Preparation and Photodegradation of TiO2 Thin Films on the Inner Wall of Quartz Tubes
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
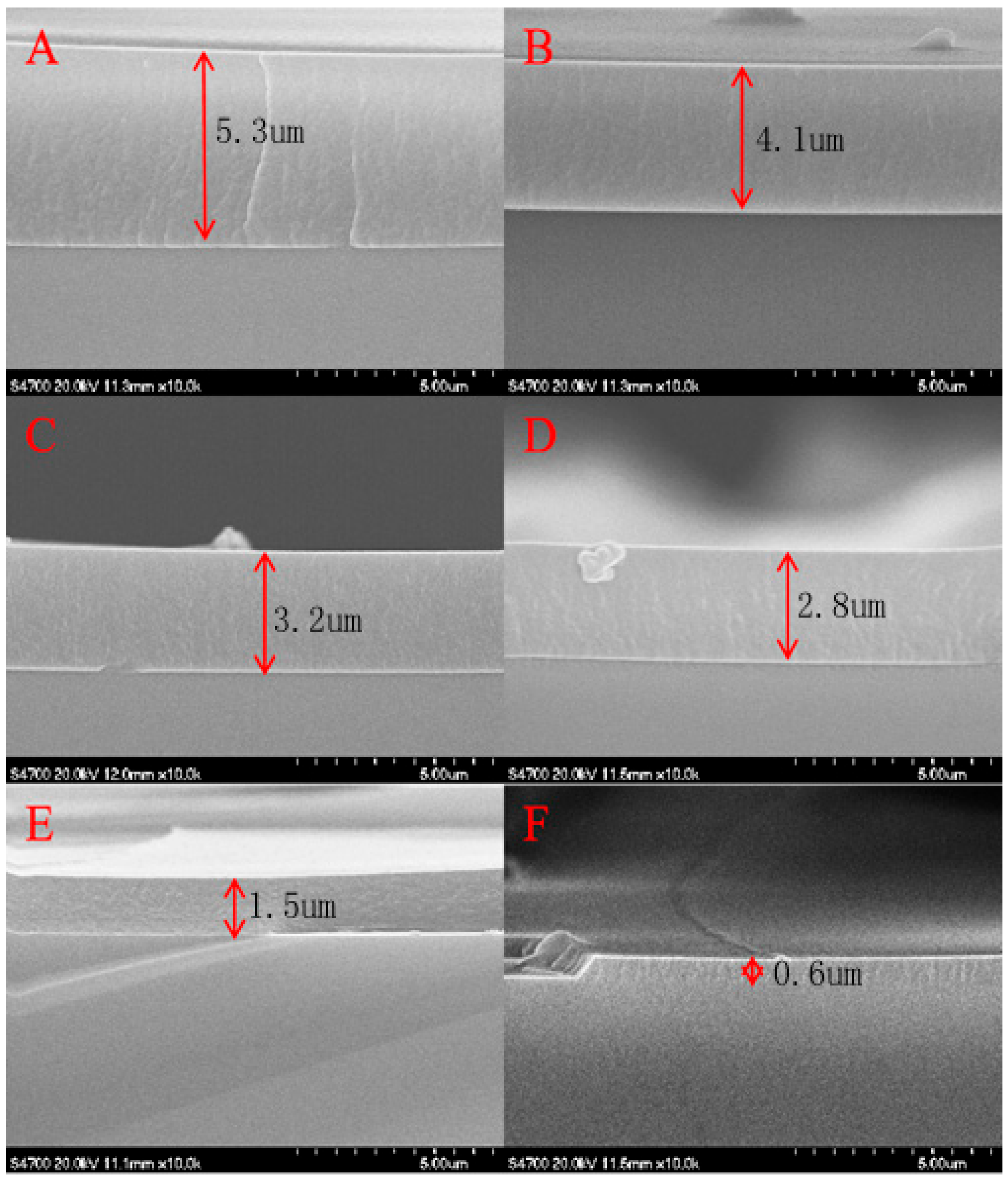
2.1.1. Cross-Sectional Morphology
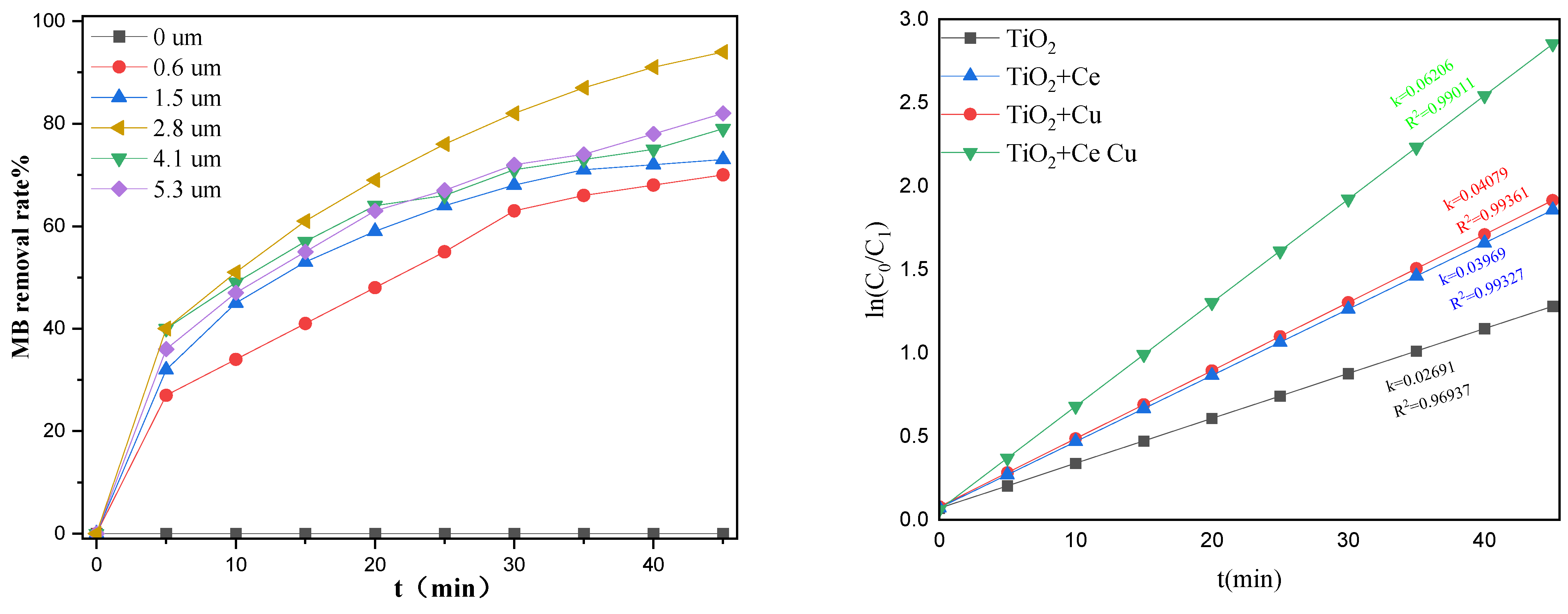
2.1.2. Effects of Ethyl Orthosilicate
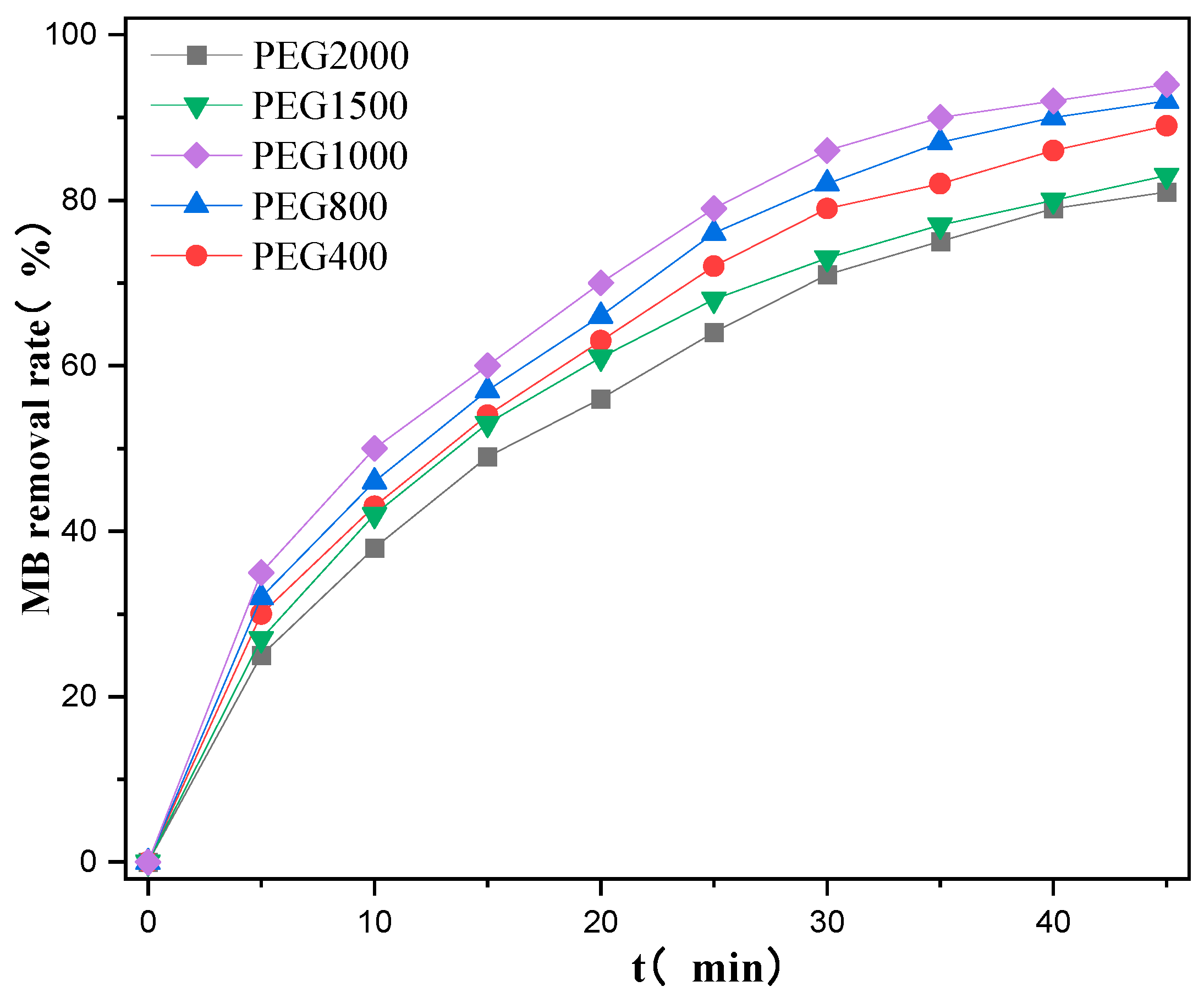
2.1.3. Effects of PEG Molecular Weights
2.1.4. Effects of Metal Doping on Surface Area
2.2. Thermogravimetric Analysis
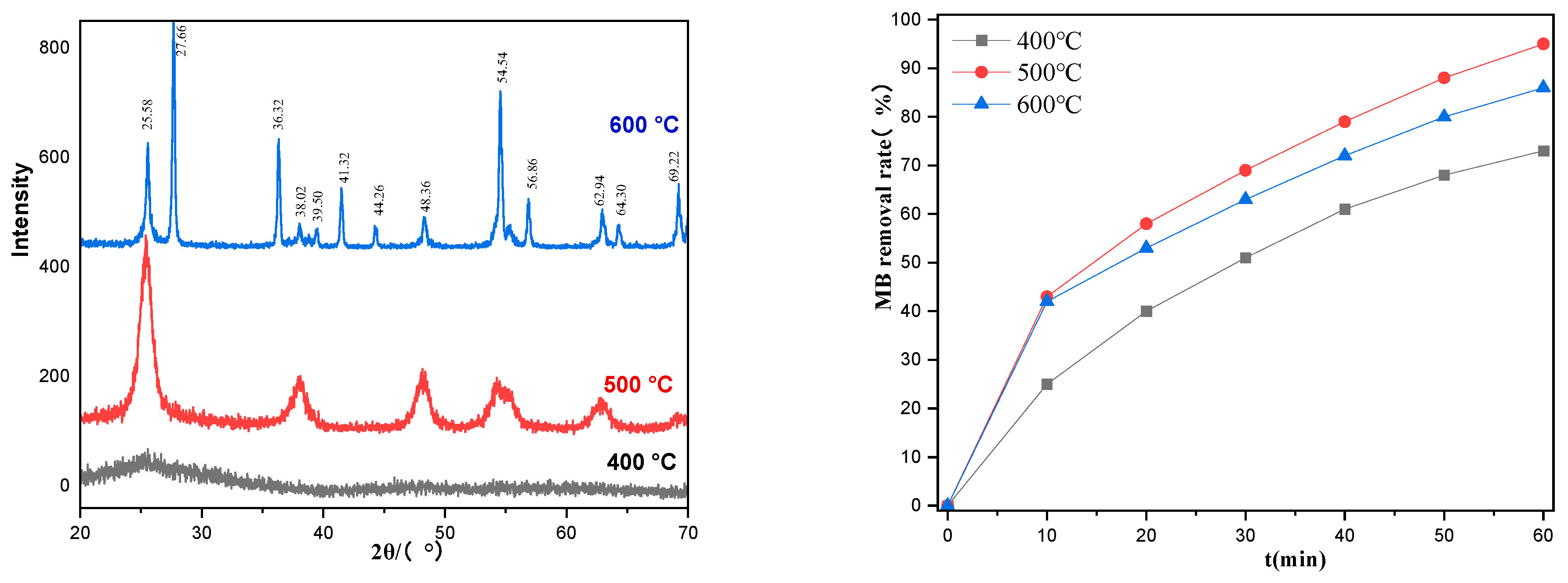
2.3. Effects of Calcination Temperature
2.4. SEM-EDS Analysis
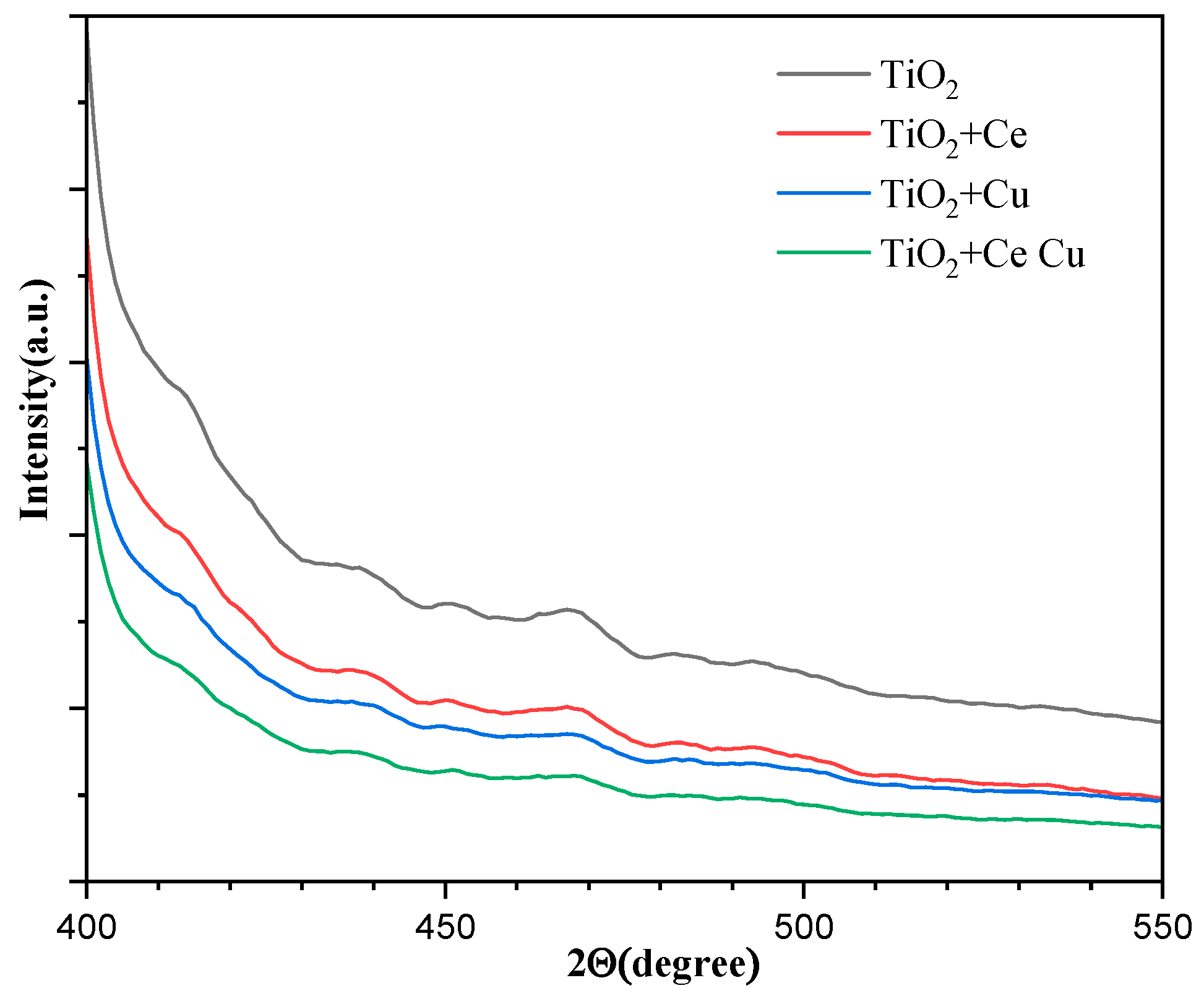
2.5. XPS Analysis
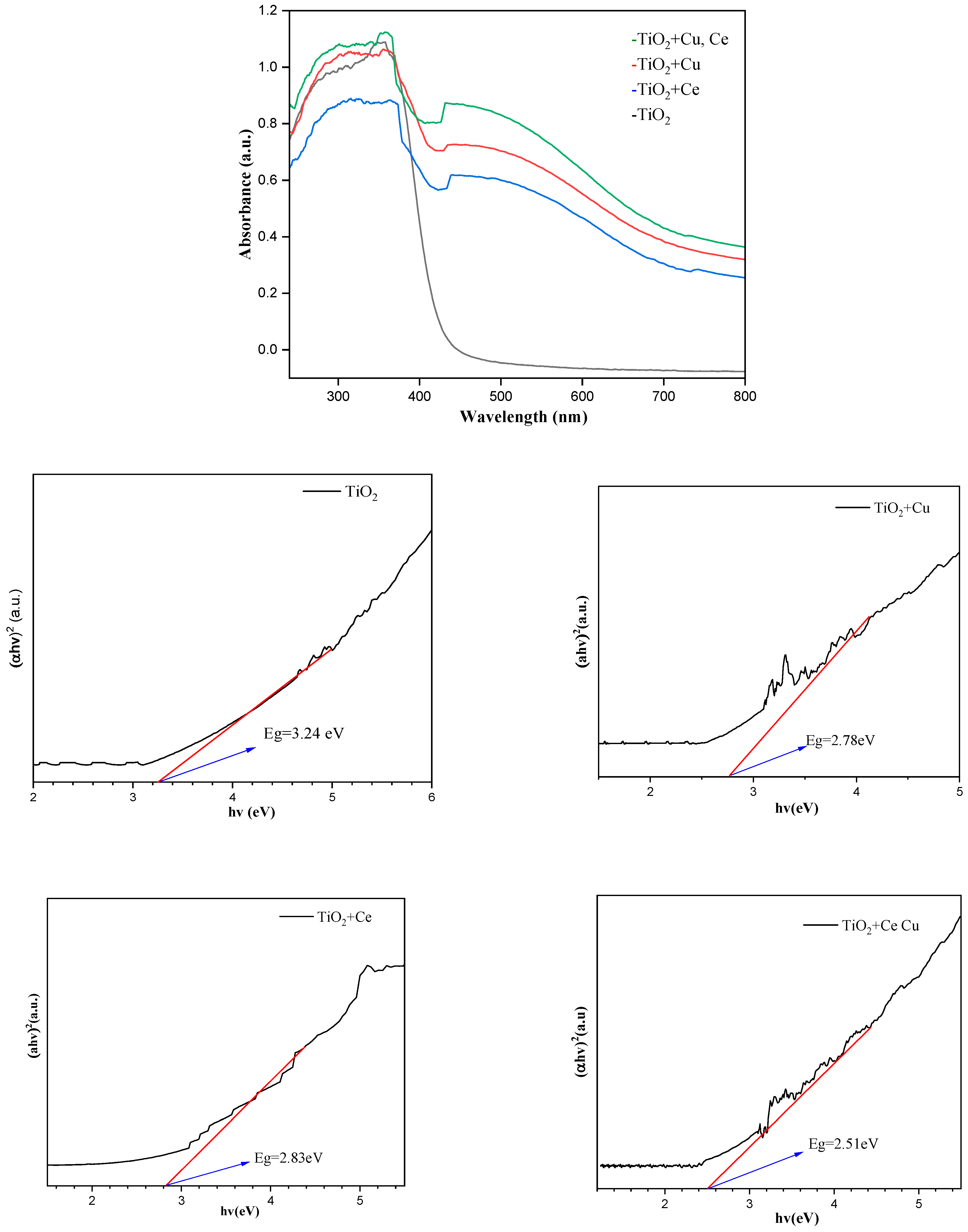
2.6. UV Diffuse Reflectance Spectroscopy Analysis
2.7. Photoluminescence Spectroscopy Analysis
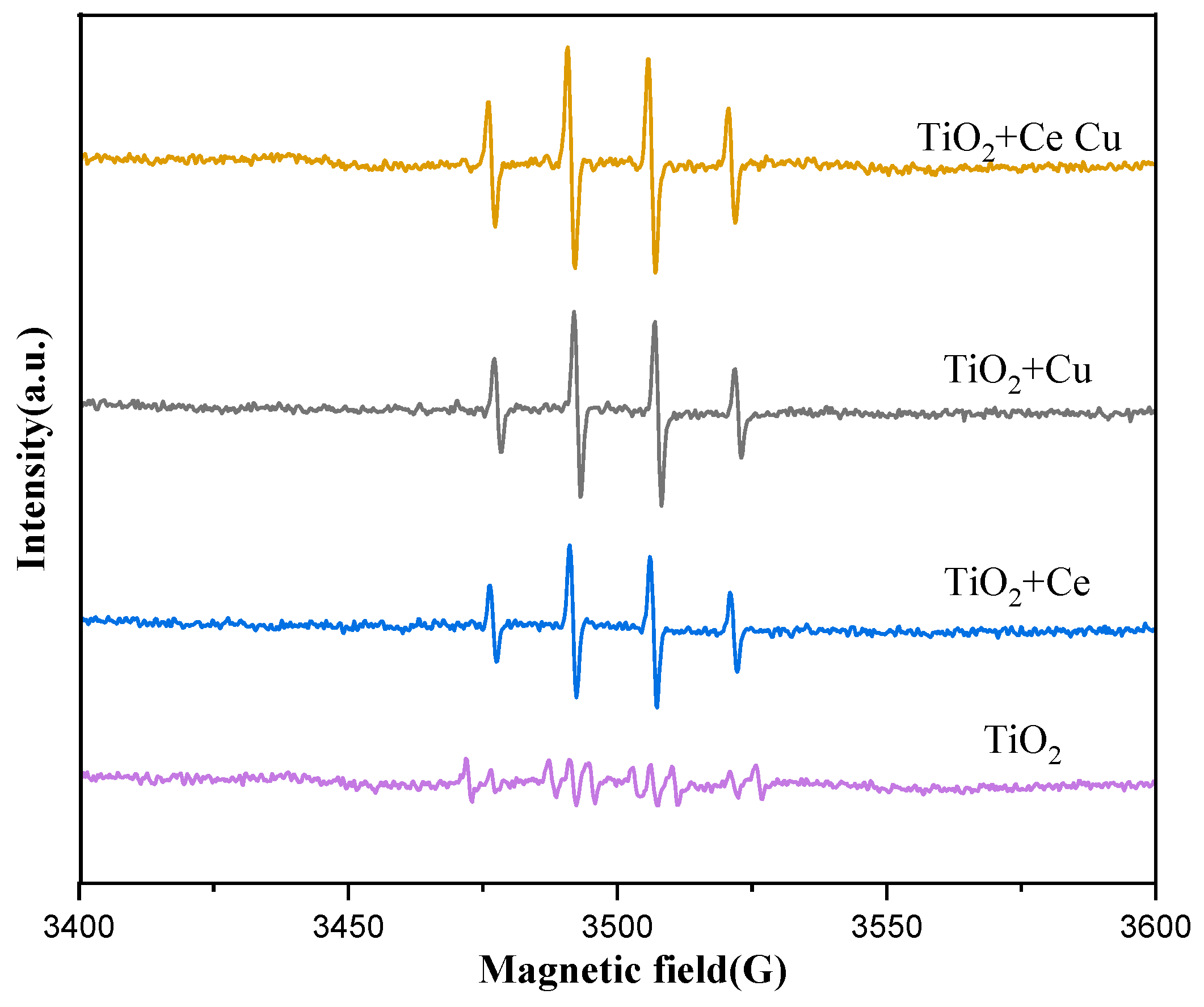
2.8. EPR Analysis
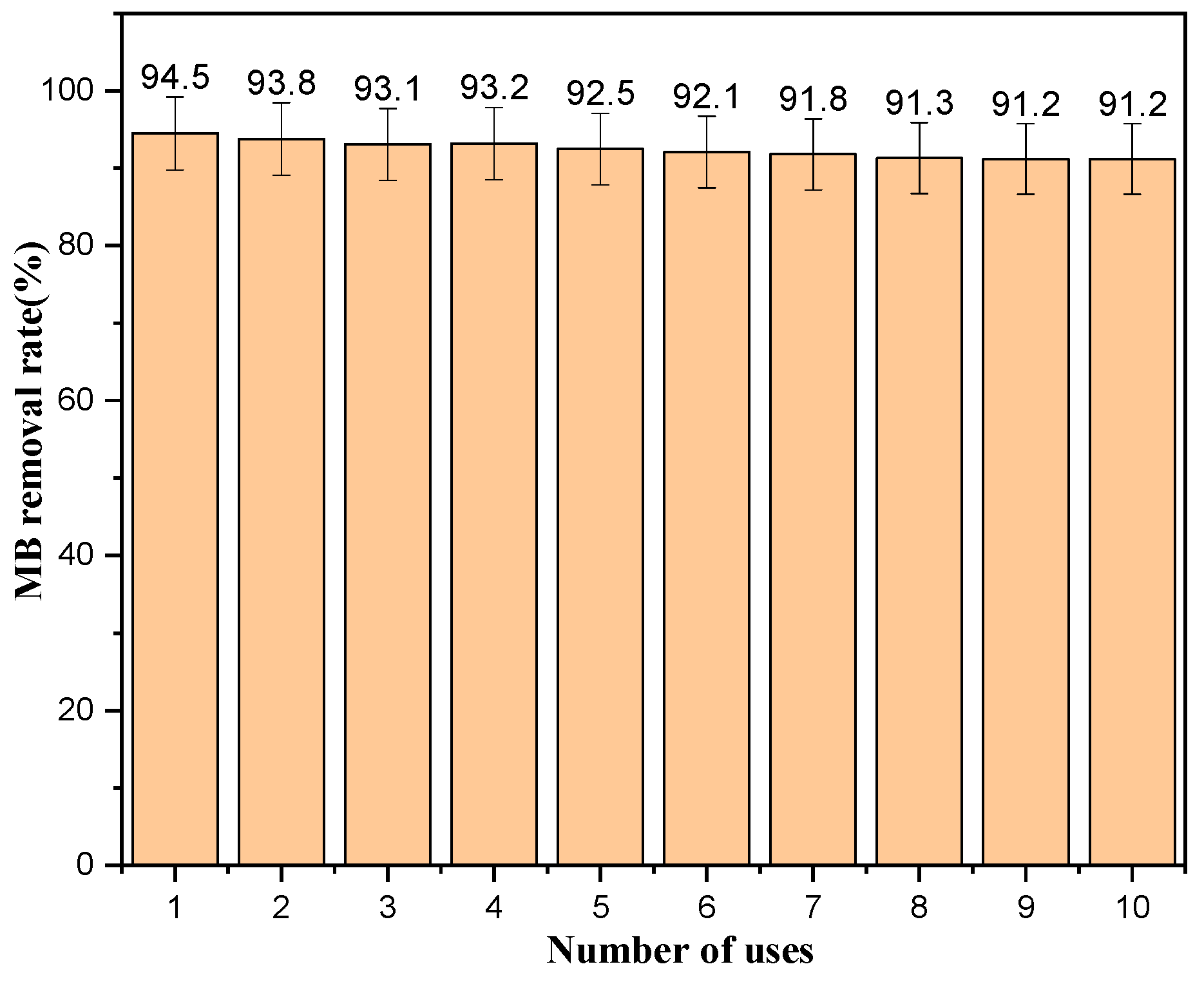
2.9. Catalyst Repeatability Stability
2.10. Degradation Products of MB
3. Materials and Methods
3.1. Reagents and Instruments
3.2. TiO2/Quartz Tube Preparation
3.2.1. Activation of Quartz Tubes
3.2.2. Coating of TiO2/Quartz Tubes
3.2.3. Modification of TiO2/Quartz Tubes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Chi, Z.; Chen, C.; Su, C.; Liu, D.; Liu, Y.; Duan, X.; Wang, S. Facet- and Defect-Dependent Activity of Perovskites in Catalytic Evolution of Sulfate Radicals. Appl. Catal. B 2020, 272, 118972. [Google Scholar] [CrossRef]
- Huan, H.; Zhang, B.-T.; Kong, H.; Li, M.; Wang, W.; Xi, B.; Wang, G. Comprehensive Assessment of Groundwater Pollution Risk Based on HVF Model: A Case Study in Jilin City of Northeast China. Sci. Total Environ. 2018, 628–629, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Dulsat-Masvidal, M.; Ciudad, C.; Infante, O.; Mateo, R.; Lacorte, S. Water Pollution Threats in Important Bird and Biodiversity Areas from Spain. J. Hazard. Mater. 2023, 448, 130938. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Wang, S.; Lu, T.; Yang, S.; Zou, L.; Ren, J.; Lu, X.; Huang, J.; Huang, C.; Yang, P. Evaluating High Temperature Photoelectrocatalysis of TiO2 Model Photoanode. J. Colloid Interface Sci. 2023, 645, 765–774. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Black TiO2 Nanomaterials: A Review of Recent Advances. Chem. Eng. J. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Safajou, H.; Khojasteh, H.; Salavati-Niasari, M.; Mortazavi-Derazkola, S. Enhanced Photocatalytic Degradation of Dyes over Graphene/Pd/TiO2 Nanocomposites: TiO2 Nanowires versus TiO2 Nanoparticles. J. Colloid Interface Sci. 2017, 498, 423–432. [Google Scholar] [CrossRef]
- Ismael, M. Highly Effective Ruthenium-Doped TiO2 Nanoparticles Photocatalyst for Visible-Light-Driven Photocatalytic Hydrogen Production. New J. Chem. 2019, 43, 9596–9605. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Antoniadou, M.; Kaltzoglou, A.; Sakellis, E.; Katsaros, F.; Sygellou, L.; Arfanis, M.K.; Salama, T.M.; Falaras, P. Silver Decorated TiO2/g-C3N4 Bifunctional Nanocomposites for Photocatalytic Elimination of Water Pollutants under UV and Artificial Solar Light. Results Eng. 2022, 14, 100470. [Google Scholar] [CrossRef]
- Ismael, M. Latest Progress on the Key Operating Parameters Affecting the Photocatalytic Activity of TiO2-Based Photocatalysts for Hydrogen Fuel Production: A Comprehensive Review. Fuel 2021, 303, 121207. [Google Scholar] [CrossRef]
- Harsha, N.; Krishna, K.V.S.; Renuka, N.K.; Shukla, S. Facile Synthesis of γ-Fe2O3 Nanoparticles Integrated H2Ti3O7 Nanotubes Structure as a Magnetically Recyclable Dye-Removal Catalyst. RSC Adv. 2015, 5, 30354–30362. [Google Scholar] [CrossRef]
- Ismael, M. A Review and Recent Advances in Solar-to-Hydrogen Energy Conversion Based on Photocatalytic Water Splitting over Doped-TiO2 Nanoparticles. Sol. Energy 2020, 211, 522–546. [Google Scholar] [CrossRef]
- García-Domínguez, Á.E.; Torres-Torres, G.; Arévalo-Pérez, J.C.; Silahua-Pavón, A.; Sánchez-Trinidad, C.; Godavarthi, S.; Ojeda-López, R.; Sierra-Gómez, U.A.; Cervantes-Uribe, A. Urea Assisted Synthesis of TiO2–CeO2 Composites for Photocatalytic Acetaminophen Degradation via Simplex-Centroid Mixture Design. Results Eng. 2022, 14, 100443. [Google Scholar] [CrossRef]
- Shukla, V.; Kumar, N. (Eds.) Environmental Concerns and Sustainable Development: Volume 1: Air, Water and Energy Resources; Springer: Singapore, 2020; ISBN 9789811358883. [Google Scholar]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic Degradation of Dyes Using Semiconductor Photocatalysts to Clean Industrial Water Pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Wang, L.; Fu, Y.; Cao, L. The Preparation and Chemical Structure of TiO2 Film Photocatalysts Supported on Stainless Steel Substrates via the Sol–Gel Method. J. Mater. Chem. 2001, 11, 1864–1868. [Google Scholar] [CrossRef]
- Weng, K.-W.; Huang, Y.-P. Preparation of TiO2 Thin Films on Glass Surfaces with Self-Cleaning Characteristics for Solar Concentrators. Surf. Coat. Technol. 2013, 231, 201–204. [Google Scholar] [CrossRef]
- Xie, T.-H.; Lin, J. Origin of Photocatalytic Deactivation of TiO2 Film Coated on Ceramic Substrate. J. Phys. Chem. C Nanomater. Interfaces 2007, 111, 9968–9974. [Google Scholar] [CrossRef]
- Huang, C.; Lv, Y.; Zhou, Q.; Kang, S.; Li, X.; Mu, J. Visible Photocatalytic Activity and Photoelectrochemical Behavior of TiO2 Nanoparticles Modified with Metal Porphyrins Containing Hydroxyl Group. Ceram. Int. 2014, 40, 7093–7098. [Google Scholar] [CrossRef]
- Lee, E.-J.; An, A.K.; He, T.; Woo, Y.C.; Shon, H.K. Electrospun Nanofiber Membranes Incorporating Fluorosilane-Coated TiO2 Nanocomposite for Direct Contact Membrane Distillation. J. Membr. Sci. 2016, 520, 145–154. [Google Scholar] [CrossRef]
- Mahy, J.G.; Lambert, S.D.; Léonard, G.L.-M.; Zubiaur, A.; Olu, P.-Y.; Mahmoud, A.; Boschini, F.; Heinrichs, B. Towards a Large Scale Aqueous Sol-Gel Synthesis of Doped TiO2: Study of Various Metallic Dopings for the Photocatalytic Degradation of p-Nitrophenol. J. Photochem. Photobiol. A Chem. 2016, 329, 189–202. [Google Scholar] [CrossRef]
- Carp, O. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Linnik, O.; Khoroshko, L. Nonporous Nitrogen and Ruthenium Co-Doped Titania Films for Photocatalysis. Int. J. Nanosci. 2019, 18, 1940043. [Google Scholar] [CrossRef]
- Hirano, M.; Nakahara, C.; Ota, K.; Tanaike, O.; Inagaki, M. Photoactivity and Phase Stability of ZrO2-Doped Anatase-Type TiO2 Directly Formed as Nanometer-Sized Particles by Hydrolysis under Hydrothermal Conditions. J. Solid State Chem. 2003, 170, 39–47. [Google Scholar] [CrossRef]
- Xu, J.; Li, K.; Wu, S.; Shi, W.; Peng, T. Preparation of Brookite Titania Quasi Nanocubes and Their Application in Dye-Sensitized Solar Cells. J. Mater. Chem. A Mater. Energy Sustain. 2015, 3, 7453–7462. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Zhukovskii, Y.F.; Piskunov, S.; Lisovski, O.; Bocharov, D.; Evarestov, R.A. Doped 1D Nanostructures of Transition-Metal Oxides: First-Principles Evaluation of Photocatalytic Suitability. Isr. J. Chem. 2017, 57, 461–476. [Google Scholar] [CrossRef]
- Magesh, G.; Viswanathan, B.; Viswanath, R.; Varadarajan, T. Photocatalytic behavior of CeO2-TiO2 system for the degradation of methylene blue. Indian J. Chem. 2009, 48, 480–488. [Google Scholar]
- Mertah, O.; Hajjaji El, K.; Amrai El, S.; Tanji, K.; Goncharova, I.; Kherbeche, A. Visible-light Cu/TiO2@Ag3PO4 heterostructure photocatalyst for selective nitrate reduction and antimicrobial activity. Opt. Mater. 2022, 129, 112549. [Google Scholar] [CrossRef]
- Reddy, N.L.; Emin, S.; Kumari, V.D.; Muthukonda Venkatakrishnan, S. CuO Quantum Dots Decorated TiO2 Nanocomposite Photocatalyst for Stable Hydrogen Generation. Ind. Eng. Chem. Res. 2018, 57, 568–577. [Google Scholar] [CrossRef]
- Shah, A.; Akhtar, S.; Mahmood, F.; Urooj, S.; Siddique, A.B.; Irfan, M.I.; Naeem-ul-Hassan, M.; Sher, M.; Alhoshani, A.; Rauf, A.; et al. Fagonia arabica extract-stabilized gold nanoparticles as a highly selective colorimetric nanoprobe for Cd2+ detection and as a potential photocatalytic and antibacterial agent. Surf. Interfaces 2024, 51, 104556–104569. [Google Scholar] [CrossRef]
- Yasin, M.; Saeed, M.; Muneer, M.; Usman, M.; ul Haq, A.; Sadia, M.; Altaf, M. Development of Bi2O3-ZnO heterostructure for enhanced photodegradation of rhodamine B and reactive yellow dyes. Surf. Interfaces 2022, 30, 101846–101858. [Google Scholar] [CrossRef]
- Ngo, H.-S.; Nguyen, T.-L.; Tran, N.-T.; Le, H.-C. Photocatalytic Removal of Ciprofloxacin in Water by Novel Sandwich-like CuFe2O4 on rGO/Halloysite Material: Insights into Kinetics and Intermediate Reactive Radicals. Water 2023, 15, 1569. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; An, Y.; Sun, X.; Wu, H.; Li, J.; Chen, X.; Li, H. Hybridization of Nanodiamond and CuFe-LDH as Heterogeneous Photoactivator for Visible-Light Driven Photo-Fenton Reaction: Photocatalytic Activity and Mechanism. Catalysts 2019, 9, 118. [Google Scholar] [CrossRef]
- Prado, T.M.; Silva, F.L.; Grosseli, G.; Fadini, P.S.; Fatibello-Filho, O.; de Moraes, F.C. Using BiVO4/CuO-Based Photoelectrocatalyzer for 4-Nitrophenol Degradation. Materials 2020, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhou, G.; Yin, J.; An, L.; Zhao, P.; Wang, T.; Rao, S.; Alireza, K.; Yang, J.; Zhang, L. Enhanced photocatalytic performance of g-C3N4@Ce-Fe bimetallic oxide with Z-scheme heterojunction for rapid degradation of tetracycline and its photodegradation pathway. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129780–129792. [Google Scholar] [CrossRef]
- Saadati, A.; Habibi-Yangjeh, A.; Khataee, A. Integration of CeO2 and Bi2O3 nanoparticles with TiO2−x to fabricate QDs-sized visible-light-triggered materials with efficient performances in the photodegradation of contaminants. Surf. Interfaces 2024, 51, 104614–104626. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M. Preparation, Characterization, and Photocatalytic Property of Cu2O-TiO2Nanocomposites. Int. J. Photoenergy 2012, 2012, 195–198. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A. TiO2/CeO2 Hybrid Photocatalyst with Enhanced Photocatalytic Activity: Optimization of Synthesis Variables. Ind. Eng. Chem. Res. 2014, 53, 7847–7855. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, Z.; Zhang, J.; Guo, W.; Li, L.; Liu, H.; Wang, Z.L. One-Step Synthesis of Ultrathin Nanobelts-Assembled Urchin-like Anatase TiO2 nanostructures for Highly Efficient Photocatalysis. CrystEngComm 2017, 19, 129–136. [Google Scholar] [CrossRef]



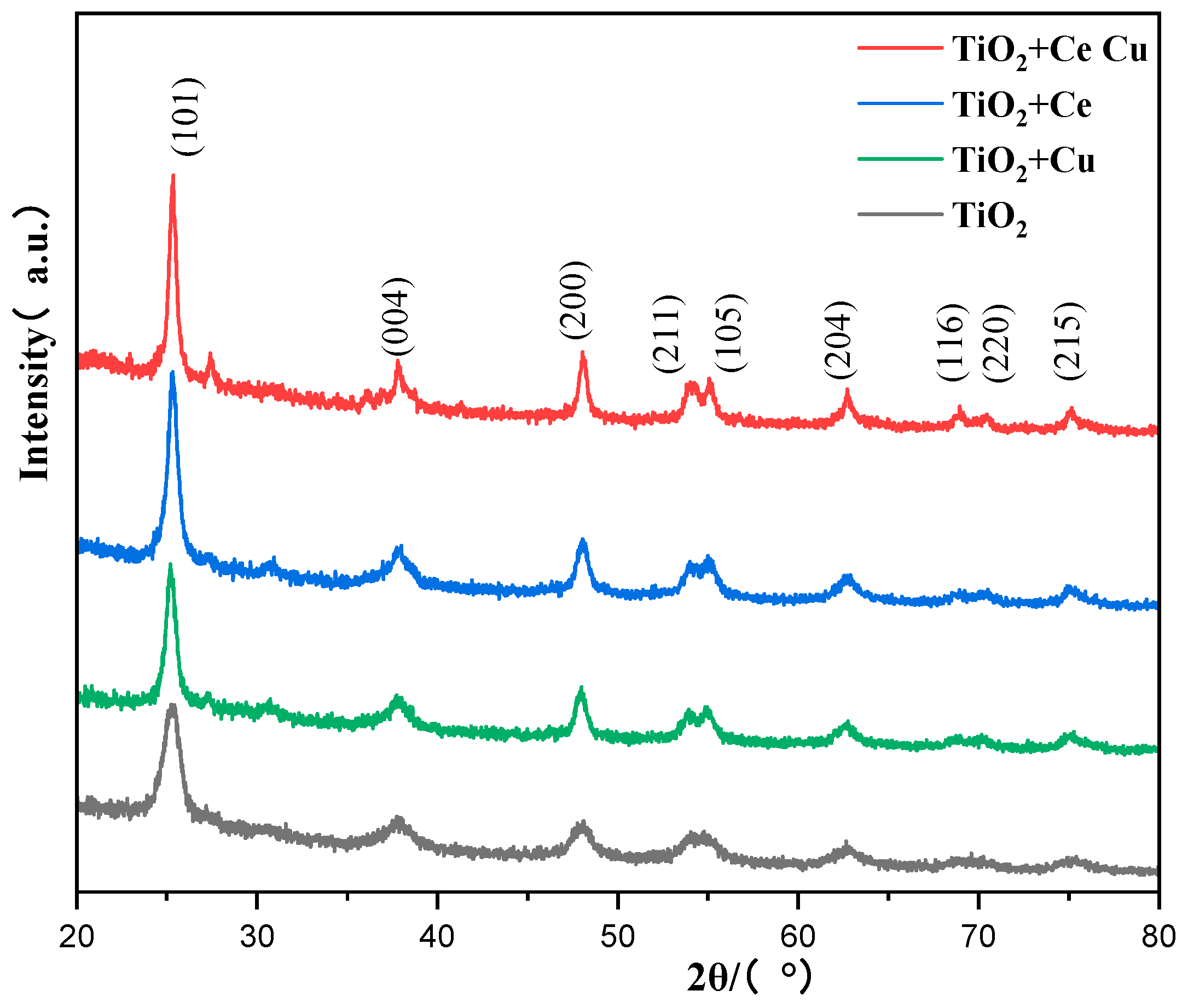
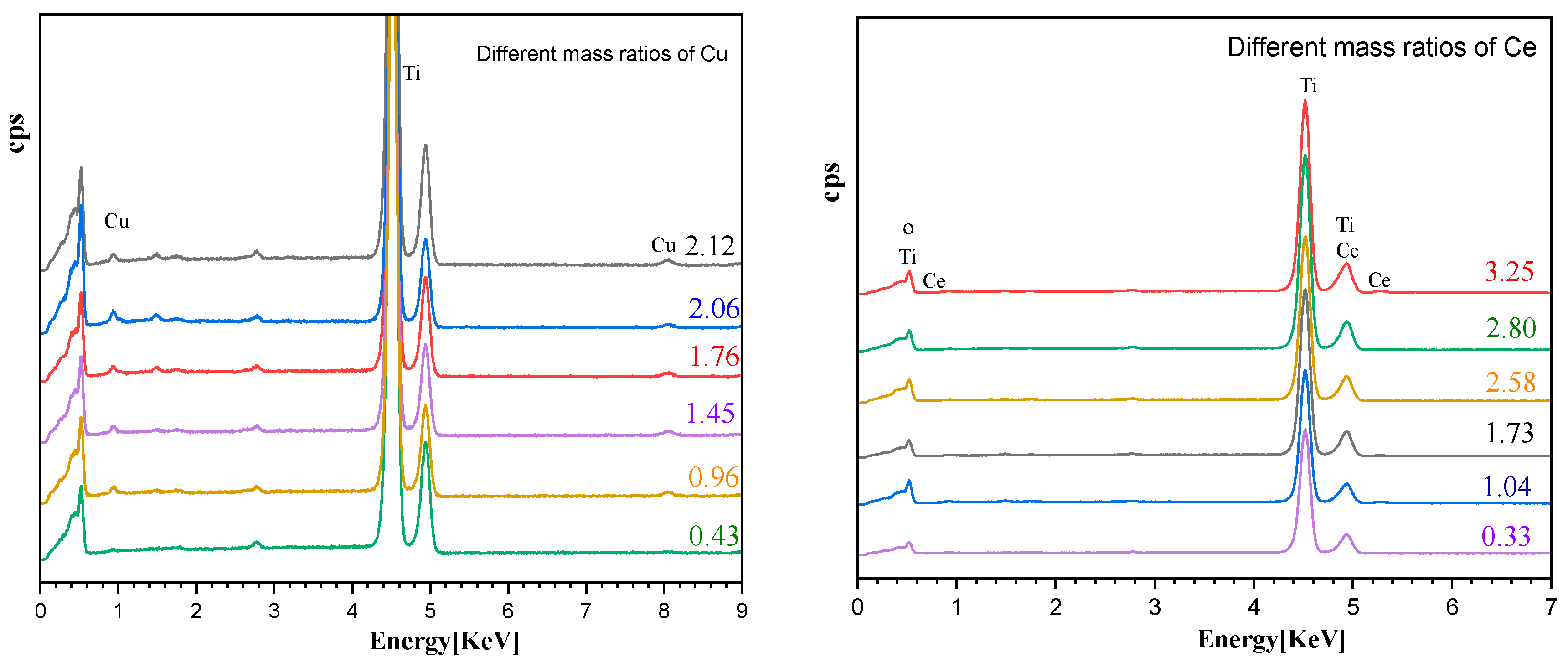

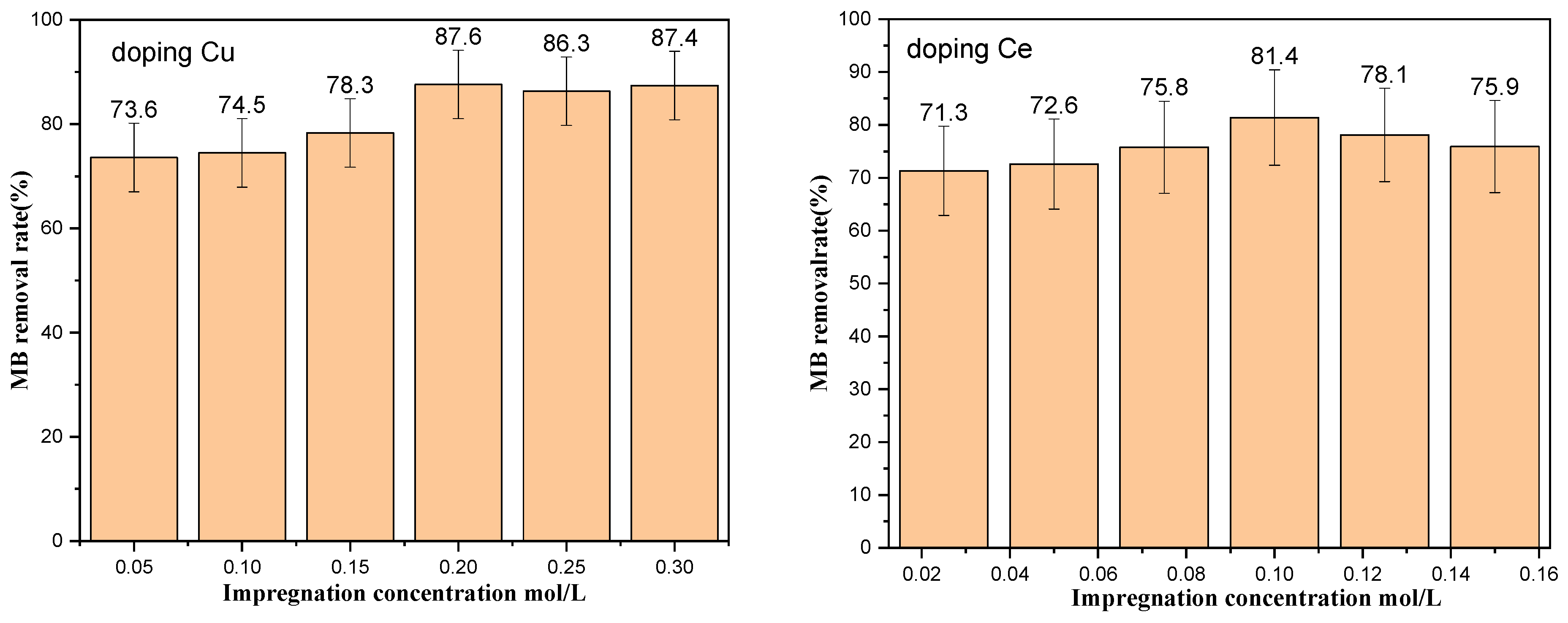

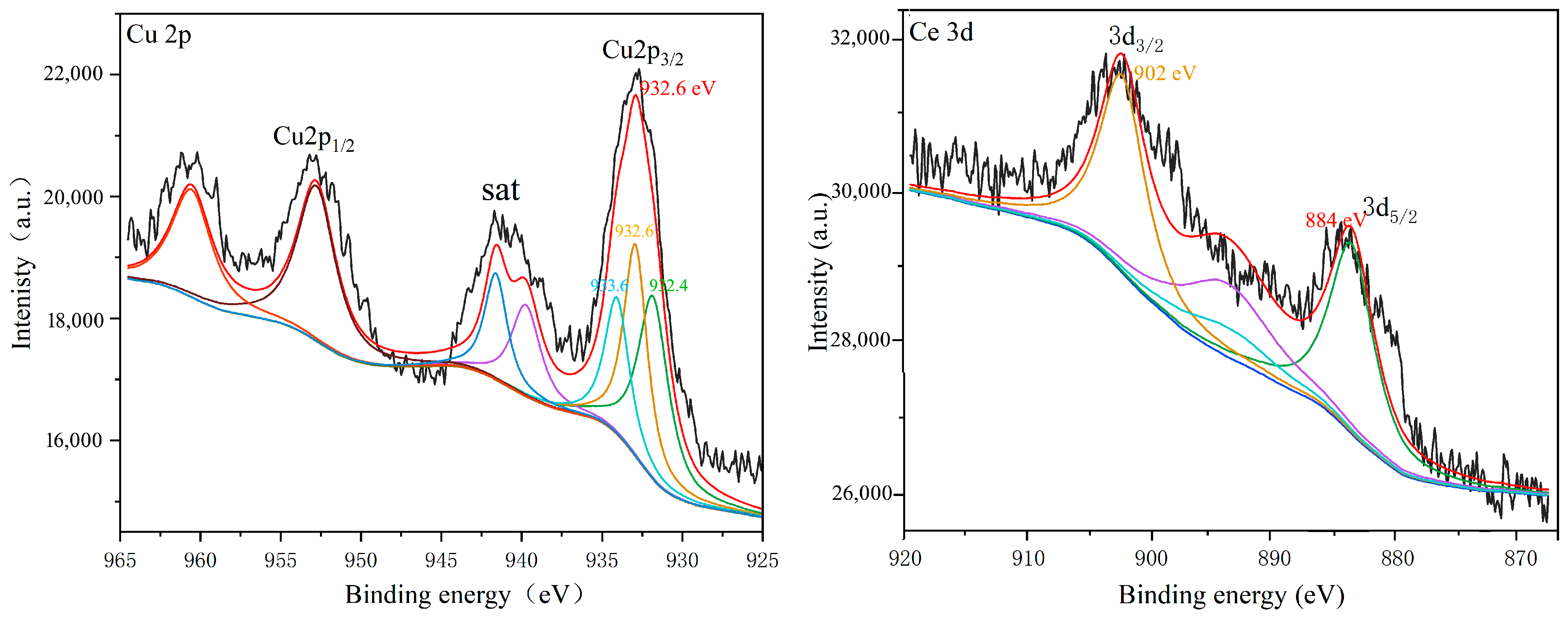

| Molecular Weight of PEG | Specific Surface Area (m2·g−1) |
|---|---|
| 400 | 261.5 |
| 800 | 297.5 |
| 1000 | 329.7 |
| 1500 | 279.6 |
| 2000 | 272.6 |
| Doped Metal | Specific Surface Area (m2·g−1) |
|---|---|
| Undoped | 329.7 |
| Cu | 311.3 |
| Ce | 430.2 |
| Cu and Ce | 337.2 |
| Structural Formula | Molecular Formula | Molecular Weight |
|---|---|---|
 | C16H18N3S+ | 284.1209 |
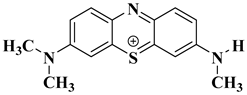 | C15H16N3S+ | 270.1054 |
 | C14H14N3S+ | 256.0898 |
| Compound Name | Chemical Formula | Manufacturer |
|---|---|---|
| Tetrabutyl titanate | Ti(OC4H9)4 | Tianjin Damao Chemical Reagent Factory (Tianjin, China) |
| Ethyl orthosilicate | (C2H5O)4Si | Tianjin Damao Chemical Reagent Factory |
| Cerium nitrates hexahydrate | Ce(NO3)3·6H2O | Shanghai Maclean’s Biochemical Technology Co., Ltd. (Shanghai, China) |
| Copper nitrate trihydrate | Cu(NO3)2·3H2O | Shanghai Maclean’s Biochemical Technology Co., Ltd. |
| Methylene blue | C16H18ClN3S·3H2O | Fuchen (Tianjin) Chemical Reagent Co., Ltd. (Tianjin, China) |
| N-butanol | C4H10O | Tianjin Damao Chemical Reagent Factory |
| Hexadecyltrimethylammonium bromide | C19H42BrN | Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) |
| Polyethylene glycol 400 | HO(CH2CH2O)nH | Tianjin Bailun Biotechnology Co., Ltd. (Shanghai, China) |
| Polyethylene glycol 800 | HO(CH2CH2O)nH | Shanghai Aladdin Biochemical Technology Co., Ltd. |
| Polyethylene glycol 1000 | HO(CH2CH2O)nH | Shanghai Aladdin Biochemical Technology Co., Ltd. |
| Polyethylene glycol 1500 | HO(CH2CH2O)nH | Shanghai Aladdin Biochemical Technology Co., Ltd. |
| Polyethylene glycol 2000 | HO(CH2CH2O)nH | Shanghai Aladdin Biochemical Technology Co., Ltd. |
| Sodium hydroxide | NaOH | Beijing Chemical Factory Co., Ltd. (Beijing, China) |
| Anhydrous ethanol | C2H6O | Beijing Chemical Factory Co., Ltd. |
| Experimental Instrument Name | Instrument Model | Producer |
|---|---|---|
| Electronic balance | AL204 | Mettler-Toledo International Trading (Shanghai) Co., Ltd. China (Shanghai, China) |
| CNC ultrasonic cleaner | KQ-400DB | KunShan Ultrasonic Instruments Co., Ltd., China (Kunshan, China) |
| 1650 °C atmosphere tube furnace | MR13 | Beijing Huace Testing Instrument Co., Ltd., China (Beijing, China) |
| Electric blast constant-temperature drying oven | 101-0B | Shaoxing Yuanmore Machine and Electrical Equipment Co., Ltd., China (Shaoxing, China) |
| Field emission scanning electron microscope | S-4700 | Hitachi, Tokyo, Japan |
| Specific surface area analyzer | ASAP 2460 2.02 | Micromeritics, Norcross, GA, USA |
| X-ray photoelectron spectrometer | ESCALAB 250 | ThermoFisher Scientific, Waltham, MA, USA |
| UV spectrophotometer | L6S | Shanghai Yidian Scientific Instrument Co., Ltd., China (Shanghai, China) |
| Fluorescence spectrometer | F-7000 | Hitachi, Tokyo, Japan |
| X-ray diffractometer | XRD-6000 | Shimadzu, Kyoto, Japan |
| Simultaneous thermal analyzer | TGA/DSC 3+ | Mettler-Toledo, Zurich, Switzerland |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Yan, Q.; Yang, J. Preparation and Photodegradation of TiO2 Thin Films on the Inner Wall of Quartz Tubes. Int. J. Mol. Sci. 2024, 25, 10253. https://doi.org/10.3390/ijms251910253
Chen W, Yan Q, Yang J. Preparation and Photodegradation of TiO2 Thin Films on the Inner Wall of Quartz Tubes. International Journal of Molecular Sciences. 2024; 25(19):10253. https://doi.org/10.3390/ijms251910253
Chicago/Turabian StyleChen, Wei, Qi Yan, and Junjiao Yang. 2024. "Preparation and Photodegradation of TiO2 Thin Films on the Inner Wall of Quartz Tubes" International Journal of Molecular Sciences 25, no. 19: 10253. https://doi.org/10.3390/ijms251910253
APA StyleChen, W., Yan, Q., & Yang, J. (2024). Preparation and Photodegradation of TiO2 Thin Films on the Inner Wall of Quartz Tubes. International Journal of Molecular Sciences, 25(19), 10253. https://doi.org/10.3390/ijms251910253





