Eucalyptus Wood Smoke Extract Elicits a Dose-Dependent Effect in Brain Endothelial Cells
Abstract
:1. Introduction
2. Results
2.1. Dose-Dependent Increase in IL-8 Proinflammatory Cytokine Production in HBMEC and hCMEC/D3 Treated with WSE
2.2. Dose-Dependent Induction of Genes Related to AhR and NRF2 Pathways in HBMEC and hCMEC/D3 Treated with WSE
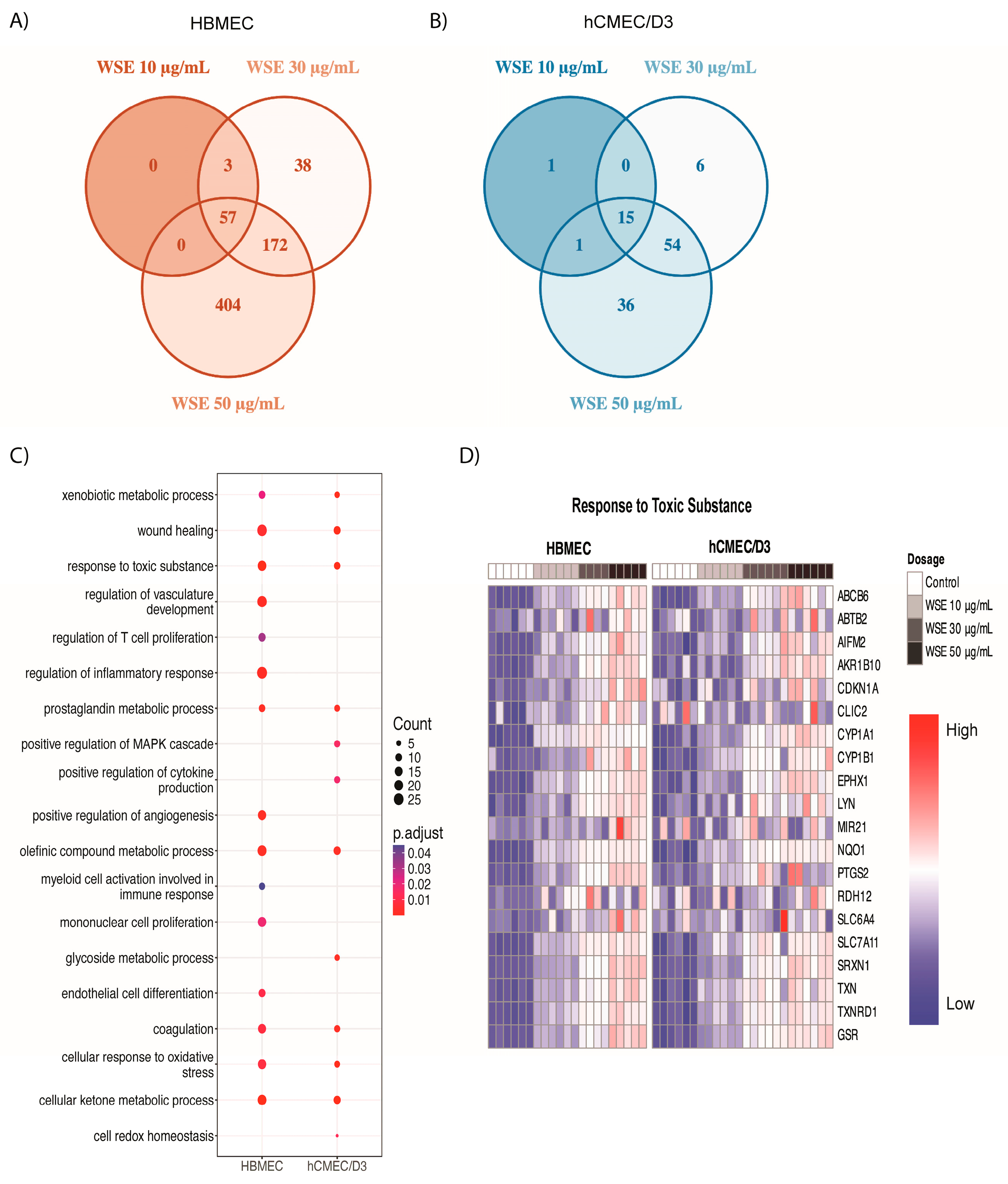
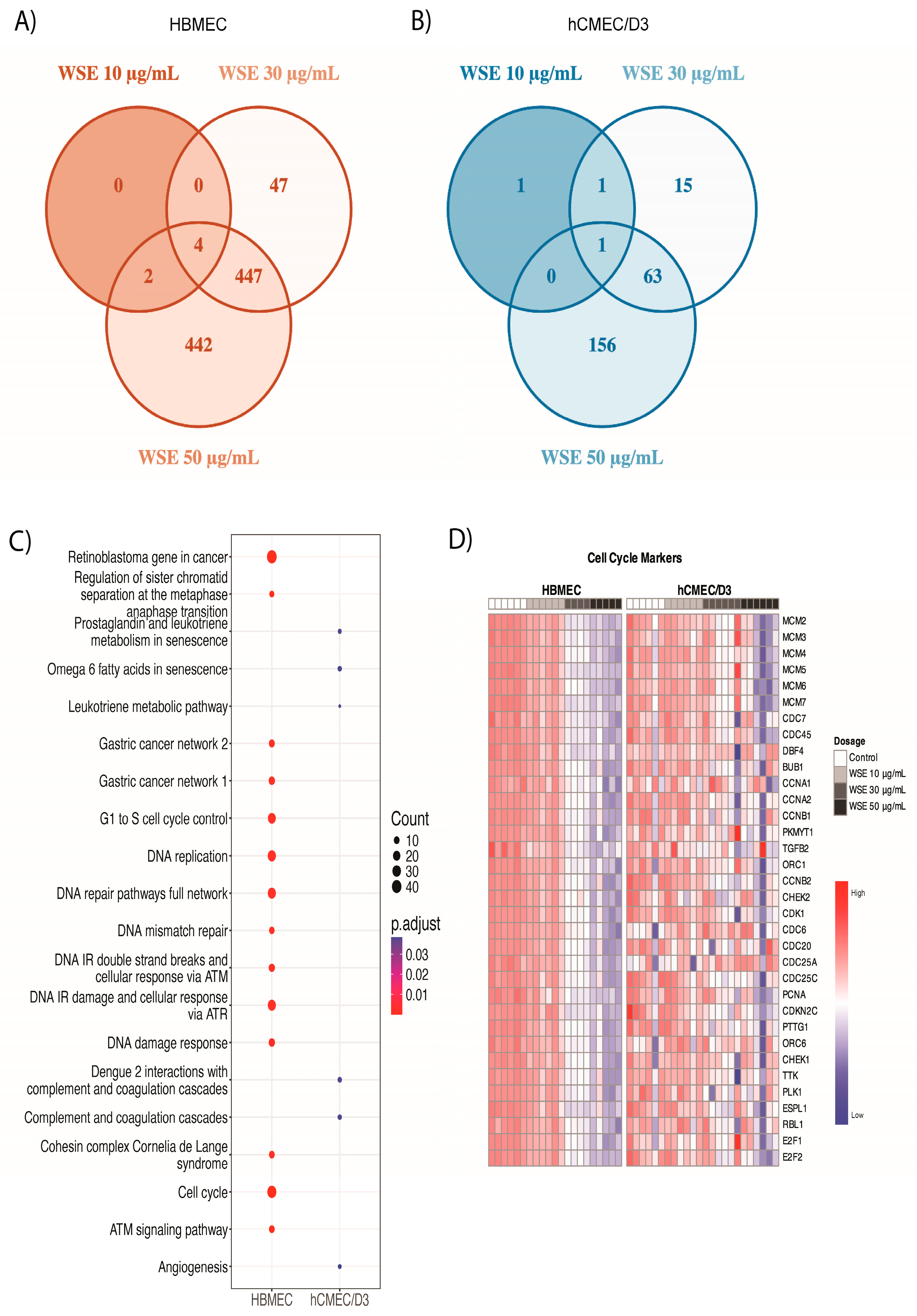
2.3. Significantly Different Gene Ontology Terms Were Detected in Downregulated Genes between HBMEC and hCMEC/D3 Treated with WSE
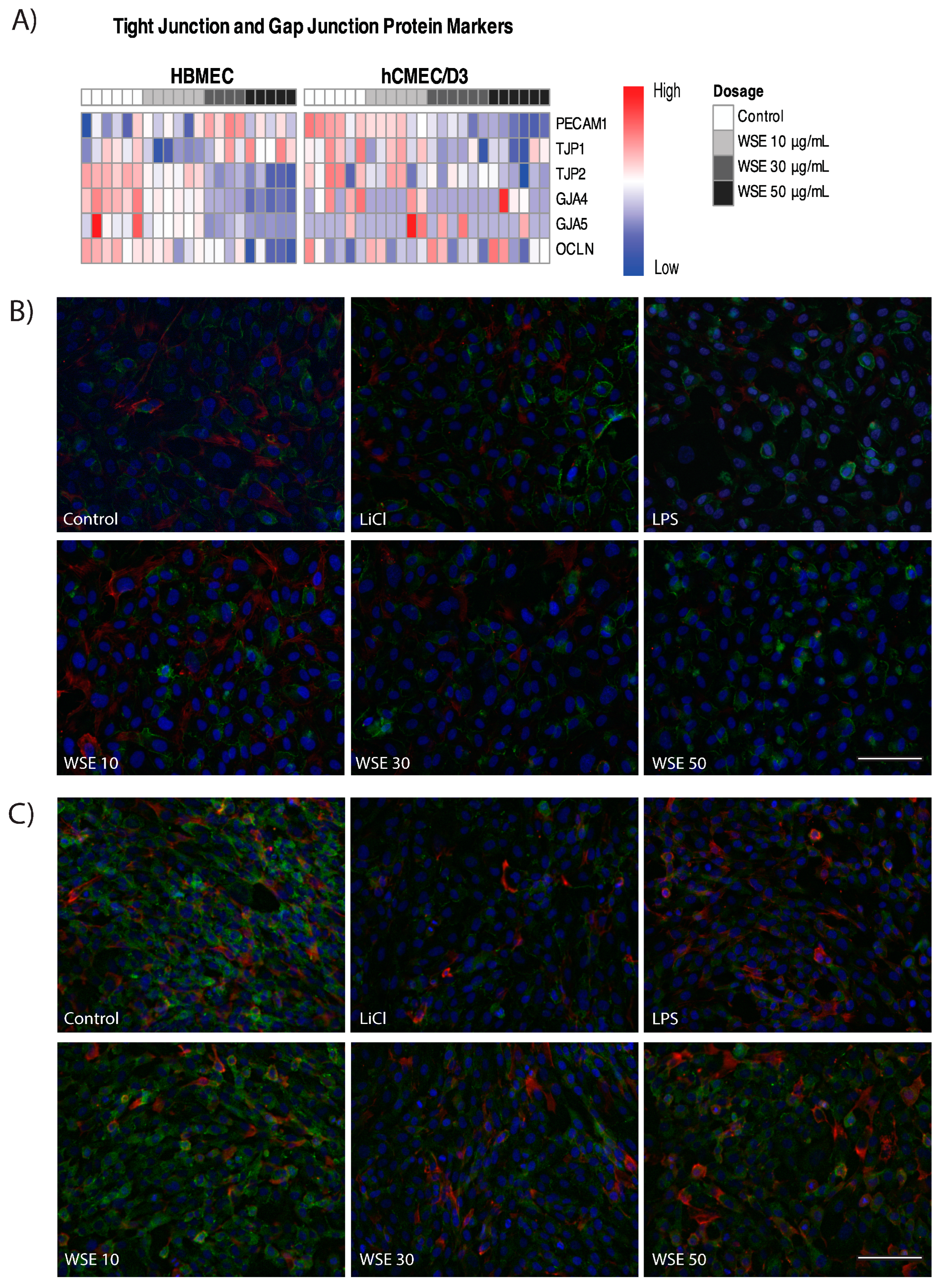
2.4. HBMEC Express Increased Levels of Tight Junction Markers Compared to hCMEC/D3
2.5. Changes in Tight Junction and Gap Junction Markers in HBMEC and hCMEC/D3 Treated with WSE
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Smoldering Eucalyptus Wood Smoke Extract
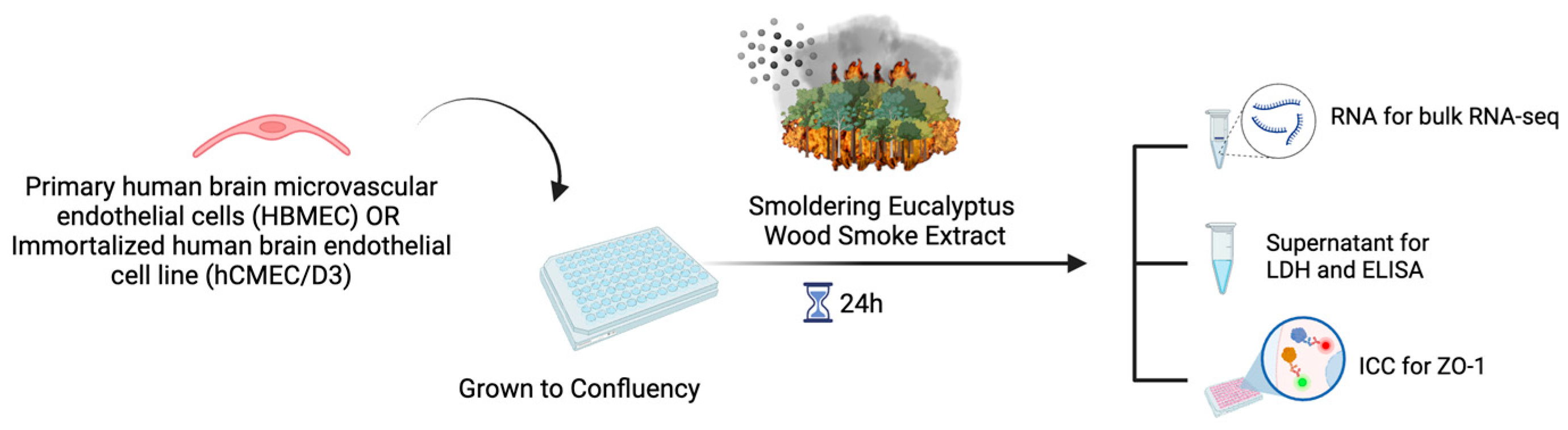
4.3. Experimental Design
4.4. Proteome Profiler Arrays
4.5. Cytokine Quantification
4.6. Cytotoxicity Assay
4.7. DCFDA Assay
4.8. Immunocytochemistry
4.9. Bulk RNA-Sequencing, Data Processing, and Analyses
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunningham, C.X.; Williamson, G.J.; Bowman, D.M.J.S. Increasing frequency and intensity of the most extreme wildfires on Earth. Nat. Ecol. Evol. 2024, 8, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ye, T.; Yue, X.; Yang, Z.; Yu, W.; Zhang, Y.; Bell, M.L.; Morawska, L.; Yu, P.; Zhang, Y.; et al. Global population exposure to landscape fire air pollution from 2000 to 2019. Nature 2023, 621, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Mickley, L.J.; Sulprizio, M.P.; Dominici, F.; Yue, X.; Ebisu, K.; Anderson, G.B.; Khan, R.F.A.; Bravo, M.A.; Bell, M.L. Particulate Air Pollution from Wildfires in the Western US under Climate Change. Clim. Chang. 2016, 138, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, J.; Li, Z.; Kondragunta, S.; Anenberg, S.; Wang, Y.; Zhang, H.; Diner, D.; Hand, J.; Lyapustin, A.; et al. Long-term mortality burden trends attributed to black carbon and PM(2.5) from wildfire emissions across the continental USA from 2000 to 2020: A deep learning modelling study. Lancet Planet Health 2023, 7, e963–e975. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM(2.5))-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public. Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Cedeno Laurent, J.G.; Parhizkar, H.; Calderon, L.; Lizonova, D.; Tsiodra, I.; Mihalopoulos, N.; Kavouras, I.; Alam, M.; Baalousha, M.; Bazina, L.; et al. Physicochemical Characterization of the Particulate Matter in New Jersey/New York City Area, Resulting from the Canadian Quebec Wildfires in June 2023. Environ. Sci. Technol. 2024, 58, 14753–14763. [Google Scholar] [CrossRef]
- Noah, T.L.; Worden, C.P.; Rebuli, M.E.; Jaspers, I. The Effects of Wildfire Smoke on Asthma and Allergy. Curr. Allergy Asthma Rep. 2023, 23, 375–387. [Google Scholar] [CrossRef]
- Chen, H.; Samet, J.M.; Bromberg, P.A.; Tong, H. Cardiovascular health impacts of wildfire smoke exposure. Part. Fibre Toxicol. 2021, 18, 2. [Google Scholar] [CrossRef]
- Cleland, S.E.; Wyatt, L.H.; Wei, L.; Paul, N.; Serre, M.L.; West, J.J.; Henderson, S.B.; Rappold, A.G. Short-Term Exposure to Wildfire Smoke and PM2.5 and Cognitive Performance in a Brain-Training Game: A Longitudinal Study of U.S. Adults. Environ. Health Perspect. 2022, 130, 67005. [Google Scholar] [CrossRef]
- Schuller, A.; Montrose, L. Influence of Woodsmoke Exposure on Molecular Mechanisms Underlying Alzheimer’s Disease: Existing Literature and Gaps in Our Understanding. Epigenet Insights 2020, 13, 2516865720954873. [Google Scholar] [CrossRef]
- Oudin, A.; Segersson, D.; Adolfsson, R.; Forsberg, B. Association between air pollution from residential wood burning and dementia incidence in a longitudinal study in Northern Sweden. PLoS ONE 2018, 13, e0198283. [Google Scholar] [CrossRef] [PubMed]
- Milton, L.A.; White, A.R. The potential impact of bushfire smoke on brain health. Neurochem. Int. 2020, 139, 104796. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Grennan, G.K.; Withers, M.C.; Ramanathan, D.S.; Mishra, J. Differences in interference processing and frontal brain function with climate trauma from California’s deadliest wildfire. PLoS Clim. 2023, 2, e0000125. [Google Scholar] [CrossRef]
- Scieszka, D.; Hunter, R.; Begay, J.; Bitsui, M.; Lin, Y.; Galewsky, J.; Morishita, M.; Klaver, Z.; Wagner, J.; Harkema, J.R.; et al. Neuroinflammatory and Neurometabolomic Consequences from Inhaled Wildfire Smoke-Derived Particulate Matter in the Western United States. Toxicol. Sci. 2022, 186, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; You, J.; Zhi, C.; Li, L. The toxicity of ambient fine particulate matter (PM2.5) to vascular endothelial cells. J. Appl. Toxicol. 2021, 41, 713–723. [Google Scholar] [CrossRef]
- Li, W.; Lin, G.; Xiao, Z.; Zhang, Y.; Li, B.; Zhou, Y.; Ma, Y.; Chai, E. A review of respirable fine particulate matter (PM(2.5))-induced brain damage. Front. Mol. Neurosci. 2022, 15, 967174. [Google Scholar] [CrossRef]
- Seo, S.B.; Choe, E.S.; Kim, K.S.; Shim, S.M. The effect of tobacco smoke exposure on the generation of reactive oxygen species and cellular membrane damage using co-culture model of blood brain barrier with astrocytes. Toxicol. Ind. Health 2017, 33, 530–536. [Google Scholar] [CrossRef]
- Mazzone, P.; Tierney, W.; Hossain, M.; Puvenna, V.; Janigro, D.; Cucullo, L. Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: Expanding the awareness of smoking toxicity in an underappreciated area. Int. J. Environ. Res. Public. Health 2010, 7, 4111–4126. [Google Scholar] [CrossRef]
- Patten, K.T.; Valenzuela, A.E.; Wallis, C.; Harvey, D.J.; Bein, K.J.; Wexler, A.S.; Gorin, F.A.; Lein, P.J. Hippocampal but Not Serum Cytokine Levels Are Altered by Traffic-Related Air Pollution in TgF344-AD and Wildtype Fischer 344 Rats in a Sex- and Age-Dependent Manner. Front. Cell Neurosci. 2022, 16, 861733. [Google Scholar] [CrossRef]
- Patten, K.T.; Valenzuela, A.E.; Wallis, C.; Berg, E.L.; Silverman, J.L.; Bein, K.J.; Wexler, A.S.; Lein, P.J. The Effects of Chronic Exposure to Ambient Traffic-Related Air Pollution on Alzheimer’s Disease Phenotypes in Wildtype and Genetically Predisposed Male and Female Rats. Environ. Health Perspect. 2021, 129, 57005. [Google Scholar] [CrossRef]
- Adivi, A.; Lucero, J.; Simpson, N.; McDonald, J.D.; Lund, A.K. Exposure to traffic-generated air pollution promotes alterations in the integrity of the brain microvasculature and inflammation in female ApoE(-/-) mice. Toxicol. Lett. 2021, 339, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Guo, M.; Mehra, M.; Royal, W., 3rd. Inflammation and oxidative stress induced by cigarette smoke in Lewis rat brains. J. Neuroimmunol. 2013, 254, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Tan, H.Y.; Lee, C.Y.; Cho, H. An Air Particulate Pollutant Induces Neuroinflammation and Neurodegeneration in Human Brain Models. Adv. Sci. 2021, 8, e2101251. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Villa, A. Cellular elements of the blood-brain barrier. Neurochem. Res. 2009, 34, 2067–2077. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef]
- Muller, N. The Role of Intercellular Adhesion Molecule-1 in the Pathogenesis of Psychiatric Disorders. Front. Pharmacol. 2019, 10, 1251. [Google Scholar] [CrossRef]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular cell adhesion molecule-1 expression and signaling during disease: Regulation by reactive oxygen species and antioxidants. Antioxid. Redox Signal 2011, 15, 1607–1638. [Google Scholar] [CrossRef]
- Qiu, Y.M.; Zhang, C.L.; Chen, A.Q.; Wang, H.L.; Zhou, Y.F.; Li, Y.N.; Hu, B. Immune Cells in the BBB Disruption After Acute Ischemic Stroke: Targets for Immune Therapy? Front. Immunol. 2021, 12, 678744. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Dore, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef]
- Suwannasual, U.; Lucero, J.; McDonald, J.D.; Lund, A.K. Exposure to traffic-generated air pollutants mediates alterations in brain microvascular integrity in wildtype mice on a high-fat diet. Environ. Res. 2018, 160, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Ku, J.M.; Vlahos, R.; Miller, A.A. Cigarette smoke extract exacerbates hyperpermeability of cerebral endothelial cells after oxygen glucose deprivation and reoxygenation. Sci. Rep. 2019, 9, 15573. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.T.; Abbruscato, T.J.; Egleton, R.D.; Brown, R.C.; Huber, J.D.; Campos, C.R.; Davis, T.P. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004, 1027, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Tobwala, S.; Zhang, X.; Zheng, Y.; Wang, H.J.; Banks, W.A.; Ercal, N. Disruption of the integrity and function of brain microvascular endothelial cells in culture by exposure to diesel engine exhaust particles. Toxicol. Lett. 2013, 220, 1–7. [Google Scholar] [CrossRef]
- Liu, F.; Huang, Y.; Zhang, F.; Chen, Q.; Wu, B.; Rui, W.; Zheng, J.C.; Ding, W. Macrophages treated with particulate matter PM2.5 induce selective neurotoxicity through glutaminase-mediated glutamate generation. J. Neurochem. 2015, 134, 315–326. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, J.Y.; Lee, H.K.; Jo, C.; Koh, Y.H. Notch1-mediated inflammation is associated with endothelial dysfunction in human brain microvascular endothelial cells upon particulate matter exposure. Arch. Toxicol. 2021, 95, 529–540. [Google Scholar] [CrossRef]
- Shih, R.-H.; Cheng, S.-E.; Hsiao, L.-D.; Kou, Y.R.; Yang, C.-M. Cigarette smoke extract upregulates heme oxygenase-1 via PKC/NADPH oxidase/ROS/PDGFR/PI3K/Akt pathway in mouse brain endothelial cells. J. Neuroinflammation 2011, 8, 104. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hong, S.; Bolormaa, O.; Seo, J.H.; Eom, S.Y.; Kim, Y.D.; Kim, H. Effects of diesel exhaust particles and urban particles on brain endothelial cells. Toxicol. Res. 2022, 38, 91–98. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Bickel, U.; Abbruscato, T.J.; Cucullo, L. Comparative assessment of in vitro BBB tight junction integrity following exposure to cigarette smoke and e-cigarette vapor: A quantitative evaluation of the protective effects of metformin using small-molecular-weight paracellular markers. Fluids Barriers CNS 2021, 18, 28. [Google Scholar] [CrossRef]
- Kaisar, M.A.; Prasad, S.; Cucullo, L. Protecting the BBB endothelium against cigarette smoke-induced oxidative stress using popular antioxidants: Are they really beneficial? Brain Res. 2015, 1627, 90–100. [Google Scholar] [CrossRef]
- Rager, J.E.; Clark, J.; Eaves, L.A.; Avula, V.; Niehoff, N.M.; Kim, Y.H.; Jaspers, I.; Gilmour, M.I. Mixtures modeling identifies chemical inducers versus repressors of toxicity associated with wildfire smoke. Sci. Total Environ. 2021, 775, 145759. [Google Scholar] [CrossRef] [PubMed]
- Boaggio, K.; LeDuc, S.D.; Rice, R.B.; Duffney, P.F.; Foley, K.M.; Holder, A.L.; McDow, S.; Weaver, C.P. Beyond Particulate Matter Mass: Heightened Levels of Lead and Other Pollutants Associated with Destructive Fire Events in California. Environ. Sci. Technol. 2022, 56, 14272–14283. [Google Scholar] [CrossRef] [PubMed]
- Simms, L.A.; Borras, E.; Chew, B.S.; Matsui, B.; McCartney, M.M.; Robinson, S.K.; Kenyon, N.; Davis, C.E. Environmental sampling of volatile organic compounds during the 2018 Camp Fire in Northern California. J. Environ. Sci. 2021, 103, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.L.; Wagner, J. Composition of particulate matter during a wildfire smoke episode in an urban area. Aerosol Sci. Technol. 2021, 55, 734–747. [Google Scholar] [CrossRef]
- California Air Resource Board. Camp Fire Air Quality Data Analysis. 2021. Available online: https://ww2.arb.ca.gov/sites/default/files/2021-07/Camp_Fire_report_July2021.pdf (accessed on 9 August 2024).
- Kim, Y.H.; Warren, S.H.; Krantz, Q.T.; King, C.; Jaskot, R.; Preston, W.T.; George, B.J.; Hays, M.D.; Landis, M.S.; Higuchi, M.; et al. Mutagenicity and Lung Toxicity of Smoldering vs. Flaming Emissions from Various Biomass Fuels: Implications for Health Effects from Wildland Fires. Environ. Health Perspect. 2018, 126, 017011. [Google Scholar] [CrossRef]
- Bertschi, I.; Yokelson, R.J.; Ward, D.E.; Babbitt, R.E.; Susott, R.A.; Goode, J.G.; Hao, W.M. Trace gas and particle emissions from fires in large diameter and belowground biomass fuels. J. Geophys. Res. Atmos. 2003, 108, 8472. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Niu, J.Z. miR-222 regulates brain injury and inflammation following intracerebral hemorrhage by targeting ITGB8. Mol. Med. Rep. 2020, 21, 1145–1153. [Google Scholar] [CrossRef]
- Chew, S.; Lampinen, R.; Saveleva, L.; Korhonen, P.; Mikhailov, N.; Grubman, A.; Polo, J.M.; Wilson, T.; Komppula, M.; Ronkko, T.; et al. Urban air particulate matter induces mitochondrial dysfunction in human olfactory mucosal cells. Part. Fibre Toxicol. 2020, 17, 18. [Google Scholar] [CrossRef]
- Karabegovic, I.; Maas, S.C.E.; Shuai, Y.; Ikram, M.A.; Stricker, B.; Aerts, J.; Brusselle, G.; Lahousse, L.; Voortman, T.; Ghanbari, M. Smoking-related dysregulation of plasma circulating microRNAs: The Rotterdam study. Hum. Genom. 2023, 17, 61. [Google Scholar] [CrossRef]
- Lemaitre, V.; Dabo, A.J.; D’Armiento, J. Cigarette smoke components induce matrix metalloproteinase-1 in aortic endothelial cells through inhibition of mTOR signaling. Toxicol. Sci. 2011, 123, 542–549. [Google Scholar] [CrossRef]
- Ock, S.A.; Knott, J.G.; Choi, I. Involvement of CDKN1A (p21) in cellular senescence in response to heat and irradiation stress during preimplantation development. Cell Stress. Chaperones 2020, 25, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Saul, D.; Kosinsky, R.L.; Atkinson, E.J.; Doolittle, M.L.; Zhang, X.; LeBrasseur, N.K.; Pignolo, R.J.; Robbins, P.D.; Niedernhofer, L.J.; Ikeno, Y.; et al. A new gene set identifies senescent cells and predicts senescence-associated pathways across tissues. Nat. Commun. 2022, 13, 4827. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lee, I.; Kang, W.K. E2F-1 is a critical modulator of cellular senescence in human cancer. Int. J. Mol. Med. 2006, 17, 715–720. [Google Scholar] [CrossRef]
- Aksoy, O.; Chicas, A.; Zeng, T.; Zhao, Z.; McCurrach, M.; Wang, X.; Lowe, S.W. The atypical E2F family member E2F7 couples the p53 and RB pathways during cellular senescence. Genes. Dev. 2012, 26, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, L.; Xiao, W.; Liu, J.; Long, C.; Zhan, W.; Cui, C.; Yang, L.; Chen, S. Alteration of E2F2 Expression in Governing Endothelial Cell Senescence. Genes 2022, 13, 1522. [Google Scholar] [CrossRef]
- Han, Y.; Kim, S.Y. Endothelial senescence in vascular diseases: Current understanding and future opportunities in senotherapeutics. Exp. Mol. Med. 2023, 55, 1–12. [Google Scholar] [CrossRef]
- Chinta, S.J.; Lieu, C.A.; Demaria, M.; Laberge, R.M.; Campisi, J.; Andersen, J.K. Environmental stress, ageing and glial cell senescence: A novel mechanistic link to Parkinson’s disease? J. Intern. Med. 2013, 273, 429–436. [Google Scholar] [CrossRef]
- Privratsky, J.R.; Newman, P.J. PECAM-1: Regulator of endothelial junctional integrity. Cell Tissue Res. 2014, 355, 607–619. [Google Scholar] [CrossRef]
- Urich, E.; Lazic, S.E.; Molnos, J.; Wells, I.; Freskgard, P.O. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS ONE 2012, 7, e38149. [Google Scholar] [CrossRef]
- Roudnicky, F.; Zhang, J.D.; Kim, B.K.; Pandya, N.J.; Lan, Y.; Sach-Peltason, L.; Ragelle, H.; Strassburger, P.; Gruener, S.; Lazendic, M.; et al. Inducers of the endothelial cell barrier identified through chemogenomic screening in genome-edited hPSC-endothelial cells. Proc. Natl. Acad. Sci. USA 2020, 117, 19854–19865. [Google Scholar] [CrossRef]
- Zou, P.; Yang, F.; Ding, Y.; Zhang, D.; Liu, Y.; Zhang, J.; Wu, D.; Wang, Y. Lipopolysaccharide downregulates the expression of ZO-1 protein through the Akt pathway. BMC Infect. Dis. 2022, 22, 774. [Google Scholar] [CrossRef] [PubMed]
- Stump, R.J.; Lovicu, F.J.; Ang, S.L.; Pandey, S.K.; McAvoy, J.W. Lithium stabilizes the polarized lens epithelial phenotype and inhibits proliferation, migration, and epithelial mesenchymal transition. J. Pathol. 2006, 210, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar] [PubMed]
- Qazi, B.S.; Tang, K.; Qazi, A. Recent advances in underlying pathologies provide insight into interleukin-8 expression-mediated inflammation and angiogenesis. Int. J. Inflam. 2011, 2011, 908468. [Google Scholar] [CrossRef] [PubMed]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef]
- Swiston, J.R.; Davidson, W.; Attridge, S.; Li, G.T.; Brauer, M.; van Eeden, S.F. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur. Respir. J. 2008, 32, 129–138. [Google Scholar] [CrossRef]
- Black, C.; Tesfaigzi, Y.; Bassein, J.A.; Miller, L.A. Wildfire smoke exposure and human health: Significant gaps in research for a growing public health issue. Environ. Toxicol. Pharmacol. 2017, 55, 186–195. [Google Scholar] [CrossRef]
- Graham, E.L.; Khaja, S.; Caban-Martinez, A.J.; Smith, D.L. Firefighters and COVID-19: An Occupational Health Perspective. J. Occup. Environ. Med. 2021, 63, e556–e563. [Google Scholar] [CrossRef]
- Black, C.; Gerriets, J.E.; Fontaine, J.H.; Harper, R.W.; Kenyon, N.J.; Tablin, F.; Schelegle, E.S.; Miller, L.A. Early Life Wildfire Smoke Exposure Is Associated with Immune Dysregulation and Lung Function Decrements in Adolescence. Am. J. Respir. Cell Mol. Biol. 2017, 56, 657–666. [Google Scholar] [CrossRef]
- Hamon, R.; Tran, H.B.; Roscioli, E.; Ween, M.; Jersmann, H.; Hodge, S. Bushfire smoke is pro-inflammatory and suppresses macrophage phagocytic function. Sci. Rep. 2018, 8, 13424. [Google Scholar] [CrossRef]
- Young, T.M.; Black, G.P.; Wong, L.; Bloszies, C.S.; Fiehn, O.; He, G.; Denison, M.S.; Vogel, C.F.A.; Durbin-Johnson, B. Identifying Toxicologically Significant Compounds in Urban Wildfire Ash Using In Vitro Bioassays and High-Resolution Mass Spectrometry. Environ. Sci. Technol. 2021, 55, 3657–3667. [Google Scholar] [CrossRef] [PubMed]
- Malany, K.; Li, X.; Vogel, C.F.A.; Ehrlich, A.K. Mechanisms underlying aryl hydrocarbon receptor-driven divergent macrophage function. Toxicol. Sci. 2024, 200, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Fulgar, C.C.; Sun, X.; Vogel, C.F.A.; Wu, C.W.; Zhang, Q.; Bein, K.J.; Young, D.E.; Li, W.; Wei, H.; et al. In vivo and in vitro inflammatory responses to fine particulate matter (PM(2.5)) from China and California. Toxicol. Lett. 2020, 328, 52–60. [Google Scholar] [CrossRef]
- Gupta, A.; Sasse, S.K.; Gruca, M.A.; Sanford, L.; Dowell, R.D.; Gerber, A.N. Deconvolution of multiplexed transcriptional responses to wood smoke particles defines rapid aryl hydrocarbon receptor signaling dynamics. J. Biol. Chem. 2021, 297, 101147. [Google Scholar] [CrossRef] [PubMed]
- Barouki, R.; Coumoul, X.; Fernandez-Salguero, P.M. The aryl hydrocarbon receptor, more than a xenobiotic-interacting protein. FEBS Lett. 2007, 581, 3608–3615. [Google Scholar] [CrossRef] [PubMed]
- Coelho, N.R.; Pimpao, A.B.; Correia, M.J.; Rodrigues, T.C.; Monteiro, E.C.; Morello, J.; Pereira, S.A. Pharmacological blockage of the AHR-CYP1A1 axis: A call for in vivo evidence. J. Mol. Med. 2022, 100, 215–243. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Kado, S.Y.; Bein, K.J.; He, Y.; Pouraryan, A.A.; Urban, A.; Haarmann-Stemmann, T.; Sweeney, C.; Vogel, C.F.A. Aryl Hydrocarbon Receptor Signaling Synergizes with TLR/NF-kappaB-Signaling for Induction of IL-22 Through Canonical and Non-Canonical AhR Pathways. Front. Toxicol. 2021, 3, 787360. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Van Winkle, L.S.; Esser, C.; Haarmann-Stemmann, T. The aryl hydrocarbon receptor as a target of environmental stressors—Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020, 34, 101530. [Google Scholar] [CrossRef]
- Guerrina, N.; Traboulsi, H.; Eidelman, D.H.; Baglole, C.J. The Aryl Hydrocarbon Receptor Suppresses Chronic Smoke-Induced Pulmonary Inflammation. Front. Toxicol. 2021, 3, 653569. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, Y.; Sun, Y.; Yang, X.; Liang, J.; Yin, Z.; Liu, Q.S.; Zhou, Q.; Jiang, G. AhR Agonistic Components in Urban Particulate Matter Regulate Astrocytic Activation and Function. Environ. Sci. Technol. 2024, 58, 4571–4580. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, K.W.; Lee, S.M.; Park, S.; Kim, B.G.; Choi, E.K.; Son, B.S.; Park, M.K. Effects of intranasal instillation of nanoparticulate matter in the olfactory bulb. Sci. Rep. 2021, 11, 16997. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, K.W.; Lee, S.M.; Lee, D.H.; Park, S.; Son, B.S.; Park, M.K. Overexpression of the Aryl Hydrocarbon Receptor (Ahr) Mediates an Oxidative Stress Response following Injection of Fine Particulate Matter in the Temporal Cortex. Oxid. Med. Cell Longev. 2020, 2020, 6879738. [Google Scholar] [CrossRef] [PubMed]
- Grishanova, A.Y.; Perepechaeva, M.L. Aryl Hydrocarbon Receptor in Oxidative Stress as a Double Agent and Its Biological and Therapeutic Significance. Int. J. Mol. Sci. 2022, 23, 6719. [Google Scholar] [CrossRef] [PubMed]
- Bortoli, S.; Boutet-Robinet, E.; Lagadic-Gossmann, D.; Huc, L. Nrf2 and AhR in metabolic reprogramming after contaminant exposure. Curr. Opin. Toxicol. 2018, 8, 34–41. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Kuang, F.; Liu, J.; Tang, D.; Kang, R. Oxidative Damage and Antioxidant Defense in Ferroptosis. Front. Cell Dev. Biol. 2020, 8, 586578. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef]
- Hughes-Fulford, M. Cell cycle arrest by prostaglandin A1 at the G1/S phase interface with up-regulation of oncogenes in S-49 cyc- cells. J. Cell Biochem. 1994, 54, 265–272. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, 15. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Y.; Yang, C.; Simeone, D.M.; Sun, T.T.; DeGraff, D.J.; Tang, M.S.; Zhang, Y.; Wu, X.R. Dominant role of CDKN2B/p15INK4B of 9p21.3 tumor suppressor hub in inhibition of cell-cycle and glycolysis. Nat. Commun. 2021, 12, 2047. [Google Scholar] [CrossRef] [PubMed]
- Dutto, I.; Tillhon, M.; Cazzalini, O.; Stivala, L.A.; Prosperi, E. Biology of the cell cycle inhibitor p21(CDKN1A): Molecular mechanisms and relevance in chemical toxicology. Arch. Toxicol. 2015, 89, 155–178. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Cam, H.; Takahashi, Y.; Volkert, T.; Terragni, J.; Young, R.A.; Dynlacht, B.D. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 2002, 16, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Salvador, E.; Burek, M.; Lohr, M.; Nagai, M.; Hagemann, C.; Forster, C.Y. Senescence and associated blood-brain barrier alterations in vitro. Histochem. Cell Biol. 2021, 156, 283–292. [Google Scholar] [CrossRef]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef]
- Martinez-Cue, C.; Rueda, N. Cellular Senescence in Neurodegenerative Diseases. Front. Cell Neurosci. 2020, 14, 16. [Google Scholar] [CrossRef]
- Heusinkveld, H.J.; Wahle, T.; Campbell, A.; Westerink, R.H.S.; Tran, L.; Johnston, H.; Stone, V.; Cassee, F.R.; Schins, R.P.F. Neurodegenerative and neurological disorders by small inhaled particles. Neurotoxicology 2016, 56, 94–106. [Google Scholar] [CrossRef]
- Fan, H.C.; Chen, C.M.; Tsai, J.D.; Chiang, K.L.; Tsai, S.C.; Huang, C.Y.; Lin, C.L.; Hsu, C.Y.; Chang, K.H. Association between Exposure to Particulate Matter Air Pollution during Early Childhood and Risk of Attention-Deficit/Hyperactivity Disorder in Taiwan. Int. J. Environ. Res. Public. Health 2022, 19, 16138. [Google Scholar] [CrossRef]
- Flanagan, E.; Malmqvist, E.; Rittner, R.; Gustafsson, P.; Kallen, K.; Oudin, A. Exposure to local, source-specific ambient air pollution during pregnancy and autism in children: A cohort study from southern Sweden. Sci. Rep. 2023, 13, 3848. [Google Scholar] [CrossRef]
- Newbury, J.B.; Stewart, R.; Fisher, H.L.; Beevers, S.; Dajnak, D.; Broadbent, M.; Pritchard, M.; Shiode, N.; Heslin, M.; Hammoud, R.; et al. Association between air pollution exposure and mental health service use among individuals with first presentations of psychotic and mood disorders: Retrospective cohort study. Br. J. Psychiatry 2021, 219, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.J.; Zarea, K.; Hatamzadeh, N.; Salahshouri, A.; Sharhani, A. Toxic Air Pollutants and Their Effect on Multiple Sclerosis: A Review Study. Front. Public. Health 2022, 10, 898043. [Google Scholar] [CrossRef] [PubMed]
- You, R.; Ho, Y.S.; Chang, R.C. The pathogenic effects of particulate matter on neurodegeneration: A review. J. Biomed. Sci. 2022, 29, 15. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, R.G.; Dorman, D.C.; Elder, A.; Veronesi, B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology 2012, 33, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R.D.; Goh, E.L. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 2017, 17, 448–459. [Google Scholar] [CrossRef]
- Biemans, E.; Jakel, L.; de Waal, R.M.W.; Kuiperij, H.B.; Verbeek, M.M. Limitations of the hCMEC/D3 cell line as a model for Abeta clearance by the human blood-brain barrier. J. Neurosci. Res. 2017, 95, 1513–1522. [Google Scholar] [CrossRef]
- Fanning, A.S.; Little, B.P.; Rahner, C.; Utepbergenov, D.; Walther, Z.; Anderson, J.M. The unique-5 and -6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol. Biol. Cell 2007, 18, 721–731. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Goodenough, D.A. Localization of the tight junction protein, ZO-1, is modulated by extracellular calcium and cell-cell contact in Madin-Darby canine kidney epithelial cells. J. Cell Biol. 1988, 107, 2389–2399. [Google Scholar] [CrossRef]
- Sugiyama, S.; Sasaki, T.; Tanaka, H.; Yan, H.; Ikegami, T.; Kanki, H.; Nishiyama, K.; Beck, G.; Gon, Y.; Okazaki, S.; et al. The tight junction protein occludin modulates blood-brain barrier integrity and neurological function after ischemic stroke in mice. Sci. Rep. 2023, 13, 2892. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef]
- Zheng, X.; Ren, B.; Gao, Y. Tight junction proteins related to blood-brain barrier and their regulatory signaling pathways in ischemic stroke. Biomed. Pharmacother. 2023, 165, 115272. [Google Scholar] [CrossRef] [PubMed]
- Daniels, B.P.; Cruz-Orengo, L.; Pasieka, T.J.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Cooper, J.A.; Doering, T.L.; Klein, R.S. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J. Neurosci. Methods 2013, 212, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Weksler, B.; Romero, I.; Couraud, P.O.; Gelli, A. Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans. Eukaryot. Cell 2009, 8, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Forster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow. Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef] [PubMed]
- Gericke, B.; Romermann, K.; Noack, A.; Noack, S.; Kronenberg, J.; Blasig, I.E.; Loscher, W. A face-to-face comparison of claudin-5 transduced human brain endothelial (hCMEC/D3) cells with porcine brain endothelial cells as blood-brain barrier models for drug transport studies. Fluids Barriers CNS 2020, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Bramley, J.C.; Drummond, C.G.; Lennemann, N.J.; Good, C.A.; Kim, K.S.; Coyne, C.B. A Three-Dimensional Cell Culture System To Model RNA Virus Infections at the Blood-Brain Barrier. mSphere 2017, 2, 206–217. [Google Scholar] [CrossRef]
- Liu, P.L.; Chen, Y.L.; Chen, Y.H.; Lin, S.J.; Kou, Y.R. Wood smoke extract induces oxidative stress-mediated caspase-independent apoptosis in human lung endothelial cells: Role of AIF and EndoG. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L739–L749. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kolde, R. Pheatmap: Pretty heatmaps. R. Package Version 2019, 1, 726. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Balmes, J.R. Where There’s Wildfire, There’s Smoke. N. Engl. J. Med. 2018, 378, 881–883. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, D.J.; Gorman, B.M.; Goshi, N.; Hum, N.R.; Sebastian, A.; Kim, Y.H.; Enright, H.A.; Buchholz, B.A. Eucalyptus Wood Smoke Extract Elicits a Dose-Dependent Effect in Brain Endothelial Cells. Int. J. Mol. Sci. 2024, 25, 10288. https://doi.org/10.3390/ijms251910288
You DJ, Gorman BM, Goshi N, Hum NR, Sebastian A, Kim YH, Enright HA, Buchholz BA. Eucalyptus Wood Smoke Extract Elicits a Dose-Dependent Effect in Brain Endothelial Cells. International Journal of Molecular Sciences. 2024; 25(19):10288. https://doi.org/10.3390/ijms251910288
Chicago/Turabian StyleYou, Dorothy J., Bria M. Gorman, Noah Goshi, Nicholas R. Hum, Aimy Sebastian, Yong Ho Kim, Heather A. Enright, and Bruce A. Buchholz. 2024. "Eucalyptus Wood Smoke Extract Elicits a Dose-Dependent Effect in Brain Endothelial Cells" International Journal of Molecular Sciences 25, no. 19: 10288. https://doi.org/10.3390/ijms251910288





