role of the Gut-Brain Axis in the Shared Genetic Etiology Between Gastrointestinal Tract Diseases and Psychiatric Disorders: A Genome-Wide Pleiotropic Analysis. JAMA Psychiatry 2023, 80, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomisation (STROBE-MR): Explanation and Elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G.; CRP CHD Genetics Collaboration. Avoiding Bias from Weak Instruments in Mendelian Randomization Studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, P.; Zhang, Y.; Liu, L.; Yan, R.; Yuan, Z.; Song, Y. Joint Analysis of Genetic Correlation, Mendelian Randomization and Colocalization Highlights the Bi-Directional Causal Association between Hypothyroidism and Primary Biliary Cirrhosis. Front. Genet. 2021, 12, 753352. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A Framework for the Investigation of Pleiotropy in Two-Sample Summary Data Mendelian Randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Erratum to: Interpreting Findings from Mendelian Randomization Using the MR-Egger Method. Eur. J. Epidemiol. 2017, 32, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C. A More Accurate Method for Colocalisation Analysis Allowing for Multiple Causal Variants. PLoS Genet. 2021, 17, e1009440. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.; Griffith, O.L.; Coffman, A.C.; Weible, J.V.; McMichael, J.F.; Spies, N.C.; Koval, J.; Das, I.; Callaway, M.B.; Eldred, J.M.; et al. DGIdb: Mining the Druggable Genome. Nat. Methods 2013, 10, 1209–1210. [Google Scholar] [CrossRef]
- Freshour, S.L.; Kiwala, S.; Cotto, K.C.; Coffman, A.C.; McMichael, J.F.; Song, J.J.; Griffith, M.; Griffith, O.L.; Wagner, A.H. Integration of the Drug-Gene Interaction Database (DGIdb 4.0) with Open Crowdsource Efforts. Nucleic Acids Res. 2021, 49, D1144–D1151. [Google Scholar] [CrossRef]



| ODs | Gene | Brain Protein | Gene | Plasma Protein | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PWAS | COLOC | PWAS | MR | COLOC | ||||||
| Z | p | PP.H4 | Z | p | OR | p | PP.H4 | |||
| Senile catarct | GSTM3 *,D | −4.55 | 5.38 × 10−6 | 0.977 | GSTM3 *,D | −5.08 | 3.74 × 10−7 | 0.93 | 4.85 × 10−7 | 0.909 |
| TOM1L2 M | −4.37 | 1.22 × 10−5 | 0.934 | - | - | - | - | - | - | |
| MXRA7 | −4.58 | 4.65 × 10−6 | 0.982 | - | - | - | - | - | - | |
| RHPN2 | −5.47 | 4.44 × 10−8 | 0.988 | - | - | - | - | - | - | |
| HINT1 | 4.33 | 1.52 × 10−5 | 0.964 | - | - | - | - | - | - | |
| AMD | WARS1 M | 3.89 | 1.00 × 10−4 | 0.880 | WARS1 D,M | −3.53 | 4.70 × 10−7 | 1.19 | 2.04 × 10−7 | 0.936 |
| CFHR1 *,D,M | 25.04 | 2.08 × 10−138 | 0.737 | C3 *,D,M | 9.03 | 1.68 × 10−19 | 2.05 | 8.71 × 10−3 | 0.999 | |
| RLBP1 *,M | 5.23 | 1.74 × 10−7 | 0.920 | IGFBP7 *,D | −3.70 | 2.16 × 10−4 | 0.88 | 3.75 × 10−5 | 0.754 | |
| TDRD7 M | 4.01 | 5.95 × 10−5 | 0.923 | PILRA * | −3.99 | 6.60 × 10−5 | 0.95 | 1.28 × 10−2 | 0.866 | |
| Glaucoma | DNM3 * | −4.72 | 2.34 × 10−6 | 0.897 | ECI1 D | −3.48 | 4.94 × 10−4 | 0.75 | 4.93 × 10−4 | 0.711 |
| PTPMT1 *,D | 4.07 | 4.65 × 10−5 | 0.861 | LCT D,M | −3.79 | 1.52 × 10−4 | 0.95 | 6.97 × 10−3 | 0.899 | |
| ISLR * | −4.11 | 3.98 × 10−5 | 0.759 | NPTXR | −3.52 | 4.37 × 10−4 | 0.94 | 3.03 × 10−4 | 0.883 | |
| MFF * | −3.76 | 1.68 × 10−4 | 0.987 | - | - | - | - | - | ||
| TXN *,D | −3.65 | 2.59 × 10−4 | 0.926 | - | - | - | - | - | - | |
| Myopia | STAT6 D | −4.40 | 1.09 × 10−5 | 0.957 | EFEMP1 *,D,M | −6.27 | 3.60 × 10−10 | 0.99 | 6.28 × 10−5 | 0.993 |
| RASGRF * | −6.77 | 1.27 × 10−11 | 0.887 | - | - | - | - | - | - | |
| ACP1 *,D | 4.55 | 5.49 × 10−6 | 0.886 | - | - | - | - | - | - | |
| DR | RAB5B * | −4.54 | 5.52 × 10−6 | 0.761 | SIRPG *,D | 3.69 | 2.20 × 10−4 | 1.18 | 6.01 × 10−5 | 0.783 |
| CTSH *,D,M | 3.76 | 1.71 × 10−4 | 0.856 | SIGLEC14 | 3.70 | 2.14 × 10−4 | 1.11 | 2.39 × 10−4 | 0.985 | |
| TPPP3 | −4.26 | 2.02 × 10−5 | 0.922 | - | - | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, Q.; Liu, X.; Gong, W.; Wang, Y.; Yuan, Z.; Sun, X.; Wang, S. Pinpointing Novel Plasma and Brain Proteins for Common Ocular Diseases: A Comprehensive Cross-Omics Integration Analysis. Int. J. Mol. Sci. 2024, 25, 10236. https://doi.org/10.3390/ijms251910236
Mo Q, Liu X, Gong W, Wang Y, Yuan Z, Sun X, Wang S. Pinpointing Novel Plasma and Brain Proteins for Common Ocular Diseases: A Comprehensive Cross-Omics Integration Analysis. International Journal of Molecular Sciences. 2024; 25(19):10236. https://doi.org/10.3390/ijms251910236
Chicago/Turabian StyleMo, Qinyou, Xinyu Liu, Weiming Gong, Yunzhuang Wang, Zhongshang Yuan, Xiubin Sun, and Shukang Wang. 2024. "Pinpointing Novel Plasma and Brain Proteins for Common Ocular Diseases: A Comprehensive Cross-Omics Integration Analysis" International Journal of Molecular Sciences 25, no. 19: 10236. https://doi.org/10.3390/ijms251910236
APA StyleMo, Q., Liu, X., Gong, W., Wang, Y., Yuan, Z., Sun, X., & Wang, S. (2024). Pinpointing Novel Plasma and Brain Proteins for Common Ocular Diseases: A Comprehensive Cross-Omics Integration Analysis. International Journal of Molecular Sciences, 25(19), 10236. https://doi.org/10.3390/ijms251910236






