Evaluating the Reparative Potential of Secretome from Patient-Derived Induced Pluripotent Stem Cells during Ischemia–Reperfusion Injury in Human Cardiomyocytes
Abstract
:1. Introduction
2. Results
2.1. iPSC-Derived Secretome from Healthy Donors Are Enriched with Proteins Involved in Calcium Handling Pathways
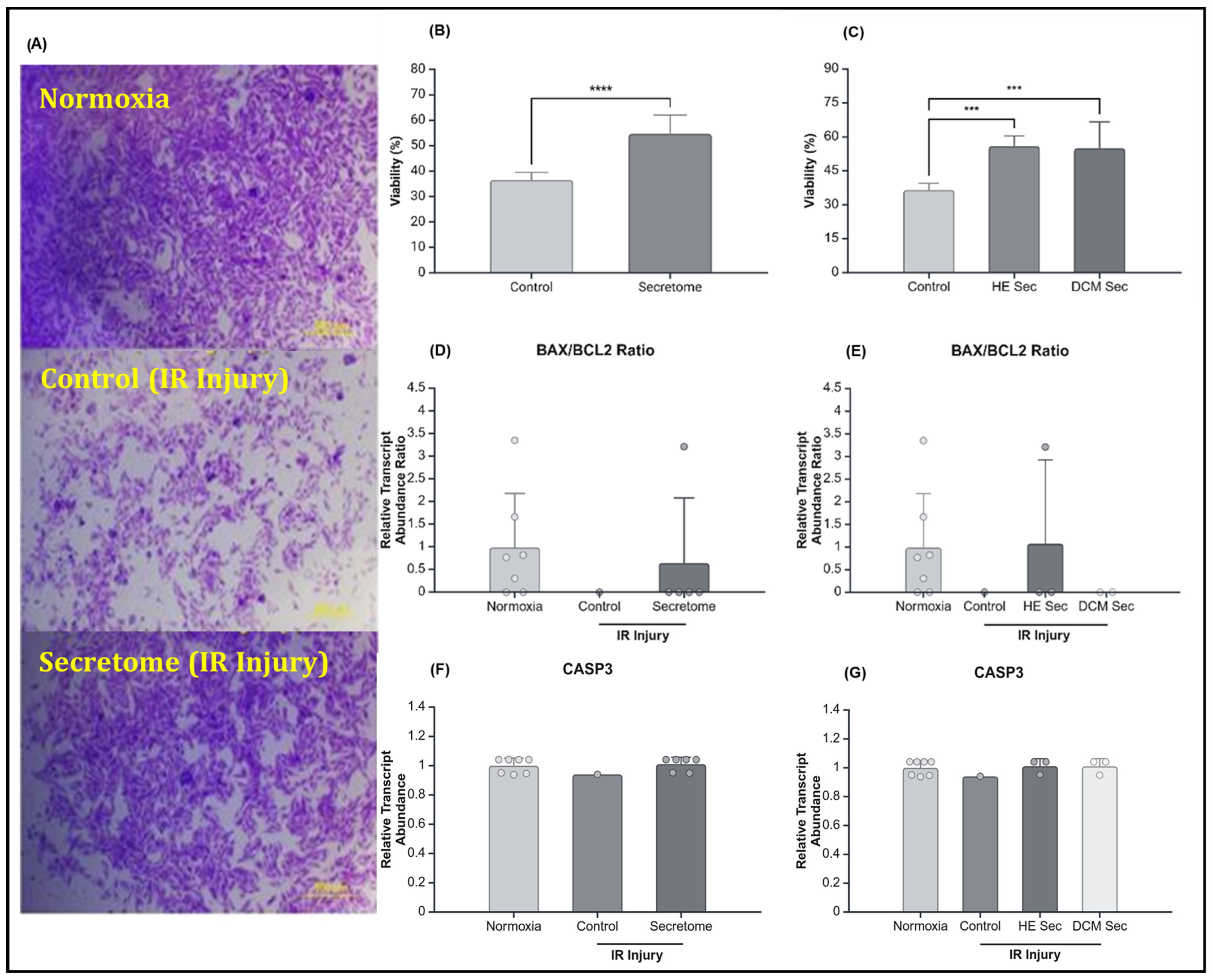
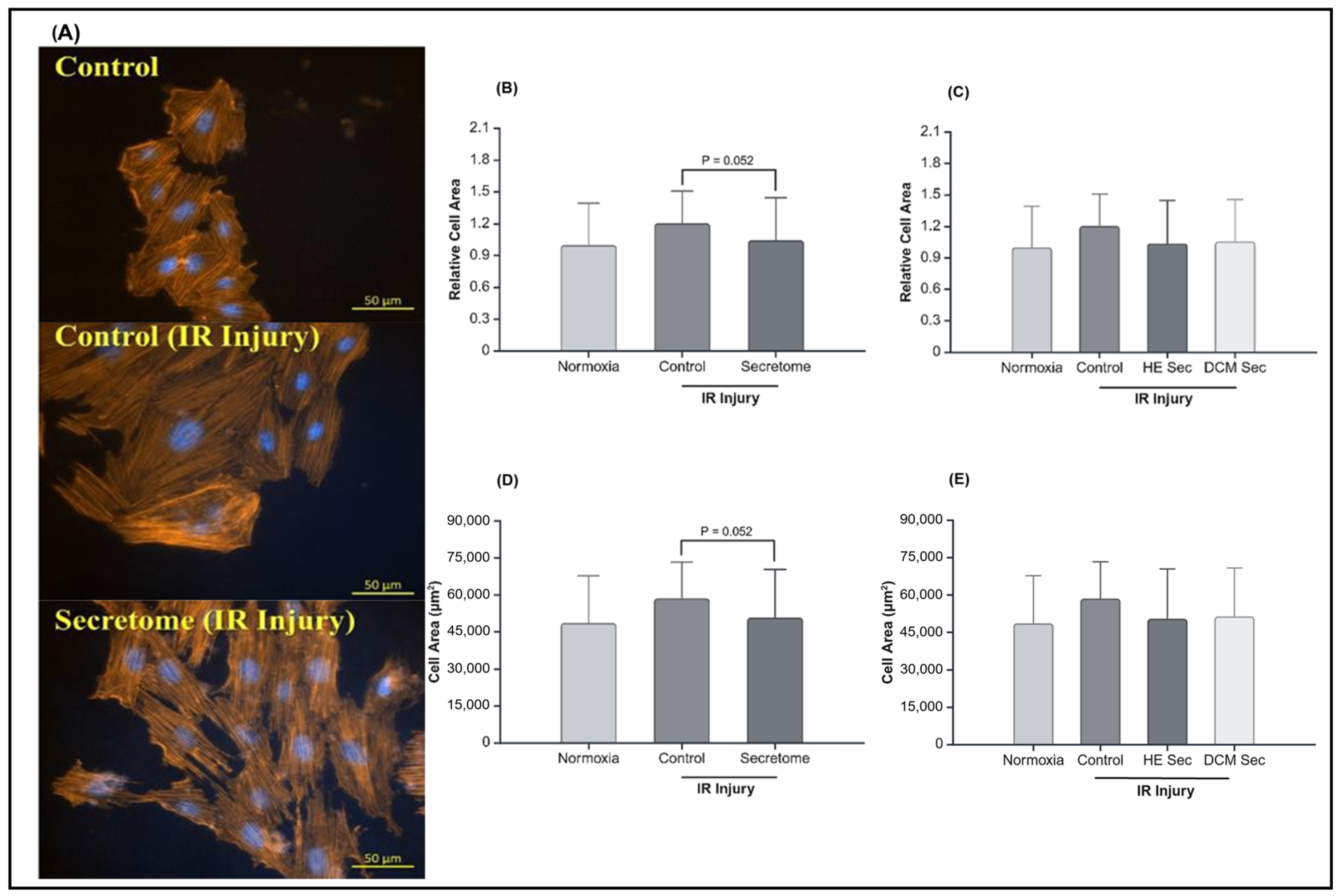
2.2. iPSC-Derived Secretome Increased CM Viability after IR Injury
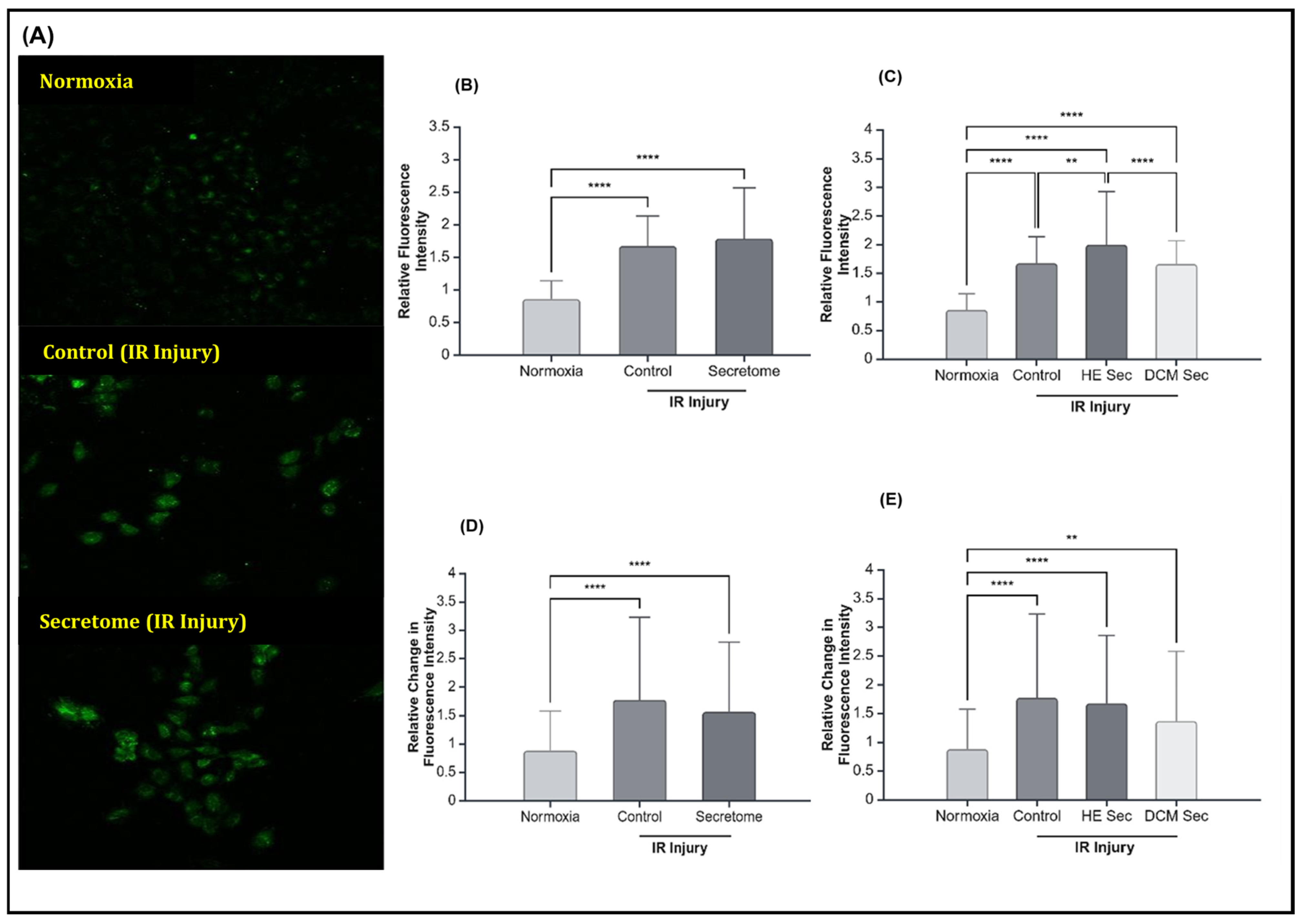
2.3. iPSC-Derived Secretome from Healthy Donors Improved Calcium Contractility
3. Discussion
4. Materials and Methods
4.1. Generating Donor-Specific iPSC-Derived Secretome
4.2. Mass Spectrometry
4.3. IR Injury Modelling
4.4. Resazurin Reduction Assay
4.5. Cell Viability
4.6. Hypertrophy Assessment
4.7. Calcium Flux Analysis
4.8. Quantitative Reverse Transcription Polymerase Chain Reaction
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| iPSC | Induced pluripotent stem cell |
| CM | Cardiomyocytes |
| MSC | Mesenchymal stem cell |
| RNA | Ribonucleic acid |
| DCM | Dilated cardiomyopathy |
| IR | Ischemia–reperfusion |
| LVEF | Left ventricular ejection fraction |
| DMEM/F12 | Dulbecco’s modified eagle medium F12 |
| FBS | Fetal bovine serum |
| ROS | Reactive oxygen species |
| HIF1A | Hypoxia inducible factor 1subunit alpha |
| SOD | Superoxide dismutase |
| BCL2 | B-cell lymphoma 2 |
| BAX | B-cell lymphoma 2-associated X |
| CASP3 | Caspase 3 |
| ANP | Atrial natriuretic peptide |
| BNP | Brain natriuretic peptide |
| SERCA2A | Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a |
| TNNT2 | Cardiac troponin T |
| CACNA1C | Calcium voltage-gated channel subunit alpha 1 C |
| CNX43 | Connexin 43 |
| RYR2 | Ryanodine receptor 2 |
| NCX1 | Sodium/calcium exchanger 1 |
References
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nguyen, H.V.; Tsang, S.H. Skin Biopsy and Patient-Specific Stem Cell Lines. Methods Mol. Biol. 2016, 1353, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B. Cellular reprogramming of human peripheral blood cells. Genom. Proteom. Bioinform. 2013, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Soda, M.; Saitoh, I.; Murakami, T.; Inada, E.; Iwase, Y.; Noguchi, H.; Shibasaki, S.; Kurosawa, M.; Sawami, T.; Terunuma, M.; et al. Repeated human deciduous tooth-derived dental pulp cell reprogramming factor transfection yields multipotent intermediate cells with enhanced iPS cell formation capability. Sci. Rep. 2019, 9, 1490. [Google Scholar] [CrossRef] [PubMed]
- Steinle, H.; Weber, M.; Behring, A.; Mau-Holzmann, U.; von Ohle, C.; Popov, A.F.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Reprogramming of Urine-Derived Renal Epithelial Cells into iPSCs Using srRNA and Consecutive Differentiation into Beating Cardiomyocytes. Mol. Ther. Nucleic Acids 2019, 17, 907–921. [Google Scholar] [CrossRef]
- Wen, W.; Zhang, J.P.; Xu, J.; Su, R.J.; Neises, A.; Ji, G.Z.; Yuan, W.; Cheng, T.; Zhang, X.B. Enhanced Generation of Integration-free iPSCs from Human Adult Peripheral Blood Mononuclear Cells with an Optimal Combination of Episomal Vectors. Stem Cell Rep. 2016, 6, 873–884. [Google Scholar] [CrossRef]
- Chen, C.X.; Abdian, N.; Maussion, G.; Thomas, R.A.; Demirova, I.; Cai, E.; Tabatabaei, M.; Beitel, L.K.; Karamchandani, J.; Fon, E.A.; et al. A Multistep Workflow to Evaluate Newly Generated iPSCs and Their Ability to Generate Different Cell Types. Methods Protoc. 2021, 4, 50. [Google Scholar] [CrossRef]
- Moretti, A.; Bellin, M.; Welling, A.; Jung, C.B.; Lam, J.T.; Bott-Flügel, L.; Dorn, T.; Goedel, A.; Höhnke, C.; Hofmann, F.; et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 2010, 363, 1397–1409. [Google Scholar] [CrossRef]
- Maizels, L.; Huber, I.; Arbel, G.; Tijsen, A.J.; Gepstein, A.; Khoury, A.; Gepstein, L. Patient-Specific Drug Screening Using a Human Induced Pluripotent Stem Cell Model of Catecholaminergic Polymorphic Ventricular Tachycardia Type 2. Circ. Arrhythm Electrophysiol. 2017, 10, e004725. [Google Scholar] [CrossRef]
- Schick, R.; Mekies, L.N.; Shemer, Y.; Eisen, B.; Hallas, T.; Ben Jehuda, R.; Ben-Ari, M.; Szantai, A.; Willi, L.; Shulman, R.; et al. Functional abnormalities in induced Pluripotent Stem Cell-derived cardiomyocytes generated from titin-mutated patients with dilated cardiomyopathy. PLoS ONE 2018, 13, e0205719. [Google Scholar] [CrossRef]
- Ahmed, R.E.; Anzai, T.; Chanthra, N.; Uosaki, H. A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pu, W.T. Cardiomyocyte Maturation: New Phase in Development. Circ. Res. 2020, 126, 1086–1106. [Google Scholar] [CrossRef] [PubMed]
- Karbassi, E.; Fenix, A.; Marchiano, S.; Muraoka, N.; Nakamura, K.; Yang, X.; Murry, C.E. Cardiomyocyte maturation: Advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 2020, 17, 341–359. [Google Scholar] [CrossRef]
- Lyra-Leite, D.M.; Gutiérrez-Gutiérrez, Ó.; Wang, M.; Zhou, Y.; Cyganek, L.; Burridge, P.W. A review of protocols for human iPSC culture, cardiac differentiation, subtype-specification, maturation, and direct reprogramming. STAR Protoc. 2022, 3, 101560–101582. [Google Scholar] [CrossRef]
- Menasché, P.; Hagège, A.A.; Scorsin, M.; Pouzet, B.; Desnos, M.; Duboc, D.; Schwartz, K.; Vilquin, J.T.; Marolleau, J.P. Myoblast transplantation for heart failure. Lancet 2001, 357, 279–280. [Google Scholar] [CrossRef]
- Perin, E.C.; Dohmann, H.F.; Borojevic, R.; Silva, S.A.; Sousa, A.L.; Mesquita, C.T.; Rossi, M.I.; Carvalho, A.C.; Dutra, H.S.; Dohmann, H.J.; et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 2003, 107, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Schächinger, V.; Erbs, S.; Elsässer, A.; Haberbosch, W.; Hambrecht, R.; Hölschermann, H.; Yu, J.; Corti, R.; Mathey, D.G.; Hamm, C.W.; et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 2006, 355, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Baharvand, H.; Ashtiani, S.K.; Soleimani, M.; Sadeghian, H.; Ardekani, J.M.; Mehrjerdi, N.Z.; Kouhkan, A.; Namiri, M.; Madani-Civi, M.; et al. Safety analysis and improved cardiac function following local autologous transplantation of CD133(+) enriched bone marrow cells after myocardial infarction. Curr. Neurovascular Res. 2007, 4, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, C.; Davidson, Y.; Cooper, K.; Tipnis, S.; Pujari, G.; Kurian, V.M. Transplantation of autologous bone marrow derived mesenchymal stem cells trans-epicardially in patients undergoing coronary bypass surgery. Indian Heart J. 2010, 62, 43–48. [Google Scholar]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.; Berman, D.; Czer, L.S.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients with Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, J.; Haack-Sørensen, M.; Juhl, M.; Harary Søndergaard, R.; Follin, B.; Drozd Lund, L.; Mønsted Johansen, E.; Ali Qayyum, A.; Bruun Mathiasen, A.; Jørgensen, E.; et al. Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure-A Safety Study. Stem Cells Transl. Med. 2017, 6, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Avilés, F.; Sanz-Ruiz, R.; Bogaert, J.; Casado Plasencia, A.; Gilaberte, I.; Belmans, A.; Fernández-Santos, M.E.; Charron, D.; Mulet, M.; Yotti, R.; et al. Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients with ST-Segment Elevation Myocardial Infarction and Left Ventricular Dysfunction. Circ. Res. 2018, 123, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, T.; Henry, T.D.; Kittleson, M.; Lima, J.; Siegel, R.J.; Slipczuk, L. Allogeneic cardiosphere-derived cells for the treatment of heart failure with reduced ejection fraction: The Dilated cardiomYopathy iNtervention with Allogeneic MyocardIally-regenerative Cells (DYNAMIC) trial. EuroIntervention 2020, 16, 293–300. [Google Scholar] [CrossRef]
- Huang, A.; Liu, Y.; Qi, X.; Chen, S.; Huang, H.; Zhang, J.; Han, Z.; Han, Z.C.; Li, Z. Intravenously transplanted mesenchymal stromal cells: A new endocrine reservoir for cardioprotection. Stem Cell Res. Ther. 2022, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Fan, M.; Zhao, Y.; Hu, X.; Zhu, Q.; Jiao, X.; Lv, Q.; Li, D.; Huang, Z.; Fu, G.; et al. Biomimetic and NOS-Responsive Nanomotor Deeply Delivery a Combination of MSC-EV and Mitochondrial ROS Scavenger and Promote Heart Repair and Regeneration. Adv. Sci. 2023, 10, 2301440. [Google Scholar] [CrossRef]
- Chen, P.; Pan, Y.; Ning, X.; Shi, X.; Zhong, J.; Fan, X.; Li, W.; Teng, Y.; Liu, X.; Yu, B.; et al. Targeted heart repair by Tβ4-loaded cardiac-resident macrophage-derived extracellular vesicles modified with monocyte membranes. Acta Biomater. 2023, 169, 372–386. [Google Scholar] [CrossRef]
- Kehl, D.; Generali, M.; Mallone, A.; Heller, M.; Uldry, A.C.; Cheng, P.; Gantenbein, B.; Hoerstrup, S.P.; Weber, B. Proteomic analysis of human mesenchymal stromal cell secretomes: A systematic comparison of the angiogenic potential. NPJ Regen. Med. 2019, 4, 8. [Google Scholar] [CrossRef]
- Papait, A.; Ragni, E.; Cargnoni, A.; Vertua, E.; Romele, P.; Masserdotti, A.; Perucca Orfei, C.; Signoroni, P.B.; Magatti, M.; Silini, A.R.; et al. Comparison of EV-free fraction, EVs, and total secretome of amniotic mesenchymal stromal cells for their immunomodulatory potential: A translational perspective. Front. Immunol. 2022, 13, 960909. [Google Scholar] [CrossRef]
- Li, L.; Cao, J.; Li, S.; Cui, T.; Ni, J.; Zhang, H.; Zhu, Y.; Mao, J.; Gao, X.; Midgley, A.C.; et al. M2 Macrophage-Derived sEV Regulate Pro-Inflammatory CCR2+ Macrophage Subpopulations to Favor Post-AMI Cardiac Repair. Adv. Sci. 2023, 10, e2202964. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- University of Ottawa Heart Institute. Cardiomyopathy. Ottawa. Available online: https://www.ottawaheart.ca/heart-condition/cardiomyopathy#top (accessed on 1 May 2024).
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Makhoul, G.; Yu, B.; Schwertani, A.; Cecere, R. The cytoprotective impact of yes-associated protein 1 after ischemia-reperfusion injury in AC16 human cardiomyocytes. Exp. Biol. Med. 2019, 244, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, R.M.; Zhang, H.; Vogel, H.; Cartwright, J., Jr.; Dionne, L.; Lu, N.; Huang, S.; Matzuk, M.M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. USA 1996, 93, 9782–9787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.; et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, R.; Xu, J.; Jiang, W.; Gong, Z.; Zhang, L.; Ning, Y.; Huang, P.; Xu, J.; Chen, G.; et al. Tongxinluo-pretreated mesenchymal stem cells facilitate cardiac repair via exosomal transfer of miR-146a-5p targeting IRAK1/NF-κB p65 pathway. Stem Cell Res. Ther. 2022, 13, 289. [Google Scholar] [CrossRef]
- Chi, B.; Zou, A.; Mao, L.; Cai, D.; Xiao, T.; Wang, Y.; Wang, Q.; Ji, Y.; Sun, L. Empagliflozin-Pretreated Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Attenuated Heart Injury. Oxidative Med. Cell. Longev. 2023, 2023, 7747727. [Google Scholar] [CrossRef]
- Ning, Y.; Huang, P.; Chen, G.; Xiong, Y.; Gong, Z.; Wu, C.; Xu, J.; Jiang, W.; Li, X.; Tang, R.; et al. Atorvastatin-pretreated mesenchymal stem cell-derived extracellular vesicles promote cardiac repair after myocardial infarction via shifting macrophage polarization by targeting microRNA-139-3p/Stat1 pathway. BMC Med. 2023, 21, 96. [Google Scholar] [CrossRef]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Pérez Lanzón, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127. [Google Scholar] [CrossRef]
- Du, W.J.; Chi, Y.; Yang, Z.X.; Li, Z.J.; Cui, J.J.; Song, B.Q.; Li, X.; Yang, S.G.; Han, Z.B.; Han, Z.C. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res. Ther. 2016, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Krishnakumar, V.; Soni, N.; Rao, E.P.; Banerjee, A.; Mohanty, S. Comparative proteomic profiling of Small Extracellular vesicles derived from iPSCs and tissue specific mesenchymal stem cells. Exp. Cell Res. 2022, 420, 113354. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Qiu, F.; Cao, H.; Li, H.; Dai, G.; Ma, T.; Gong, Y.; Luo, W.; Zhu, D.; Qiu, Z.; et al. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics 2023, 13, 685–703. [Google Scholar] [CrossRef]
- Turlo, A.J.; Hammond, D.E.; Ramsbottom, K.A.; Soul, J.; Gillen, A.; McDonald, K.; Peffers, M.J. Mesenchymal Stromal Cell Secretome Is Affected by Tissue Source and Donor Age. Stem Cells 2023, 41, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Lowsky, D.J.; Olshansky, S.J.; Bhattacharya, J.; Goldman, D.P. Heterogeneity in healthy aging. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 640–649. [Google Scholar] [CrossRef]
- Pavanello, S.; Campisi, M.; Fabozzo, A.; Cibin, G.; Tarzia, V.; Toscano, G.; Gerosa, G. The biological age of the heart is consistently younger than chronological age. Sci. Rep. 2020, 10, 10752. [Google Scholar] [CrossRef]
- Xie, M.; Burchfield, J.S.; Hill, J.A. Pathological Ventricular Remodeling: Mechanisms: Part 1 of 2. Circulation 2014, 128, 388–400. [Google Scholar] [CrossRef]
- Calderone, A.; Thaik, C.M.; Takahashi, N.; Chang, D.L.; Colucci, W.S. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J. Clin. Investig. 1998, 101, 812–818. [Google Scholar] [CrossRef]
- Horio, T.; Nishikimi, T.; Yoshihara, F.; Matsuo, H.; Takishita, S.; Kangawa, K. Inhibitory regulation of hypertrophy by endogenous atrial natriuretic peptide in cultured cardiac myocytes. Hypertension 2000, 35 Pt 1, 19–24. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Kubo, H.; Harris, D.M.; Mills, G.D.; Moyer, J.; Berretta, R.; Potts, S.T.; Marsh, J.D.; Houser, S.R. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ. Res. 2005, 97, 1009–1017. [Google Scholar] [CrossRef]
- Wang, C.; Liu, N.; Luan, R.; Li, Y.; Wang, D.; Zou, W.; Xing, Y.; Tao, L.; Cao, F.; Wang, H. Apelin protects sarcoplasmic reticulum function and cardiac performance in ischaemia-reperfusion by attenuating oxidation of sarcoplasmic reticulum Ca2+-ATPase and ryanodine receptor. Cardiovasc. Res. 2013, 100, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, M.; Zhou, J.; Dai, Z.; Sun, G.; Sun, X. Calenduloside E suppresses calcium overload by promoting the interaction between L-type calcium channels and Bcl2-associated athanogene 3 to alleviate myocardial ischemia/reperfusion injury. J. Adv. Res. 2020, 34, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Schellenberg, D.; Weinberg, L.; Singal, P.K. Effects of ouabain on calcium paradox in rat hearts. Can. J. Physiol. Pharmacol. 1986, 64, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, M.; Cheema, Y.; Shahbaz, A.U.; Bhattacharya, S.K.; Weber, K.T. Intracellular calcium overloading and oxidative stress in cardiomyocyte necrosis via a mitochondriocentric signal-transducer-effector pathway. Exp. Clin. Cardiol. 2011, 16, 109–115. [Google Scholar]
- Ryter, S.W.; Kim, H.P.; Hoetzel, A.; Park, J.W.; Nakahira, K.; Wang, X.; Choi, A.M. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007, 9, 49–89. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; He, S.; Sun, G.; Sun, X. Targeting Calcium Homeostasis in Myocardial Ischemia/Reperfusion Injury: An Overview of Regulatory Mechanisms and Therapeutic Reagents. Front. Pharmacol. 2020, 11, 872. [Google Scholar] [CrossRef]
- Maqsood, M.I.; Matin, M.M.; Bahrami, A.R.; Ghasroldasht, M.M. Immortality of cell lines: Challenges and advantages of establishment. Cell Biol. Int. 2013, 37, 1038–1045. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Lozano, J.; Rai, A.; Lees, J.G.; Fang, H.; Claridge, B.; Lim, S.Y.; Greening, D.W. Scalable Generation of Nanovesicles from Human-Induced Pluripotent Stem Cells for Cardiac Repair. Int. J. Mol. Sci. 2022, 23, 14334. [Google Scholar] [CrossRef]
- Lee, W.H.; Chen, W.Y.; Shao, N.Y.; Xiao, D.; Qin, X.; Baker, N.; Bae, H.R.; Wei, T.T.; Wang, Y.; Shukla, P.; et al. Comparison of Non-Coding RNAs in Exosomes and Functional Efficacy of Human Embryonic Stem Cell- versus Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cells 2017, 35, 2138–2149. [Google Scholar] [CrossRef]
- Adamiak, M.; Cheng, G.; Bobis-Wozowicz, S.; Zhao, L.; Kedracka-Krok, S.; Samanta, A.; Karnas, E.; Xuan, Y.T.; Skupien-Rabian, B.; Chen, X.; et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ. Res. 2018, 122, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Vaskova, E.; Tada, Y.; von Bornstaedt, D.; Woo, Y.; Yang, P. Pleiotropic effects of the exosomes from IPSC-derivatives in restoring injured myocardium. J. Am. Coll. Cardiol. 2018, 71 (Suppl. S11), A80. [Google Scholar] [CrossRef]
- El Harane, N.; Kervadec, A.; Bellamy, V.; Pidial, L.; Neametalla, H.J.; Perier, M.C.; Lima Correa, B.; Thiébault, L.; Cagnard, N.; Duché, A.; et al. Acellular therapeutic approach for heart failure: In vitro production of extracellular vesicles from human cardiovascular progenitors. Eur. Heart J. 2018, 39, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zhang, E.; Joshi, J.; Yang, J.; Zhang, J.; Zhu, W. Utilization of Human Induced Pluripotent Stem Cells for Cardiac Repair. Front. Cell Dev. Biol. 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tominaga, Y.; Kawamura, T.; Ito, E.; Takeda, M.; Harada, A.; Torigata, K.; Sakaniwa, R.; Sawa, Y.; Miyagawa, S. Pleiotropic effects of extracellular vesicles from induced pluripotent stem cell-derived cardiomyocytes on ischemic cardiomyopathy: A preclinical study. J. Heart Lung Transpl. 2024, 43, 85–99. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Casati, S.; Giannasi, C.; Niada, S.; Della Morte, E.; Orioli, M.; Brini, A.T. Lipidomics of Cell Secretome Combined with the Study of Selected Bioactive Lipids in an In Vitro Model of Osteoarthritis. Stem Cells Transl. Med. 2022, 11, 959–970. [Google Scholar] [CrossRef]



| Sample ID | Age (Years) | Sex | Ethnicity | Healthy or DCM |
|---|---|---|---|---|
| HID041004 | 46 | Female | Caucasian | Healthy |
| HID041032 | 55 | Male | Black, other | Healthy |
| AIW001-02 | 48 | Female | Caucasian | Healthy |
| HID041019 | 41 | Female | Caucasian | DCM |
| HID041021 | 45 | Female | Caucasian | DCM |
| HID041031 | 39 | Male | Asian (East or Southeast Asian) | DCM |
| Average | 45.66 | 66% (F) | - | 50% (DCM) |
| Gene Name | Abbreviation | Forward Sequence | Reverse Sequence |
|---|---|---|---|
| Hypoxia inducible factor 1 subunit alpha | HIF1A | TATGAGCCAGAAGAACTTTTAGGC | CACCTCTTTTGGCAAGCATCCTG |
| Superoxide dismutase 1 | SOD1 | TTTCGAGCAGAAGGAAAGTAATGGA | CAACATGCCTCTCTTCATCCTTTGG |
| Superoxide dismutase 2 | SOD2 | GATGTTACAGCCCAGATAGCTCTTC | CGTCAGCTTCTCCTTAAACTTGTCA |
| B-cell lymphoma 2 | BCL2 | AATTGCCGAGAAGGGGAAAACA | CGATTCCCAGACTTCTGCTTCA |
| B-cell lymphoma 2-associated X | Bax | GTTTTCTGACGGCAACTTCAACTG | AATGTCCAGCCCATGATGGTTC |
| Caspase-3 | CASP3 | GGAAGCGAATCAATGGACTCTGG | GCATCGACATCTGTACCAGACC |
| Atrial natriuretic peptide | ANP | GGTTCTGGTTGCCTTGGTAGGA | CCATGGCAACAAGATGACACAAATG |
| B-type natriuretic peptide | BNP | TCTGATCGATCTGCCCTCCTAAAAA | CAGGGTGTAGAGGACCATTTTGC |
| Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2a | SERCA2a | GGACTTTGAAGGCGTGGATTGTG | CTCAGCAAGGACTGGTTTTCGG |
| Cardiac troponin T | TNNT2 | GGAGGAGTCCAAACCAAAGCC | TCAAAGTCCACTCTCTCTCCATC |
| Calcium voltage-gated channel subunit Alpha1C | CACNA1C | TGATTCCAACGCCACCAATTC | GAGGAGTCCATAGGCGATTACT |
| Connexin 43 | Cx43 | CAATCTCTCATGTGCGCTTCT | GGCAACCTTGAGTTCTTCCTCT |
| Ryanodine receptor 2 | RYR2 | CATCGAACACTCCTCTACGGA | GGACACGCTAACTAAGATGAGGT |
| Solute carrier family 8 member A1 | NCX1 | CATTAAGAAGACAAACCTGGCCCTT | ACATTCATCGTCGTCATCATCTTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rody, E.; Zwaig, J.; Derish, I.; Khan, K.; Kachurina, N.; Gendron, N.; Giannetti, N.; Schwertani, A.; Cecere, R. Evaluating the Reparative Potential of Secretome from Patient-Derived Induced Pluripotent Stem Cells during Ischemia–Reperfusion Injury in Human Cardiomyocytes. Int. J. Mol. Sci. 2024, 25, 10279. https://doi.org/10.3390/ijms251910279
Rody E, Zwaig J, Derish I, Khan K, Kachurina N, Gendron N, Giannetti N, Schwertani A, Cecere R. Evaluating the Reparative Potential of Secretome from Patient-Derived Induced Pluripotent Stem Cells during Ischemia–Reperfusion Injury in Human Cardiomyocytes. International Journal of Molecular Sciences. 2024; 25(19):10279. https://doi.org/10.3390/ijms251910279
Chicago/Turabian StyleRody, Elise, Jeremy Zwaig, Ida Derish, Kashif Khan, Nadezda Kachurina, Natalie Gendron, Nadia Giannetti, Adel Schwertani, and Renzo Cecere. 2024. "Evaluating the Reparative Potential of Secretome from Patient-Derived Induced Pluripotent Stem Cells during Ischemia–Reperfusion Injury in Human Cardiomyocytes" International Journal of Molecular Sciences 25, no. 19: 10279. https://doi.org/10.3390/ijms251910279






