Assessing Biomarkers of Porcine Kidneys under Normothermic Machine Perfusion—Can We Gain Insight into a Marginal Organ?
Abstract
1. Introduction
2. Results
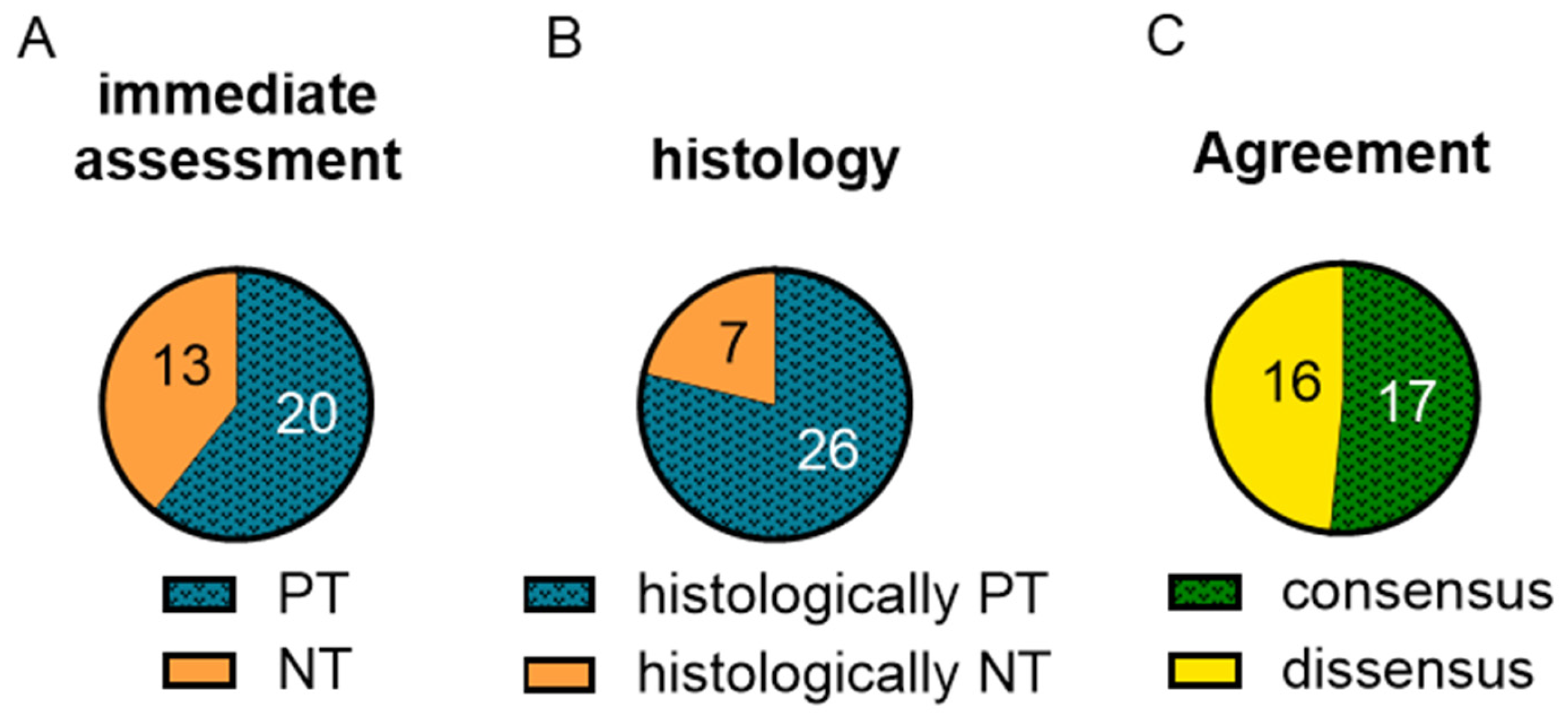
2.1. Kidney Classification Based on Macroscopy and Remuzzi-Score Was Inhomogeneous
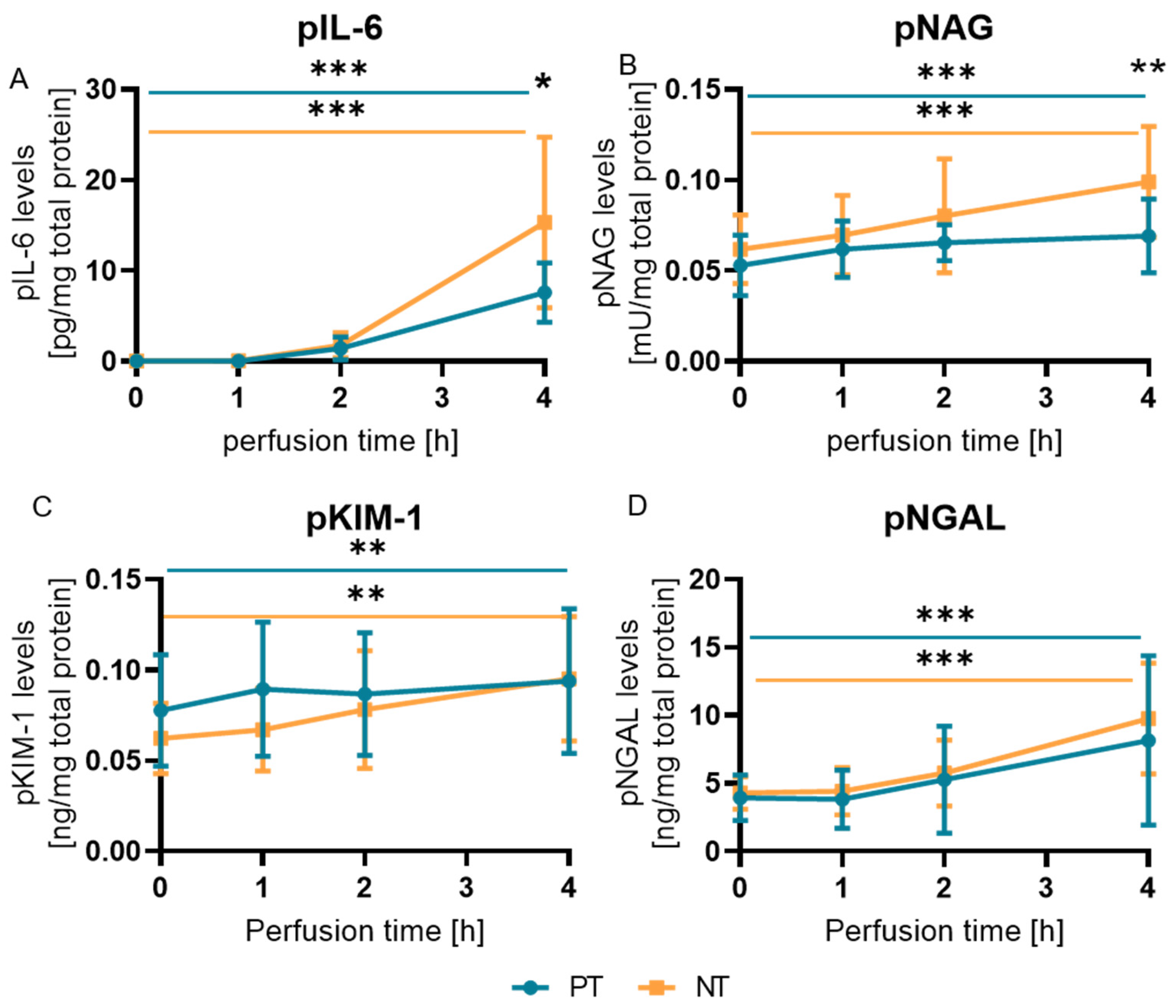
2.2. pNAG and pIL-6 Correlated with the Macroscopic Clinical Classification under NMP
2.3. Biomarkers Did Not Reflect Histological Changes
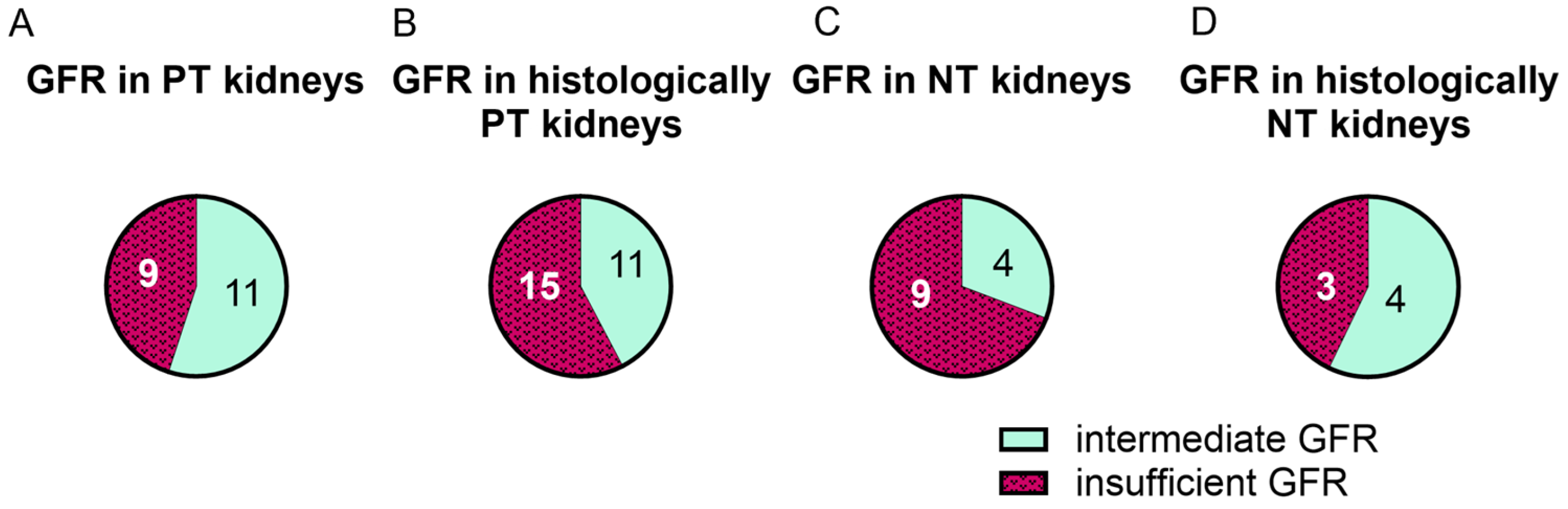
2.4. Functionality Did Not Correlate with Macroscopic or Histologic Assessment
2.5. GFR in PT Kidneys Correlated with uIL-6
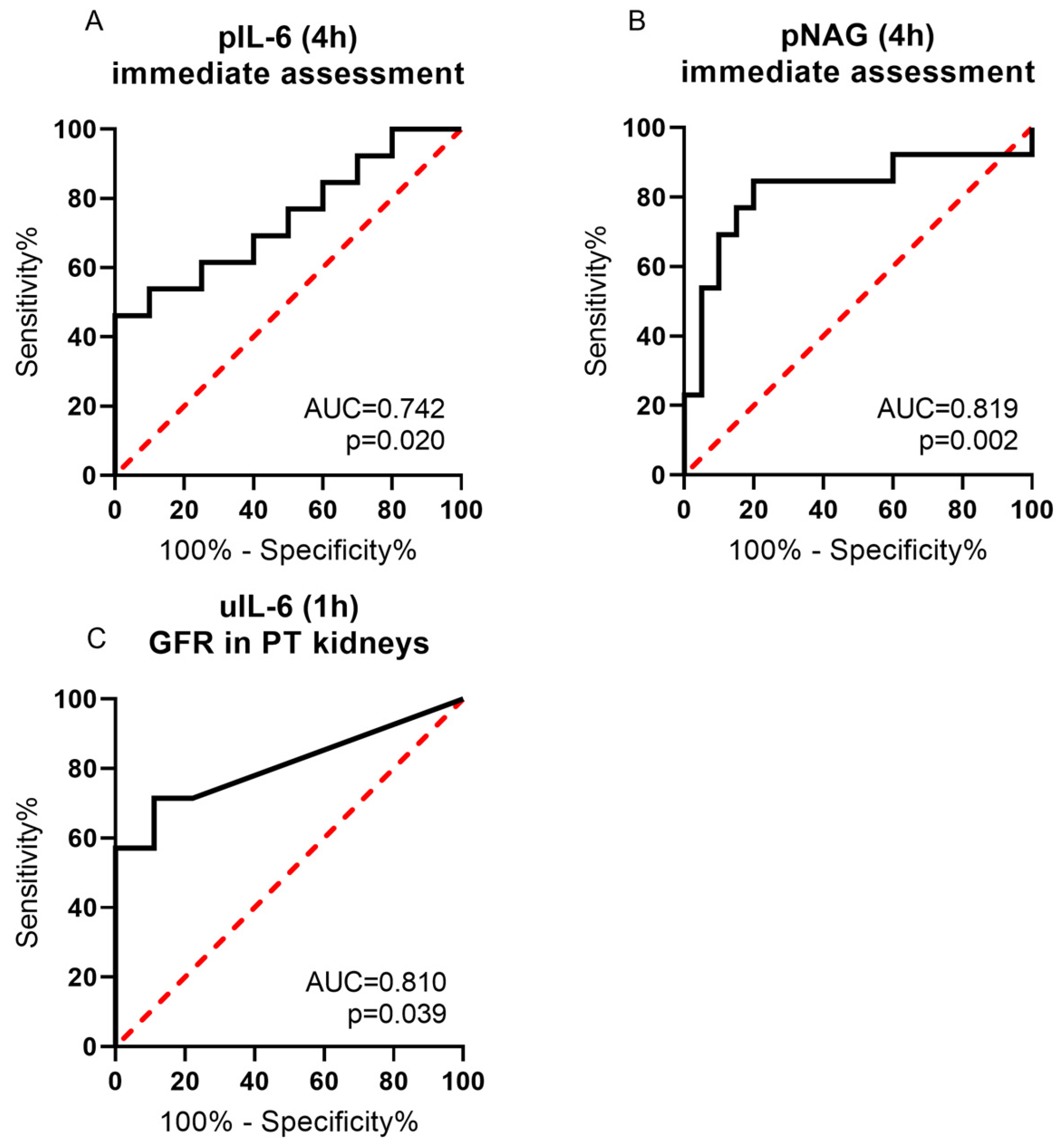
2.6. ROC Analysis of pIL-6, uIL-6 and pNAG Supported Performance Prediction
3. Discussion
3.1. EDN-1 as a Biomarker
3.2. TLR-4 as a Biomarker
3.3. IL-6 as a Biomarker
3.4. NAG as a Biomarker
3.5. KIM-1 as a Biomarker
3.6. NGAL as a Biomarker
3.7. Aptitude of the Classifications
3.8. NMP Setting
4. Materials and Methods
4.1. Setting
4.2. Normothermic Machine Perfusion with Whole Blood
4.3. Macroscopic Classification of Kidneys
4.4. Histological Classification of Kidneys
4.5. Functional Classification of the Kidneys
4.6. Analysis of Markers in Blood and Urine
4.7. Gene Expression in Biopsies
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ojo, A.O.; Hanson, J.A.; Meier-Kriesche, H.U.; Okechukwu, C.N.; Wolfe, R.A.; Leichtman, A.B.; Agodoa, L.Y.; Kaplan, B.; Port, F.K. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J. Am. Soc. Nephrol. 2001, 12, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Urawa, A.; Watanabe, S.; Hasegawa, T.; Ogura, T.; Nishikawa, K.; Sugimura, Y.; Komori, T.; Okada, M. Mood Status and Quality of Life in Kidney Recipients After Transplantation. Transplant. Proc. 2018, 50, 2521–2525. [Google Scholar] [CrossRef]
- Wang, Y.; Hemmelder, M.H.; Bos, W.J.W.; Snoep, J.D.; de Vries, A.P.J.; Dekker, F.W.; Meuleman, Y. Mapping health-related quality of life after kidney transplantation by group comparisons: A systematic review. Nephrol. Dial. Transplant. 2021, 36, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Eurotransplant. Annual Report 2021; Eurotransplant: Leiden, The Netherlands, 2022. [Google Scholar]
- Melih, K.V.; Boynuegri, B.; Mustafa, C.; Nilgun, A. Incidence, Risk Factors, and Outcomes of Delayed Graft Function in Deceased Donor Kidney Transplantation. Transplant. Proc. 2019, 51, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Mor, E.; Michowiz, R.; Ashkenazi, T.; Shabtai, E.; Nakache, R.; Eid, A.; Hoffman, A.; Mizrahi, S.; Shabtai, M.; Shapira, Z. Extension of the organ pool in kidney transplantation: First year experience of the Israel Transplant Center. Isr. Med. Assoc. J. 2000, 2, 302–305. [Google Scholar]
- Klein, A.S.; Messersmith, E.E.; Ratner, L.E.; Kochik, R.; Baliga, P.K.; Ojo, A.O. Organ Donation and Utilization in the United States, 1999–2008. Am. J. Transplant. 2010, 10, 973–986. [Google Scholar] [CrossRef]
- Mittal, S.; Adamusiak, A.; Horsfield, C.; Loukopoulos, I.; Karydis, N.; Kessaris, N.; Drage, M.; Olsburgh, J.; Watson, C.J.; Callaghan, C.J. A Re-evaluation of Discarded Deceased Donor Kidneys in the UK: Are Usable Organs Still Being Discarded? Transplantation 2017, 101, 1698–1703. [Google Scholar] [CrossRef]
- Brat, A.; de Vries, K.M.; van Heurn, E.W.E.; Huurman, V.A.L.; de Jongh, W.; Leuvenink, H.G.D.; van Zuilen, A.D.; Haase-Kromwijk, B.; de Jonge, J.; Berger, S.P.; et al. Hypothermic Machine Perfusion as a National Standard Preservation Method for Deceased Donor Kidneys. Transplantation 2022, 106, 1043–1050. [Google Scholar] [CrossRef]
- De Sandes-Freitas, T.V.; Costa, S.D.; de Andrade, L.G.M.; Girão, C.M.; Fernandes, P.F.C.B.C.; de Oliveira, C.M.C.; Esmeraldo, R.d.M. The Impact of Hypothermic Pulsatile Machine Perfusion Versus Static Cold Storage: A Donor-Matched Paired Analysis in a Scenario of High Incidence of Delayed Kidney Graft Function. Ann. Transplant. 2020, 25, e927010. [Google Scholar] [CrossRef]
- Moers, C.; Pirenne, J.; Paul, A.; Ploeg, R.J. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2012, 366, 770–771. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Saeb-Parsy, K.; Hamed, M.O.; Nicholson, M.L. Successful Transplantation of Human Kidneys Deemed Untransplantable but Resuscitated by Ex Vivo Normothermic Machine Perfusion. Am. J. Transplant. 2016, 16, 3282–3285. [Google Scholar] [CrossRef] [PubMed]
- Kaths, J.M.; Hamar, M.; Echeverri, J.; Linares, I.; Urbanellis, P.; Cen, J.Y.; Ganesh, S.; Dingwell, L.S.; Yip, P.; John, R.; et al. Normothermic ex vivo kidney perfusion for graft quality assessment prior to transplantation. Am. J. Transplant. 2018, 18, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Georgiades, F.; Hosgood, S.A.; Butler, A.J.; Nicholson, M.L. Use of ex vivo normothermic machine perfusion after normothermic regional perfusion to salvage a poorly perfused DCD kidney. Am. J. Transplant. 2019, 19, 3415–3419. [Google Scholar] [CrossRef] [PubMed]
- Rijkse, E.; de Jonge, J.; Kimenai, H.; Hoogduijn, M.J.; de Bruin, R.W.F.; van den Hoogen, M.W.F.; IJzermans, J.N.M.; Minnee, R.C. Safety and feasibility of 2 h of normothermic machine perfusion of donor kidneys in the Eurotransplant Senior Program. BJS Open 2021, 5, zraa024. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Barlow, A.D.; Hunter, J.P.; Nicholson, M.L. Ex vivo normothermic perfusion for quality assessment of marginal donor kidney transplants. Br. J. Surg. 2015, 102, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Markgraf, W.; Mühle, R.; Lilienthal, J.; Kromnik, S.; Thiele, C.; Malberg, H.; Janssen, M.; Putz, J. Inulin Clearance during Ex vivo Normothermic Machine Perfusion as a Marker of Renal Function. ASAIO J. 2022, 68, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Messner, F.; Soleiman, A.; Öfner, D.; Neuwirt, H.; Schneeberger, S.; Weissenbacher, A. 48 h Normothermic Machine Perfusion with Urine Recirculation for Discarded Human Kidney Grafts. Transpl. Int. 2023, 36, 11804. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef]
- Marsden, P.A.; Dorfman, D.M.; Collins, T.; Brenner, B.M.; Orkin, S.H.; Ballermann, B.J. Regulated expression of endothelin 1 in glomerular capillary endothelial cells. Am. J. Physiol. 1991, 261, F117–F125. [Google Scholar] [CrossRef]
- Sakamoto, H.; Sasaki, S.; Hirata, Y.; Imai, T.; Ando, K.; Ida, T.; Sakurai, T.; Yanagisawa, M.; Masaki, T.; Marumo, F. Production of endothelin-1 by rat cultured mesangial cells. Biochem. Biophys. Res. Commun. 1990, 169, 462–468. [Google Scholar] [CrossRef]
- Kohan, D.E. Endothelin synthesis by rabbit renal tubule cells. Am. J. Physiol. 1991, 261, F221–F226. [Google Scholar] [CrossRef] [PubMed]
- Cybulsky, A.V.; Stewart, D.J.; Cybulsky, M.I. Glomerular epithelial cells produce endothelin-1. J. Am. Soc. Nephrol. 1993, 3, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, W.; Pittrow, D. Endothelin receptor selectivity in chronic kidney disease: Rationale and review of recent evidence. Eur. J. Clin. Investig. 2009, 39 (Suppl. S2), 50–67. [Google Scholar] [CrossRef] [PubMed]
- Kohan, D.E.; Inscho, E.W.; Wesson, D.; Pollock, D.M. Physiology of endothelin and the kidney. Compr. Physiol. 2011, 1, 883–919. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Burkart, V.; Flohé, S.; Kolb, H. Cutting Edge: Heat Shock Protein 60 Is a Putative Endogenous Ligand of the Toll-Like Receptor-4 Complex. J. Immunol. 2000, 164, 558–561. [Google Scholar] [CrossRef]
- Vabulas, R.M.; Ahmad-Nejad, P.; Ghose, S.; Kirschning, C.J.; Issels, R.D.; Wagner, H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002, 277, 15107–15112. [Google Scholar] [CrossRef]
- Okamura, Y.; Watari, M.; Jerud, E.S.; Young, D.W.; Ishizaka, S.T.; Rose, J.; Chow, J.C.; Strauss, J.F., 3rd. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 2001, 276, 10229–10233. [Google Scholar] [CrossRef]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 2002, 195, 99–111. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Van Oers, M.H.; Van der Heyden, A.A.; Aarden, L.A. Interleukin 6 (IL-6) in serum and urine of renal transplant recipients. Clin. Exp. Immunol. 1988, 71, 314–319. [Google Scholar]
- Nechemia-Arbely, Y.; Barkan, D.; Pizov, G.; Shriki, A.; Rose-John, S.; Galun, E.; Axelrod, J.H. IL-6/IL-6R axis plays a critical role in acute kidney injury. J. Am. Soc. Nephrol. 2008, 19, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Ranganathan, P.; Jayakumar, C.; Ramesh, G. Proximal tubule-specific overexpression of netrin-1 suppresses acute kidney injury-induced interstitial fibrosis and glomerulosclerosis through suppression of IL-6/STAT3 signaling. Am. J. Physiol. Ren. Physiol. 2013, 304, F1054–F1065. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, F.; Ruggenenti, P.; Noris, M.; Locatelli, G.; Perico, N.; Perna, A.; Remuzzi, G. Sequential monitoring of urine-soluble interleukin 2 receptor and interleukin 6 predicts acute rejection of human renal allografts before clinical or laboratory signs of renal dysfunction. Transplantation 1997, 63, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Sonkar, G.K.; Singh, S.; Sonkar, S.K.; Singh, U.; Singh, R.G. Evaluation of serum interleukin 6 and tumour necrosis factor alpha levels, and their association with various non-immunological parameters in renal transplant recipients. Singap. Med. J. 2013, 54, 511–515. [Google Scholar] [CrossRef]
- Wellwood, J.M.; Ellis, B.G.; Price, R.G.; Hammond, K.; Thompson, A.E.; Jones, N.F. Urinary N-Acetyl-β-D-Glucosaminidase Activities in Patients with Renal Disease. Br. Med. J. 1975, 3, 408–411. [Google Scholar] [CrossRef]
- Bíró, E.; Szegedi, I.; Kiss, C.; Oláh, A.V.; Dockrell, M.; Price, R.G.; Szabó, T. The role of urinary N-acetyl-β-D-glucosaminidase in early detection of acute kidney injury among pediatric patients with neoplastic disorders in a retrospective study. BMC Pediatr. 2022, 22, 429. [Google Scholar] [CrossRef]
- Herget-Rosenthal, S.; Poppen, D.; Hüsing, J.; Marggraf, G.; Pietruck, F.; Jakob, H.G.; Philipp, T.; Kribben, A. Prognostic value of tubular proteinuria and enzymuria in nonoliguric acute tubular necrosis. Clin. Chem. 2004, 50, 552–558. [Google Scholar] [CrossRef]
- Chew, S.L.; Lins, R.L.; Daelemans, R.; Nuyts, G.D.; De Broe, M.E. Urinary enzymes in acute renal failure. Nephrol. Dial. Transplant. 1993, 8, 507–511. [Google Scholar] [CrossRef]
- Bourbouze, R.; Baumann, F.-C.; Bonvalet, J.-P.; Farman, N. Distribution of N-acetyl-β-D-glucosaminidase isoenzymes along the rabbit nephron. Kidney Int. 1984, 25, 636–642. [Google Scholar] [CrossRef]
- Hosohata, K.; Jin, D.; Takai, S. In Vivo and In Vitro Evaluation of Urinary Biomarkers in Ischemia/Reperfusion-Induced Kidney Injury. Int. J. Mol. Sci. 2021, 22, 11448. [Google Scholar] [CrossRef]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Bailly, V.; Zhang, Z.; Meier, W.; Cate, R.; Sanicola, M.; Bonventre, J.V. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J. Biol. Chem. 2002, 277, 39739–39748. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Yi, J.; Ha, J.; Shi, H.; Ismail, O.; Nathoo, S.; Bonventre, J.V.; Zhang, X.; Gunaratnam, L. Accelerated receptor shedding inhibits kidney injury molecule-1 (KIM-1)-mediated efferocytosis. Am. J. Physiol.-Ren. Physiol. 2014, 307, F205–F221. [Google Scholar] [CrossRef]
- Paragas, N.; Qiu, A.; Zhang, Q.; Samstein, B.; Deng, S.X.; Schmidt-Ott, K.M.; Viltard, M.; Yu, W.; Forster, C.S.; Gong, G.; et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat. Med. 2011, 17, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, H.; Slyne, J.; Higgins, M.; Radford, R.; Conlon, P.J.; Watson, A.J.; Ryan, M.P.; McMorrow, T.; Slattery, C. Neutrophil gelatinase-associated lipocalin (NGAL) is localised to the primary cilium in renal tubular epithelial cells—A novel source of urinary biomarkers of renal injury. Biochim. Biophys. Acta-Mol. Basis Dis. 2019, 1865, 165532. [Google Scholar] [CrossRef]
- Ikeda, M.; Prachasilchai, W.; Burne-Taney, M.J.; Rabb, H.; Yokota-Ikeda, N. Ischemic acute tubular necrosis models and drug discovery: A focus on cellular inflammation. Drug Discov. Today 2006, 11, 364–370. [Google Scholar] [CrossRef]
- Remuzzi, G.; Grinyò, J.; Ruggenenti, P.; Beatini, M.; Cole, E.H.; Milford, E.L.; Brenner, B.M.; The Double Kidney Transplant Group (DKG). Early Experience with Dual Kidney Transplantation in Adults using Expanded Donor Criteria. J. Am. Soc. Nephrol. 1999, 10, 2591–2598. [Google Scholar] [CrossRef]
- Dhaun, N.; Lilitkarntakul, P.; Macintyre, I.M.; Muilwijk, E.; Johnston, N.R.; Kluth, D.C.; Webb, D.J.; Goddard, J. Urinary endothelin-1 in chronic kidney disease and as a marker of disease activity in lupus nephritis. Am. J. Physiol. Ren. Physiol. 2009, 296, F1477–F1483. [Google Scholar] [CrossRef]
- Firth, J.D.; Ratcliffe, P.J. Organ distribution of the three rat endothelin messenger RNAs and the effects of ischemia on renal gene expression. J. Clin. Investig. 1992, 90, 1023–1031. [Google Scholar] [CrossRef]
- Gallinat, A.; Fox, M.; Lüer, B.; Efferz, P.; Paul, A.; Minor, T. Role of Pulsatility in Hypothermic Reconditioning of Porcine Kidney Grafts by Machine Perfusion After Cold Storage. Transplantation 2013, 96, 538–542. [Google Scholar] [CrossRef]
- Kron, P.; Schlegel, A.; de Rougemont, O.; Oberkofler, C.E.; Clavien, P.-A.; Dutkowski, P. Short, Cool, and Well Oxygenated–HOPE for Kidney Transplantation in a Rodent Model. Ann. Surg. 2016, 264, 815–822. [Google Scholar] [CrossRef]
- Patel, M.; Hosgood, S.; Nicholson, M.L. The effects of arterial pressure during normothermic kidney perfusion. J. Surg. Res. 2014, 191, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Von Horn, C.; Minor, T. Isolated kidney perfusion: The influence of pulsatile flow. Scand J. Clin. Lab. Investig. 2018, 78, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Hunter, J.P.; Nicholson, M.L. Early urinary biomarkers of warm and cold ischemic injury in an experimental kidney model. J. Surg. Res. 2012, 174, e85–e90. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Nicholson, M.L. An assessment of urinary biomarkers in a series of declined human kidneys measured during ex vivo normothermic kidney perfusion. Transplantation 2017, 101, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Krüger, B.; Krick, S.; Dhillon, N.; Lerner, S.M.; Ames, S.; Bromberg, J.S.; Lin, M.; Walsh, L.; Vella, J.; Fischereder, M.; et al. Donor Toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 3390–3395. [Google Scholar] [CrossRef]
- Pulskens, W.P.; Teske, G.J.; Butter, L.M.; Roelofs, J.J.; van der Poll, T.; Florquin, S.; Leemans, J.C. Toll-Like Receptor-4 Coordinates the Innate Immune Response of the Kidney to Renal Ischemia/Reperfusion Injury. PLoS ONE 2008, 3, e3596. [Google Scholar] [CrossRef]
- Wu, H.; Chen, G.; Wyburn, K.R.; Yin, J.; Bertolino, P.; Eris, J.M.; Alexander, S.I.; Sharland, A.F.; Chadban, S.J. TLR4 activation mediates kidney ischemia/reperfusion injury. J. Clin. Investig. 2007, 117, 2847–2859. [Google Scholar] [CrossRef]
- Minor, T.; Sutschet, K.; Witzke, O.; Paul, A.; Gallinat, A. Prediction of renal function upon reperfusion by ex situ controlled oxygenated rewarming. Eur. J. Clin. Investig. 2016, 46, 1024–1030. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Moore, T.; Kleverlaan, T.; Adams, T.; Nicholson, M.L. Haemoadsorption reduces the inflammatory response and improves blood flow during ex vivo renal perfusion in an experimental model. J. Transl. Med. 2017, 15, 216. [Google Scholar] [CrossRef]
- Bhattacharjee, R.N.; Patel, S.V.B.; Sun, Q.; Jiang, L.; Richard-Mohamed, M.; Ruthirakanthan, A.; Aquil, S.; Al-Ogaili, R.; Juriasingani, S.; Sener, A.; et al. Renal Protection Against Ischemia Reperfusion Injury: Hemoglobin-based Oxygen Carrier-201 Versus Blood as an Oxygen Carrier in Ex Vivo Subnormothermic Machine Perfusion. Transplantation 2020, 104, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Dominitzki, S.; Fantini, M.C.; Neufert, C.; Nikolaev, A.; Galle, P.R.; Scheller, J.; Monteleone, G.; Rose-John, S.; Neurath, M.F.; Becker, C. Cutting edge: Trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+ CD25− T cells. J. Immunol. 2007, 179, 2041–2045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Boenisch, O.; Yeung, M.; Mfarrej, B.; Yang, S.; Turka, L.A.; Sayegh, M.H.; Iacomini, J.; Yuan, X. Critical role of proinflammatory cytokine IL-6 in allograft rejection and tolerance. Am. J. Transplant. 2012, 12, 90–101. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Agorogiannis, E.I.; Regateiro, F.S.; Howie, D.; Waldmann, H.; Cobbold, S.P. Th17 Cells Induce a Distinct Graft Rejection Response That Does Not Require IL-17A. Am. J. Transplant. 2012, 12, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Wing, J.B.; Sakaguchi, S. Two modes of immune suppression by Foxp3+ regulatory T cells under inflammatory or non-inflammatory conditions. Semin. Immunol. 2011, 23, 424–430. [Google Scholar] [CrossRef]
- San Segundo, D.; Fernández-Fresnedo, G.; Rodrigo, E.; Ruiz, J.C.; González, M.; Gómez-Alamillo, C.; Arias, M.; López-Hoyos, M. High regulatory T-cell levels at 1 year posttransplantation predict long-term graft survival among kidney transplant recipients. Transplant. Proc. 2012, 44, 2538–2541. [Google Scholar] [CrossRef]
- Moers, C.; Varnav, O.C.; Van Heurn, E.; Jochmans, I.; Kirste, G.R.; Rahmel, A.; Leuvenink, H.G.D.; Squifflet, J.P.; Paul, A.; Pirenne, J.; et al. The value of machine perfusion perfusate biomarkers for predicting kidney transplant outcome. Transplantation 2010, 90, 966–973. [Google Scholar] [CrossRef]
- Jochmans, I.; Lerut, E.; van Pelt, J.; Monbaliu, D.; Pirenne, J. Circulating AST, H-FABP, and NGAL are Early and Accurate Biomarkers of Graft Injury and Dysfunction in a Preclinical Model of Kidney Transplantation. Ann. Surg. 2011, 254, 784–792. [Google Scholar] [CrossRef]
- Pool, M.B.F.; Hamelink, T.L.; van Goor, H.; van den Heuvel, M.C.; Leuvenink, H.G.D.; Moers, C. Prolonged ex-vivo normothermic kidney perfusion: The impact of perfusate composition. PLoS ONE 2021, 16, e0251595. [Google Scholar] [CrossRef]
- Venema, L.H.; van Leeuwen, L.L.; Posma, R.A.; van Goor, H.; Ploeg, R.J.; Hannaert, P.; Hauet, T.; Minor, T.; Leuvenink, H.G.D. Impact of Red Blood Cells on Function and Metabolism of Porcine Deceased Donor Kidneys during Normothermic Machine Perfusion. Transplantation 2022, 106, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Asseldonk, E.J.P.V.; Humphreys, B.D.; Gunaratnam, L.; Duffield, J.S.; Bonventre, J.V. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Investig. 2008, 118, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.R.; Yeung, M.Y.; Brooks, Y.S.; Chen, H.; Ichimura, T.; Henderson, J.M.; Bonventre, J.V. KIM-1-/TIM-1-mediated phagocytosis links ATG5-/ULK1-dependent clearance of apoptotic cells to antigen presentation. EMBO J. 2015, 34, 2441–2464. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Brooks, C.R.; Xiao, S.; Sabbisetti, V.; Yeung, M.Y.; Hsiao, L.L.; Ichimura, T.; Kuchroo, V.; Bonventre, J.V. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J. Clin. Investig. 2015, 125, 1620–1636. [Google Scholar] [CrossRef]
- Bank, J.R.; Van Der Pol, P.; Vreeken, D.; Monge-Chaubo, C.; Bajema, I.M.; Schlagwein, N.; Van Gijlswijk, D.J.; Van Der Kooij, S.W.; Reinders, M.E.J.; De Fijter, J.W.; et al. Kidney injury molecule-1 staining in renal allograft biopsies 10 days after transplantation is inversely correlated with functioning proximal tubular epithelial cells. Nephrol. Dial. Transplant. 2017, 32, 2132–2141. [Google Scholar] [CrossRef]
- Hollmen, M.E.; Kyllönen, L.E.; Inkinen, K.A.; Lalla, M.L.; Merenmies, J.; Salmela, K.T. Deceased donor neutrophil gelatinase-associated lipocalin and delayed graft function after kidney transplantation: A prospective study. Crit. Care 2011, 15, R121. [Google Scholar] [CrossRef]
- Hollmen, M.E.; Kyllönen, L.E.; Merenmies, J.; Salmela, K.T. Serum neutrophil gelatinase-associated lipocalin and recovery of kidney graft function after transplantation. BMC Nephrol. 2014, 15, 123. [Google Scholar] [CrossRef]
- Kielar, M.; Dumnicka, P.; Gala-Błądzińska, A.; Będkowska-Prokop, A.; Ignacak, E.; Maziarz, B.; Ceranowicz, P.; Kuśnierz-Cabala, B. Urinary NGAL Measured after the First Year Post Kidney Transplantation Predicts Changes in Glomerular Filtration over One-Year Follow-Up. J. Clin. Med. 2020, 10, 43. [Google Scholar] [CrossRef]
- Korbély, R.; Wilflingseder, J.; Perco, P.; Kainz, A.; Langer, R.M.; Mayer, B.; Oberbauer, R. Molecular biomarker candidates of acute kidney injury in zero-hour renal transplant needle biopsies. Transpl. Int. 2011, 24, 143–149. [Google Scholar] [CrossRef][Green Version]
- Parikh, C.R.; Jani, A.; Mishra, J.; Ma, Q.; Kelly, C.; Barasch, J.; Edelstein, C.L.; Devarajan, P. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am. J. Transplant. 2006, 6, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, E.R.P.; de Vries, E.E.; Christiaans, M.H.L.; Winkens, B.; Snoeijs, M.G.J.; van Heurn, L.W.E. The Value of Machine Perfusion Biomarker Concentration in DCD Kidney Transplantations. Transplantation 2013, 95, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; De Pace, V.; Angeletti, A.; Comai, G.; Vasuri, F.; Baldassarre, M.; Maroni, L.; Odaldi, F.; Fallani, G.; Caraceni, P.; et al. Hypothermic Oxygenated New Machine Perfusion System in Liver and Kidney Transplantation of Extended Criteria Donors:First Italian Clinical Trial. Sci. Rep. 2020, 10, 6063. [Google Scholar] [CrossRef]
- Mishra, J.; Mori, K.; Ma, Q.; Kelly, C.; Yang, J.; Mitsnefes, M.; Barasch, J.; Devarajan, P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J. Am. Soc. Nephrol. 2004, 15, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Mazilescu, L.I.; Urbanellis, P.; Kaths, M.J.; Ganesh, S.; Goto, T.; Noguchi, Y.; John, R.; Konvalinka, A.; Mucsi, I.; Ghanekar, A.; et al. Prolonged Normothermic Ex Vivo Kidney Perfusion is Superior to Cold Nonoxygenated and Oxygenated Machine Perfusion for the Preservation of DCD Porcine Kidney Grafts. Transplant. Direct 2021, 7, e751. [Google Scholar] [CrossRef]
- Pool, M.B.F.; Hartveld, L.; Leuvenink, H.G.D.; Moers, C. Normothermic machine perfusion of ischaemically damaged porcine kidneys with autologous, allogeneic porcine and human red blood cells. PLoS ONE 2020, 15, e0229566. [Google Scholar] [CrossRef]
- Vallant, N.; Wolfhagen, N.; Sandhu, B.; Hamaoui, K.; Cook, T.; Pusey, C.; Papalois, V. A Comparison of Pulsatile Hypothermic and Normothermic Ex Vivo Machine Perfusion in a Porcine Kidney Model. Transplantation 2021, 105, 1760–1770. [Google Scholar] [CrossRef]
- Mellati, A.; Lo Faro, L.; Dumbill, R.; Meertens, P.; Rozenberg, K.; Shaheed, S.; Snashall, C.; McGivern, H.; Ploeg, R.; Hunter, J. Kidney Normothermic Machine Perfusion Can Be Used as a Preservation Technique and a Model of Reperfusion to Deliver Novel Therapies and Assess Inflammation and Immune Activation. Front. Immunol. 2022, 13, 850271. [Google Scholar] [CrossRef]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015, 4, 20–27. [Google Scholar] [CrossRef]
- Thurman, J.M. Triggers of inflammation after renal ischemia/reperfusion. Clin. Immunol. 2007, 123, 7–13. [Google Scholar] [CrossRef] [PubMed]
| Gene | Gene Abbreviation | Assay-ID |
|---|---|---|
| Endothelin 1 | EDN-1 | Ss03392453_m1 |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | Ss03375629_u1 |
| Hepatitis A virus cellular receptor 1 | HAVCR-1 (KIM-1) | Ss04245599_m1 |
| Interleukin 6 | IL-6 | Ss03384604_u1 |
| Lipocalin 2 | LCN2 (NGAL) | Ss04327246_m1 |
| Ribosomal protein L19 | RPL19 | Ss03375624_g1 |
| Toll-like receptor 4 | TLR-4 | Ss03389780_m1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steinhauser, C.; Yakac, A.; Markgraf, W.; Kromnik, S.; Döcke, A.; Talhofer, P.; Thiele, C.; Malberg, H.; Sommer, U.; Baretton, G.B.; et al. Assessing Biomarkers of Porcine Kidneys under Normothermic Machine Perfusion—Can We Gain Insight into a Marginal Organ? Int. J. Mol. Sci. 2024, 25, 10280. https://doi.org/10.3390/ijms251910280
Steinhauser C, Yakac A, Markgraf W, Kromnik S, Döcke A, Talhofer P, Thiele C, Malberg H, Sommer U, Baretton GB, et al. Assessing Biomarkers of Porcine Kidneys under Normothermic Machine Perfusion—Can We Gain Insight into a Marginal Organ? International Journal of Molecular Sciences. 2024; 25(19):10280. https://doi.org/10.3390/ijms251910280
Chicago/Turabian StyleSteinhauser, Carla, Abdulbaki Yakac, Wenke Markgraf, Susanne Kromnik, Andreas Döcke, Philipp Talhofer, Christine Thiele, Hagen Malberg, Ulrich Sommer, Gustavo B. Baretton, and et al. 2024. "Assessing Biomarkers of Porcine Kidneys under Normothermic Machine Perfusion—Can We Gain Insight into a Marginal Organ?" International Journal of Molecular Sciences 25, no. 19: 10280. https://doi.org/10.3390/ijms251910280
APA StyleSteinhauser, C., Yakac, A., Markgraf, W., Kromnik, S., Döcke, A., Talhofer, P., Thiele, C., Malberg, H., Sommer, U., Baretton, G. B., Füssel, S., Thomas, C., & Putz, J. (2024). Assessing Biomarkers of Porcine Kidneys under Normothermic Machine Perfusion—Can We Gain Insight into a Marginal Organ? International Journal of Molecular Sciences, 25(19), 10280. https://doi.org/10.3390/ijms251910280








